Abstract
Purpose
Plasmacytoid urothelial carcinoma (PUC) is a rare variant histology with poorly defined clinical behavior. We report clinical outcomes information on patients with predominant PUC.
Materials and Methods
Retrospective analysis of treatments and outcomes in patients with predominant PUC seen at MD Anderson Cancer Center from 1990–2010. Kaplan-Meier method was used to calculate Overall (OS) and progression-free survival (PFS).
Results
31 patients were identified (median age:63.5yrs; 83.3% male; TNM stage:cT1N0,n=4;cT2N0,n=7;cT3b-4aN0,n=5; cT4b, N+ or M+ n = 15). Median OS for all patients was 17.7months (Stage I-III vs IV: 45.8 vs 13.3mo). Of 16 patients with potentially surgically resectable PUC (<=pT4aN0M0) 5 received neo-adjuvant chemotherapy, 10 had initial surgery, and one was treated with TURBT alone. Despite pathologic downstaging in 80% of patients treated with neo-adjuvant chemotherapy, relapses were common and there was no difference in survival between patients treated with neo-adjuvant chemotherapy compared to initial surgery, even though adjuvant chemotherapy was given in 7 patients. Surgical upstaging with positive margins was also common with surgery alone. The most common site of recurrence was in the peritoneum (19/23), with relapses occurring even in those with pCR at surgery. In patients presenting with metastatic disease who were treated with chemotherapy, the median survival was 12.6 months.
Conclusions
PUC is a very aggressive subset with overall poor outcomes. Although downstaging is seen with neoadjuvant chemotherapy, there are few long-term survivors. There is a strong predilection for recurrences along the peritoneal lining.
Keywords: survival, plasmacytoid, urothelial carcinoma
1. INTRODUCTION
Plasmacytoid urothelial carcinoma (PUC) is a recognized, yet poorly understood variant histology1. The rarity of these tumors is emphasized by the very small case series, usually focusing on pathological features, that have been published to date 2–8. While plasmacytoid tumors are thought to exhibit more aggressive behavior compared to more traditional high grade urothelial tumors, 2–8 the clinical significance of the plasmacytoid variant has not been well-understood.
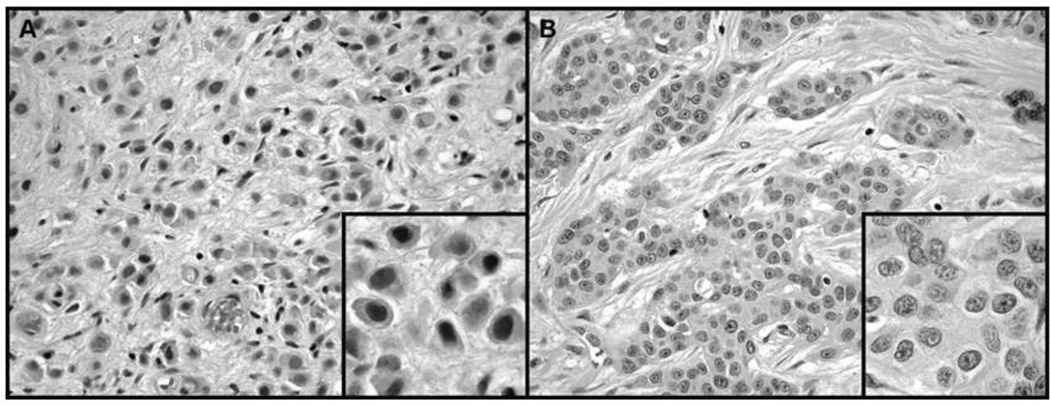
More is known about the histologic characteristics of plasmacytoid urothelial cancer. Maintaining features reminiscent of plasma cells (Figure 1), these tumors exhibit eccentrically placed nuclei with abundant eosinophilic cytoplasm 3, 5, 7 and express CD138, a marker shared with myeloma cells. However, MUM-1, a marker expressed by myeloma cells, is lacking in all examined plasmacytoid bladder cancer cases reported 5. Plasmacytoid urothelial carcinoma cells are highly proliferative and exhibit abundant mitoses with the majority of the cells labeling for Ki-67 7. The cell adhesion marker E-cadherin is downregulated or negative in the majority of plasmacytoid urothelial carcinomas, consistent with its observed mesenchymal phenotype 3, 5, 7. One unique aspect of the latter is a characteristic spread pattern which has been described as “coat sleeve pattern”, along nerve bundles 7.
Figure 1.
(A) Plasmacytoid urothelial carcinoma showing typical dyscohesive tumor cells with perinuclear clearing reminiscent of plasma cells; H&E, ×200 and inset, H&E, ×400. (B) Typical urothelial carcinoma showing cohesive nests of tumor cells with prominent nuclear pleomorphism and conspicuous nucleoli; H&E, ×200 and inset, H&E, ×400.
Given the limited number of cases reported to date, there is a paucity of published data regarding the clinical behavior of this malignancy. We now report clinical outcomes information on patients with predominant plasmacytoid urothelial carcinomas seen at University of Texas MD Anderson Cancer Center (MDACC) during 1990–2010.
2. METHODS
Patients
After obtaining approval from MD Anderson’s institutional review board, we performed a retrospective chart review of all patients with carcinomas of bladder with predominant plasmacytoid histology who had been seen from January 1, 1990, through December 31, 2010. Since the significance of small foci of plasmacytoid histology in the background of urothelial carcinoma is unclear, we only included cases where plasmacytoid morphology was the predominant component, estimated as comprising the majority of the tumor (i.e. >50%). All pathology slides were reviewed and interpreted by experienced, subspecialized genitourinary pathologists at MDACC. The plasmacytoid variant of urothelial carcinoma can be readily diagnosed by pathologists when admixed with more typical urothelial carcinoma in resection specimens. Appropriate stains (including cytokeratins, mucin, and CD138) were performed when needed to differentiate predominant PUC from other variant histologies (e.g. signet ring carcinoma) and to confirm their epithelial derivation. All chart reviews were conducted by the same author (FD), and the diagnosis of predominant PUC was based on the official pathology reports and re-review of sections from two dedicated genitourinary pathologists (BAC, KS).
Survival outcome and statistical analyses
Charts were reviewed for clinical and pathologic staging, surgical procedures, sites of metastases, chemotherapy regimens, survival, and time of last follow-up. Clinical staging was determined using radiographic imaging, and in surgically resectable tumors, using an exam under anesthesia (EUA) following thorough TUR to document cT3b disease or extension to local organs. Data for surviving patients were censored at the time of their last documented clinical assessment. Overall survival (OS) and disease-free survival (DFS) were calculated from the date of diagnosis of urothelial carcinoma to the date of death or last follow-up for all patients. The productlimit estimator of Kaplan and Meier9 was used to calculate OS and DFS. Cox proportional hazards regression analysis10 was used to model the effect of potential prognostic factors on OS from the start of chemotherapy. All analyses were performed with S-PLUS 7.0 for Windows software (Insightful Corp., Seattle, WA).
3. RESULTS
Patients
The characteristics at presentation of all 31 patients are shown in Table 1. More than 85% of the patients had at least muscle-invasive disease, and 48% presented initially with metastatic or locally unresectable disease. The primary tumor was located in the bladder in all cases. Ninety percent of the patients (28/31) received systemic therapy, in about half of the cases for non-metastatic potentially curable disease (either pre-surgical or adjuvant chemotherapy) (Table 2). Interestingly, 7 patients (23%) presented with locally advanced fixed T4b lesions, involving mainly the pelvic side wall and the rectum. On cystoscopic examination under anesthesia, these lesions were frequently described as “linitis plastica”, indicating their infiltrative nature of growth.
TABLE 1.
Characteristics of all patients at presentation.
| Total No. pts (%) | 31 | (100) |
| Age (yrs) | ||
| Median | 63 | |
| Range | 45–78 | |
| No. gender (%) | ||
| Male | 27 | (87) |
| Female | 4 | (13) |
| No. race (%) | ||
| White | 30 | (97) |
| Black | 0 | (0) |
| Other | 1 | (3) |
| No. clinical stage (%) | ||
| I | 4 | (13) |
| II | 7 | (23) |
| III | 5 | (16) |
| IV | 15 | (48) |
| T1N0 | 4 | (13) |
| T2N0 | 7 | (23) |
| T3N0 | 4 | (13) |
| T4aN0 | 1 | (3) |
| T4bN0 | 7 | (23) |
| N1M0 | 4 | (13) |
| M1 | 4 | (13) |
| Histologic variants (No.; %) | ||
| None | 14 | (45) |
| Signet ring cell | 11 | (35) |
| Adenocarcinoma | 2 | (6) |
| Micropapillary | 2 | (6) |
| Other1 | 2 | (6) |
Includes one patient with foci of spindle cells, and one patient who had glandular and micropapillary features.
Table 2.
Surgery and chemotherapy outcomes in 31 patients with predominant plasmacytoid histology
| No. Pts (%) | |
|---|---|
| Surgery type: | |
| No surgery | 9 (29) |
| Cystectomy/cystoprostatectomy | 17 (55) |
| Exenteration | 2 (6) |
| Transurethral bladder tumor resection | 2 (6) |
| Surgery aborted due to T4b stage | 3 (10) |
| Unknown | 1 (3) |
| Lymph node dissection: | |
| Yes | 20 (65) |
| No | 9 (29) |
| Unknown | 2 (6) |
| Lymph node status at surgery: | |
| Pos | 11 (35) |
| Neg | 9 (29) |
| Not applicable (no surgery or no lymph node dissection) | 10 (32) |
| Unknown | 1 (3) |
| Margin status at surgery: | |
| Pos | 2 (6) |
| Neg | 18 (58) |
| Not applicable (no surgery or unreported margins) | 10 (32) |
| Unknown | 1 (3) |
| Pathological CR: | |
| Yes | 4 (13) |
| No | 17 (55) |
| Not applicable (no or aborted surgery) | 9 (29) |
| Unknown | 1 (3) |
| Chemotherapy setting: | |
| None | 3 (10) |
| Neoadjuvant | 7 (23) |
| Adjuvant | 7 (23) |
| Metastatic | 14 (45) |
| Best chemotherapy response: | |
| CR (tumor resolution on imaging) | 6 (19) |
| Less than CR on imaging | 10 (32) |
| Stable disease | 1 (3) |
| Progressive disease | 1 (3) |
| Unknown | 2 (6) |
| Not applicable (adjuvant or no chemotherapy) | 11 (35) |
| Chemotherapy type:* | |
| Dose dense MVAC | 14 (45) |
| Ifosfamide, doxorubicin + gemcitabine | 8 (26) |
| GC based | 6 (19) |
| Other | 6 (19) |
| Unknown | 1 (3) |
| None | 3 (10) |
| Post-chemotherapy progression site:† | |
| Peritoneum | 19 (61) |
| Retroperitoneal lymph nodes | 3 (10) |
| Bladder | 3 (10) |
| Bone, lungs, scrotum | 3 (10) |
| Unknown | 3 (10) |
| No progression | 5 (16) |
CR plus partial response in 7 of 9 patients with dose dense MVAC, 6 of 7 (86%) with ifosfamide, doxorubicin and gemcitabine, all 5 (100%) with GC based chemotherapy and 3 of 4 (75%) with other chemotherapy, not including those with adjuvant chemotherapy or missing response data and with percents totaling greater than 100% since some patients received more than 1 regimen.
Some patients had progression at different sites.
Resectable Disease
Sixteen patients had surgically resectable disease (stage I-III) at the time of presentation, and 14 of them underwent chemotherapy (neoadjuvant, n=5; adjuvant, n=7). In addition, seven patients with stage IV at presentation (two had clinically positive lymph nodes) received presurgical chemotherapy followed by salvage surgery (one patient declined surgery). While in patients with stage IV who underwent chemotherapy followed by surgery there was no downstaging, we observed in four of five (80%) of patients who were treated with neo-adjuvant chemotherapy pathologic downstaging, including three complete responses (pT0N0). Pathologic upstaging was frequent in patients who underwent upfront surgery. All neoadjuvant therapies included cisplatin-containing regimens (either methotrexate, vinblastine, doxorubicin, cisplatin [MVAC] or gemcitabine, cisplatin [GC]) or an ifosfamide containing regimen ifosfamide, doxorubicin, and gemcitabine (IAG)). Six patients were treated with MVAC in the adjuvant setting, and one patient received adjuvant chemo-radiation with cisplatin.
In all available specimens from patients who underwent surgery, the rate of negative margins and negative lymph node status at the time of surgery were 90% (18/20) and 45% (9/20), respectively (Table 2).
Metastatic disease
Fifteen patients presented with metastatic or locally unresectable (i.e. cT4b) disease, 13 of them were treated with chemotherapy. Treatment information was not available for one patient. Nine of fifteen (60%) of treatment regimens included MVAC or GC-based combinations. Overall response rate (CR+PR) was 53% (8/15), with two patients having a minimal response. Only one patient experienced disease progression (7%) and response data were missing for the remaining patients. As mentioned above, 4/15 (29%) patients with stage 4 disease had salvage surgery after upfront chemotherapy (one patient had declined surgery, in two other patients surgery was aborted). Three underwent pelvic exenteration and one patient had radical cystoprostatectomy and bilateral pelvic lymph node dissection. All four have died after a median of 11.6 months (range 6–23 months).
Survival
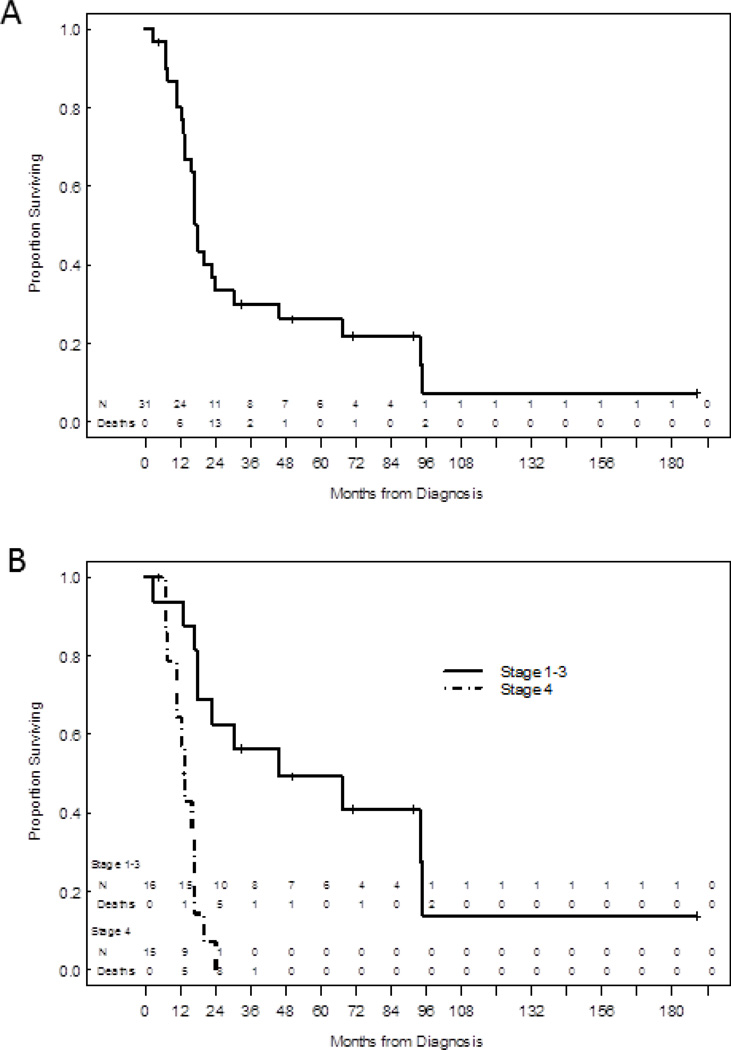
The overall survival (OS) for all 31 patients was 17.7 months (Figure 2A). As expected, patients with non-metastatic (stage I-III) disease had a better OS compared to those with stage IV disease (45.8 vs. 13.4 months; P<0.001; Figure 2B). Those patients with de novo metastatic bladder cancer had a median survival of 12.9 months from the initiation of chemotherapy, while survival from the first cycle of pre-surgical chemotherapy (or from the date of surgery for those who were not treated with chemotherapy) was 37.3 months. Out of 4 patients with a pathologic complete response at the time of surgery, one remains without evidence of disease with a follow-up of more than 18 months, and another one died from an unrelated cause.
Figure 2.
(A) Overall survival (OS) for all patients (n=31) was 17.7 months. (B) OS by stage (I-III [45.8 months] vs. IV [13.4 months]; P<0.001).
Three of 31 patients were lost to follow-up and data on progression of their disease was not available. Among the remaining 28, at the time of analysis (November 2011), objective progression of disease was documented in 23 patients (82%). Interestingly, 19 of these 23 patients (83%) developed recurrent disease in the peritoneum, while 3 of 23 (13%) had local recurrence in the bladder (Table 2). In 11 of 19 patients (58%) with peritoneal disease involvement, the peritoneum was the only site of the initial recurrence. Recurrence was diagnosed in all except two patients based on computed tomography and characteristic findings of peritoneal thickening consistent with carcinomatosis. One patient with peritoneal metastasis exhibited a rise in serum CA-125 levels prior to detection of macroscopic metastatic disease, and another one presented with a malignant effusion. Three patients (10%) developed leptomeningeal involvement during the course of their disease.
4. DISCUSSION
Plasmacytoid urothelial carcinomas (PUC) are an aggressive subset of bladder cancers. Initial response rates to chemotherapy are relatively high, as supported by our finding that only one patient had primary refractory disease to upfront chemotherapy. However, the majority of cases of PUC in our series recurred rapidly, with a remarkable predilection for peritoneal carcinomatosis. Even with neo-adjuvant surgery, the outcomes are modest and survival is around three years.11, 12 . At this point it is not clear whether the poor outcomes of PUC are due to the underlying biology or because the conventional cytotoxic regimens for urothelial cancers are less effective in PUC.
This series of 31 consecutive patients with predominant PUC represents the largest cohort of patients with PUC reported in the literature to date, and is the first to focus on clinical phenotype and treatment outcome. The clinical pattern of extensive local involvement with linitus plastica, and extension to surrounding tissues with circumferential thickening in both bladder and rectum, provides some insight into the biology of these tumors. It is possible that this reflects the archetype of a bladder tumor that has undergone EMT (epithelial-mesenchymal transition), a feature associated with a tumor’s capacity to invade through tissue. The mesenchymal phenotype in more traditional urothelial cancers is associated with a loss of e-cadherin and increased expression of vimentin, ZEB1, and Zeb213. Previous reports suggest a lack of e-cadherin expression in plasmacytoid tumors 14, providing some support for this hypothesis. Additional studies of plasmacytoid histology may provide some insight into the clinical behavior of other urothelial malignancies that behave in a locally infiltrating fashion.
Previous reports have all described an aggressive clinical behavior of PUCs3, 5, 6. The largest series thus far was published by Keck et al., and described the morphologic features of 32 patients with PUC3. Like other reports8, we could confirm a male predominance in patients with PUC. Fifty percent of our cohort presented with locally advanced disease (>T3 stage), one third of the patients had metastatic disease. Unlike other reported cases 3, 5, 6, 14, most of our patients with predominant PUC received cisplatin-based systemic therapy. Although the majority of the tumors responded to upfront chemotherapy, our results indicate that outcomes are still unsatisfactory and long-term survival is only seen in relatively few patients with ≤T4a non-metastatic tumors (stage I-III). These outcomes are much worse than what would typically be expected in patients with more traditional urothelial histology where the 5 year OS and DSS is around 63% and 68%, respectively, even in patients with clinical features placing them at a high risk of stage III or greater disease 15.
For patients with resectable bladder cancer who underwent cystectomy without chemotherapy, median OS of 5–15 years have been reported, depending on the T-stage of the tumor16. Although it is difficult to compare survival rates between our cohort and the reported cohort because of sample size and other factors, it is still remarkable that in our cohort, despite neoadjuvant chemotherapy, the patients with resectable disease had only a median OS of less than four years. Compared with non-PUC bladder cancers, there is also an inferior survival for stage IV patients. While up to 20% of stage IV patients with conventional urothelial carcinomas might be alive at 5 years 16, none of the patients with stage IV PUC in our study survived beyond 24 months.
Interestingly, the most common site of recurrence in these patients was the peritoneum, and in some an initial surge in the serum CA-125 levels preceded radiologic and symptomatic findings of progression. Thus, our description of the peritoneum as the primary site of recurrence might suggest follow-up with serial serum CA-125 measurements17 which will aid in recognizing early disease progression and possibly initiating second-line therapies sooner before the patients become symptomatic.
5. CONCLUSIONS
In conclusion, PUC is a rare tumor with a locally infiltrative pattern. The prognosis remains poor, with few long-term survivors despite neo-adjuvant chemotherapy. Patients are at a high risk of relapse in the peritoneal lining. The diagnosis of peritoneal carcinomatosis should be considered in patients presenting with abdominal symptoms. Tumor markers, including CEA, CA125, and CA19-9 may be useful in this disease. Responses to traditional urothelial cancer regimens, including DD-MVAC, are of short duration. It is still unknown whether this is the optimal chemotherapy regimen for this cancer.
Acknowledgments
Funding/Support and role of the sponsor: F.D. was supported by NIH grant T32 CA009666; 1 P50 CA140388-01; F.D. was also supported from a grant by the Prostate Cancer Foundation. Also supported by NIH/NCI under award number P30CA016672 and used the Biostatistics Resource Group shared resources.
Abbreviations
- PUC
Plasmacytoid urothelial carcinoma
Footnotes
Author contributions: FD and ASR had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: FD, ASR.
Acquisition of data: FD, ASR.
Analysis and interpretation of data: All authors.
Drafting of the manuscript: FD.
Critical revision of the manuscript for important intellectual content: All authors.
Statistical analysis: MM, ASR.
Obtaining funding: None.
Administrative, technical, or material support: None.
Supervision: FD, ASR.
Other (specify): None.
Financial disclosures: All authors declare no conflict of interest.
REFERENCES
- 1.Montironi R, Lopez-Beltran A. The 2004 WHO classification of bladder tumors: a summary and commentary. International journal of surgical pathology. 2005;13:143–153. doi: 10.1177/106689690501300203. [DOI] [PubMed] [Google Scholar]
- 2.Mai KT, Park PC, Yazdi HM, et al. Plasmacytoid urothelial carcinoma of the urinary bladder report of seven new cases. Eur Urol. 2006;50:1111–1114. doi: 10.1016/j.eururo.2005.12.047. [DOI] [PubMed] [Google Scholar]
- 3.Keck B, Stoehr R, Wach S, et al. The plasmacytoid carcinoma of the bladder-rare variant of aggressive urothelial carcinoma. Int J Cancer. doi: 10.1002/ijc.25700. [DOI] [PubMed] [Google Scholar]
- 4.Kohno T, Kitamura M, Akai H, et al. Plasmacytoid urothelial carcinoma of the bladder. Int J Urol. 2006;13:485–486. doi: 10.1111/j.1442-2042.2006.01338.x. [DOI] [PubMed] [Google Scholar]
- 5.Lopez-Beltran A, Requena MJ, Montironi R, et al. Plasmacytoid urothelial carcinoma of the bladder. Hum Pathol. 2009;40:1023–1028. doi: 10.1016/j.humpath.2009.01.001. [DOI] [PubMed] [Google Scholar]
- 6.Nigwekar P, Tamboli P, Amin MB, et al. Plasmacytoid urothelial carcinoma: detailed analysis of morphology with clinicopathologic correlation in 17 cases. Am J Surg Pathol. 2009;33:417–424. doi: 10.1097/PAS.0b013e318186c45e. [DOI] [PubMed] [Google Scholar]
- 7.Raspollini MR, Sardi I, Giunti L, et al. Plasmacytoid urothelial carcinoma of the urinary bladder: clinicopathologic, immunohistochemical, ultrastructural, and molecular analysis of a case series. Hum Pathol. doi: 10.1016/j.humpath.2010.11.011. [DOI] [PubMed] [Google Scholar]
- 8.Ro JY, Shen SS, Lee HI, et al. Plasmacytoid transitional cell carcinoma of urinary bladder: a clinicopathologic study of 9 cases. Am J Surg Pathol. 2008;32:752–757. doi: 10.1097/PAS.0b013e318159af9e. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. Journal of the American Statistical Association. 1958;53:457–481. [Google Scholar]
- 10.Cox D. Regression models and life tables (with discussion) Journal of the Royal Statistical Society B. 1972;34:187–220. [Google Scholar]
- 11.Siefker-Radtke AO, Dinney CP, Abrahams NA, et al. Evidence supporting preoperative chemotherapy for small cell carcinoma of the bladder: a retrospective review of the M. D. Anderson cancer experience. J Urol. 2004;172:481–484. doi: 10.1097/01.ju.0000132413.85866.fc. [DOI] [PubMed] [Google Scholar]
- 12.Siefker-Radtke AO, Kamat AM, Grossman HB, et al. Phase II clinical trial of neoadjuvant alternating doublet chemotherapy with ifosfamide/doxorubicin and etoposide/cisplatin in small-cell urothelial cancer. J Clin Oncol. 2009;27:2592–2597. doi: 10.1200/JCO.2008.19.0256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.McConkey DJ, Lee S, Choi W, et al. Molecular genetics of bladder cancer: Emerging mechanisms of tumor initiation and progression. Urol Oncol. 2010;28:429–440. doi: 10.1016/j.urolonc.2010.04.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Fritsche HM, Burger M, Denzinger S, et al. Plasmacytoid urothelial carcinoma of the bladder: histological and clinical features of 5 cases. J Urol. 2008;180:1923–1927. doi: 10.1016/j.juro.2008.07.035. [DOI] [PubMed] [Google Scholar]
- 15.Siefker-Radtke AO, Dinney CP, Shen Y, et al. A phase 2 clinical trial of sequential neoadjuvant chemotherapy with ifosfamide, doxorubicin, and gemcitabine followed by cisplatin, gemcitabine, and ifosfamide in locally advanced urothelial cancer: Final results. Cancer. 2012 doi: 10.1002/cncr.27751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stein JP, Lieskovsky G, Cote R, et al. Radical cystectomy in the treatment of invasive bladder cancer: long-term results in 1,054 patients. J Clin Oncol. 2001;19:666–675. doi: 10.1200/JCO.2001.19.3.666. [DOI] [PubMed] [Google Scholar]
- 17.Topalak O, Saygili U, Soyturk M, et al. Serum, pleural effusion, and ascites CA-125 levels in ovarian cancer and nonovarian benign and malignant diseases: a comparative study. Gynecol Oncol. 2002;85:108–113. doi: 10.1006/gyno.2001.6575. [DOI] [PubMed] [Google Scholar]