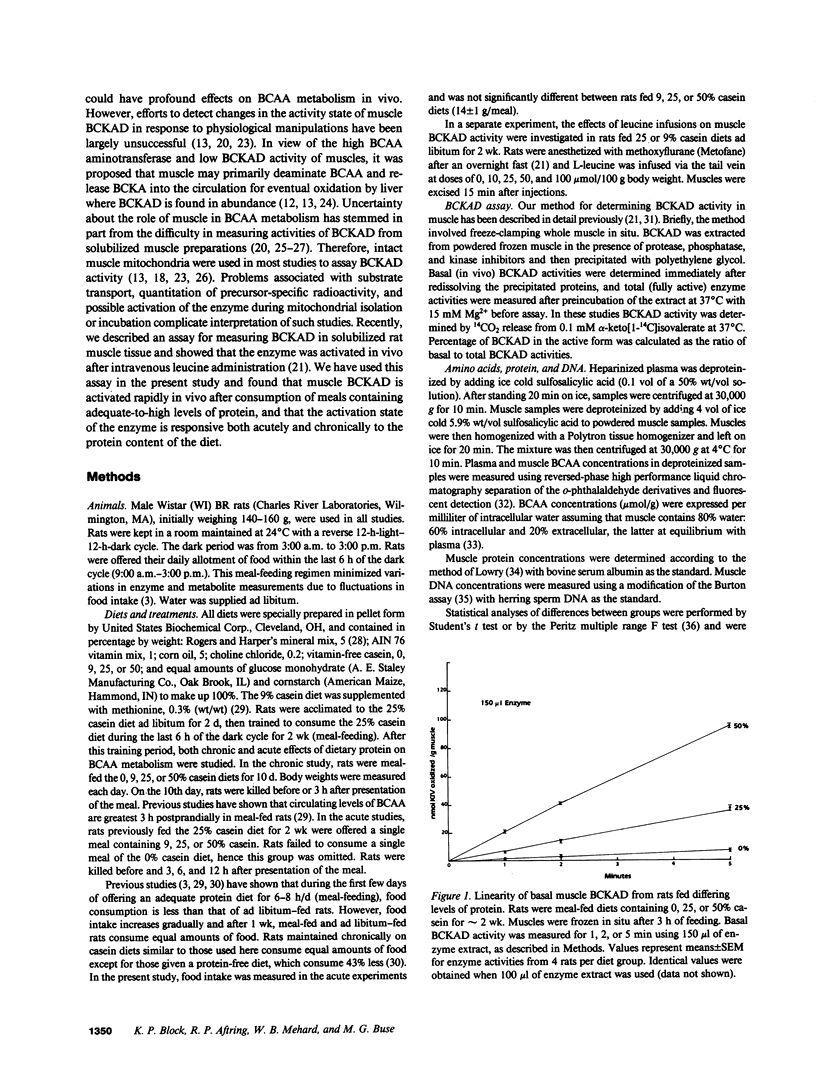
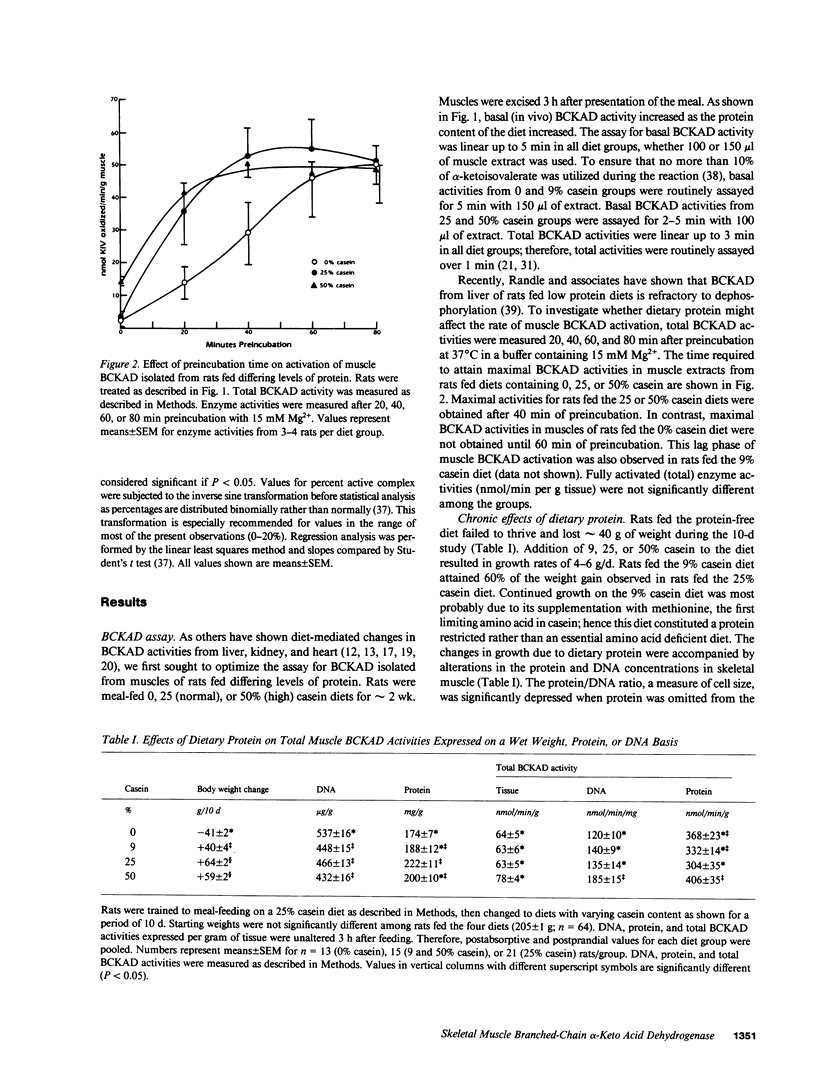
Abstract
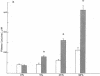
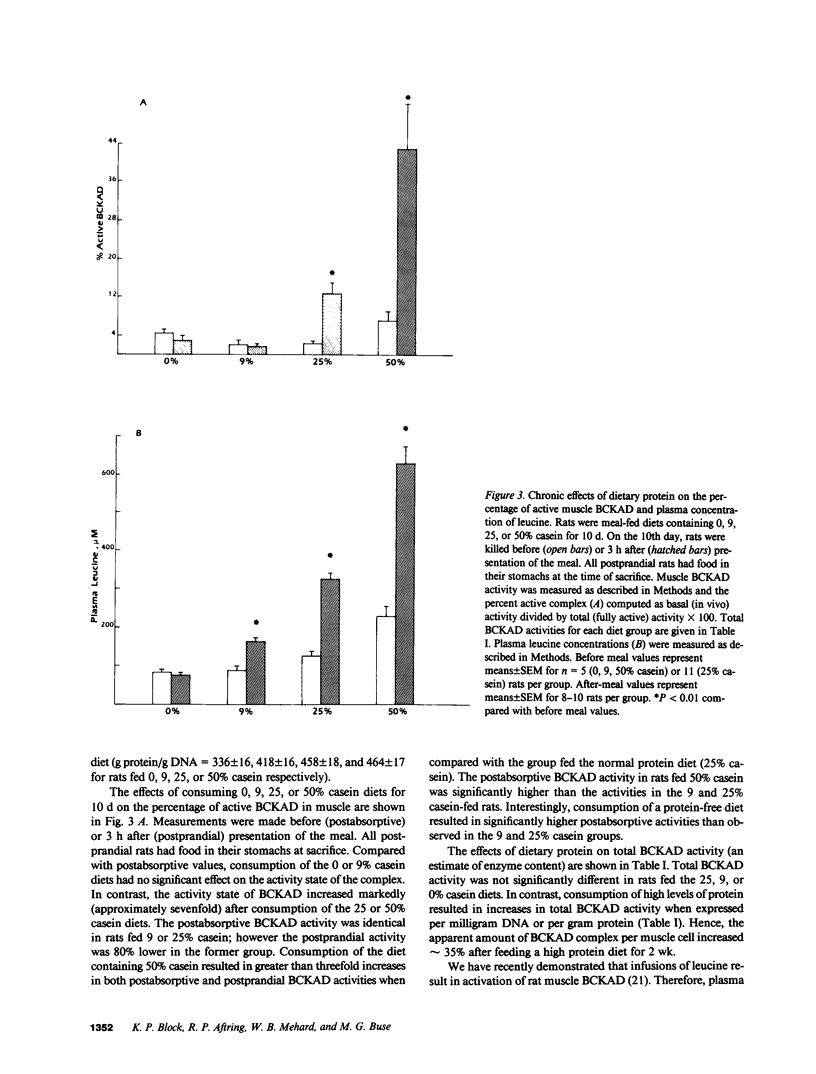
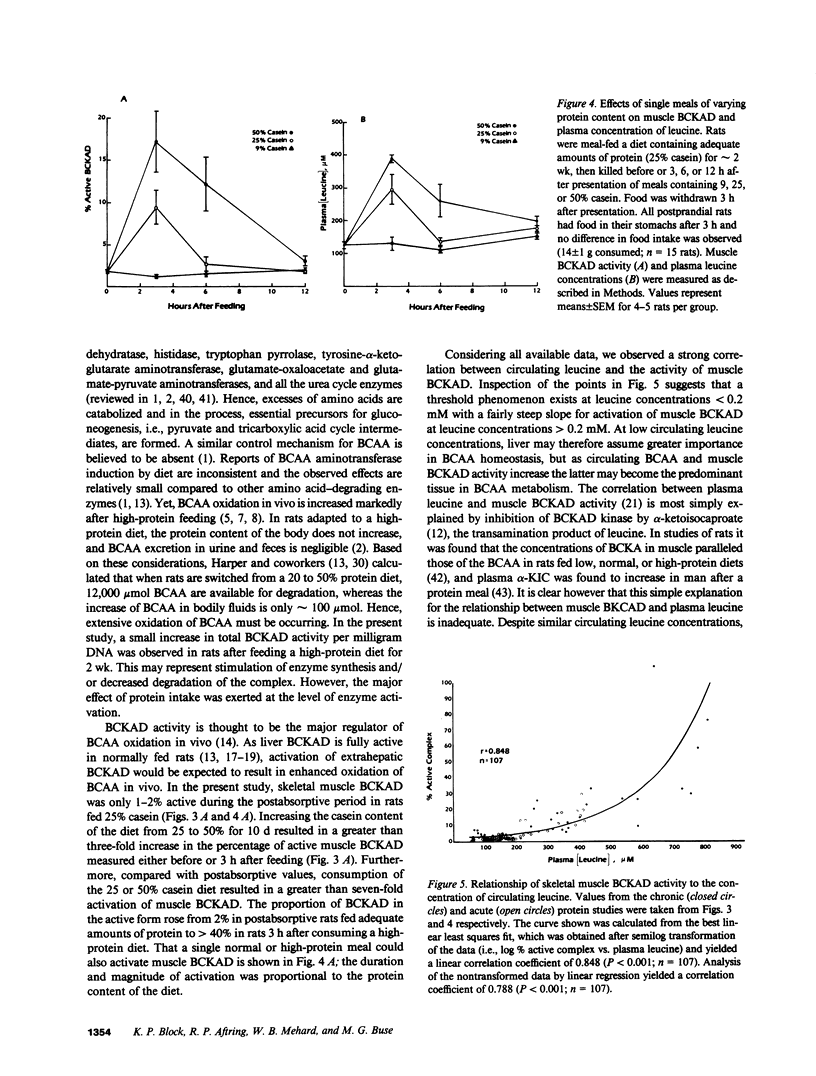
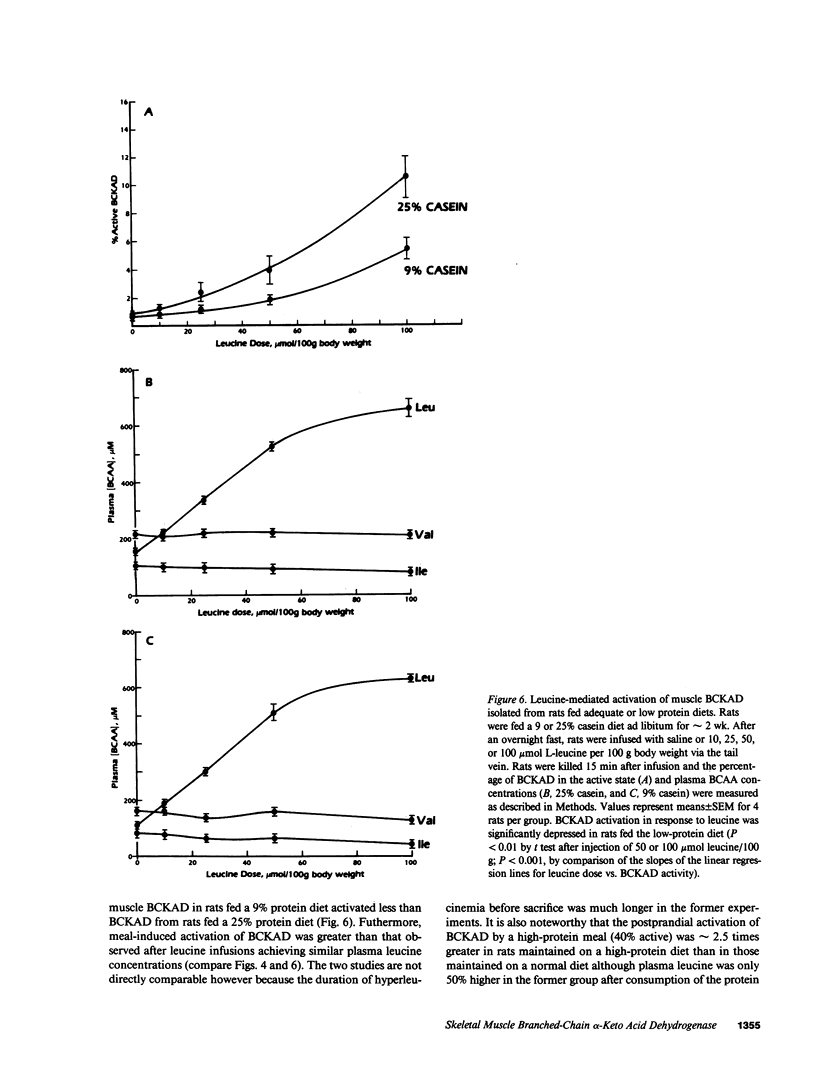
The effects of dietary protein on the activity of skeletal muscle branched-chain alpha-keto acid dehydrogenase (BCKAD) were investigated. BCKAD is rate-limiting for branched-chain amino acid (BCAA) catabolism by muscle; its activity is modulated by phosphorylation-dephosphorylation. In rats fed an adequate protein (25% casein) diet, BCKAD was approximately 2% active postabsorptively and increased to 10% or 16% active after a 25% or 50% protein meal, respectively. Prolonged feeding of a 50% protein diet increased postabsorptive BCKAD activity to 7% with further increases to 40% active postprandially. On a low protein (9% casein) diet BCKAD remained approximately 2% active regardless of meal-feeding. Dose-dependent activation of BCKAD by intravenous leucine in postabsorptive rats was blunted by a low protein diet. We conclude that excesses of dietary protein enhance the capacity of skeletal muscle to oxidize BCAA, muscle conserves BCAA when protein intake is inadequate, and skeletal muscle may play an important role in whole-body BCAA homeostasis.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aftring R. P., Block K. P., Buse M. G. Leucine and isoleucine activate skeletal muscle branched-chain alpha-keto acid dehydrogenase in vivo. Am J Physiol. 1986 May;250(5 Pt 1):E599–E604. doi: 10.1152/ajpendo.1986.250.5.E599. [DOI] [PubMed] [Google Scholar]
- Aftring R. P., Manos P. N., Buse M. G. Catabolism of branched-chain amino acids by diaphragm muscles of fasted and diabetic rats. Metabolism. 1985 Aug;34(8):702–711. doi: 10.1016/0026-0495(85)90018-6. [DOI] [PubMed] [Google Scholar]
- Benevenga N. J., Steele R. D. Adverse effects of excessive consumption of amino acids. Annu Rev Nutr. 1984;4:157–181. doi: 10.1146/annurev.nu.04.070184.001105. [DOI] [PubMed] [Google Scholar]
- Berry H. K., Bofinger M. K., Hunt M. M., Phillips P. J., Guilfoile M. B. Reduction of cerebrospinal fluid phenylalanine after oral administration of valine, isoleucine, and leucine. Pediatr Res. 1982 Sep;16(9):751–755. doi: 10.1203/00006450-198209000-00009. [DOI] [PubMed] [Google Scholar]
- Block K. P., Richmond W. B., Mehard W. B., Buse M. G. Glucocorticoid-mediated activation of muscle branched-chain alpha-keto acid dehydrogenase in vivo. Am J Physiol. 1987 Mar;252(3 Pt 1):E396–E407. doi: 10.1152/ajpendo.1987.252.3.E396. [DOI] [PubMed] [Google Scholar]
- Bower R. H., Fischer J. E. Nutritional management of hepatic encephalopathy. Adv Nutr Res. 1983;5:1–11. doi: 10.1007/978-1-4613-9937-7_1. [DOI] [PubMed] [Google Scholar]
- Buse M. G., Weigand D. A., Peeler D., Hedden M. P. The effect of diabetes and the redox potential on amino acid content and release by isolated rat hemidiaphragms. Metabolism. 1980 Jul;29(7):605–616. doi: 10.1016/0026-0495(80)90104-3. [DOI] [PubMed] [Google Scholar]
- Dixon J. L., Harper A. E. Effects on plasma amino acid concentrations and hepatic branched-chain alpha-keto acid dehydrogenase activity of feeding rats diets containing 9 or 50% casein. J Nutr. 1984 Jun;114(6):1025–1034. doi: 10.1093/jn/114.6.1025. [DOI] [PubMed] [Google Scholar]
- Espinal J., Beggs M., Patel H., Randle P. J. Effects of low-protein diet and starvation on the activity of branched-chain 2-oxo acid dehydrogenase kinase in rat liver and heart. Biochem J. 1986 Jul 1;237(1):285–288. doi: 10.1042/bj2370285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fernstrom J. D. Dietary effects on brain serotonin synthesis: relationship to appetite regulation. Am J Clin Nutr. 1985 Nov;42(5 Suppl):1072–1082. doi: 10.1093/ajcn/42.5.1072. [DOI] [PubMed] [Google Scholar]
- Fernstrom J. D., Larin F., Wurtman R. J. Daily variations in the concentrations of individual amino acids in rat plasma. Life Sci I. 1971 Jul 15;10(14):813–819. doi: 10.1016/0024-3205(71)90036-1. [DOI] [PubMed] [Google Scholar]
- Gillim S. E., Paxton R., Cook G. A., Harris R. A. Activity state of the branched chain alpha-ketoacid dehydrogenase complex in heart, liver, and kidney of normal, fasted, diabetic, and protein-starved rats. Biochem Biophys Res Commun. 1983 Feb 28;111(1):74–81. doi: 10.1016/s0006-291x(83)80119-3. [DOI] [PubMed] [Google Scholar]
- Harper A. E. Effect of variations in protein intake on enzymes of amino acid metabolism. Can J Biochem. 1965 Sep;43(9):1589–1603. doi: 10.1139/o65-176. [DOI] [PubMed] [Google Scholar]
- Harper A. E., Miller R. H., Block K. P. Branched-chain amino acid metabolism. Annu Rev Nutr. 1984;4:409–454. doi: 10.1146/annurev.nu.04.070184.002205. [DOI] [PubMed] [Google Scholar]
- Harper J. F. Peritz' F test: basic program of a robust multiple comparison test for statistical analysis of all differences among group means. Comput Biol Med. 1984;14(4):437–445. doi: 10.1016/0010-4825(84)90044-1. [DOI] [PubMed] [Google Scholar]
- Harris R. A., Paxton R., Goodwin G. W., Powell S. M. Regulation of the branched-chain 2-oxo acid dehydrogenase complex in hepatocytes isolated from rats fed on a low-protein diet. Biochem J. 1986 Mar 1;234(2):285–294. doi: 10.1042/bj2340285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hutson S. M. Branched chain alpha-keto acid oxidative decarboxylation in skeletal muscle mitochondria. Effect of isolation procedure and mitochondrial delta pH. J Biol Chem. 1986 Apr 5;261(10):4420–4425. [PubMed] [Google Scholar]
- Hutson S. M., Harper A. E. Blood and tissue branched-chain amino and alpha-keto acid concentrations: effect of diet, starvation, and disease. Am J Clin Nutr. 1981 Feb;34(2):173–183. doi: 10.1093/ajcn/34.2.173. [DOI] [PubMed] [Google Scholar]
- Hutson S. M., Zapalowski C., Cree T. C., Harper A. E. Regulation of leucine and alpha-ketoisocaproic acid metabolism in skeletal muscle. Effects of starvation and insulin. J Biol Chem. 1980 Mar 25;255(6):2418–2426. [PubMed] [Google Scholar]
- Krebs H. A., Lund P. Aspects of the regulation of the metabolism of branched-chain amino acids. Adv Enzyme Regul. 1976;15:375–394. doi: 10.1016/0065-2571(77)90026-7. [DOI] [PubMed] [Google Scholar]
- Krebs H. A. Some aspects of the regulation of fuel supply in omnivorous animals. Adv Enzyme Regul. 1972;10:397–420. doi: 10.1016/0065-2571(72)90025-8. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laurent B. C., Moldawer L. L., Young V. R., Bistrian B. R., Blackburn G. L. Whole-body leucine and muscle protein kinetics in rats fed varying protein intakes. Am J Physiol. 1984 May;246(5 Pt 1):E444–E451. doi: 10.1152/ajpendo.1984.246.5.E444. [DOI] [PubMed] [Google Scholar]
- Livesey G., Lund P. Enzymic determination of branched-chain amino acids and 2-oxoacids in rat tissues. Transfer of 2-oxoacids from skeletal muscle to liver in vivo. Biochem J. 1980 Jun 15;188(3):705–713. doi: 10.1042/bj1880705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. E., Bier D. M., Rennie M. J., Edwards R. H., Halliday D., Millward D. J., Clugston G. A. Regulation of leucine metabolism in man: a stable isotope study. Science. 1981 Dec 4;214(4525):1129–1131. doi: 10.1126/science.7302583. [DOI] [PubMed] [Google Scholar]
- Matthews D. E., Schwarz H. P., Yang R. D., Motil K. J., Young V. R., Bier D. M. Relationship of plasma leucine and alpha-ketoisocaproate during a L-[1-13C]leucine infusion in man: a method for measuring human intracellular leucine tracer enrichment. Metabolism. 1982 Nov;31(11):1105–1112. doi: 10.1016/0026-0495(82)90160-3. [DOI] [PubMed] [Google Scholar]
- McFarlane I. G., Von Holt C. Metabolism of amino acids in protein-calorie-deficient rats. Biochem J. 1969 Feb;111(4):557–563. doi: 10.1042/bj1110557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motil K. J., Matthews D. E., Bier D. M., Burke J. F., Munro H. N., Young V. R. Whole-body leucine and lysine metabolism: response to dietary protein intake in young men. Am J Physiol. 1981 Jun;240(6):E712–E721. doi: 10.1152/ajpendo.1981.240.6.E712. [DOI] [PubMed] [Google Scholar]
- Nissen S., Haymond M. W. Changes in leucine kinetics during meal absorption: effects of dietary leucine availability. Am J Physiol. 1986 Jun;250(6 Pt 1):E695–E701. doi: 10.1152/ajpendo.1986.250.6.E695. [DOI] [PubMed] [Google Scholar]
- Odessey R., Goldberg A. L. Leucine degradation in cell-free extracts of skeletal muscle. Biochem J. 1979 Feb 15;178(2):475–489. doi: 10.1042/bj1780475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardridge W. M. Kinetics of competitive inhibition of neutral amino acid transport across the blood-brain barrier. J Neurochem. 1977 Jan;28(1):103–108. doi: 10.1111/j.1471-4159.1977.tb07714.x. [DOI] [PubMed] [Google Scholar]
- Patston P. A., Espinal J., Randle P. J. Effects of diet and of alloxan-diabetes on the activity of branched-chain 2-oxo acid dehydrogenase complex and of activator protein in rat tissues. Biochem J. 1984 Sep 15;222(3):711–719. doi: 10.1042/bj2220711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patston P. A., Espinal J., Shaw J. M., Randle P. J. Rat tissue concentrations of branched-chain 2-oxo acid dehydrogenase complex. Re-evaluation by immunoassay and bioassay. Biochem J. 1986 Apr 15;235(2):429–434. doi: 10.1042/bj2350429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul H. S., Adibi S. A. Role of ATP in the regulation of branched-chain alpha-keto acid dehydrogenase activity in liver and muscle mitochondria of fed, fasted, and diabetic rats. J Biol Chem. 1982 May 10;257(9):4875–4881. [PubMed] [Google Scholar]
- Peters J. C., Harper A. E. Adaptation of rats to diets containing different levels of protein: effects on food intake, plasma and brain amino acid concentrations and brain neurotransmitter metabolism. J Nutr. 1985 Mar;115(3):382–398. doi: 10.1093/jn/115.3.382. [DOI] [PubMed] [Google Scholar]
- Reeds P. J. The catabolism of valine in the malnourished rat. Studies in vivo and in vitro with different labelled forms of valine. Br J Nutr. 1974 Mar;31(2):259–270. doi: 10.1079/bjn19740031. [DOI] [PubMed] [Google Scholar]
- Rogers Q. R., Harper A. E. Amino acid diets and maximal growth in the rat. J Nutr. 1965 Nov;87(3):267–273. doi: 10.1093/jn/87.3.267. [DOI] [PubMed] [Google Scholar]
- Schauder P., Schröder K., Langenbeck U. Serum branched-chain amino and keto acid response to a protein-rich meal in man. Ann Nutr Metab. 1984;28(6):350–356. doi: 10.1159/000176843. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Control of enzyme levels in mammalian tissues. Adv Enzymol Relat Areas Mol Biol. 1973;37:135–187. doi: 10.1002/9780470122822.ch3. [DOI] [PubMed] [Google Scholar]
- Sketcher R. D., James W. P. Branched-chain amino acid oxidation in relation to catabolic enzyme activities in rats given a protein-free diet at different stages of development. Br J Nutr. 1974 Nov;32(3):615–623. doi: 10.1079/bjn19740114. [DOI] [PubMed] [Google Scholar]
- Van Hinsbergh V. W., Veerkamp J. H., Glatz J. F. 4-Methyl-2-oxopentanoate oxidation by rat skeletal-muscle mitochondria. Biochem J. 1979 Aug 15;182(2):353–360. doi: 10.1042/bj1820353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez J. A., Morse E. L., Adibi S. A. Effect of dietary fat, carbohydrate, and protein on branched-chain amino acid catabolism during caloric restriction. J Clin Invest. 1985 Aug;76(2):737–743. doi: 10.1172/JCI112029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers A. J., Schepens J. T., Veerkamp J. H. Effect of starvation and exercise on actual and total activity of the branched-chain 2-oxo acid dehydrogenase complex in rat tissues. Biochem J. 1984 Nov 1;223(3):815–821. doi: 10.1042/bj2230815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wagenmakers A. J., Schepens J. T., Veldhuizen J. A., Veerkamp J. H. The activity state of the branched-chain 2-oxo acid dehydrogenase complex in rat tissues. Biochem J. 1984 May 15;220(1):273–281. doi: 10.1042/bj2200273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahren J., Felig P., Hagenfeldt L. Effect of protein ingestion on splanchnic and leg metabolism in normal man and in patients with diabetes mellitus. J Clin Invest. 1976 Apr;57(4):987–999. doi: 10.1172/JCI108375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walser M. Therapeutic aspects of branched-chain amino and keto acids. Clin Sci (Lond) 1984 Jan;66(1):1–15. doi: 10.1042/cs0660001. [DOI] [PubMed] [Google Scholar]
- Waterlow J. C. Metabolic adaptation to low intakes of energy and protein. Annu Rev Nutr. 1986;6:495–526. doi: 10.1146/annurev.nu.06.070186.002431. [DOI] [PubMed] [Google Scholar]
- Wieland O. H. The mammalian pyruvate dehydrogenase complex: structure and regulation. Rev Physiol Biochem Pharmacol. 1983;96:123–170. doi: 10.1007/BFb0031008. [DOI] [PubMed] [Google Scholar]
- Wurtman R. J., Hefti F., Melamed E. Precursor control of neurotransmitter synthesis. Pharmacol Rev. 1980 Dec;32(4):315–335. [PubMed] [Google Scholar]