Abstract
Macrophages infiltrate hypoxic tumor regions where they promote angiogenesis and immunosuppression. In this issue of Cancer Cell, Mazzone and colleagues report that tumor–associated macrophage (TAM)entry into avascular tumor areas is regulated by Semaphorin 3A/Neuropilin-1 signaling;interference with this pathway entraps TAMs in oxygenated areas preventing their tumorigenic function.
Tumor–associated macrophages (TAMs) are tissue-resident cells that differentiate from circulating monocytes in peripheral blood. They can constitute the major leukocytic infiltrate found within the stroma of many tumor types. Although macrophages in normal tissues are implicated in phagocytosis of microbes and antigen presentation to T cells, TAMs have two opposing phenotypes,they can eitherendorse proimmune and tumoricidal processes orpromote tumor growth and metastasis by suppressing immunity and promoting angiogenesis.The phenotype of TAMs is regulated by specific tumor-derived chemokines and cytokines that polarize macrophages to a proimmune ‘M1’ or immunesuppressive/proangiogenic ‘M2’ phenotype. The dichotomousTAMphenotypes may explain why TAMs can elicit a poor prognosis in some tumors including glioma and breast cancers and a better prognosis in others such as stomach andcolon cancers and some prostate and non-small cell lungcancers(Allavena et al., 2008; Bingle et al., 2002).Macrophage polarization is also in part regulated by intratumoral hypoxia, in which infiltrating myeloid cells accumulate and are stimulated to secrete various immune suppressive and proangiogenic factors (De Palma and Lewis, 2013; Qian and Pollard, 2010).
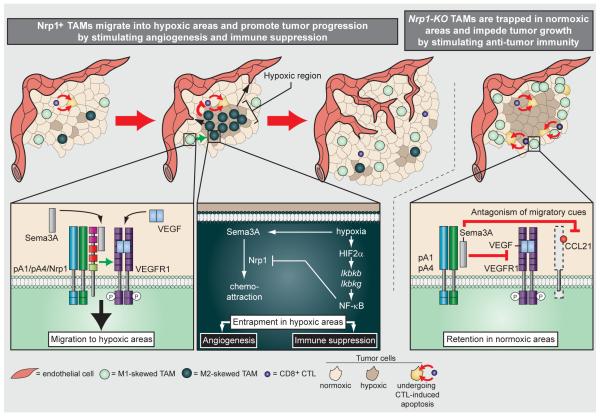
In this issue of Cancer CellCasazza et al. describe a Neuropilin-1 (Nrp1)-dependent guidance mechanism by which macrophages enter hypoxic areasto elicit proangiogenic and immune suppressive functions (Figure 1)(Casazza et al. 2013).Utilizing elegant genetic tools to interfere with Nrp1 function in TAMs in various mouse tumor models they demonstrated that Semaphorin3A (Sema3A) mediates Nrp-1-dependent signaling of a PlexinA1/PlexinA4/VEGFR1 holoreceptorcomplexthat leads toVEGFR1 activation in TAMsand their subsequent migration into hypoxic regions. Notably, although Sema3A and VEGF levels are both increased under hypoxic conditions, Sema3A, but not VEGF, was sufficient to attract TAMs.They tested this by generating TAMs with a Sema3A-binding mutant of Nrp1 that was still able to bind to VEGF. These macrophages failed to enter hypoxic regions of the tumor similarly to Nrp1-KO TAMs.As soon as TAMs were positioned in the hypoxic environment, Nrp1 expression was repressed;this terminated the migratory response of TAMs to Sema3A.Interestingly, hypoxia-dependent Nrp1 repression was facilitated by HIF2α –mediatedactivation oftheNF-κB pathway. The loss of Nrp1 switched Sema3A to mediating a PlexinA1/PlexinA4-mediated TAM arrest antagonizing VEGFR1-induced attraction and entrapping TAMs in hypoxic regions (Figure 1).As TAMs shift from an anti- to a pro-tumoralphenotype upon association with hypoxic environments, the authors then asked how loss of Nrp1 in TAMs and their subsequent differing positioning within tumors would affect tumor propagation and progression.
Sema3A/Nrp1 signaling regulatesTAM entry into hypoxic regions and thereby promotes tumor progression.
Intratumoral hypoxia enhances the expression of VEGF and Sema3A. Sema3A binds to the Nrp1/PlexinA1 (pA1)/PlexinA4 (pA4)holoreceptor complex at the TAM surface, resulting in VEGFR1/Nrp1-dependent migration towards the Sema3A-expressing hypoxic area. Hypoxia-associated TAMs experience stabilization of HIF2α, which induces expression of Ikbkb and Ikbkg, ultimately leading to phosphorylation of IkB and nuclear translocation of NF-κB. NF-κB then repressesexpression of Nrp1. In the absence of Nrp1, Sema3A antagonizes migration signals through PlexinA1/PlexinA4 signaling, thus retaining and entrapping TAMs within hypoxic areas. Here, TAMs are “educated” to endorse angiogenesis and suppress anti-tumor immunity, thus facilitating tumor progression. Sema3A/PlexinA1/PlexinA4 retention signals entrap Nrp1-KO TAMs in normoxic areas by blocking VEGF-mediated migration into hypoxic regions. Nrp1-KO TAMs therefore do not attain a tumor promoting phenotype and suppress tumor growth by stimulating anti-tumor immunity.
Casazza et al. explored the function of Nrp1 on TAMs by creating conditional TAM-specificNrp1-knockout (KO) mice. Orthotopic lung and pancreatic tumors, and tumors from a transgenic breast cancer mouse model,in Nrp1-KO mice grew to only a fraction of the size of tumors in wildtype (WT) mice. Nrp-1 deficiency in TAMs yielded tumors with nearly double the number of TAMs, likely due to increased tumor hypoxia.Surprisingly, however, TAMs solely accumulated within normoxicregions. Moreover, despite the increase in TAMs, endstage tumors exhibited reduced vessel density and perfusion, suggesting that Nrp1-KO TAMs were impaired in their angiogenicfunctions compared to their WT counterparts. Indeed, isolated WT TAMs induced more robust endothelial cell migration and capillary formation compared to Nrp1-KO TAMs. In addition, Nrp1-KO TAMs secreted more nitric oxide, increased Tcell proliferation and were more cytotoxic. Interestingly, Casazza et al. found that the acquired “M1” TAM phenotype was not endorsed by the lack of Nrp1 per se because WT and Nrp1-KOmacrophages obtained from bone marrow were equally able to switch between proimmune and immune suppressive phenotypes upon appropriate stimulation in vitro. Further Nrp-1 deficiency in TAMs neither affected the numbers of circulating or resident monocyte numbers nor changed proliferation and apoptosisof macrophages precluding aNrp1-dependent regulation of monocyte/TAM recruitment or differentiation. Rather, these elegant studies revealedthat blocking Nrp1 in TAMs was sufficient to keep the cells in a tumor-suppressive state by solely entrapping the cells in vascularized normoxictumor areas.
These studies support the concept of macrophage “reprogramming” as a sufficient and feasible approach to abrogate angiogenesis and restore T cell-mediated antitumor immunity(Coussens et al., 2013). Further, Casazza et al. provide a new therapeutic opportunity to turn TAMsagainst cancer by modulating their intratumoral location via inhibition of Nrp1.Such an approach is advantageous over those that target total TAM infiltration as it harnesses the tumor suppressing capacities of TAMs.
These studies also have important clinical applications. Although historically successful tumor eradication had been linked with tumor necrosis, various studies have demonstrated that hypoxia- generating drugs cause amore aggressive disease in part by accumulating more immune suppressive innate immune cells that facilitate angiogenesis, tumor invasion and metastasis. Emerging data support the notion that normalization of the tumor vasculature provides beneficial effects enabling better drug delivery and enhanced influx of T cells.A recent study by Klug et al. demonstrated that low-dose irradiation and T cell transfer normalized the tumor vasculature and enhanced the recruitment CD8+ T cells and TAMs expressing high levels of the M1 marker iNOS(Klug et al., 2013). Similarly, Casazza et al. found that normoxia enhanced secretion of nitric oxide by TAMs and induced CD8+ T cell expansion. Thus oxygenation of the tumor should also help to redirectmacrophagedifferentiation to facilitate anti-tumor immunity. In addition, targeting Nrp1 would restrict the TAMs to oxygenated areas even during hypoxia-inducing therapies including standard chemo- and radiation therapy.
The authors confirm that exposure of TAMs to hypoxia is a requisite for their acquisition of a tumor promoting phenotype; however, whether or not hypoxia directly regulates M2 reprogramming is unclear. A recent study by Laoui et al. suggests hypoxia plays a supportive rather than a direct role in driving M2 functions by TAMs (Laoui et al., 2013). Using prolyly-4 hydroxylase 2-haplodeficient mice, this group found that reduced tumor hypoxia resulted in downregulated TAM expression of genes involved in glycolysis, angiogenesis, and metastasis, and not in typical M2 markers including mannose receptor and arginase. This suggests targeting the Nrp1/Sema3A axis can synergize with reprogramming approaches to provide better TAM-mediated anti-tumor responses.
In support of these hypotheses, blockade of Nrp1 in preclinical tumor models has been encouraging, suppressing both angiogenesis and tumor growth, and clinical trials are currently ongoing(Pan et al., 2007).As Nrp1 is expressed in a variety of cell types besides TAMs, including endothelial cellsand tumorcells, it will be pivotal to analyze whetherthe mechanism proposed by this studyis still evident when Nrp1 activity is broadly abrogated in murine tumor models and human tumors. Whether TAMlocation and activity is similarly regulated in other hypoxia-generating pathologiesalso warrants further investigation.For example, in a mouse model of cerebral stroke, microglia and macrophages were found to undergo M2 polarization immediately after ischemic insult, but eventually underwent M1 polarization induced by ischemic neurons (Huang and Feng, 2013). The M2 polarized cells were found to have a protective effect on neurons whereas M1 polarized cells promoted neuronal destruction; therefore preventing microglia and macrophages from associating with ischemic areas might maintain their neuronal-protective phenotypes. If validated, manipulation of the Nrp1/Sema3A axis could become a valuable agent for diseaseslike ischemia and stroke to redirect macrophage function and improve patient outcome.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Allavena P, Sica A, Garlanda C, Mantovani A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunological reviews. 2008;222:155–161. doi: 10.1111/j.1600-065X.2008.00607.x. [DOI] [PubMed] [Google Scholar]
- Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. The Journal of pathology. 2002;196:254–265. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- Casazza A, Laoui D, Wenes M, Rizzolio S, Bassani N, Mambretti M, Deschoemaeker S, Van Ginderacter JA, Tamagnone L, Mazzone M. Impeding macrophage entry into hypoxic tumor areas by SemasA/Nrp1 signaling blockade inhibits angiogenesis and restores anti-tumor immunity. Cancer Cell. 2013 doi: 10.1016/j.ccr.2013.11.007. this issue. [DOI] [PubMed] [Google Scholar]
- Coussens LM, Zitvogel L, Palucka AK. Neutralizing tumor-promoting chronic inflammation: a magic bullet? Science. 2013;339:286–291. doi: 10.1126/science.1232227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Palma M, Lewis CE. Macrophage regulation of tumor responses to anticancer therapies. Cancer Cell. 2013;23:277–286. doi: 10.1016/j.ccr.2013.02.013. [DOI] [PubMed] [Google Scholar]
- Huang YC, Feng ZP. The good and bad of microglia/macrophages: new hope in stroke therapeutics. Acta Pharmacologica Sinica. 2013;34:6–7. doi: 10.1038/aps.2012.178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klug F, Prakash H, Huber PE, Seibel T, Bender N, Halama N, Pfirschke C, Voss RH, Timke C, Umansky L, et al. Low-Dose Irradiation Programs Macrophage Differentiation to an iNOS(+)/M1 Phenotype that Orchestrates Effective T Cell Immunotherapy. Cancer Cell. 2013;24:589–602. doi: 10.1016/j.ccr.2013.09.014. [DOI] [PubMed] [Google Scholar]
- Laoui D, Van Overmeire E, Di Conza G, Aldeni C, Keirsse J, Morias Y, Movahedi K, Houbracken I, Schouppe E, Elkrim Y, et al. Tumor hypoxia does not drive differentiation of tumor-associated macrophages but rather fine-tunes the M2-like macrophage population. Cancer Research. 2013 doi: 10.1158/0008-5472.CAN-13-1196. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- Pan Q, Chanthery Y, Liang WC, Stawicki S, Mak J, Rathore N, Tong RK, Kowalski J, Yee SF, Pacheco G, et al. Blocking neuropilin-1 function has an additive effect with anti-VEGF to inhibit tumor growth. Cancer Cell. 2007;11:53–67. doi: 10.1016/j.ccr.2006.10.018. [DOI] [PubMed] [Google Scholar]
- Qian BZ, Pollard JW. Macrophage diversity enhances tumor progression and metastasis. Cell. 2010;141:39–51. doi: 10.1016/j.cell.2010.03.014. [DOI] [PMC free article] [PubMed] [Google Scholar]