Abstract
Background and Purpose
Oxidative stress is an important pathogenic factor in the development of hypertension. Resveratrol, the main antioxidant in red wine, improves NO bioavailability and prevents cardiovascular disease. The aim of this study was to examine whether resveratrol decreases the generation of reactive oxygen species (ROS), thereby reducing BP in rats with fructose-induced hypertension.
Experimental Approach
Rats were fed 10% fructose with or without resveratrol (10 mg·kg−1·day−1) for 1 week or for 4 weeks with resveratrol treatment beginning at week 2; systolic BP (SBP) was measured by tail-cuff method. Endogenous in vivo O2− production in the nucleus tractus solitarii (NTS) was determined with dihydroethidium. Real-time PCR and immunoblotting analyses were used to quantify RNA and protein expression levels.
Key Results
In fructose-fed rats, ROS levels in the NTS were higher, whereas the NO level was significantly decreased. Also, RNA and protein levels of NADPH oxidase subunits (p67, p22-phox) were elevated, superoxide dismutase 2 (SOD2) reduced and AMP-activated PK (AMPK) T172 phosphorylation levels in the NTS were lower in fructose-fed rats. Treatment with the AMPK activator resveratrol decreased levels of NADPH oxidase subunits and ROS, and increased NO and SOD2 levels in the NTS of fructose-fed rats. Administration of resveratrol, in combination with fructose at week 0 and later at week 2, significantly reduced the SBP of fructose-fed rats.
Conclusions and Implications
Collectively, resveratrol decreased BP through the phosphorylation of AMPK, Akt and neuronal NOS in fructose-fed rats. These novel findings suggest that resveratrol may be a potential pharmacological candidate for the treatment of hypertension.
Keywords: AMPK, hypertension, nucleus tractus solitarii, nitrogen oxides, resveratrol
Introduction
At the end of 2011, the World Health Organization warned that high fructose consumption, mainly in the form of sweetened beverages, is a risk factor for several metabolic diseases (Aller et al., 2011). Fructose-fed rats are a model of acquired systolic hypertension, which displays many of the features symptomatic of metabolic syndrome in humans (Tran et al., 2009).
The nucleus tractus solitarii (NTS) is located in the dorsal medulla of the brainstem, which is the primary integrating centre for cardiovascular regulation and other autonomic functions of the CNS. Moreover, NO has important modulatory functions in the NTS, including the modulation of arterial BP and sympathetic nerve activity (Ho et al., 2008). Previous studies have indicated that fructose causes oxidative stress and sympathetic overactivity. Oxidative stress presents as chronically elevated levels of reactive oxygen species (ROS) and is associated with cardiovascular disease (Paravicini and Touyz, 2006). One possible mechanism that causes hypertension is that ROS interact with the vasodilator NO and decrease its bioavailability (Zhang et al., 2004). Recent studies have demonstrated that the phagocyte-type NADPH oxidase is a major source of ROS in the vasculature (Bendall et al., 2007). NADPH oxidase is composed of two catalytic subunits (gp91phox and p22phox) and four regulatory subunits (p47phox, p40phox, p67phox and Rac1). Superoxide dismutases (SODs), antioxidant enzymes, are the first and most important line of defence against ROS, particularly against superoxide anion radicals. Three distinct isoforms of SOD (CuZn-SOD or SOD1; Mn-SOD or SOD2; EC-SOD or SOD3) have been identified in mammals.
Resveratrol has neuroprotective properties and is a potent activator of AMPK in neuronal cell lines, primary neurons and the brain (Dasgupta and Milbrandt, 2007). Resveratrol also promotes antioxidant defences by regulating a host of antioxidant enzymes (Li et al., 2006; Robb et al., 2008b). AMP-activated PK (AMPK) is a serine/threonine PK that functions as an energy sensor in the regulation of cellular metabolism (see Alexander et al., 2013b). AMPK isoforms α1 and α2 consist of a catalytic α subunit together with β and γ non-catalytic subunits (Stapleton et al., 1996), which requires phosphorylation of Thr172 in the activation loop of the catalytic α subunit (Hardie et al., 1999). Inhibition of AMPK by an AMPK inhibitor leads to increases in Rac1 and NADPH oxidase activities and decreases in SOD2 expression in human aortic endothelial cells (Wang et al., 2012).
The mechanisms of brain oxidative stress-induced ROS generation that cause NOx dysfunction in fructose-induced hypertension remain unclear. Therefore, the aim of this study was to examine whether resveratrol acts through the AMPK pathway to decrease ROS generation and increase NOx in the NTS, thereby reducing BP in rats with fructose-induced hypertension. Our results suggest that in the NTS, fructose induces NADPH oxidase activity and reduces SOD2 activity, which contributes to increase BP in fructose-fed rats. Resveratrol decreases the BP in these fructose-induced hypertensive rats through the activation of AMPK, which, by reducing the activity of NADPH oxidase, activates the Akt-neuronal NOS (nNOS) signal pathway in the brain and leads to a decrease in the generation of ROS and an increase in SOD2 levels.
Methods
Reagents and chemicals
Experimental drugs including pentobarbital sodium salt, fructose, resveratrol, DMSO, mouse anti-actin antibody and goat anti-rabbit and goat anti-mouse IgG secondary antibodies were all obtained from Sigma-Aldrich (Sigma Chemical Co., St. Louis, MO, USA). Anti-p-AMPKT172, anti-AMPK, anti-nNOSS1416 and anti-nNOS antibodies were purchased from Cell Signaling Technology (Beverly, MA, USA). Anti-p22-phox antibody was purchased from Santa Cruz Biotechnology (Santa Cruz, CA, USA). Anti-p47-phox and anti-p67-phox antibodies were purchased from Millipore (Bedford, MA, USA). Anti-Cu/Zn-SOD and anti-Mn-SOD antibodies were obtained from StressGen Biotechnologies (La Jolla, CA, USA) and Abcam (Cambridge, UK) respectively.
Animals
Sixteen-week-old male Wistar-Kyoto rats (WKY) were obtained from the National Science Council Animal Facility (Taipei, Taiwan) and housed in the animal room of Kaohsiung Veterans General Hospital (Kaohsiung, Taiwan). The rats were kept in individual cages in a room with controlled lighting (12 h light/12 h dark cycle), and the temperature was maintained at 23–24°C. The rats were given normal rat chow (Purina; St. Louis, MO, USA) and tap water ad libitum. All animal research protocols had been approved by the Research Animal Facility Committee of Kaohsiung Veterans General Hospital and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication no. 85-23, revised 1996; A5047-01). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). The animals were killed with pentobarbital sodium salt (200 mg·kg−1, i.p.) at the end of the experiment. The rats were acclimatized to the housing conditions for 1 week. They were then trained for 1 week to accustom them to the procedure of indirect BP measurement. Then, the rats were randomly divided into five groups (control; fed with 10% fructose; fed with resveratrol 10 mg·kg−1·day−1; fed with 10% fructose and resveratrol 10 mg·kg−1·day−1; fructose-fed for 2 weeks then fructose + resveratrol for 2 weeks); there were six rats in each group. The fructose solution was prepared every 2 days by dissolving the fructose in tap water. Ordinary tap water was given to control animals to drink throughout the whole experimental period.
BP measurement
Using a tail-cuff method (Noninvasive Blood Pressure System, SINGA, Taipei, Taiwan) (Cheng et al., 2010), the systolic BP (SBP) of the rats was measured before the start of the fructose or resveratrol treatment (week 0). The rats were placed in the holder for 30 min at 37°C. With this method, the reappearance of pulsation on a digital display of the BP cuff was detected by a pressure transducer and amplified and recorded as the SBP. During the measurement, 10 individual readings were obtained in rapid sequence. The highest and lowest readings were discarded, and the average of the remaining eight readings was used. The SBP of the rats was measured every day.
Determination of NOx in the NTS and cerebellum
The cerebellum was chosen as a negative control because previous studies found that it was responsible for cognitive, sensory and motor function (Bavithra et al., 2013). The brain was excised immediately; cerebellum regions were separated according to the rat brain in stereotaxic coordinates, sixth edition (Paxinos and Watson, 2006). The NTS or cerebellum (10–50 mg) was deproteinized using a Microcon YM-30 filter unit (Millipore). The total amount of NOx in the samples was determined by use of a modified chemiluminescence-based procedure using a Sievers Nitric Oxide Analyzer (NOA 280i; Sievers Instruments, Boulder, CO, USA) purge system (Li et al., 2006; Cheng et al., 2010). The sample (10 µL) was injected into a reflux column containing 0.1 mol·L−1 of VCl3 in 1 mol·L−1 of HCl at 90°C to reduce any nitrates and nitrites into NO. The NOx was then combined with the O3 produced by the analyser to form NO2. The emission resulting from the excited NO2 was detected by a photomultiplier tube and digitally recorded (mV). The values were then interpolated to a standard curve of concurrently determined NaNO2 concentrations. The measurements were recorded in triplicate for each sample. The NOx levels measured were corrected for the NTS and cerebellum of the rats.
ROS production in the NTS and cerebellum
Endogenous in vivo O2− production in the NTS or cerebellum was determined by staining the NTS and cerebellum slices with dihydroethidium (DHE, Invitrogen, Carlsbad, CA, USA; Gao et al., 2004; Cheng et al., 2010). The NTS and cerebellum were dissected from the rats, placed in optimum cutting temperature (tissue freezing medium) compound (Shandon Cryomatrix; Thermo Electron Co., Pittsburgh, PA, USA), flash-frozen in a methylbutane-chilled bath, and then placed in liquid nitrogen. Cryostat slices (10 µm) were stained in the dark for 20 min at 37°C in a humidified 5% CO2 incubator with a 1 µM solution of DHE. The samples were analysed by fluorescence microscopy and Zeiss LSM Image Browser (Carl Zeiss MicroImaging, Jena, Germany).
Real-time reverse transcriptase-PCR
Total RNA was extracted from the tissue samples using the Trizol reagent (Invitrogen) according to the manufacturer's instructions, and cDNA was synthesized with SuperScript (Invitrogen). As a control for intact RNA and cDNA, a PCR to amplify the housekeeping gene GAPDH was performed for all tissue samples. Data obtained from the real-time PCR for p22phox, p47phox, p67phox, SOD1, SOD2 and SOD3 were normalized to the housekeeping gene GAPDH content. Amplification was for 30 s at 94°C, 30 s at 69°C, and 1 min at 72°C. The initial denaturation was 2 min at 95°C, and the final elongation was 10 min at 72°C.
Immunoblotting analysis
All groups of rats (six rats per group) were used in this study. The NTS was dissected under a microscope using a micro punch (1 mm inner diameter) from a 1 mm thick brainstem slice at the level of the obex. Total protein extract was prepared by homogenization of the NTS in lysis buffer with a protease inhibitor cocktail and a phosphatase inhibitor cocktail. The homogenate was incubated for 1 h at 4°C. Protein extracts (20 g per sample assessed by bicinchoninic acid protein assay; Pierce) were subjected to 7.5–10.0% SDS-Tris glycerin gel electrophoresis and transferred to a PVDF membrane (GE Healthcare, Buckingamshire, UK). The membrane was blocked with 5% non-fat milk in TBS/Tween 20 buffer (10 mmol·L−1 Tris, 150 mmol·L−1 NaCl, 0.1% Tween 20, pH 7.4); incubated with anti-p22-phox, anti-p67-phox, anti-Cu/Zn-SOD, anti-Mn-SOD, anti-p-AMPKT172, anti-AMPK, anti-p-AKTS473, anti-AKT, anti-p-nNOSS1416 (Abcam, Cambridge, UK) or anti-nNOS antibody at 1:1000 in phosphate buffer saline Tween-20 with 5% BSA; and incubated at 4°C; overnight. Peroxidase-conjugated anti-mouse or anti-rabbit antibody (1:5000) was used as the secondary antibody. The proteins were detected with an ECL-Plus detection kit (GE Healthcare) and film. The films were scanned by photo scanner (4490, Epson, Long Beach, CA, USA) and analysed with NIH Image densitometry analysis software (NIH, Bethesda, MD, USA).
Immunofluorescent staining analysis
The rats were perfused with saline, a solution of 4% formaldehyde, and finally with a 30% sucrose solution. Sections (20 µm) of the brainstem were stained. Brain stem sections were incubated with rabbit-anti-phospho-AMPKT172 antibody (1:100). After washing with PBS, the sections were incubated with green-fluorescent Alexa Fluor488 donkey anti-rabbit IgG (1:200; Invitrogen) at 25°C for 2 h. Sections were analysed by fluorescence microscopy and Zeiss LSM Image Browser (Carl Zeiss MicroImaging).
Statistical analysis
The BP measurements (fructose-treated and no treatment groups) were analysed with two-way anova for repeated measurements and Bonferroni's post hoc tests. Student's unpaired t-test was used to compare ROS and NOx levels (fructose-treated and no treatment groups), and a one-way anova with Scheffe's post hoc comparison were applied to compare group differences. Differences with P < 0.05 were considered significant. All data are expressed as the means ± SEM.
Results
Fructose reduces the systemic vasodepressor effect of NO by increasing ROS production in the NTS of fructose-induced hypertensive rats
To determine whether ROS-dependent NO release elevates SBP in fructose-induced hypertension, we examined the SBP, nitrate levels and ROS in animals fed fructose for 2 weeks. Our results showed that the SBP was significantly elevated in a time-dependent manner (days 2, 7 and 14) in the fructose-fed rats (Supporting Information Figure S1A). However, the NOx levels in the NTS were significantly decreased, whereas the levels of DHE fluorescence in the NTS sections were significantly increased in the fructose-fed group on days 7 and 14 (Supporting Information Figure S1B and C). Therefore, we used the rats fed fructose for 7 days to investigate the mechanism whereby ROS-dependent release of NO elevated the SBP during fructose-induced hypertension. NOx and DHE stain levels in the cerebellum sections did not change in the fructose-fed group compared with in the control groups (Figure 1, lanes 1 and 2; *P < 0.05, n = 6).
Figure 1.
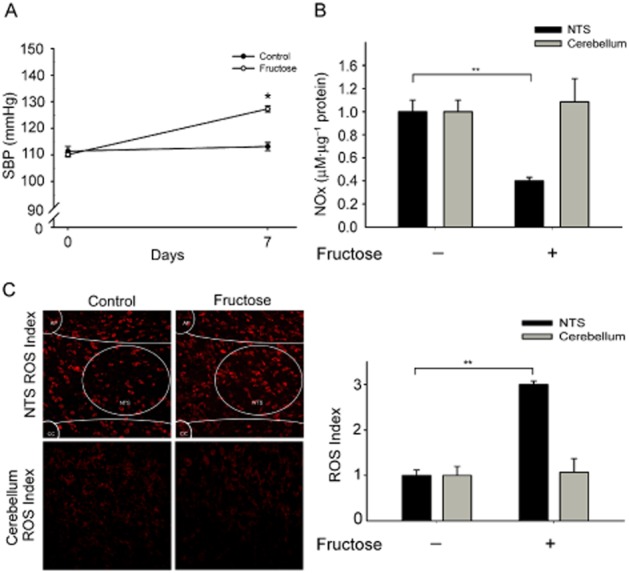
Superoxide-dependent NO production elevates SBP in the NTS of fructose-induced hypertensive rats. (A) The graph shows the SBP in the fructose groups after 1 week. The SBP was significantly increased in the fructose group compared with the control group. (B) Quantification of NOx concentrations in the NTS and cerebellum of fructose-fed rats. The bar graph shows the NOx concentration (as µM nitrate µg-1 of total protein). NOx levels significantly decreased in the NTS of the fructose-fed rats compared with the control rats. However, NOx levels in the cerebellum sections did not change in the fructose-fed group compared with in the control groups. (C) Representative images of DHE-treated brain sections. The images were photographed at ×280 magnification. Bar graph representation of ROS index in the NTS and cerebellum of the fructose groups as compared with WKY controls. The ROS index is the relative mean intensity of fluorescence of DHE. Sections including the NTS of fructose-fed rats displayed a significant increase in DHE fluorescence compared with the control group sections. However, DHE stain levels in the cerebellum sections did not change in the fructose-fed group compared with the control groups. Values are shown as the means ± SEM, n = 6. *P < 0.05, **P < 0.01.
Fructose functions through receptor for advanced glycation end products (RAGE) to modulate the elevation of NADPH oxidases and the decrease in SOD1/2 levels in the NTS of fructose-induced hypertensive rats
The interaction between RAGE and its ligands may directly induce the generation of ROS via NADPH oxidases (Wautier et al., 2001). ROS in the brain are thought to contribute to the neuropathogenesis of hypertension by enhancing sympathetic nervous system (SNS) activity. We investigated whether fructose promotes an imbalance by simultaneously enhancing the production of NADPH oxidase and down-regulating the main antioxidant enzymes, such as SOD1, SOD2 and SOD3. As shown in Figure 2A, real-time PCR analysis demonstrated that the expression of p22-phox and p67-phox mRNA in the NTS was significantly increased in fructose-fed rats compared with the control group (P < 0.05, n = 6). We recorded significantly higher relative protein expression levels of gp22-phox, p67-phox and RAGE in the NTS of fructose-fed rats compared with control rats (Figure 2B). However, relative mRNA and protein expression values of SOD2 were significantly lower in the NTS of fructose-fed rats compared with control rats (Figure 2D). These results indicate that fructose functions through RAGE to modulate the elevation in NADPH oxidase levels and the reduction in SOD1/2 levels in the NTS of fructose-induced hypertensive rats.
Figure 2.
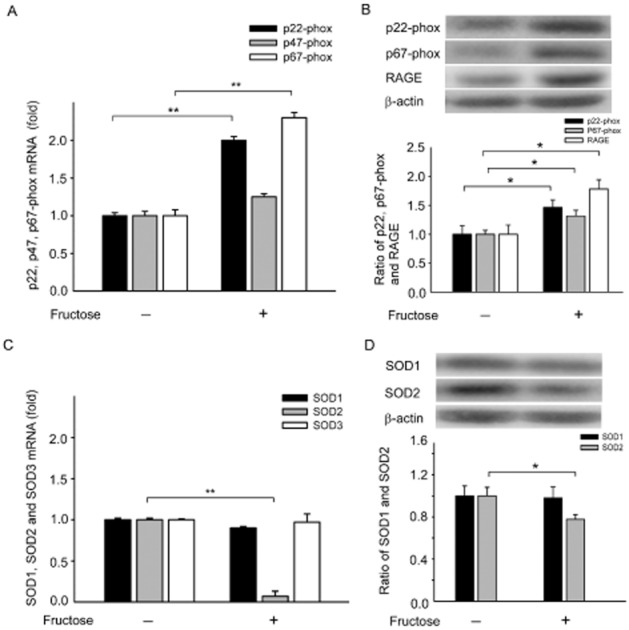
Fructose increases RAGE expression, which increases NADPH oxidases and decreases SOD1/2 in the NTS of fructose-induced hypertensive rats. (A) In the bar graph showing the real-time PCR analysis of relative NADPH oxidase subunits p22-phox p47-phox and p67-phox mRNA expression levels in the NTS of fructose groups after 1 week. Note the significant increase in the mRNA expression of the NADPH oxidase subunits p22-phox and p67-phox in fructose-fed rats compared with control rats. (B) Quantitative immunoblotting analysis demonstrates that the ratio of NADPH oxidase subunits p22-phox and p67-phox was significantly increased in the NTS of the fructose-fed group after 1 week. RAGE, was significantly increased in the NTS of fructose-fed rats compared with control rats. (C) Bar graph showing the effect of after fructose-fed 1 week on SOD1, SOD2 and SOD3 mRNA expression. Note the significant decrease in SOD2 mRNA expression in fructose-fed rats compared with control rats. (D) Quantitative immunoblotting analysis demonstrates that the SOD2 ratio was significantly decreased in the NTS of fructose-fed rats after 1 week. The data shown are the means ± SEM of six independent experiments. *P < 0.05, **P < 0.01.
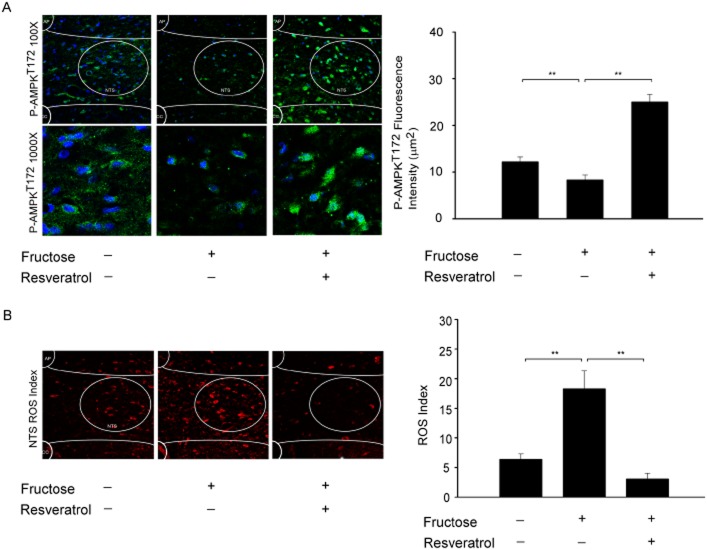
Resveratrol may activate AMPK by eliminating ROS production in the NTS of rats with fructose-induced hypertension. AMPKα2 deletion increases the expression of NADPH oxidase subunits and oxidative stress in vascular endothelial cells (Wang et al., 2010), suggesting that AMPK is an important regulator of NADPH oxidase activity. Using immunofluorescent staining, we demonstrated that AMPKT172 phosphorylation levels in the NTS were significantly reduced by the administration of fructose (Figure 3A). DHE fluorescence was used to estimate the superoxide levels in the NTS of animals fed fructose for 1 week. Representative images are shown in Figure 3B. The levels of DHE fluorescence in the NTS were significantly higher in the fructose-fed group than in the control groups. Furthermore, the DHE fluorescence levels in the NTS were significantly attenuated in the animals that received both fructose and resveratrol. These results suggest that resveratrol reduces ROS production in fructose-fed rats by activating AMPK.
Figure 3.
AMPK is significantly decreased in the NTS of fructose-induced hypertensive rats. (A) Confocal analysis of green fluorescence was used to estimate p-AMPKT172 levels in the NTS of fructose-fed rats after 1 week. The representative images demonstrate that the AMPK phosphorylation ratio was reduced in the NTS by fructose. (B) DHE fluorescence was use to estimate the ROS levels in the NTS of animals fed fructose or resveratrol for 1 week. We examined three groups (control; 10% fructose-treated; and 10% fructose + resveratrol; n = 6 for each). The images were photographed at ×100 and ×200 magnifications. Bar graph representation of ROS index in the NTS of the fructose groups as compared with WKY controls or 10% fructose + resveratrol group compared with fructose groups. The ROS index is the relative mean intensity of fluorescence of DHE. Sections of the NTS from the fructose group showed significant increases in DHE fluorescence compared with the control group sections. Furthermore, the DHE fluorescence in the NTS was significantly attenuated by the resveratrol treatment. Data are expressed as the means ± SEM (n = 6). **P < 0.01.
Resveratrol induces a systemic vasodepressor effect and NO release through AMPK activation in the NTS of fructose-induced hypertensive rats
AMPK is an important regulator of NADPH oxidase activity. In the previous experiment, we demonstrated that AMPK dysfunction could be responsible for the increase in NADPH oxidase and the decrease in SOD2 in fructose-fed rats. To study the effect of resveratrol on BP regulation in fructose-fed rats, SBP was measured every week in each group from week 0 to 4. As shown in Figure 4A, there were no differences in the baseline SBP among the five groups (control; fructose; resveratrol; fructose + resveratrol; fructose-fed for 2 weeks then fructose + resveratrol for 2 weeks) at week 0. However, resveratrol attenuated the increase in SBP induced by fructose when administered together with fructose from weeks 1 to 4 and when administered from weeks 3 to 4 to rats fed fructose for 2 weeks previously (Figure 4A).
Figure 4.
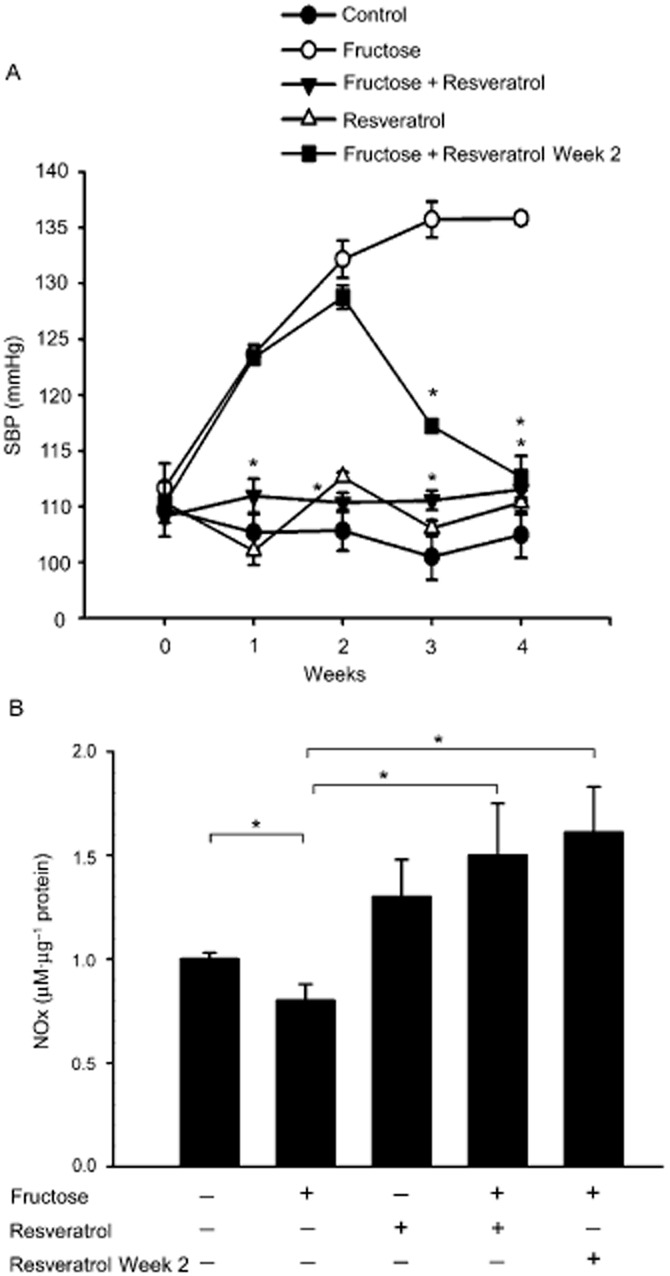
Resveratrol elevates SBP and reverses superoxide-dependent NO production in the NTS of rats with fructose-induced hypertension. (A) The graph shows the effects of resveratrol on SBP in the study groups during weeks 1–4 of treatment. We examined five groups (control group, fructose-fed group, resveratrol group, fructose + resveratrol group, fructose-fed week 2 + resveratrol group; n = 6 for each). The SBP significantly recovered after resveratrol treatment compared with the fructose group. (B) Quantification of the NOx concentration in the NTS of rats. The rats were randomly divided into five groups (control; fed with 10% fructose; fed with resveratrol 10 mg·kg−1·day−1; fed with 10% fructose and resveratrol 10 mg·kg−1·day−1; fructose-fed for 2 weeks then fructose + resveratrol for 2 weeks) of six rats each. The bar graph shows the NOx concentration (as µM nitrate µg-1 of total protein). Fructose significantly decreased the NOx levels in the NTS of the fructose group compared with the control group. However, treatment with resveratrol significantly increased the NOx levels in the NTS of the fructose + resveratrol group or fructose-fed week 2 + resveratrol group compared with the frucotse group. Data are expressed as the means ± SEM (n = 6). *P < 0.05.
As shown in Figure 4B, nitrate levels in the NTS were significantly decreased in fructose-fed rats compared with control groups. Interestingly, treatment with resveratrol markedly enhanced NOx levels in the NTS of both the fructose + resveratrol-fed rats and fructose-fed rats for 2 weeks followed by fructose + resveratrol for 2 weeks (Figure 4B). These results indicate that an attenuation of ROS is required for AMPK-induced NO release and the depressor response.
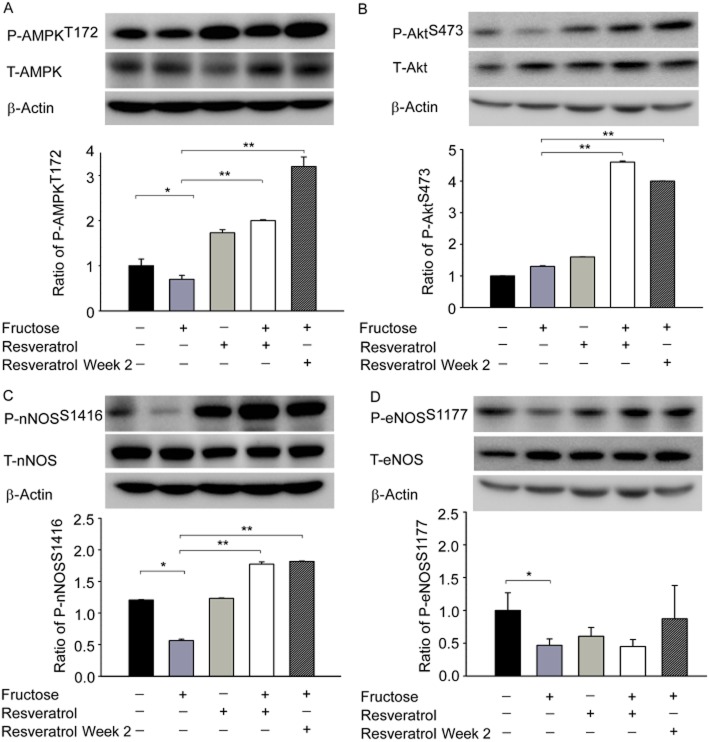
Resveratrol overexpression of AMPK enhances Akt-nNOS pathway activity in the NTS of fructose-induced hypertension
Our results suggested that AMPK-induced NO release eliminates NADPH oxidase and induces a depressor response. However, the mechanisms by which brain oxidative stress induces sympathoexcitation that then causes NO dysfunction in fructose-induced hypertension remain unclear. AMPK has been shown to modulate the Akt axis by regulating endothelial NOS (eNOS) in endothelial cells (Levine et al., 2007). We used a pharmacological approach to further elucidate the involvement of the PI3K/Akt cascades in AMPK-induced nNOS phosphorylation. As depicted in Figure 5, immunoblotting analyses of proteins extracted from the NTS demonstrated that treatment with the AMPK activator resveratrol increased the phosphorylation of AktS473 and nNOSS1416 but not eNOSS1177 in fructose-fed rats. These results indicate that the Akt-nNOS pathways may play a role in the AMPK-mediated depressor response in the NTS.
Figure 5.
Resveratrol increases the activity of the AMPK-Akt-nNOS pathway in the NTS of rats with fructose-induced hypertension. (A–D) Immunoblot showing the levels of P-AMPKT172, P-AktS473, P-nNOSS1416 and P-eNOSS1177 after treatment with resveratrol. The AMPK, Akt and nNOS phosphorylation ratios were significantly higher in the NTS after treatment with resveratrol. The values shown are the means ± SEM, n = 6. *P < 0.05, **P < 0.01.
Discussion and conclusions
Recent studies have shown that a high-fructose diet/solution is associated with increased BP in rats (Dimo et al., 2002). Studies in yeast have suggested that high fructose consumption is a risk factor for several metabolic diseases in humans through the resultant increase in ROS levels (Hecker et al., 2012; Rebollo et al., 2012). ROS in the brain are thought to contribute to the neuropathogenesis of hypertension by enhancing SNS activity (Paravicini and Touyz, 2006). Dysregulation or elimination of ROS production in brain plays a pivotal role in the pathophysiology of a number of cardiovascular diseases associated with metabolic disorders, including hypertension, obesity, metabolic syndrome, type-2 diabetes and dyslipidaemia (Chan et al., 2009; Farina et al., 2013; Ilkun and Boudina, 2013). The major findings of the present study are that the activation of AMPK decreases BP, decreases the production of ROS, and enhances the activity of the Akt-nNOS pathway by down-regulating the levels of NADPH oxidase and up-regulating the levels of SOD2 in the NTS of oxidative stress-associated hypertensive rats (Figure 6).
Figure 6.

The proposed mechanism by which the AMPK signalling pathway regulates BP in the NTS of rats with fructose-induced hypertension. Treatment with an AMPK activator (resveratrol) demonstrated that AMPK acts as an important regulator of BP through NADPH oxidase activity. AMPK decreased BP, decreased the generation of ROS, and enhanced the activity of the Akt-nNOS pathway by down-regulating NADPH oxidase levels and up-regulating SOD2 levels in the NTS of rats with oxidative stress-induced hypertension.
Hyperglycaemia, a consequence of diabetes, increases the formation of AGEs, which are senescent protein derivatives that result from the auto-oxidation of glucose and fructose (Guglielmotto et al., 2012). However, AGEs and their receptor, RAGE, may directly induce the generation of ROS via NADPH oxidases and/or other identified mechanisms (Wautier et al., 2001). In the present study, we observed a significant increase in the expression of RAGE and the NADPH oxidase subunits p22-phox and p67-phox in the NTS of fructose-fed rats compared with control rats (Figure 2). NADPH oxidase subunits such as p22-phox, p67-phox and p47-phox may be subjected to ubiquitination and proteasomal degradation by the 26S proteasome. Thus, an increased level of NADPH oxidase is unlikely to results from a decrease in its rate degradation. In addition, the NADPH oxidase-derived superoxide anion in the rostroventrolateral medulla mediates the chronic pressor response induced by angiotensin II (Ang II; Chan et al., 2007). As an important antioxidant in red wine, resveratrol is likely to contribute to the ability of red wine to prevent cardiovascular disease (Siemann and Creasy, 1992). By inhibiting the Ang II-induced production of ROS, resveratrol inhibits Ang-II-induced cardiomyocyte hypertrophy, which is clearly linked to its antioxidant effects (Cheng et al., 2004). Resveratrol has been shown to have potent antioxidant and antitumorigenic activities as well as important protective effects on the nervous system (Baur and Sinclair, 2006). In addition, the major pathways by which the numerous biological effects attributed to resveratrol occur include activation of AMPK and Sirt1 (Chong et al., 2012). Overexpression of Sirt1 has also been demonstrated to promote recruitment of PI3K/Akt and NO. FOXO1-dependent gene expression appears to be regulated by the NAD+-dependent deacetylase Sirt1, a key mechanism in the progression of many diseases, including cardiovascular disorders (Hughes et al., 2011). Mammalian Sirt1 is a protein deacetylase that has been shown to be involved in resveratrol-mediated protection from high-fat diet-induced metabolic damage (Pfluger et al., 2008). Furthermore, resveratrol has been shown to reduce the expression of AT1 receptors (for nomenclature see Alexander et al., 2013a) through Sirt1 activation both in vivo and in vitro. This inhibition of the renin–angiotensin system may contribute, at least in part, to the ability of resveratrol to suppress Ang II-induced hypertension (Miyazaki et al., 2008).
There is accumulating evidence that fructose promotes an ROS imbalance by simultaneously enhancing ROS production and down-regulation of the main antioxidant enzymes, such as SOD1 and SOD2. In the present study, we also demonstrated that the relative protein expression levels of SOD1 and SOD2 were significantly lower in the NTS of fructose-fed rats than in control rats. Nozoe et al. reported that the transfection of SOD1, which scavenges ROS generation, into the NTS decreased BP and HR (Nozoe et al., 2007). Recently, AMPKα1 or AMPKα2 silencing was shown to elevate oxidative stress through the down-regulation of genes involved in antioxidant defence, including SOD2, catalase, γ-glutamylcysteine synthase and thioredoxin (Colombo and Moncada, 2009; Fisslthaler and Fleming, 2009; Dong et al., 2010). However, activation of AMPK by agents such as metformin or AICAR (5-amino-1-β-D-ribofuranosyl-imidazole-4-carboxamide) inhibits hyperglycaemia-induced intracellular and mitochondrial ROS production and increases the expression of PPARγ coactivator-1α and SOD2. Therefore, we hypothesized that AMPK is an important regulator of NADPH oxidase activity. In the present study, we used the AMPK activator resveratrol to demonstrate that AMPK is an important regulator of the ability of NADPH oxidase to affect BP (Figure 4).
NO is synthesized within cardiac myocytes and plays a key role in modulating cardiovascular signalling. In the present study, we demonstrated that ROS levels of fructose-fed rats (1 week) were significantly higher in the NTS, leading to the down-regulation of NO release and the development of hypertension (Figure 1). We also used rats fed fructose from weeks 1 to 2 to investigate the mechanism of the elevated SBP induced by the ROS-dependent reduction in the release of NO. For several years, a number of laboratories have focused on the interplay between NO and ROS in the CVS, as well as the brain, organs that play a critical role in the regulation of BP. Several studies have determined that NO plays an important role in the modulation of sympathetic activity, and hence central regulation of arterial pressure. Superoxide derived from NAD(P)H oxidase avidly reacts with and inactivates NO and, thereby, modulates its bioavailability (Campese et al., 2007). Resveratrol treatment from 3 to 4 weeks to 11 to 12 weeks of age has been shown to reduce H2O2 content and elevate SOD activity and significantly attenuate the rise in BP in spontaneously hypertensive rats (SHR; Bhatt et al., 2011). In our studies, we also demonstrated that treatment with resveratrol attenuated the increase in SBP induced by fructose when administered from weeks 1 to 4 and also when administered from weeks 3 to 4 to rats that had been fed fructose for the previous 2 weeks (Figure 4). Therefore, early treatment with resveratrol lowers oxidative stress, preserves SOD function and attenuates the development of hypertension.
H2O2-activated AMPK phosphorylates eNOS on serine 1177, but does not phosphorylate nNOS in cardiac myocytes (Sartoretto et al., 2011) and phosphorylates eNOS on serine 633 in vascular endothelial cells (Chen et al., 2009). NO inhibits the activation of the SNS in the brain, which modulates BP. nNOS is present in neurons found in specific regions of the brain including the NTS, RVLM, paraventricular nucleus and the caudal ventrolateral medulla (CVLM) (Kantzides and Badoer, 2005). However, AMPK plays a key role in modulating NO, but its signalling mechanisms in the NTS remain unclear. In the present study, we demonstrated that the overexpression of AMPK affects nNOS but not eNOS in the depressor response mediated by the PI3K/Akt signalling pathway in the NTS of fructose-induced hypertensive rats (Figure 5). Thus, we propose that AMPK lowers BP, decreases the generation of ROS, and enhances Akt-nNOS pathway activity by down-regulation of NADPH oxidase levels and up-regulation of SOD2 levels in the NTS of oxidative stress-associated hypertensive rats.
Thandapilly et al.'s study demonstrates that 10 weeks of resveratrol treatment significantly improved the NO levels in plasma of SHR. Treatment with resveratrol did not affect NO levels and P-AMPK/T-AMPK ratio in normotensive WKY rats. NO-AMPK signalling has also been shown to be linked to the anti-hypertrophic effect of resveratrol in the SHR model in vivo (Thandapilly et al., 2011). In addition, Bhatt et al., using SHR and WKY rats (3–4 weeks old), showed that untreated control SHR exhibited increased BP, oxidative stress and attenuated endothelium-dependent relaxation in comparison to WKY rats. The impaired endothelium function in SHR was associated with lower nitrite/nitrate levels. Resveratrol treatment, in drinking water for 10 weeks, attenuated the hypertension development in SHR, as indicated by lower BP in resveratrol-treated SHR (SHR-R) compared with control SHR. SHR-R also had reduced H2O2 content and elevated SOD activity. However, resveratrol did not affect SBP, H2O2 and NO levels in WKY rats (Bhatt et al., 2011). Taken together, these findings show that resveratrol treatment normalized the SOD activity and H2O2 levels in SHR rats, but did not affect SOD, H2O2 and NO levels in WKY rats (normotensive rats). Resveratrol has also been shown to induce a 14-fold increase in the action of SOD2 in cells (Robb et al., 2008a). SOD2 reduces superoxide to hydrogen peroxide (H2O2), but the levels of H2O2 did not increase because of other cellular activities. There is also evidence suggesting that SOD2 is up-regulated following resveratrol supplementation (Qiu et al., 2010). In our opinion, resveratrol alone may be able to increase SOD2 levels in the control group (normotensive rats). Therefore, early treatment with resveratrol lowers oxidative stress, preserves endothelial function and attenuates the development of hypertension.
In conclusion, apart from its well-characterized actions as an antioxidant, accumulating evidence suggests that resveratrol can eliminate ROS and has cardioprotective and chemopreventive effects. In addition, the CNS is a target of resveratrol, which can cross the blood–brain barrier and have neuroprotective effects (Quincozes-Santos and Gottfried, 2011). The beneficial effects of resveratrol may be mediated by the activation of AMPK, which down-regulates several downstream factors, such as Rac1, NADPH oxidases and SOD2, leading to CNS protection. These novel findings suggest that the AMPK activator resveratrol is a potential pharmacological candidate for the treatment of hypertension.
Acknowledgments
The authors gratefully acknowledge the technical assistance and the invaluable input and support of Mr. Chia-Yu Lee. This work was supported by funding from the National Science Council (NSC100-2321-B-075B-002, 101-2320-B-075B-002-MY3) and the Kaohsiung Veterans General Hospital (VGHKS 100-101) to Dr. C. J. Tseng.
Glossary
- AMPK
AMP-activated PK
- eNOS
endothelial NOS
- nNOS
neuronal NOS
- NOx
nitrogen oxides
- NTS
nucleus tractus solitarii
- ROS
reactive oxygen species
- SHRs
spontaneously hypertensive rats
- WKY
Wistar-Kyoto rats
Conflict of interest
None.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Superoxide-dependent NO production elevated SBP has significantly time-dependent manner in the NTS of fructose-induced hypertensive rats. (A) The graph shows the SBP in the fructose groups after 2 weeks. The SBP's time-dependent manner (day 2, 7 and 14) significantly increased in the fructose group compared with the day 0 group. (B) Quantification of NOx concentrations in the NTS of fructose-fed rats. The bar graph shows the NOx concentration (as µM nitrate µg-1 of total protein). NOx levels significantly decreased in the NTS of the fructose-fed rats (day 7 and 14) compared with the day 0 group. (C) Representative images of DHE-treated brain sections. The images were photographed at ×100 magnification. Bar graph representation of ROS index in the NTS of tShe fructose groups. The ROS index is the relative mean intensity of fluorescence of DHE. Sections including the NTS of fructose-fed rats (days 7 and 14) displayed a significant increase in DHE fluorescence compared with the day 0 sections. Values are shown as the means ± SEM, n = 6. *P < 0.05, **P < 0.01.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G protein-coupled receptors. Br J Pharmacol. 2013a;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Enzymes. Br J Pharmacol. 2013b;170:1797–1867. doi: 10.1111/bph.12451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aller EE, Abete I, Astrup A, Martinez JA, van Baak MA. Starches, sugars and obesity. Nutrients. 2011;3:341–369. doi: 10.3390/nu3030341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baur JA, Sinclair DA. Therapeutic potential of resveratrol: the in vivo evidence. Nat Rev Drug Discov. 2006;5:493–506. doi: 10.1038/nrd2060. [DOI] [PubMed] [Google Scholar]
- Bavithra S, Selvakumar K, Krishnamoorthy G, Venkataraman P, Arunakaran J. Melatonin attenuates polychlorinated biphenyls induced apoptosis in the neuronal cells of cerebral cortex and cerebellum of adult male rats – in vivo. Environ Toxicol Pharmacol. 2013;36:152–163. doi: 10.1016/j.etap.2013.03.005. [DOI] [PubMed] [Google Scholar]
- Bendall JK, Rinze R, Adlam D, Tatham AL, de Bono J, Wilson N, et al. Endothelial Nox2 overexpression potentiates vascular oxidative stress and hemodynamic response to angiotensin II: studies in endothelial-targeted Nox2 transgenic mice. Circ Res. 2007;100:1016–1025. doi: 10.1161/01.RES.0000263381.83835.7b. [DOI] [PubMed] [Google Scholar]
- Bhatt SR, Lokhandwala MF, Banday AA. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur J Pharmacol. 2011;667:258–264. doi: 10.1016/j.ejphar.2011.05.026. [DOI] [PubMed] [Google Scholar]
- Campese VM, Sindhu RK, Ye S, Bai Y, Vaziri ND, Jabbari B. Regional expression of NO synthase, NAD(P)H oxidase and superoxide dismutase in the rat brain. Brain Res. 2007;1134:27–32. doi: 10.1016/j.brainres.2006.11.067. [DOI] [PubMed] [Google Scholar]
- Chan SH, Wang LL, Tseng HL, Chan JY. Upregulation of AT1 receptor gene on activation of protein kinase Cbeta/nicotinamide adenine dinucleotide diphosphate oxidase/ERK1/2/c-fos signaling cascade mediates long-term pressor effect of angiotensin II in rostral ventrolateral medulla. J Hypertens. 2007;25:1845–1861. doi: 10.1097/HJH.0b013e328217b286. [DOI] [PubMed] [Google Scholar]
- Chan SH, Wu CA, Wu KL, Ho YH, Chang AY, Chan JY. Transcriptional upregulation of mitochondrial uncoupling protein 2 protects against oxidative stress-associated neurogenic hypertension. Circ Res. 2009;105:886–896. doi: 10.1161/CIRCRESAHA.109.199018. [DOI] [PubMed] [Google Scholar]
- Chen Z, Peng IC, Sun W, Su MI, Hsu PH, Fu Y, et al. AMP-activated protein kinase functionally phosphorylates endothelial nitric oxide synthase Ser633. Circ Res. 2009;104:496–505. doi: 10.1161/CIRCRESAHA.108.187567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng TH, Liu JC, Lin H, Shih NL, Chen YL, Huang MT, et al. Inhibitory effect of resveratrol on angiotensin II-induced cardiomyocyte hypertrophy. Naunyn Schmiedebergs Arch Pharmacol. 2004;369:239–244. doi: 10.1007/s00210-003-0849-6. [DOI] [PubMed] [Google Scholar]
- Cheng WH, Lu PJ, Ho WY, Tung CS, Cheng PW, Hsiao M, et al. Angiotensin II inhibits neuronal nitric oxide synthase activation through the ERK1/2-RSK signaling pathway to modulate central control of blood pressure. Circ Res. 2010;106:788–795. doi: 10.1161/CIRCRESAHA.109.208439. [DOI] [PubMed] [Google Scholar]
- Chong ZZ, Wang S, Shang YC, Maiese K. Targeting cardiovascular disease with novel SIRT1 pathways. Future Cardiol. 2012;8:89–100. doi: 10.2217/fca.11.76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colombo SL, Moncada S. AMPKalpha1 regulates the antioxidant status of vascular endothelial cells. Biochem J. 2009;421:163–169. doi: 10.1042/BJ20090613. [DOI] [PubMed] [Google Scholar]
- Dasgupta B, Milbrandt J. Resveratrol stimulates AMP kinase activity in neurons. Proc Natl Acad Sci U S A. 2007;104:7217–7222. doi: 10.1073/pnas.0610068104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dimo T, Rakotonirina SV, Tan PV, Azay J, Dongo E, Cros G. Leaf methanol extract of Bidens pilosa prevents and attenuates the hypertension induced by high-fructose diet in Wistar rats. J Ethnopharmacol. 2002;83:183–191. doi: 10.1016/s0378-8741(02)00162-9. [DOI] [PubMed] [Google Scholar]
- Dong Y, Zhang M, Liang B, Xie Z, Zhao Z, Asfa S, et al. Reduction of AMP-activated protein kinase alpha2 increases endoplasmic reticulum stress and atherosclerosis in vivo. Circulation. 2010;121:792–803. doi: 10.1161/CIRCULATIONAHA.109.900928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farina JP, Garcia ME, Alzamendi A, Giovambattista A, Marra CA, Spinedi E, et al. Antioxidant treatment prevents the development of fructose-induced abdominal adipose tissue dysfunction. Clin Sci (Lond) 2013;125:87–97. doi: 10.1042/CS20120470. [DOI] [PubMed] [Google Scholar]
- Fisslthaler B, Fleming I. Activation and signaling by the AMP-activated protein kinase in endothelial cells. Circ Res. 2009;105:114–127. doi: 10.1161/CIRCRESAHA.109.201590. [DOI] [PubMed] [Google Scholar]
- Gao L, Wang W, Li YL, Schultz HD, Liu D, Cornish KG, et al. Superoxide mediates sympathoexcitation in heart failure: roles of angiotensin II and NAD(P)H oxidase. Circ Res. 2004;95:937–944. doi: 10.1161/01.RES.0000146676.04359.64. [DOI] [PubMed] [Google Scholar]
- Guglielmotto M, Aragno M, Tamagno E, Vercellinatto I, Visentin S, Medana C, et al. AGEs/RAGE complex upregulates BACE1 via NF-kappaB pathway activation. Neurobiol Aging. 2012;33:196. doi: 10.1016/j.neurobiolaging.2010.05.026. e113–127. [DOI] [PubMed] [Google Scholar]
- Hardie DG, Salt IP, Hawley SA, Davies SP. AMP-activated protein kinase: an ultrasensitive system for monitoring cellular energy charge. Biochem J. 1999;338(Pt 3):717–722. [PMC free article] [PubMed] [Google Scholar]
- Hecker PA, Galvao TF, O'Shea KM, Brown BH, Henderson R, Jr, Riggle H, et al. High-sugar intake does not exacerbate metabolic abnormalities or cardiac dysfunction in genetic cardiomyopathy. Nutrition. 2012;28:520–526. doi: 10.1016/j.nut.2011.09.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho WY, Lu PJ, Hsiao M, Hwang HR, Tseng YC, Yen MH, et al. Adenosine modulates cardiovascular functions through activation of extracellular signal-regulated kinases 1 and 2 and endothelial nitric oxide synthase in the nucleus tractus solitarii of rats. Circulation. 2008;117:773–780. doi: 10.1161/CIRCULATIONAHA.107.746032. [DOI] [PubMed] [Google Scholar]
- Hughes KJ, Meares GP, Hansen PA, Corbett JA. FoxO1 and SIRT1 regulate beta-cell responses to nitric oxide. J Biol Chem. 2011;286:8338–8348. doi: 10.1074/jbc.M110.204768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ilkun O, Boudina S. cardiac dysfunction and oxidative stress in the metabolic syndrome: an update on antioxidant therapies. Curr Pharm Des. 2013;19:4806–4817. doi: 10.2174/1381612811319270003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kantzides A, Badoer E. nNOS-containing neurons in the hypothalamus and medulla project to the RVLM. Brain Res. 2005;1037:25–34. doi: 10.1016/j.brainres.2004.11.032. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levine YC, Li GK, Michel T. Agonist-modulated regulation of AMP-activated protein kinase (AMPK) in endothelial cells. Evidence for an AMPK → Rac1 → Akt → endothelial nitric-oxide synthase pathway. J Biol Chem. 2007;282:20351–20364. doi: 10.1074/jbc.M702182200. [DOI] [PubMed] [Google Scholar]
- Li Y, Cao Z, Zhu H. Upregulation of endogenous antioxidants and phase 2 enzymes by the red wine polyphenol, resveratrol in cultured aortic smooth muscle cells leads to cytoprotection against oxidative and electrophilic stress. Pharmacol Res. 2006;53:6–15. doi: 10.1016/j.phrs.2005.08.002. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki R, Ichiki T, Hashimoto T, Inanaga K, Imayama I, Sadoshima J, et al. SIRT1, a longevity gene, downregulates angiotensin II type 1 receptor expression in vascular smooth muscle cells. Arterioscler Thromb Vasc Biol. 2008;28:1263–1269. doi: 10.1161/ATVBAHA.108.166991. [DOI] [PubMed] [Google Scholar]
- Nozoe M, Hirooka Y, Koga Y, Sagara Y, Kishi T, Engelhardt JF, et al. Inhibition of Rac1-derived reactive oxygen species in nucleus tractus solitarius decreases blood pressure and heart rate in stroke-prone spontaneously hypertensive rats. Hypertension. 2007;50:62–68. doi: 10.1161/HYPERTENSIONAHA.107.087981. [DOI] [PubMed] [Google Scholar]
- Paravicini TM, Touyz RM. Redox signaling in hypertension. Cardiovasc Res. 2006;71:247–258. doi: 10.1016/j.cardiores.2006.05.001. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotaxic Coordinates. 6th edn. Elsevier: London; 2006. [Google Scholar]
- Pfluger PT, Herranz D, Velasco-Miguel S, Serrano M, Tschop MH. Sirt1 protects against high-fat diet-induced metabolic damage. Proc Natl Acad Sci U S A. 2008;105:9793–9798. doi: 10.1073/pnas.0802917105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qiu X, Brown K, Hirschey MD, Verdin E, Chen D. Calorie restriction reduces oxidative stress by SIRT3-mediated SOD2 activation. Cell Metab. 2010;12:662–667. doi: 10.1016/j.cmet.2010.11.015. [DOI] [PubMed] [Google Scholar]
- Quincozes-Santos A, Gottfried C. Resveratrol modulates astroglial functions: neuroprotective hypothesis. Ann N Y Acad Sci. 2011;1215:72–78. doi: 10.1111/j.1749-6632.2010.05857.x. [DOI] [PubMed] [Google Scholar]
- Rebollo A, Roglans N, Alegret M, Laguna JC. Way back for fructose and liver metabolism: bench side to molecular insights. World J Gastroenterol. 2012;18:6552–6559. doi: 10.3748/wjg.v18.i45.6552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robb EL, Page MM, Wiens BE, Stuart JA. Molecular mechanisms of oxidative stress resistance induced by resveratrol: specific and progressive induction of MnSOD. Biochem Biophys Res Commun. 2008a;367:406–412. doi: 10.1016/j.bbrc.2007.12.138. [DOI] [PubMed] [Google Scholar]
- Robb EL, Winkelmolen L, Visanji N, Brotchie J, Stuart JA. Dietary resveratrol administration increases MnSOD expression and activity in mouse brain. Biochem Biophys Res Commun. 2008b;372:254–259. doi: 10.1016/j.bbrc.2008.05.028. [DOI] [PubMed] [Google Scholar]
- Sartoretto JL, Kalwa H, Pluth MD, Lippard SJ, Michel T. Hydrogen peroxide differentially modulates cardiac myocyte nitric oxide synthesis. Proc Natl Acad Sci U S A. 2011;108:15792–15797. doi: 10.1073/pnas.1111331108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siemann EH, Creasy LL. Concentration of the phytoalexin resveratrol in wine. Am J Enol Vitic. 1992;43:49–52. [Google Scholar]
- Stapleton D, Mitchelhill KI, Gao G, Widmer J, Michell BJ, Teh T, et al. Mammalian AMP-activated protein kinase subfamily. J Biol Chem. 1996;271:611–614. doi: 10.1074/jbc.271.2.611. [DOI] [PubMed] [Google Scholar]
- Thandapilly SJ, Louis XL, Yang T, Stringer DM, Yu L, Zhang S, et al. Resveratrol prevents norepinephrine induced hypertrophy in adult rat cardiomyocytes, by activating NO-AMPK pathway. Eur J Pharmacol. 2011;668:217–224. doi: 10.1016/j.ejphar.2011.06.042. [DOI] [PubMed] [Google Scholar]
- Tran LT, Yuen VG, McNeill JH. The fructose-fed rat: a review on the mechanisms of fructose-induced insulin resistance and hypertension. Mol Cell Biochem. 2009;332:145–159. doi: 10.1007/s11010-009-0184-4. [DOI] [PubMed] [Google Scholar]
- Wang S, Zhang M, Liang B, Xu J, Xie Z, Liu C, et al. AMPKalpha2 deletion causes aberrant expression and activation of NAD(P)H oxidase and consequent endothelial dysfunction in vivo: role of 26S proteasomes. Circ Res. 2010;106:1117–1128. doi: 10.1161/CIRCRESAHA.109.212530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Wang Q, Sun Z. Normal IgG downregulates the intracellular superoxide level and attenuates migration and permeability in human aortic endothelial cells isolated from a hypertensive patient. Hypertension. 2012;60:818–826. doi: 10.1161/HYPERTENSIONAHA.112.199281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wautier MP, Chappey O, Corda S, Stern DM, Schmidt AM, Wautier JL. Activation of NADPH oxidase by AGE links oxidant stress to altered gene expression via RAGE. Am J Physiol Endocrinol Metab. 2001;280:E685–E694. doi: 10.1152/ajpendo.2001.280.5.E685. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Croft KD, Mori TA, Schyvens CG, McKenzie KU, Whitworth JA. The antioxidant tempol prevents and partially reverses dexamethasone-induced hypertension in the rat. Am J Hypertens. 2004;17:260–265. doi: 10.1016/j.amjhyper.2003.11.004. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Superoxide-dependent NO production elevated SBP has significantly time-dependent manner in the NTS of fructose-induced hypertensive rats. (A) The graph shows the SBP in the fructose groups after 2 weeks. The SBP's time-dependent manner (day 2, 7 and 14) significantly increased in the fructose group compared with the day 0 group. (B) Quantification of NOx concentrations in the NTS of fructose-fed rats. The bar graph shows the NOx concentration (as µM nitrate µg-1 of total protein). NOx levels significantly decreased in the NTS of the fructose-fed rats (day 7 and 14) compared with the day 0 group. (C) Representative images of DHE-treated brain sections. The images were photographed at ×100 magnification. Bar graph representation of ROS index in the NTS of tShe fructose groups. The ROS index is the relative mean intensity of fluorescence of DHE. Sections including the NTS of fructose-fed rats (days 7 and 14) displayed a significant increase in DHE fluorescence compared with the day 0 sections. Values are shown as the means ± SEM, n = 6. *P < 0.05, **P < 0.01.