Abstract
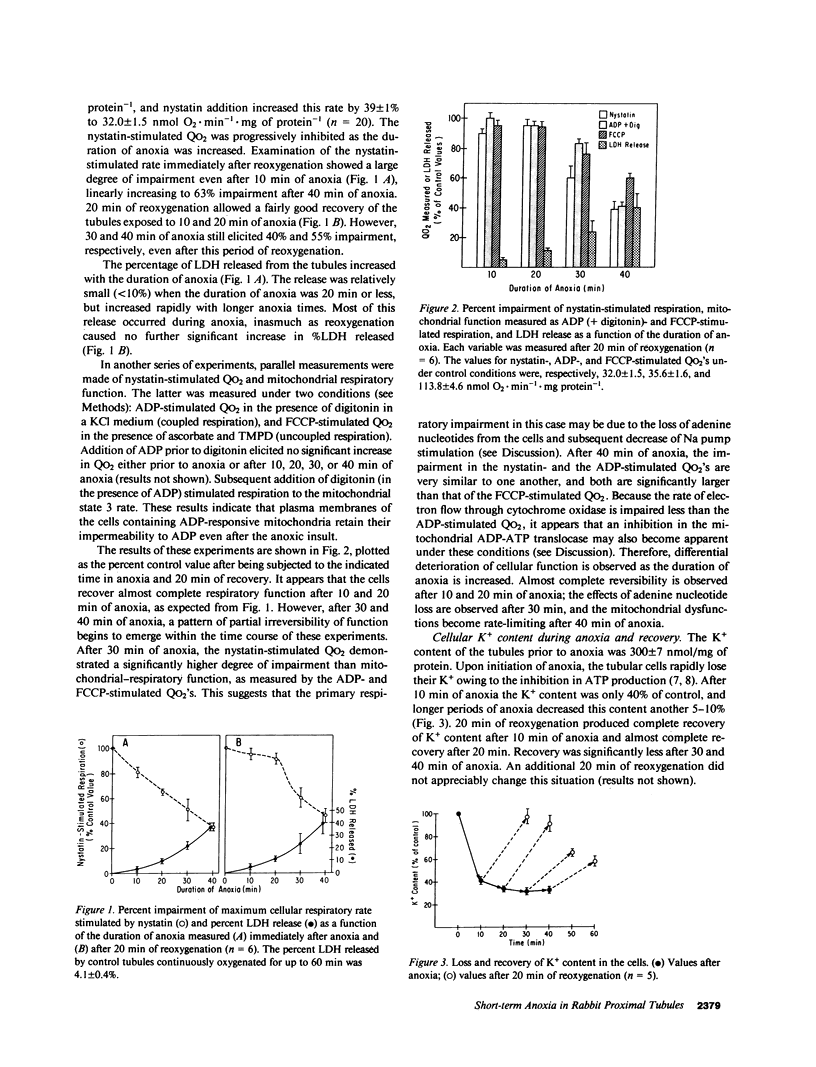
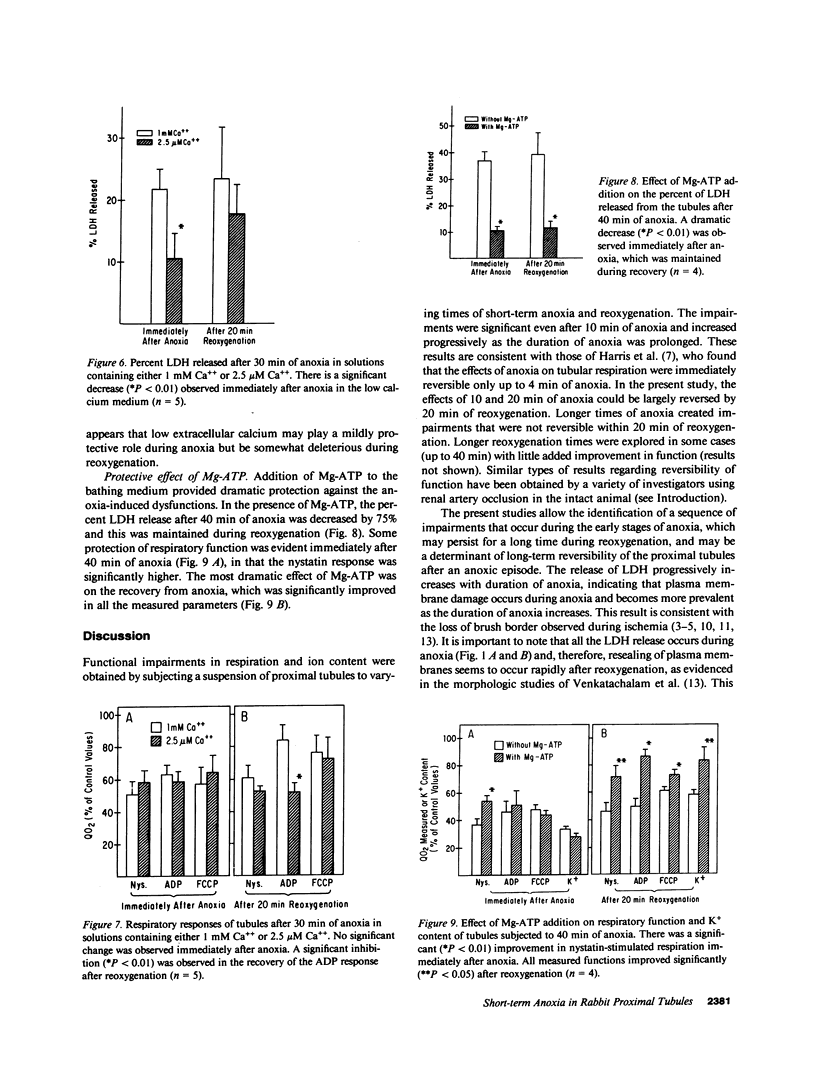
The effects of short-term anoxia and hypoxia were studied in a rabbit proximal renal tubule suspension in order to avoid the hemodynamic consequences of clamp-induced ischemia. The suspension was subjected to anoxia for 10-40 min and the effects on a number of cellular transport and respiratory parameters were monitored. Cellular respiration was measured upon addition of nystatin (Nys) to maximally stimulate Na pump activity. Mitochondrial respiration was measured in the tubules by addition of digitonin and ADP to obtain the state 3 respiratory rate. The release of lactate dehydrogenase (LDH) was measured as an index of plasma membrane damage. The cellular contents of K and Ca were also measured. Results show that 10 and 20 min of anoxia partially inhibited Nys-stimulated and mitochondrial respiration, and partially decreased the K contents, but all these effects were largely reversible after 20 min of reoxygenation. After 40 min of anoxia and 20 min of reoxygenation, all these variables remained irreversibly inhibited: Nys-stimulated respiration by 54%, mitochondrial respiration by 50%, K content by 42%, and LDH release was 40% of total. Ca content decreased slightly during anoxia, but increased up to fourfold during severe hypoxia; the excess Ca was released during the first 10 min of reoxygenation. The degree of respiratory impairment was identical during anoxia or hypoxia, suggesting that Ca accumulation was not associated with the impairment. Decreasing the extracellular Ca to 2.5 microM decreased LDH release significantly during anoxia, suggesting that plasma membrane damage during anoxia may be associated with increased intracellular free Ca. Addition of Mg-adenosine triphosphate during anoxia dramatically improved recovery of all the measured parameters after the anoxic period.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson R. J., Gordon J. A., Kim J., Peterson L. M., Gross P. A. Renal concentration defect following nonoliguric acute renal failure in the rat. Kidney Int. 1982 Apr;21(4):583–591. doi: 10.1038/ki.1982.65. [DOI] [PubMed] [Google Scholar]
- Arendshorst W. J., Finn W. F., Gottschalk C. W. Pathogenesis of acute renal failure following temporary renal ischemia in the rat. Circ Res. 1975 Nov;37(5):558–568. doi: 10.1161/01.res.37.5.558. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Mandel L. J., Soltoff S. P., Storey J. M. Coupling of active ion transport and aerobic respiratory rate in isolated renal tubules. Proc Natl Acad Sci U S A. 1980 Jan;77(1):447–451. doi: 10.1073/pnas.77.1.447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balaban R. S., Soltoff S. P., Storey J. M., Mandel L. J. Improved renal cortical tubule suspension: spectrophotometric study of O2 delivery. Am J Physiol. 1980 Jan;238(1):F50–F59. doi: 10.1152/ajprenal.1980.238.1.F50. [DOI] [PubMed] [Google Scholar]
- Burke T. J., Arnold P. E., Gordon J. A., Bulger R. E., Dobyan D. C., Schrier R. W. Protective effect of intrarenal calcium membrane blockers before or after renal ischemia. Functional, morphological, and mitochondrial studies. J Clin Invest. 1984 Nov;74(5):1830–1841. doi: 10.1172/JCI111602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke T. J., Arnold P. E., Schrier R. W. Prevention of ischemic acute renal failure with impermeant solutes. Am J Physiol. 1983 Jun;244(6):F646–F649. doi: 10.1152/ajprenal.1983.244.6.F646. [DOI] [PubMed] [Google Scholar]
- Burke T. J., Cronin R. E., Duchin K. L., Peterson L. N., Schrier R. W. Ischemia and tubule obstruction during acute renal failure in dogs: mannitol in protection. Am J Physiol. 1980 Apr;238(4):F305–F314. doi: 10.1152/ajprenal.1980.238.4.F305. [DOI] [PubMed] [Google Scholar]
- CHANCE B., WILLIAMS G. R. The respiratory chain and oxidative phosphorylation. Adv Enzymol Relat Subj Biochem. 1956;17:65–134. doi: 10.1002/9780470122624.ch2. [DOI] [PubMed] [Google Scholar]
- Chien K. R., Abrams J., Serroni A., Martin J. T., Farber J. L. Accelerated phospholipid degradation and associated membrane dysfunction in irreversible, ischemic liver cell injury. J Biol Chem. 1978 Jul 10;253(13):4809–4817. [PubMed] [Google Scholar]
- Doucet A., Katz A. I. High-affinity Ca-Mg-ATPase along the rabbit nephron. Am J Physiol. 1982 Apr;242(4):F346–F352. doi: 10.1152/ajprenal.1982.242.4.F346. [DOI] [PubMed] [Google Scholar]
- Farber J. L., Chien K. R., Mittnacht S., Jr Myocardial ischemia: the pathogenesis of irreversible cell injury in ischemia. Am J Pathol. 1981 Feb;102(2):271–281. [PMC free article] [PubMed] [Google Scholar]
- Gaudio K. M., Ardito T. A., Reilly H. F., Kashgarian M., Siegel N. J. Accelerated cellular recovery after an ischemic renal injury. Am J Pathol. 1983 Sep;112(3):338–346. [PMC free article] [PubMed] [Google Scholar]
- Gaudio K. M., Ardito T. A., Reilly H. F., Kashgarian M., Siegel N. J. Accelerated cellular recovery after an ischemic renal injury. Am J Pathol. 1983 Sep;112(3):338–346. [PMC free article] [PubMed] [Google Scholar]
- Glaumann B., Glaumann H., Berezesky I. K., Trump B. F. Studies on cellular recovery from injury. II. Ultrastructural studies on the recovery of the pars convoluta of the proximal tubule of the rate kidney from temporary ischemia. Virchows Arch B Cell Pathol. 1977 May 3;24(1):1–18. [PubMed] [Google Scholar]
- Gmaj P., Murer H., Kinne R. Calcium ion transport across plasma membranes isolated from rat kidney cortex. Biochem J. 1979 Mar 15;178(3):549–557. doi: 10.1042/bj1780549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanley M. J., Davidson K. Prior mannitol and furosemide infusion in a model of ischemic acute renal failure. Am J Physiol. 1981 Nov;241(5):F556–F564. doi: 10.1152/ajprenal.1981.241.5.F556. [DOI] [PubMed] [Google Scholar]
- Hanley M. J. Isolated nephron segments in a rabbit model of ischemic acute renal failure. Am J Physiol. 1980 Jul;239(1):F17–F23. doi: 10.1152/ajprenal.1980.239.1.F17. [DOI] [PubMed] [Google Scholar]
- Harris S. I., Balaban R. S., Barrett L., Mandel L. J. Mitochondrial respiratory capacity and Na+- and K+-dependent adenosine triphosphatase-mediated ion transport in the intact renal cell. J Biol Chem. 1981 Oct 25;256(20):10319–10328. [PubMed] [Google Scholar]
- Johnston P. A., Rennke H., Levinsky N. G. Recovery of proximal tubular function from ischemic injury. Am J Physiol. 1984 Feb;246(2 Pt 2):F159–F166. doi: 10.1152/ajprenal.1984.246.2.F159. [DOI] [PubMed] [Google Scholar]
- Jones D. P., Kennedy F. G. Intracellular oxygen supply during hypoxia. Am J Physiol. 1982 Nov;243(5):C247–C253. doi: 10.1152/ajpcell.1982.243.5.C247. [DOI] [PubMed] [Google Scholar]
- Kahng M. W., Berezesky I. K., Trump B. F. Metabolic and ultrastructural response of rat kidney cortex to in vitro ischemia. Exp Mol Pathol. 1978 Oct;29(2):183–198. doi: 10.1016/0014-4800(78)90038-2. [DOI] [PubMed] [Google Scholar]
- Katz A. M., Reuter H. Cellular calcium and cardiac cell death. Am J Cardiol. 1979 Jul;44(1):188–190. doi: 10.1016/0002-9149(79)90270-4. [DOI] [PubMed] [Google Scholar]
- Lötscher H. R., Winterhalter K. H., Carafoli E., Richter C. The energy-state of mitochondria during the transport of Ca2+. Eur J Biochem. 1980 Sep;110(1):211–216. doi: 10.1111/j.1432-1033.1980.tb04857.x. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Balaban R. S. Stoichiometry and coupling of active transport to oxidative metabolism in epithelial tissues. Am J Physiol. 1981 May;240(5):F357–F371. doi: 10.1152/ajprenal.1981.240.5.F357. [DOI] [PubMed] [Google Scholar]
- Mandel L. J., Murphy E. Regulation of cytosolic free calcium in rabbit proximal renal tubules. J Biol Chem. 1984 Sep 25;259(18):11188–11196. [PubMed] [Google Scholar]
- Mason J., Beck F., Dörge A., Rick R., Thurau K. Intracellular electrolyte composition following renal ischemia. Kidney Int. 1981 Jul;20(1):61–70. doi: 10.1038/ki.1981.105. [DOI] [PubMed] [Google Scholar]
- Mergner W. J., Marzella L., Mergner C., Kahng M. W., Smith M. W., Trump B. F. Studies on the pathogenesis of ischemic cell injury. VII. Proton gradient and respiration of renal tissue cubes, renal mitochondrial and submitochondrial particles following ischemic cell injury. Beitr Pathol. 1977 Nov;161(3):230–243. doi: 10.1016/s0005-8165(77)80079-6. [DOI] [PubMed] [Google Scholar]
- Mittnacht S., Jr, Sherman S. C., Farber J. L. Reversal of ischemic mitochondrial dysfunction. J Biol Chem. 1979 Oct 10;254(19):9871–9878. [PubMed] [Google Scholar]
- Miyahara M., Okimasu E., Mikasa H., Terada S., Kodama H., Utsumi K. Improvement of the anoxia-induced mitochondrial dysfunction by membrane modulation. Arch Biochem Biophys. 1984 Aug 15;233(1):139–150. doi: 10.1016/0003-9861(84)90610-6. [DOI] [PubMed] [Google Scholar]
- Murphy E., Aiton J. F., Horres C. R., Lieberman M. Calcium elevation in cultured heart cells: its role in cell injury. Am J Physiol. 1983 Nov;245(5 Pt 1):C316–C321. doi: 10.1152/ajpcell.1983.245.5.C316. [DOI] [PubMed] [Google Scholar]
- Nakazawa T., Nunokawa T. Energy transduction and adenine nucleotides in mitochondria from rat liver after hypoxic perfusion. J Biochem. 1977 Dec;82(6):1575–1583. doi: 10.1093/oxfordjournals.jbchem.a131852. [DOI] [PubMed] [Google Scholar]
- ROSSI C. S., LEHNINGER A. L. STOICHIOMETRY OF RESPIRATORY STIMULATION, ACCUMULATION OF CA++ AND PHOSPHATE, AND OXIDATIVE PHOSPHORYLATION IN RAT LIVER MITOCHONDRIA. J Biol Chem. 1964 Nov;239:3971–3980. [PubMed] [Google Scholar]
- Schrier R. W., Arnold P. E., Gordon J. A., Burke T. J. Protection of mitochondrial function by mannitol in ischemic acute renal failure. Am J Physiol. 1984 Aug;247(2 Pt 2):F365–F369. doi: 10.1152/ajprenal.1984.247.2.F365. [DOI] [PubMed] [Google Scholar]
- Siegel N. J., Glazier W. B., Chaudry I. H., Gaudio K. M., Lytton B., Baue A. E., Kashgarian M. Enhanced recovery from acute renal failure by the postischemic infusin of adenine nucleotides and magnesium chloride in rats. Kidney Int. 1980 Mar;17(3):338–349. doi: 10.1038/ki.1980.39. [DOI] [PubMed] [Google Scholar]
- Snowdowne K. W., Borle A. B. Measurement of cytosolic free calcium in mammalian cells with aequorin. Am J Physiol. 1984 Nov;247(5 Pt 1):C396–C408. doi: 10.1152/ajpcell.1984.247.5.C396. [DOI] [PubMed] [Google Scholar]
- Soltoff S. P., Mandel L. J. Active ion transport in the renal proximal tubule. I. Transport and metabolic studies. J Gen Physiol. 1984 Oct;84(4):601–622. doi: 10.1085/jgp.84.4.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soltoff S. P., Mandel L. J. Active ion transport in the renal proximal tubule. III. The ATP dependence of the Na pump. J Gen Physiol. 1984 Oct;84(4):643–662. doi: 10.1085/jgp.84.4.643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venkatachalam M. A., Bernard D. B., Donohoe J. F., Levinsky N. G. Ischemic damage and repair in the rat proximal tubule: differences among the S1, S2, and S3 segments. Kidney Int. 1978 Jul;14(1):31–49. doi: 10.1038/ki.1978.87. [DOI] [PubMed] [Google Scholar]
- Venkatachalam M. A., Jones D. B., Rennke H. G., Sandstrom D., Patel Y. Mechanism of proximal tubule brush border loss and regeneration following mild renal ischemia. Lab Invest. 1981 Oct;45(4):355–365. [PubMed] [Google Scholar]
- Wilson D. R., Arnold P. E., Burke T. J., Schrier R. W. Mitochondrial calcium accumulation and respiration in ischemic acute renal failure in the rat. Kidney Int. 1984 Mar;25(3):519–526. doi: 10.1038/ki.1984.48. [DOI] [PubMed] [Google Scholar]


