Abstract
Background and Purpose
Endothelins act via two receptor subtypes, ETA and ETB. Under physiological conditions in coronary arteries, ETA receptors expressed in smooth muscle cells mediate vasoconstriction whereas ETB receptors mainly found in endothelial cells mediate vasorelaxation. However, under pathophysiological conditions, ETB receptors may also be expressed in vascular smooth muscle cells mediating vasoconstriction. Here, we have investigated whether vasoconstrictor ETB receptors are up-regulated in coronary arteries after experimental myocardial ischaemia in rats.
Experimental Approach
Male Sprague-Dawley rats were subjected to either heart ischaemia–reperfusion (15 min ischaemia and 22 h reperfusion), permanent ischaemia (22 h) by ligation of the left anterior descending coronary artery, or sham operation. Using wire myography, the endothelin receptor subtypes mediating vasoconstriction were examined in isolated segments of the left anterior descending and the non-ligated septal coronary arteries. Endothelin receptor-mediated vasoconstriction was examined with cumulative administration of sarafotoxin 6c (ETB receptor agonist) and endothelin-1 (with or without ETA or ETB receptor blockade). The distribution of ETB receptors was localized with immunohistochemistry and quantified by Western blot.
Key Results
Endothelin ETB receptor-mediated vasoconstriction and receptor protein levels were significantly augmented in coronary arteries situated downstream of the occlusion after ischaemia–reperfusion compared with non-ischaemic arteries. In contrast, the ETA receptor-mediated vasoconstriction was unaltered in all groups.
Conclusions and Implications
Ischaemia–reperfusion induced local up-regulation of ETB receptors in the smooth muscle cells of coronary arteries in the post-ischaemic area. In contrast, in non-ischaemic areas, ETB receptor function was unaltered.
Keywords: endothelin receptor, endothelin, coronary artery, sarafotoxin 6c, vascular smooth muscle
Introduction
Vasoactive peptides play a significant role in the regulation of coronary blood flow. One of the most important groups of vasoactive peptides is the family of endothelins. The family comprises three 21-amino acid peptides: endothelin-1, -2 and -3 (ET-1, -2,-3; Yanagisawa et al., 1988b; Inoue et al., 1989). The principal isoform in the heart is ET-1 and this peptide is the most potent endogenous vasoconstrictor yet discovered (Yanagisawa et al., 1988a). In the heart, ET-1 is secreted by both vascular and endocardial endothelial cells, as well as by myocytes (Wagner et al., 1992; Suzuki et al., 1993; Saetrum et al., 2001).
The actions of ET-1 are mediated via ETA and ETB receptors (nomenclature follows Alexander et al., 2013) which belong to the 7-transmembrane domain receptor family (Arai et al., 1990; Sakurai et al., 1990). In vasculature, ETA receptors are located in vascular smooth muscle cells (VSMCs) which mediate potent vasoconstriction. In contrast, ETB receptors in healthy vessels are located in the endothelium where they cause vasorelaxation through release of prostacyclin and NO (Seo et al., 1994; Bacon et al., 1996; Alonso and Radomski, 2013). However, ETB receptor distribution is not restricted to endothelial cells. Besides its primary physiological function as vasodilator, ETB receptors have been shown to mediate vasoconstriction in VSMCs (Seo et al., 1994; Adner et al., 1998).
The degree of functional ETB receptor expression in coronary artery VSMC may be subjected to modulation and possibly related to pathophysiological conditions of coronary arteries. A binding study suggests increased ETB receptor expression in VSMC in human atherosclerotic coronary arteries (Dagassan et al., 1996) while another has shown elevated ETB receptor mRNA levels in coronary arteries from patients after myocardial infarction (Wackenfors et al., 2004). These interesting observations suggest that altered expression of contractile ETB receptors in coronary arteries may participate in and augment the vascular tone which consequently could impair myocardial blood flow. However, the ETB receptor expression studies need further validation and the mechanisms involved need elucidation, as the coronary arteries mentioned above were obtained by autopsy 10–24 h post mortem from patients with chronic pathological conditions. In addition, the above studies neither reported the vasoactivity mediated by the endothelin ETB receptor nor the receptors' exact localization in the coronary artery wall. Furthermore, the studies did not address whether an acute cessation of blood flow initiates an acute ETB receptor up-regulation, or if this is restricted to a slowly progressing and chronic condition, atherosclerosis.
In order to address these questions, we used a non-atherosclerotic heart ischaemia model in rats to examine the hypothesis that coronary arteries show altered expression of endothelin receptors acutely after myocardial ischaemia–reperfusion (IR) and permanent ischaemia (PI), using functional and molecular methods.
Methods
Animals
All animal care and experimental procedures were performed in accordance with the national laws and guidelines and approved by the Danish Animal Experimentation Board (2006/561-1234 and 2012/561-162). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). A total of 85 animals were used in the experiments described here. Male Sprague-Dawley rats (9–11 weeks, weight 295–355 g) were obtained from Taconic, Denmark. Rats were provided with standard rat chow and water ad libitum, and were housed under a 12 h light and 12 h dark cycle condition.
Surgery
The rats were divided at random into three groups in which they were either subjected to heart ischaemia followed by reperfusion (n = 37), permanent heart ischaemia (n = 5) or sham (n = 33) operation procedures.
The rats were anaesthetized with a 2.5 mL·kg−1 mixture of hypnorm-midazolam (1:1:2) in sterile water (containing 0.079 mg·mL−1 fentanyl, 2.50 mg·mL−1 fluanison, Hypnorm®; VetaPharma Ltd, Leeds, UK, and 1.25 mg·mL−1 midazolam, ‘Hameln’; Hameln, Germany). During the surgical procedure, rats were artificially ventilated (75 cycles/min, tidal volume 10 mL air/kg) with a rodent ventilator (Ugo Basile Rodent Ventilator, Comerio, Italy) and body temperature (37°C) was maintained by keeping the rats on a heating pad.
The chest was opened through a left thoracotomy, and the heart was exposed by making an incision in the pericardium. A 6-0 prolene suture was passed around the left anterior descending coronary artery (LAD) approximately 4–5 mm away from its origin. In the IR group, ischaemia was achieved by passing the ends of the suture through a plastic pearl and tightening it against polyethylene tubing. After 15 min of ischaemia, the ligature around the LAD was released and the tissue was reperfused for 22 h. Regional myocardial ischaemia was confirmed by visual observation of cyanosis over the left ventricular surface and ST segment elevation was seen on the ECG obtained from a precordial lead and recorded via a bio-amplifier (ADInstruments, Oxford, UK) and collected by a PowerLab unit (ADInstruments). After the ligature was loosened, the ST elevation returned to normal after about 30 min of reperfusion. The suture was kept in the heart to mark the occlusion site.
The chest was closed by approximating the edges of the ribs and by stitching the musculature with a 4-0 suture. When the last suture was placed, air was sucked out of the thorax via a polyvinyl catheter. Skin was closed with a 4-0 suture after the removal of the catheter. As post-operative analgesia and fluid supplement, the rats were given (s.c.) 5 mg·kg−1 carprofen (Rimadyl Vet; Pfizer, Helsinki, Finland), 0.03 mg·kg−1 buprenorphin (Temgesic; Schering-Plough, Brussels, Belgium) and 5 mL 0.9% NaCl isotonic solution. Rats were allowed to recover on a heating pad (37°C) and were extubated when normal spontaneous breathing resumed.
In PI, the suture was kept tight to maintain occlusion for 24 h. Sham-operated rats underwent identical surgical procedure with the exception of the suture being loosely placed around the LAD.
Vessel isolation
The rats were sedated with CO2 and killed by decapitation, at 22 h after the surgery. The heart was immediately excised and placed in oxygenated ice cold physiological saline solution (PSS): 119 mM NaCl, 4.7 mM KCl, 2.5 mM CaCl2, 25 mM NaHCO3, 1.17 mM MgSO4, 1.18 mM KH2PO4, 5.5 mM glucose and 0.03 mM EDTA, with 5% CO2. The LAD and septal coronary arteries (SCAs) were removed by dissecting the tissue around the vessel wall. The LAD was divided into two, approximately 2 mm, segments: one upstream and one downstream of the ligature. The SCA segment was dissected out approximately 2–4 mm away from its origin.
Wire myography
Coronary artery segments were mounted in a wire myograph and stretched to their optimal lumen diameter in order to obtain optimal conditions for active tension development, as described previously (Skovsted et al., 2012).
Effect of myograph incubation time on ET receptor-mediated responses
ETB receptor-mediated responses were investigated using the peptide sarafotoxin 6c (S6c), which exhibits high selectivity for the ETB receptor over the ETA receptor. The ETA receptor-mediated response was investigated on the same artery segments after S6c treatment by adding ET-1 ranging from 1 pM to 30 nM. Before adding ET-1, the arteries were pretreated with the selective ETB receptor antagonist BQ788 (30 nM) for 30 min. Other segments were stimulated with ET-1 in the presence of BQ788 (0.9 μM) but without prior desensitization with S6c. In other coronary segments, dual ETA- and ETB-mediated responses were investigated using the endogenous ligands, ET-1 (1 pM–30 nM) with or without the presence of the selective ETA receptor antagonist BQ123 (10 μM). Before each concentration response curve, the VSMC contractile function was confirmed by challenging the segments two or three times with KPSS (PSS containing 125 mM K+; NaCl was exchanged for KCl on an equimolar basis).
Endothelial function and maximal contractile capacity
Endothelial function was determined on all artery segments by assessing the relaxation to carbachol (10 μM) after obtaining steady-state pre-contraction tone with PGF2α (10 μM). Normal endothelial function (100%) was defined as a carbachol-induced relaxation of the PGF2α-induced contraction to the baseline.
Maximal relaxation was determined by exposing the vessels to calcium free buffer solution (Ca2+-free PSS) buffer. After the last concentration–response curve, the maximal contractile capacity of the arterial segments was assessed by addition of a ‘cocktail’ solution (KPSS with 10 μM PGF2α and 10 μM 5-HT).
Immunohistochemistry
Immediately after dissection coronary arteries were embedded in Tissue-Tek®, frozen on dry ice and stored at −80°C until further processing. The frozen coronary arteries were sectioned (10 μm) on a cryostat (Leica, Denmark) and mounted on microscope slides (SuperFrost®, Menzel, Germany). The sections were fixed for 20 min using Stefanini's fixative (2% paraformaldehyde and 0.2% picric acid in phosphate buffer, pH 7.2) and permeabilized in PBS containing 0.25% Triton X-100 (T-PBS). To prevent non-specific staining, the sections were blocked with 2% donkey serum and 1% BSA in T-PBS for 1 h. Samples were incubated at 4°C overnight with primary antibodies: mouse anti-actin (1:500, AB11003; Abcam, Cambridge, UK) and sheep anti-ETB receptor (1:250, alx-210-506a; Alexis Biochemicals, Nottingham, UK) diluted in T-PBS. The following day, the slides were rinsed in 3x PBS and incubated with secondary antibodies: donkey anti-mouse IgG DyLight™ 549 (1:200, 715-505-150; Jackson ImmunoResearch, Suffolk, UK) and donkey anti-sheep DyLight™ 488 (1:200, 713-485-003; Jackson ImmunoResearch) for 1 h followed by 3x PBS before mounting with ClearMount™ (Invitrogen™, Cavarillo, CA, USA). For negative control staining, the anti-ETB receptor primary antibody was omitted.
In order to confirm the immunohistochemical results, the staining was repeated using another primary antibody (1:400, rabbit anti-ETB receptor, AER-002; Alomone Labs Ltd., Jerusalem, Israel) targeting a different epitope of the ETB receptor. Here, donkey anti-rabbit DyLightTM 488 (1:200, 711-485-152; Jackson ImmunoResearch) was used as secondary antibody. Preabsorption control was performed using an antigenic peptide (CEMLR KKSGM QIALN D, corresponding to amino acid residues 298-314 of the ETB receptor). The ratio between blocking peptide and antibody was 1 μg peptide/1 μg antibody, giving an approximately 30 times molar excess of peptide. Blocking peptides were preincubated in dilution buffer with 2% donkey serum with the corresponding primary antibody at +4°C for 24 h and then used in immunostaining procedures as described above, in parallel with the staining without pre-adsorption. Omission of primary antibodies and preabsorption with blocking peptides served as negative controls. The immunostaining protocol used here was the same as described above. Immunostaining with both primary antibodies revealed similar staining patterns in terms of receptor localization (data not shown).
Confocal microscopy
The images were taken using a 60× oil immersion lens NA 1.4 on a Nikon C1 confocal microscope (LRI, Lund, Sweden). The laser channels used were 488 nm excitation (with filter 515/30) and 543 nm excitation (with filter 605/75). The gain and laser intensities were kept constant between the samples. Fluorescence intensity measurements were performed as a region of interest consisting of the entire vessel area staining for both actin and the ETB receptor in question from four vessel sections of each vessel sample. Lining tissue and lamina elastica was omitted from intensity measurements. Image analyses were conducted using NIS basic research software (Nikon D-Eclipse C1; Nikon Instruments, Amsterdam, the Netherlands). All quantifications were done without knowledge of the treatments. The fluorescence intensity of each group is given as the percentage fluorescence of IR LAD downstream or upstream group compared with the sham LAD downstream groups, where the LAD downstream group was set to 100%, and the mean value for each of the other groups was used for comparison.
Western blot
Coronary artery segments (upstream, downstream LAD) were pooled from four IR-operated rats and from five sham-operated rats for each blot. Three blots were performed as described below.
After isolation the arteries were stored at −80°C and subsequently immersed in 50 μL modified RIPA (50 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 50 mM β -glycerolphosphate, 1% NP-40, 0.1% deoxycholate, 0.1% SDS, 0.5% Triton X-100) containing Complete® Protease Inhibitor and PhosStop cocktails (both from Roche, Hvidovre, Denmark) before sonication (2 × 10 1 s pulses, with intermittent cooling on ice, and then 15 s constant). The resulting lysate was pre-cleared by centrifugation (18 000× g at 4°C for 15 min). The supernatant was transferred to new tubes and total protein concentration was determined using the Bio-Rad DC kit (Bio-Rad Laboratories, Copenhagen, Denmark) and a Tecan M200 spectrophotometer at 750 nm. Samples were adjusted to 20 μg of total protein and dissolved in LDS buffer (Expedeon, San Diego, CA, USA) containing 50 mM DTT. Proteins were separated by gel electrophoresis on 4–20% RunBlue gradient gels (Expedeon) at a maximum of 180 V, 150 mA and 15 W for approximately 1 h and 10 min. After electrophoresis, gels were briefly equilibrated in Tris-glycine transfer buffer [2.9 g Trizma-base and 14.4 g glycine L-1, 20% ethanol and 0.1% SDS diluted in MilliQ water (ELGA LabWater Global Operations Centre, Bucks, UK)] before transfer to ECL Hybond nitrocellulose membranes (GE Lifesciences, Brøndby, Denmark) using wet blotting for 1 h and 20 min (maximum settings of 180 V and 350 mA). Membranes were subsequently blocked for 1 h in 2% ECL Advance Blocking Agent (GE Lifesciences) in TBS-T (TBS and 0.1% Tween 20) before incubation overnight at 4°C on a rotor with primary antibody (polyclonal rabbit anti-ETB receptor, 1:200 in TBS-T containing 0.02% sodium azide, AER-002; Alomone Laboratories). The next day, membranes were briefly washed in TBS-T before 1 h of incubation at room temperature with secondary antibodies (HRP-conjugated donkey anti-rabbit, 1:40.000 in TBS-T, NA9340V; GE Lifesciences). Finally, membranes were washed a minimum of 5 × 5 min in large volumes of TBS-T before development using the ECL Advance Western Blot Detection Kit (GE Lifesciences) for 5 min at room temperature. Image capture was performed using a Fujifilm LAS-4000 imaging unit (Fujifilm, Tokyo, Japan).
To quantify the relative band intensity signal, it was first normalized to GAPDH as internal loading control and then compared using MultiGauge 3.2 software (Fujifilm). GAPDH was chosen as loading control as it had been tested for variability in a screen of five possible loading control candidates.
Triphenyltetrazolium chloride (TTC) staining
TTC staining was used to assess viability of the myocardial tissue and identification of myocardial infarction. After dissection of the coronary arteries, the heart was wrapped in clear food plastic and semifrozen in −20°C for 30 min and cut into 2 mm slices. The slices were incubated in 1% TTC (Catalogue No. 103 126; Biomedicals LLC, Aurora, OH, USA) at 37°C for 15–20 min and then rinsed briefly in PBS and the infarcted area (unstained by TTC) and viable (red) areas were visualized using a digital camera (Infinity 2; LRI Instruments). TTC staining was done in five to seven hearts of each group.
Data analysis
Results are given as mean ± SEM and n denotes the number of rats. All concentration–response curves were analysed by non-linear regression analysis using GraphPad Prism 5.03 (GraphPad Corp, San Diego, CA, USA). Each regression line was fitted to sigmoid equation: Y = bottom + (top bottom)/(1 + 10∧((LogEC50-X)*Hill Slope)), where X is the log of agonist concentration, Y is the contractile response developed by the agonist and normalized to the cocktail response, bottom is the initial contractile response (basal tone), top is the top plateau of the concentration–response curve induced by the agonist. Hill Slope describes the steepness of the curve. Emax is the difference between the top and bottom normalized to cocktail response and sensitivity to the agonist is expressed as the negative logarithm of the molar concentration of the agonist that elicited 50% of maximum contraction (pEC50). Staining intensities for ETB receptors and the coronary contraction to S6c or endothelin-1 were compared in the different vascular segments by Student's t-test or two-way repeated-measures anova followed by Bonferroni post-test respectively. Statistical significance was accepted when P < 0.05.
Materials
The materials used in these experiments were supplied as follows: BQ788 (30 nM; Sigma, St. Louis, MO, USA); BQ123, 5-HT, carbachol (Sigma-Aldrich Chemie GmbH, Schnelldorf, Germany); ET-1, sarafotoxin 6c (NeoMPS, Strasbourg, France); PGF2α (Dinolytic®, Pfizer, Helsinki, Finland)
Results
Infarction
Rats that underwent IR showed scattered subendocardial infarctions located in the myocardium downstream to the occlusion (Figure 1B and E). This was in contrast to the solid infarction displayed after PI (Figure 1C and F). The sham-operated hearts did not show any infarction (Figure 1A and D).
Figure 1.
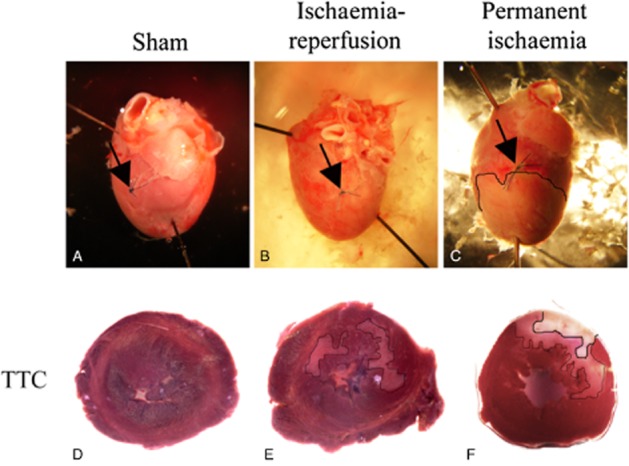
Montage demonstrating representative (A) sham-operated, (B) ischaemia-reperfused and (C) permanent ischaemic hearts. Upper panel shows hearts with the surgical ligature (arrows) and in the lower panel, TTC staining of heart slices. The thin line (in D, E) outlines areas with diffuse ischaemic appearance and the thicker line (only in permanent ischaemic hearts; F) with clear infarction.
General vasomotor response
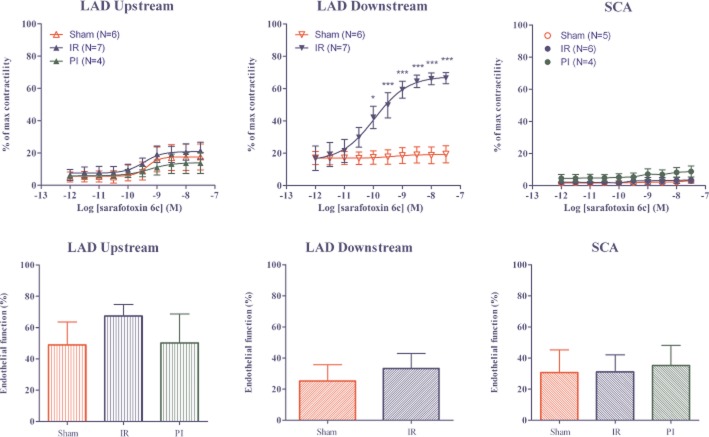
Coronary arteries, isolated from the infarcted area of hearts subjected to PI, showed no contractile responses after addition of KPSS, the cocktail[KPSS+ 5-HT (10 μM) and PGF2α (10 μM)], S6c or ET-1. Therefore, these arteries were excluded from the further functional studies. In contrast, segments of the LAD downstream of the ligature after IR exhibited sufficient contractile capacity and were included for further functional studies. The maximal contraction induced by the cocktail was slightly higher in segments of the SCA compared with those from the LAD. The endothelial function was similar within all three artery segments (LAD upstream, LAD downstream and SCA) of IR- (LAD upstream and SCA), PI- and sham-operated rats (Figures 2 and 3B). However, LAD upstream segments from IR hearts revealed a significantly higher endothelial function, compared with that in both LAD downstream and SCA segments.
Figure 2.
Upper panel: Concentration–response curves of increasing concentrations of S6c in LAD upstream to occlusion, LAD downstream to occlusion, and SCA after IR, sham or PI procedures. The results are shown as mean values ± SEM (n = 4–7). ***P < 0.001 and *P < 0.05; two-way anova with repeated measurements and Bonferroni multiple comparison test. Characteristics of the arteries used are shown in Table 1. Lower panel: Percentage of endothelium-mediated relaxation induced by carbachol (10 μM) after precontraction with PGF2α (0.3 μM) in LAD upstream to occlusion, LAD downstream to occlusion, and SCA after sham operation, IR or PI. NS = P > 0.05; one-way anova with Newman–Keuls multiple comparison test or Student's t-test.
Figure 3.
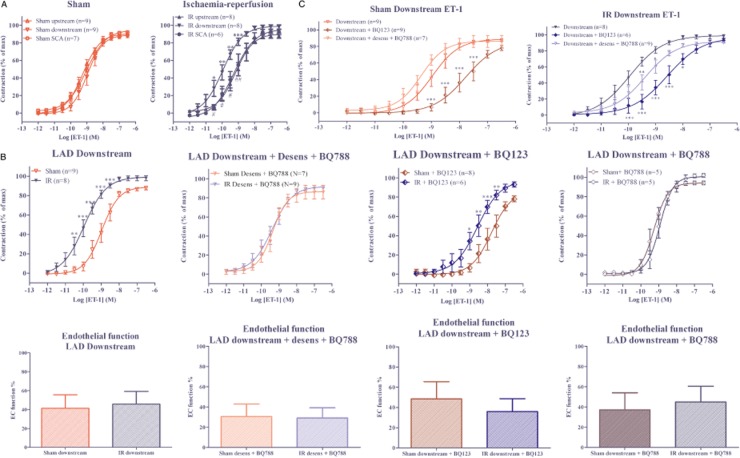
(A) Concentration–response curves of increasing concentrations of ET-1 in LAD upstream to ligature, LAD downstream to ligature, and SCA after IR or sham procedures. The results are shown as mean values ± SEM (n = 6–9). ***P < 0.001, **P < 0.01, *P < 0.05; downstream vs. upstream; ##P < 0.01. # P < 0.05 downstream vs. SCA; two-way anova with repeated measurements and Bonferroni multiple comparison test. Characteristics of the arteries used are shown in Table 1. (B) Concentration–response curves in LAD downstream segments after IR or sham procedures induced by ET-1, ET-1 after S6c induced ETB desensitization and addition of the selective endothelin ETB receptor antagonist BQ788 (30 nM), ET-1 after addition of the selective endothelin ETA receptor antagonist BQ123 (10 μM) and ET-1 after addition of the selective endothelin ETB receptor antagonist BQ788. The results are shown as mean values ± SEM (n = 5–9). ***P < 0.001, **P < 0.01 and *P < 0.05; two-way anova with repeated measurements and Bonferroni multiple comparison test. Characteristics of the arteries used are shown in Table 1. Lower panel: Percentage of endothelium mediated relaxation induced by carbachol (10 μM) after precontraction with PGF2α (10 μM) in LAD upstream to occlusion, LAD downstream to occlusion and SCA after sham operation, IR or PI. (C) Concentration–response curves of increasing concentrations of ET-1 in LAD downstream to ligature, ET-1 after S6c induced ETB desensitization and addition of the selective endothelin ETB receptor antagonist BQ788 (30nM) and ET-1 after addition of the selective endothelin ETA receptor antagonist BQ123 (10 μM) after IR or sham procedures. The results are shown as mean values ± SEM (n = 6-9). ***P < 0.001 and *P < 0.05; compared with ET-1 curve without antagonists; two-way anova with repeated measurements and Bonferroni multiple comparison test.
Endothelin receptor pharmacology
The ETB receptor-mediated vasoconstriction was studied using the selective ETB receptor agonist S6c (Kloog et al., 1988). The ETB receptor-mediated vasoconstriction was very weak (<15% of the cocktail-induced contraction) in SCA and LAD from sham-operated rats, as well as in arteries from the non-ischaemic areas of both PI hearts and IR hearts (Table 1). In contrast, arteries situated downstream of the occlusion in IR hearts showed a significantly augmented ETB receptor-mediated vasoconstriction (Emax = 52 ± 10% of cocktail response) (Figure 2 and Table 1).
Table 1.
Contractile properties of isolated coronary arteries from sham-, IR- or PI-operated rats
| Surgery | Segment | Endothelin-1 | Endothelin-1 +desensitization +BQ788 (30 nM) | Endothelin-1 +BQ788 (0.9 μM) | Endothelin-1 +BQ123 (10 μM) | S6c | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Emax | pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | pEC50 | Emax | pEC50 | ||
| Sham | Upstream | 91.1 ± 4.0 | 9.2 ± 0.1 | 80.6 ± 3.3 | 9.2 ± 0.1 | – | – | – | – | 11.9±4.6 | 9.3 ± 0.5 |
| Downstream | 89.8 ± 5.0 | 9.0 ± 0.1 | 83.6 ± 5.8 | 9.4 ± 0.1 | 95.2 ± 3.8 | 9.2 ± 0.1 | 86.8 ± 13.5 | 7.8 ± 0.2 | 0.8 ± 3.6 | ND | |
| SCA | 92.7 ± 4.8 | 9.0 ± 0.1 | 86.8 ± 4.4 | 9.1 ± 0.1 | – | – | – | – | 4.5 ± 1.5 | ND | |
| IR | Upstream | 89.0 ± 4.8 | 9.2 ± 0.1 | 86.3 ± 6.5 | 9.3 ± 0.1 | – | – | – | – | 13.9 ± 4.3 | 9.4 ± 0.4 |
| Downstream | 102.4 ± 8.7 | 10.0 ± 0.1 | 89.6 ± 6.0 | 9.4 ± 0.1 | 99.6 ± 4.1 | 9.0 ± 0.1 | 96.8 ± 10.1 | 8.6 ± 0.2 | 52.3 ± 10.0 | 10.0 ± 0.3 | |
| SCA | 101.3 ± 8.7 | 9.3 ± 0.1 | 89.5 ± 6.2 | 9.4 ± 0.1 | – | – | – | – | ND | ND | |
| PI | Upstream | – | – | 85.7 ± 6.9 | 8.8 ± 0.2 | – | – | – | – | 14.1 ± 3.1 | 9.2 ± 0.7 |
| SCA | – | – | 83.5 ± 5.3 | 9.2 ± 0.1 | – | – | – | – | 4.8 ± 2.7 | ND | |
As shown in Figure 2, there was no significant difference in endothelial function in the downstream arteries of sham and IR hearts. Hence, the enhanced ETB receptor-mediated vasoconstriction in the downstream artery segments were not associated with differences in endothelial function between the segments.
Dual ETA and ETB receptor-mediated responses induced by ET-1
The dual ETA and ETB receptor-mediated vasoconstrictor responses were measured by stimulating the arteries with ET-1. As seen in Figure 3A, ET-1 induced a sigmoid concentration–response curve, with almost equal potency and maximal contraction in the three segments from sham-operated hearts and LAD upstream and SCA from IR hearts. In contrast, ET-1 induced a significantly potentiated contractile response in LAD downstream segments compared with the upstream and SCA segments from the same IR hearts (Figure 3A). This increased contractile sensitivity towards ET-1 in downstream segments was also significantly higher than that in downstream segments of sham-operated hearts (Figure 3B). However, we found no significant differences in endothelial integrity between the artery segments (Figure 3B).
Effects of ETA receptor antagonism
The presence of the selective ETA receptor antagonist BQ123 (10 μM) reduced the sensitivity to ET-1 in LAD downstream segments from both IR- and sham-operated hearts (Figure 3B and Table 1). Despite ETA receptor antagonism, the ET-1-induced contractions were still greater in IR downstream segments compared with sham downstream segments.
Effects of ETB receptor antagonism
The ETA receptor-mediated vasoconstriction was studied with ET-1 in artery segments after abolishing ETB receptor responses by desensitization with S6c (Adner et al., 1996), followed by blockade of any remaining ETB receptors with the selective ETB receptor antagonist BQ788 (30 nM) (Korzick et al., 2005) or in the presence of BQ788, without prior desensitization. We found no differences in ETA receptor-mediated responses after either IR or PI, compared with sham-operated heart (Table 1, Figure 3B), suggesting that IR did not alter ETA receptor-mediated vasoconstriction.
The findings indicate that the endothelial vasodilatation is not altered in segments from IR, compared with those from sham-operated animals. Thus, the enhanced contractile responses to ET-1 in post-ischaemic arteries were not due to uneven endothelial function (Figure 3B) but reflected changes in the ETB receptors in VSMCs.
Immunohistochemistry
Low levels of immunoreactivity for ETB receptors was observed in VSMCs in arteries of sham-operated rats. After IR, immunoreactivity for ETB receptors was increased in VSMC of LAD segments situated downstream of the occlusion, compared with arteries upstream to occlusion, and compared with LAD segments situated downstream to the loose ligature in sham-operated hearts (Figure 4A).
Figure 4.
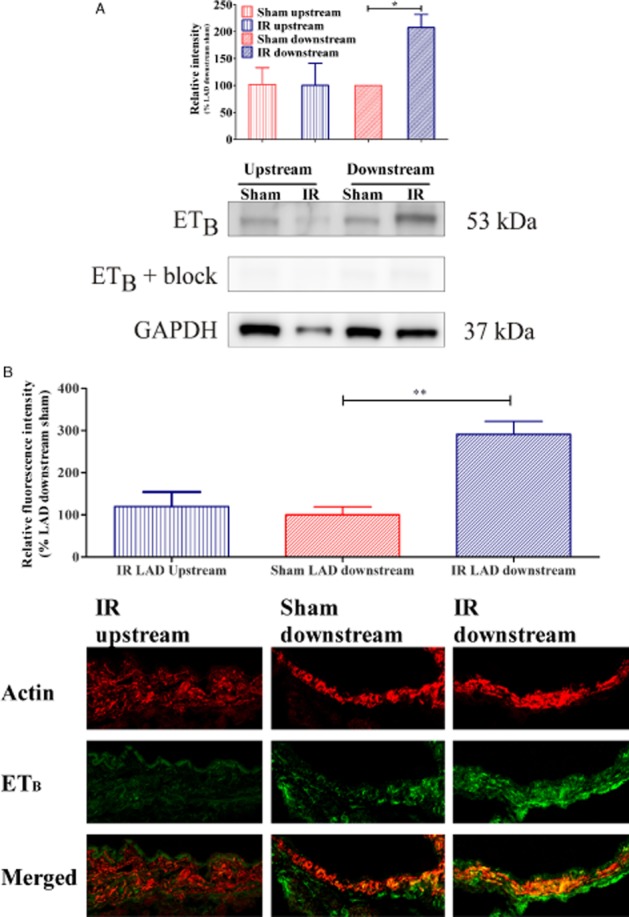
(A) Western blot of three coronary artery homogenates pooled from IR- (n = 10) and sham- (n = 9) operated rats. Results revealed endothelin ETB receptor band at approximately 50 kDa. Quantification of ETB immunoreactivity normalized to GAPDH revealed increased ETB receptor protein levels in downstream LAD segments after IR. Results are expressed as mean ± SEM of three different homogenates where mean of (ETB immunoreactivity)/(GAPDH immunoreactivity) from sham LAD downstream was set to 100%. *P < 0.05, comparing LAD downstream IR versus LAD downstream sham; Student's t-test. (B) The fluorescence intensity of each group is given as the percentage fluorescence of IR LAD downstream (n = 5) or IR LAD upstream (n = 3) group compared with the sham LAD downstream (n = 3) groups, where the LAD downstream group was set to 100%, and the mean value for each of the other groups was used for comparison. Confocal images showing representative examples of immunofluorescence staining of rat coronary arteries (n = 3–5) of actin (upper row), ETB receptor (middle row), and merged pictures that show the location of endothelin ETB receptors in VSMC (bottom row). **P < 0.01, comparing LAD downstream IR versus LAD downstream sham; Student's t-test.
Western blots
In support of the immunohistochemical and functional results, the Western blot experiments revealed a significant strong band corresponding to the ETB receptor (with an approximate molecular weight around 53 kDa) in segments of the downstream coronary artery (207 ± 24%), compared with the downstream segments of sham-operated rats (100%) (Figure 4B).
Discussion
The main novel findings of the present study were: (i) ET-1-induced vasoconstriction was augmented in rat coronary arteries downstream of occlusion after 15 min of ischaemia followed by 22 h of reperfusion; (ii) the IR-induced changes in coronary arteries were associated with increased ETB receptor-mediated vasoconstriction and enhanced ETB receptor protein levels in the VSMC; (iii) the ETA receptor-mediated constriction was unaltered in non-ischaemic and post-ischaemic coronary artery segments. (iv) coronary arteries located in non-ischaemic areas of hearts subjected to either PI or IR expressed no changes in ETB receptor-mediated vasoconstriction.
Vasomotor responses to ET-1
The peptide ET-1 is a major regulator of vascular function acting via ETA and ETB receptors (Yanagisawa et al., 1988a; Arai et al., 1990; Sakurai et al., 1990). ET-1 is formed in the coronary vessel endothelium (Saetrum et al., 1994; 2001) and in the endocardium (Resink et al., 1990; Suzuki et al., 1993; Saetrum et al., 2001), and contributes to regulation of both coronary and peripheral vascular tone (Haynes and Webb, 1994; MacCarthy et al., 2001). Continuous systemic infusion of ET-1 in conscious dogs causes a sustained coronary artery constriction (Wang et al., 1991), and during intra-coronary infusion ET-1 induces a concentration-dependent decrease in coronary artery blood flow and myocardial perfusion (Larkin et al., 1989; Domenech et al., 1991). The response to ET-1 is primarily mediated by contractile ETA receptors located in the VSMCs and vasodilatory ETB receptors located in the endothelial cells (Takamura et al., 2000; Merkus et al., 2005; de Beer et al., 2010). Few studies have indicated the presence of ETB receptors in VSMC of coronary arteries (Wendel-Wellner et al., 2002; Wendel et al., 2005). Characterization of constrictor ET receptor responses in human isolated coronary arteries has shown that ET-1 elicits vasoconstriction via ETA receptor activation whereas ETB receptor-mediated vasoconstriction is minor, as shown by small responses to S6c and ET-3 (Maguire and Davenport, 1995). The present results are in accordance with these findings. In sham-operated rats and coronary arteries from non-ischaemic areas, S6c induced only a negligible ETB receptor-mediated contraction whereas ET-1 induced strong contractile responses that were sensitive to ETA receptor blockade. We found no vasodilator effect of either ET-1 or S6c in precontracted vessels (data not shown), which is consistent with previous studies of canine isolated coronary arteries (Thorin et al., 1999).
Local ET-1 production and vasomotor responses after IR
There is substantial evidence that ET-1 release is enhanced during myocardial ischaemia and/or reperfusion. Plasma levels of ET-1 are increased in patients with coronary artery spasm (Matsuyama et al., 1991; Toyo-Oka et al., 1991), following myocardial infarction (Lerman et al., 1991; Stewart et al., 1991), and in congestive heart failure (Wei et al., 1994). Furthermore, elevated plasma levels of ET-1 can serve as a prognostic marker in coronary artery disease (Zouridakis et al., 2001). Enhanced ET-1 levels during ischaemia may result in increased coronary tone and impair coronary blood flow to the myocardium.
Two recent case studies have reported that patients with severe, treatment-resistant, coronary vasospasm were successfully treated with the dual ETA/B receptor antagonist bosentan (Vermeltfoort et al., 2009; Krishnan et al., 2010). Furthermore, the effect of BQ123 has currently been tested in a randomized, placebo-controlled, double-blind pilot study in patients with acute posterior wall ST elevation (Adlbrecht et al., 2012). The results showed that acute short-term intravenous administration of BQ123 during percutaneous coronary intervention improved myocardial perfusion and left ventricular ejection fraction at 6 months. Furthermore, in patients with atherosclerosis but with unobstructed coronary arteries, treatment with BQ123 i.c. improved myocardial epicardial and microvascular coronary circulation (Halcox et al., 2001).
Enhanced constrictor responses to ET-1 have been reported in coronary arteries acutely (between minutes and few hours) after ischaemia and reperfusion in several animal models (Neubauer et al., 1991; Watts et al., 1992; Lockowandt et al., 2001; Climent et al., 2005; Garcia-Villalon et al., 2008). The underlying mechanisms behind this increased response to ET-1 have been attributed to up-regulation of ETA receptors (Neubauer et al., 1991) or reduced ETB receptor-mediated relaxation due to endothelial dysfunction (Watts et al., 1992; Lockowandt et al., 2001; Climent et al., 2005). However, the role of endothelin receptors in coronary arteries, sub-acutely (one day) after myocardial ischaemia, has only been sparsely investigated. In the present study, we found enhanced ET-1-induced vasoconstriction in the post-ischaemic coronary arteries, situated downstream to the occlusion site. The enhanced vasoconstrictor responses were associated with augmented ETB receptor-mediated vasoconstriction, as seen by significant vasoconstrictor responses to S6c. Interestingly, the S6c-induced vasoconstriction was not associated with reduction in endothelial function, but largely due to an enhanced density of ETB receptors in the VSMCs of the post-ischaemic arteries. To our knowledge, this is the first study to demonstrate that contractile ETB receptors are de novo highly expressed in the VSMCs locally in coronary artery segments downstream to the ligature after 15 min of ischaemia, followed by 22 h of reperfusion.
ET receptor blockade and coronary artery responses
In the present study, we found that ETA receptor blockade reversed the ET-1 vasoconstriction to an equal extent, in both control and post-ischaemic coronary arteries. Consequently, despite ETA receptor blockade, the IR downstream segments were more sensitive to ET-1 compared with sham downstream segments. In contrast, after block of ETB receptors (with or without prior desensitization with S6c), ET-1-induced vasoconstriction was almost equal in control and post-ischaemic artery segments. These results further confirm that ETB receptors in post-ischaemic coronary arteries show potentiated vasoconstrictor responses to ET-1. Based on our findings, we suggest that pharmacological treatment with ETB receptor blockers could hypothetically improve blood flow in post-ischaemic and reperfused coronary arteries. However, ETB receptor antagonism may block the ET-1 clearance leading to further enhanced local concentrations of ET-1 in the myocardium.
Human studies
ETA and ETB receptor mRNA levels are significantly higher in coronary arteries from patients with ischaemic heart disease (Wackenfors et al., 2004). In addition, a binding study performed by Degassan and co-workers reported up-regulation of ETB receptors in atherosclerotic human coronary arteries (Dagassan et al., 1996). In contrast, Maguire and Davenport found no increase in vasoconstrictive ETB receptors in human coronary arteries, from patients undergoing transplantation for either cardiomyopathy or ischaemic heart disease (Maguire and Davenport, 2000). The discrepancy between these studies could be that ETB receptor up-regulation is a transient phenomenon arising immediately after IR, but lost over longer periods of time. Thus, the present findings may have important pathophysiological and therapeutic implications in the early stages of IR injury.
Clinical impact
Little is known about the transduction pathways involved in ETB receptor up-regulation in coronary arteries. The mechanisms involved in the de novo formation of ETB receptors have to some extent only been studied after organ culture of coronary arteries (Skovsted et al., 2012), a method that mimics a no-flow condition. Thus, after 7 and 24 h of organ culture of coronary arteries, the ETB receptor-mediated vasoconstriction is significantly augmented, compared with fresh arteries. These studies showed that the up-regulation of coronary artery ETB receptors was associated with elevated levels of phosphorylated ERK1/2 located in the VSMC, and that the selective MEK1/2 inhibitor U0126 was able to attenuate expression of ETB receptors. Another study has shown that the increased ET-1 vasoconstriction after IR in mesenteric arteries involves both up-regulation of VSMC and ETB receptors, together with a decrease in NO bioavailability (Martinez-Revelles et al., 2012).
Conclusions
The present study has revealed local changes in ETB receptor expression after myocardial ischaemia and reperfusion. We have demonstrated augmented ETB receptor-mediated vasoconstrictor responses in coronary arteries situated downstream of the occlusion after myocardial IR. This augmented response was associated with increased protein levels of ETB receptors in the VSMCs, which suggests the formation of more ETB receptors. An up-regulation of contractile ETB receptors may shift the vascular tone in the post-ischaemic coronary arties towards a contractile state and attenuate the blood flow in the ischaemic myocardium.
Acknowledgments
We thank Professor Karin Warfvinge (http://www.sciencesupport.se) for fruitful discussions and valuable comments on the manuscript. This work was supported by the Swedish Heart and Lung Foundation, Sweden, and the Lundbeck Foundation, Denmark.
Glossary
- BQ788
N-cis-2,6-dimethylpiperidinocarbonyl-L- γ-methylleucyl- D-1-methoxycarboyl-D-norleucine
- BQ123
2-[(3R,6R,9S,12R,15S)-6-(1H-indol-3-ylmethyl)-9-(2-methylpropyl)-2,5,8,11,14-pentaoxo-12-propan-2-yl-1,4,7,10,13 pentazabicyclo[13.3.0]octadecan-3-yl]acetic acid
- ETA receptor
endothelin receptor type A
- ETB receptor
endothelin receptor type B
- ET-1
endothelin-1
- IR
ischaemia–reperfusion
- LAD
left anterior descending coronary artery
- PI
permanent ischaemia
- S6c
sarafotoxin 6c
- SCA
septal coronary artery
- TTC
triphenyltetrazolium chloride
- VSMC
vascular smooth muscle cell
Conflict of interest
None.
References
- Adlbrecht C, Andreas M, Redwan B, Distelmaier K, Mascherbauer J, Kaider A, et al. Systemic endothelin receptor blockade in ST-segment elevation acute coronary syndrome protects the microvasculature: a randomised pilot study. EuroIntervention. 2012;7:1386–1395. doi: 10.4244/EIJV7I12A218. [DOI] [PubMed] [Google Scholar]
- Adner M, Cantera L, Ehlert F, Nilsson L, Edvinsson L. Plasticity of contractile endothelin-B receptors in human arteries after organ culture. Br J Pharmacol. 1996;119:1159–1166. doi: 10.1111/j.1476-5381.1996.tb16018.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adner M, Geary GG, Edvinsson L. Appearance of contractile endothelin-B receptors in rat mesenteric arterial segments following organ culture. Acta Physiol Scand. 1998;163:121–129. doi: 10.1046/j.1365-201X.1998.00369.x. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alonso D, Radomski MW. The nitric oxide-endothelin-1 connection. Heart Fail Rev. 2013;8:107–115. doi: 10.1023/a:1022155206928. 2003. [DOI] [PubMed] [Google Scholar]
- Arai H, Hori S, Aramori I, Ohkubo H, Nakanishi S. Cloning and expression of a cDNA encoding an endothelin receptor. Nature. 1990;348:730–732. doi: 10.1038/348730a0. [DOI] [PubMed] [Google Scholar]
- Bacon CR, Cary NR, Davenport AP. Endothelin peptide and receptors in human atherosclerotic coronary artery and aorta. Circ Res. 1996;79:794–801. doi: 10.1161/01.res.79.4.794. [DOI] [PubMed] [Google Scholar]
- de Beer VJ, de Graaff HJ, Hoekstra M, Duncker DJ, Merkus D. Integrated control of pulmonary vascular tone by endothelin and angiotensin II in exercising swine depends on gender. Am J Physiol Heart Circ Physiol. 2010;298:H1976–H1985. doi: 10.1152/ajpheart.00459.2009. [DOI] [PubMed] [Google Scholar]
- Climent B, Fernandez N, Sanz E, Sanchez A, Monge L, Garcia-Villalon AL, et al. Enhanced response of pig coronary arteries to endothelin-1 after ischemia-reperfusion. Role of endothelin receptors, nitric oxide and prostanoids. Eur J Pharmacol. 2005;524:102–110. doi: 10.1016/j.ejphar.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Dagassan PH, Breu V, Clozel M, Kunzli A, Vogt P, Turina M, et al. Up-regulation of endothelin-B receptors in atherosclerotic human coronary arteries. J Cardiovasc Pharmacol. 1996;27:147–153. doi: 10.1097/00005344-199601000-00023. [DOI] [PubMed] [Google Scholar]
- Domenech R, Macho P, Gonzalez R, Huidobro-Toro JP. Effect of endothelin on total and regional coronary resistance and on myocardial contractility. Eur J Pharmacol. 1991;192:409–416. doi: 10.1016/0014-2999(91)90233-g. [DOI] [PubMed] [Google Scholar]
- Garcia-Villalon AL, Amezquita YM, Monge L, Fernandez N, Salcedo A, Dieguez G. Endothelin-1 potentiation of coronary artery contraction after ischemia-reperfusion. Vascul Pharmacol. 2008;48:109–114. doi: 10.1016/j.vph.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Halcox JP, Nour KR, Zalos G, Quyyumi AA. Coronary vasodilation and improvement in endothelial dysfunction with endothelin ET(A) receptor blockade. Circ Res. 2001;89:969–976. doi: 10.1161/hh2301.100980. [DOI] [PubMed] [Google Scholar]
- Haynes WG, Webb DJ. Contribution of endogenous generation of endothelin-1 to basal vascular tone. Lancet. 1994;344:852–854. doi: 10.1016/s0140-6736(94)92827-4. [DOI] [PubMed] [Google Scholar]
- Inoue A, Yanagisawa M, Kimura S, Kasuya Y, Miyauchi T, Goto K, et al. The human endothelin family: three structurally and pharmacologically distinct isopeptides predicted by three separate genes. Proc Natl Acad Sci U S A. 1989;86:2863–2867. doi: 10.1073/pnas.86.8.2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kloog Y, Ambar I, Sokolovsky M, Kochva E, Wollberg Z, Bdolah A. Sarafotoxin, a novel vasoconstrictor peptide: phosphoinositide hydrolysis in rat heart and brain. Science. 1988;242:268–270. doi: 10.1126/science.2845579. [DOI] [PubMed] [Google Scholar]
- Korzick DH, Muller-Delp JM, Dougherty P, Heaps CL, Bowles DK, Krick KK. Exaggerated coronary vasoreactivity to endothelin-1 in aged rats: role of protein kinase C. Cardiovasc Res. 2005;66:384–392. doi: 10.1016/j.cardiores.2005.01.015. [DOI] [PubMed] [Google Scholar]
- Krishnan U, Win W, Fisher M. First report of the successful use of bosentan in refractory vasospastic angina. Cardiology. 2010;116:26–28. doi: 10.1159/000313365. [DOI] [PubMed] [Google Scholar]
- Larkin SW, Clarke JG, Keogh BE, Araujo L, Rhodes C, Davies GJ, et al. Intracoronary endothelin induces myocardial ischemia by small vessel constriction in the dog. Am J Cardiol. 1989;64:956–958. doi: 10.1016/0002-9149(89)90855-2. [DOI] [PubMed] [Google Scholar]
- Lerman A, Edwards BS, Hallett JW, Heublein DM, Sandberg SM, Burnett JC., Jr Circulating and tissue endothelin immunoreactivity in advanced atherosclerosis. N Engl J Med. 1991;325:997–1001. doi: 10.1056/NEJM199110033251404. [DOI] [PubMed] [Google Scholar]
- Lockowandt U, Liska J, Franco-Cereceda A. Short ischemia causes endothelial dysfunction in porcine coronary vessels in an in vivo model. Ann Thorac Surg. 2001;71:265–269. doi: 10.1016/s0003-4975(00)02253-0. [DOI] [PubMed] [Google Scholar]
- MacCarthy PA, Pegge NC, Prendergast BD, Shah AM, Groves PH. The physiological role of endogenous endothelin in the regulation of human coronary vasomotor tone. J Am Coll Cardiol. 2001;37:137–143. doi: 10.1016/s0735-1097(00)01042-1. [DOI] [PubMed] [Google Scholar]
- Maguire JJ, Davenport AP. ETA receptor-mediated constrictor responses to endothelin peptides in human blood vessels in vitro. Br J Pharmacol. 1995;115:191–197. doi: 10.1111/j.1476-5381.1995.tb16338.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maguire JJ, Davenport AP. No alteration in vasoconstrictor endothelin-B-receptor density or function in human coronary artery disease. J Cardiovasc Pharmacol. 2000;36:S380–S381. doi: 10.1097/00005344-200036051-00110. [DOI] [PubMed] [Google Scholar]
- Martinez-Revelles S, Caracuel L, Marquez-Martin A, Dantas A, Oliver E, D'Ocon P, et al. Increased endothelin-1 vasoconstriction in mesenteric resistance arteries after superior mesenteric ischaemia-reperfusion. Br J Pharmacol. 2012;165:937–950. doi: 10.1111/j.1476-5381.2011.01617.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuyama K, Yasue H, Okumura K, Saito Y, Nakao K, Shirakami G, et al. Increased plasma level of endothelin-1-like immunoreactivity during coronary spasm in patients with coronary spastic angina. Am J Cardiol. 1991;68:991–995. doi: 10.1016/0002-9149(91)90484-3. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkus D, Houweling B, van den Meiracker AH, Boomsma F, Duncker DJ. Contribution of endothelin to coronary vasomotor tone is abolished after myocardial infarction. Am J Physiol Heart Circ Physiol. 2005;288:H871–H880. doi: 10.1152/ajpheart.00429.2004. [DOI] [PubMed] [Google Scholar]
- Neubauer S, Zimmermann S, Hirsch A, Pulzer F, Tian R, Bauer W, et al. Effects of endothelin-1 in the isolated heart in ischemia/reperfusion and hypoxia/reoxygenation injury. J Mol Cell Cardiol. 1991;23:1397–1409. doi: 10.1016/0022-2828(91)90186-p. [DOI] [PubMed] [Google Scholar]
- Resink TJ, Hahn AW, Scott-Burden T, Powell J, Weber E, Buhler FR. Inducible endothelin mRNA expression and peptide secretion in cultured human vascular smooth muscle cells. Biochem Biophys Res Commun. 1990;168:1303–1310. doi: 10.1016/0006-291x(90)91171-n. [DOI] [PubMed] [Google Scholar]
- Saetrum OO, Adner M, Gulbenkian S, Edvinsson L. Localization of endothelin immunoreactivity and demonstration of constrictory endothelin-A receptors in human coronary arteries and veins. J Cardiovasc Pharmacol. 1994;23:576–583. doi: 10.1097/00005344-199404000-00009. [DOI] [PubMed] [Google Scholar]
- Saetrum OO, Adner M, Peters TH, Xu CB, Stavenow L, Gulbenkian S, et al. Endocardial expression and functional characterization of endothelin-1. Mol Cell Biochem. 2001;224:151–158. doi: 10.1023/a:1011952504093. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Yanagisawa M, Takuwa Y, Miyazaki H, Kimura S, Goto K, et al. Cloning of a cDNA encoding a non-isopeptide-selective subtype of the endothelin receptor. Nature. 1990;348:732–735. doi: 10.1038/348732a0. [DOI] [PubMed] [Google Scholar]
- Seo B, Oemar BS, Siebenmann R, von Segesser L, Luscher TF. Both ETA and ETB receptors mediate contraction to endothelin-1 in human blood vessels. Circulation. 1994;89:1203–1208. doi: 10.1161/01.cir.89.3.1203. [DOI] [PubMed] [Google Scholar]
- Skovsted GF, Pedersen AF, Larsen R, Sheykhzade M, Edvinsson L. Rapid functional upregulation of vasocontractile endothelin ET(B) receptors in rat coronary arteries. Life Sci. 2012;91:593–599. doi: 10.1016/j.lfs.2012.02.009. [DOI] [PubMed] [Google Scholar]
- Stewart DJ, Kubac G, Costello KB, Cernacek P. Increased plasma endothelin-1 in the early hours of acute myocardial infarction. J Am Coll Cardiol. 1991;18:38–43. doi: 10.1016/s0735-1097(10)80214-1. [DOI] [PubMed] [Google Scholar]
- Suzuki T, Kumazaki T, Mitsui Y. Endothelin-1 is produced and secreted by neonatal rat cardiac myocytes in vitro. Biochem Biophys Res Commun. 1993;191:823–830. doi: 10.1006/bbrc.1993.1291. [DOI] [PubMed] [Google Scholar]
- Takamura M, Parent R, Cernacek P, Lavallee M. Influence of dual ET(A)/ET(B)-receptor blockade on coronary responses to treadmill exercise in dogs. J Appl Physiol. 2000;89:2041–2048. doi: 10.1152/jappl.2000.89.5.2041. [DOI] [PubMed] [Google Scholar]
- Thorin E, Parent R, Ming Z, Lavallee M. Contribution of endogenous endothelin to large epicardial coronary artery tone in dogs and humans. Am J Physiol. 1999;277:H524–H532. doi: 10.1152/ajpheart.1999.277.2.H524. [DOI] [PubMed] [Google Scholar]
- Toyo-Oka T, Aizawa T, Suzuki N, Hirata Y, Miyauchi T, Shin WS, et al. Increased plasma level of endothelin-1 and coronary spasm induction in patients with vasospastic angina pectoris. Circulation. 1991;83:476–483. doi: 10.1161/01.cir.83.2.476. [DOI] [PubMed] [Google Scholar]
- Vermeltfoort IA, Raijmakers PG, Kamphuisen PW. Improved myocardial perfusion preceding clinical response on bosentan treatment for coronary vasospasm. Acta Cardiol. 2009;64:415–417. doi: 10.2143/AC.64.3.2038032. [DOI] [PubMed] [Google Scholar]
- Wackenfors A, Emilson M, Ingemansson R, Hortobagyi T, Szok D, Tajti J, et al. Ischemic heart disease induces upregulation of endothelin receptor mRNA in human coronary arteries. Eur J Pharmacol. 2004;484:103–109. doi: 10.1016/j.ejphar.2003.11.001. [DOI] [PubMed] [Google Scholar]
- Wagner OF, Christ G, Wojta J, Vierhapper H, Parzer S, Nowotny PJ, et al. Polar secretion of endothelin-1 by cultured endothelial cells. J Biol Chem. 1992;267:16066–16068. [PubMed] [Google Scholar]
- Wang J, Zeballos GA, Kaley G, Hintze TH. Dilation and constriction of large coronary arteries in conscious dogs by endothelin. Am J Physiol. 1991;261:H1379–H1386. doi: 10.1152/ajpheart.1991.261.5.H1379. [DOI] [PubMed] [Google Scholar]
- Watts JA, Chapat S, Johnson DE, Janis RA. Effects of nisoldipine upon vasoconstrictor responses and binding of endothelin-1 in ischemic and reperfused rat hearts. J Cardiovasc Pharmacol. 1992;19:929–936. doi: 10.1097/00005344-199206000-00014. [DOI] [PubMed] [Google Scholar]
- Wei CM, Lerman A, Rodeheffer RJ, McGregor CG, Brandt RR, Wright S, et al. Endothelin in human congestive heart failure. Circulation. 1994;89:1580–1586. doi: 10.1161/01.cir.89.4.1580. [DOI] [PubMed] [Google Scholar]
- Wendel M, Kummer W, Knels L, Schmeck J, Koch T. Muscular ETB receptors develop postnatally and are differentially distributed in specific segments of the rat vasculature. J Histochem Cytochem. 2005;53:187–196. doi: 10.1369/jhc.4A6474.2005. [DOI] [PubMed] [Google Scholar]
- Wendel-Wellner M, Noll T, Konig P, Schmeck J, Koch T, Kummer W. Cellular localization of the endothelin receptor subtypes ET(A) and ET(B) in the rat heart and their differential expression in coronary arteries, veins, and capillaries. Histochem Cell Biol. 2002;118:361–369. doi: 10.1007/s00418-002-0457-4. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Goto K, Masaki T. A novel peptide vasoconstrictor, endothelin, is produced by vascular endothelium and modulates smooth muscle Ca2+ channels. J Hypertens Suppl. 1988a;6:S188–S191. doi: 10.1097/00004872-198812040-00056. [DOI] [PubMed] [Google Scholar]
- Yanagisawa M, Kurihara H, Kimura S, Tomobe Y, Kobayashi M, Mitsui Y, et al. A novel potent vasoconstrictor peptide produced by vascular endothelial cells. Nature. 1988b;332:411–415. doi: 10.1038/332411a0. [DOI] [PubMed] [Google Scholar]
- Zouridakis EG, Schwartzman R, Garcia-Moll X, Cox ID, Fredericks S, Holt DW, et al. Increased plasma endothelin levels in angina patients with rapid coronary artery disease progression. Eur Heart J. 2001;22:1578–1584. doi: 10.1053/euhj.2000.2588. [DOI] [PubMed] [Google Scholar]