Abstract
Background and Purpose
Cardiovascular risk is higher in men and postmenopausal women compared with premenopausal women. This may be due to sex differences in endothelial function. Here, sex differences in endothelial function of porcine coronary arteries (PCAs) were investigated.
Experimental Approach
Distal PCAs were studied under myographic conditions and after precontraction with U46619. Concentration-response curves to bradykinin were constructed in the presence of a range of inhibitors.
Key Results
In male and female PCAs, bradykinin produced comparable vasorelaxant responses. Inhibition of NO and prostanoid synthesis produced greater inhibition in males compared with females. Removing H2O2 with PEG-catalase reduced the maximum relaxation in the absence, but not the presence of L-NAME and indomethacin in females, and had no effect in males. Blocking gap junctions with 100 µM carbenoxolone or 18α-glycyrrhetinic acid further inhibited the endothelium-derived hyperpolarization (EDH)-mediated response in females but not in males. In female PCAs, the maximum EDH-mediated response was reduced by inhibiting SKCa with apamin and by inhibiting IKCa with TRAM-34, or with both. In male PCAs, at maximum bradykinin concentration, the EDH-mediated response was reduced in the presence of apamin but not TRAM-34. Western blot did not detect any differences in connexins 40 or 43 or in IKCa expression between male and female PCAs.
Conclusions and Implications
H2O2 mediated some part of endothelium-dependent vasorelaxation in female PCAs and EDH was more important in females, with differences in the contribution of gap junctions and IKCa channels. These findings may contribute to understanding vascular protection in premenopausal women.
Keywords: endothelium-derived hyperpolarization (EDH), sex differences, gap junctions, hydrogen peroxide (H2O2), nitric oxide (NO), porcine coronary artery (PCA), intermediate-conductance calcium-activated K+ channel (IKca), small-conductance calcium-activated K+ channel (SKca), bradykinin, connexin (Cx)
Introduction
Regulation of vascular tone is controlled by endothelium-derived relaxants including NO (Furchgott and Zawadzki, 1980), prostacyclin (Moncada et al., 1976) and endothelium-dependent hyperpolarization (EDH)-type mechanisms (Taylor and Weston, 1988; Edwards et al., 2010; Feletou and Vanhoutte, 2013). Many mediators have been proposed to be responsible for EDH over the past decade, yet none has appeared to be a ‘universal EDH’ (Griffith, 2004). However, in 2010, the EDH-mediated responses were classified into two categories (Edwards et al., 2010): the first category is the ‘classical’ EDH pathway in which an increase in intracellular Ca2+ concentration hyperpolarizes the endothelial cells leading to activation of small (SKCa) and intermediate (IKCa) conductance Ca2+-activated potassium channels on endothelial cells (Busse et al., 2002; Gluais et al., 2005a; Edwards et al., 2010; channel nomenclature follows Alexander et al., 2013). Activation of these potassium channels in turn hyperpolarizes the vascular smooth muscle either through the transfer of electrical signalling via myoendothelial gap junctions (MEGJs) (Chaytor et al., 1998; Edwards et al., 2000; Harris et al., 2000; Kenny et al., 2002; Sandow et al., 2002; de Wit and Griffith, 2010; Chadha et al., 2011; Kerr et al., 2012) or through efflux of K+ ions from endothelial SKCa and IKCa channels acting on barium-sensitive inwardly rectifying potassium channels and the ouabain-sensitive Na+/K+ ATPase pump respectively (Edwards et al., 1998; 2010). Gap junctions are formed from two docking hemichannels with each hemichannel made up of six connexin (Cx) proteins (Griffith, 2004). In blood vessels, four subtypes of Cx proteins have been identified, Cx 37, 40, 43 and 45 (Chaytor et al., 2003; Lang et al., 2007; de Wit and Griffith, 2010). The Cx 43 subtype in particular has been demonstrated to play a functional role in the EDH-mediated vasorelaxation in subcutaneous resistance arteries from pregnant women (Lang et al., 2007).
In the second category of the EDH-mediated pathway, smooth muscles cells are hyperpolarized by endothelium-derived mediators (Edwards et al., 2010) such as H2O2 (Hayabuchi et al., 1998; Yada et al., 2003; Shimokawa, 2010) or arachidonic acid derivatives (epoxyeicosatrienoic acids) (Campbell et al., 1996). H2O2 has been reported to act as a factor responsible for EDH in porcine coronary arteries (PCAs), human, murine and rat mesenteric arteries (Matoba et al., 2000; 2002; 2003; Wheal et al., 2012) (see Shimokawa, 2010). However, the responses to H2O2 may vary between species, vascular beds and experimental conditions (Chaytor et al., 2003; Gluais et al., 2005b; Lucchesi et al., 2005).
Cardiovascular risk in men and postmenopausal women is higher than premenopausal women and sex differences in endothelial function have been suggested to contribute to this difference in risk (McCulloch and Randall, 1998; Villar et al., 2008). To date, most studies on endothelial function have been conducted on either arteries from male animals only (Edwards et al., 1998; Harris et al., 2000; Matoba et al., 2003; Leung et al., 2006; Garry et al., 2009) or from both sexes (Quignard et al., 1999; Edwards et al., 2000; Yang et al., 2003; Chadha et al., 2011; Huang et al., 2011). However, previous studies have demonstrated clear sex differences in vascular function of the EDH-mediated pathways (see Feletou and Vanhoutte, 2006; Villar et al., 2008). EDH-mediated responses have been reported to be up-regulated to compensate for the loss of NO (McCulloch et al., 1997; Yada et al., 2003; Wheal et al., 2012) and this compensation was greater in females than males (McCulloch and Randall, 1998; White et al., 2000; Garry et al., 2009). Furthermore, in endothelial NO synthase (eNOS) and COX-1 double-knockout mice (‘EDH mice’), the male mice were hypertensive while female mice were normotensive with greater endothelium-dependent vasorelaxation in female mice (Scotland et al., 2005). However, in rat cerebral arteries, the EDH-mediated responses were greater in males compared with females (Sokoya et al., 2007). In a previous study on mesenteric arteries from rats, EDH-mediated responses in females were partly dependent on increased expression of Cx 43, which was driven by oestrogen (Liu et al., 2002).
The aim of the present study was to investigate the effects of sex on endothelium-dependent vasorelaxation of isolated coronary arteries from male or female pigs (PCAs), specifically the contributions of gap junction communication, endogenous H2O2 and Ca2+-activated K+ channels to the vasorelaxation induced by bradykinin.
Methods
Preparation of rings of distal PCAs
Hearts from male and female pigs (large white hybrid pigs, 4–6 months old, weighing ∼50 kg) were collected from a local abattoir and transported to the laboratory in ice-cold modified Krebs'-Henseleit solution (118 mM NaCl, 4.8 mM KCl, 1.1 mM MgSO4, 25 mM NaHCO3, 1.2 mM KH2PO4, 12 mM D-glucose, 1.25 mM CaCl2) previously gassed with 5% CO2 and 95% O2. The distal part of the coronary artery was then dissected and placed in 2% w/v Ficoll in Krebs'-Henseleit solution for overnight storage at 4°C. The following day, tissues were finely dissected, cleaned of adherent connective and fatty tissues. PCAs were then cut into rings of about 2 mm in length and mounted in a multichannel wire myograph (Model 610 M, DMT, Aarhus N, Denmark) filled with 5 mL Krebs'-Henseleit solution gassed with 5% CO2 and 95% O2 and maintained at 37°C. Vessels were tensioned to 24.5 mN and left to equilibrate for approximately 30 min. Tension was measured and recorded using a PowerLab recording system (AD instruments, Oxfordshire, UK). The distal part of the PCAs was used because it has previously been reported that the EDH response is greater with decreasing vessel size (Shimokawa et al., 1996) and the number of MEGJs appears to be greater in the distal part of the rat mesenteric arteries, compared with the proximal (Sandow and Hill, 2000). Here, the mean vessel size of PCAs from female pigs (0.86 ± 0.02 mm) did not differ significantly from the mean vessel size from male pigs (0.89 ± 0.02 mm) (two-tailed, unpaired Student's t-test). Seasonal variations in responses were not allowed for the present study design but each set of experiment has been carried out with an internal control.
Experimental protocol
Wire myography
After 30 min of equilibration, responses to 60 mM KCl were determined twice. The vascular tone was then raised to about 50–80% of the second KCl contraction tone by the addition of the thromboxane A2 mimetic, 9,11-dideoxy-9a,11a-epoxymethanoprostaglandin F2α (U46619; 2 nM–50 µM). Once stable tone was achieved, concentration-response curves to bradykinin, an endothelium-dependent relaxant (0.01 nM–1 µM) or NS309, (6,7-dichloro-1H-indole-2,3-dione 3-oxime), a positive modulator of SKCa and IKCa channels (Leuranguer et al., 2008; Dalsgaard et al., 2009; Brondum et al., 2010) (0.1 µM–0.1 mM) were constructed. To examine the selectivity of NS309, some experiments were precontracted with 60 mM KCl with their respective controls raised to the same tone with U46619. All inhibitors were incubated with the tissues for 1 h before precontraction with U46619. Vasorelaxation to bradykinin was studied in the absence or presence of NG-nitro-L-arginine methyl ester (L-NAME) (300 µM) which is a NO synthase inhibitor to determine the NO-mediated component. Indomethacin (10 µM) was used to inhibit the synthesis of prostanoids. In some experiments, polyethelene glycol-catalase (PEG-catalase; 300 U mL−1) (Hedegaard et al., 2011) was added to remove intracellular H2O2. To study the role of gap junctions, a non-selective gap junction inhibitor carbenoxolone (100 µM) (Harris et al., 2002; Tang and Vanhoutte, 2008) or 18α-glycyrrhetinic acid (18α-GA) (100 µM) (Kenny et al., 2002; Matoba et al., 2003) was used. Apamin (500 nM) and TRAM-34 (10 µM) (Gluais et al., 2005a), SKCa and IKCa inhibitors, respectively, were used to study the role of K+ channels in the bradykinin-induced vasorelaxation.
Western blotting
Western blot studies were carried out to determine the relative expression levels of Cx 37, 40, 43 and IKCa in PCAs from male and female pigs. PCAs from male and female pigs were finely dissected and cut into rings of about 1 cm in length. Vessels were then gassed with 5% CO2 and 95% O2 in Krebs'-Henseleit solution at 37°C for 1 h. Segments (designated F1–F5 for samples from females and M1–M5 for samples from males) were then homogenized on ice in lysis buffer (80 mM sodium β-glycerophosphate, 20 mM imidazole, 1 mM dithiothreitol, 1 mM sodium fluoride, pH 7.6) containing protease inhibitor cocktail (Calbiochem, VWR International Ltd, Lutterworth, Leicestershire, UK). Samples (F1–F5 and M1–M5) were diluted 1:1 in 2× Laemmli sample buffer and heated at 95°C for 5 min. After centrifugation at 13 000× g for 1 min, 5 µg of protein were loaded on a 4–20% Mini-PROTEAN TGX precast gel (Bio-Rad, Hemel Hempstead, Hertfordshire, UK) and transferred onto nitrocellulose membrane (GE Healthcare, Little Chalfont, Buckinghamshire, UK) using a Bio-Rad mini-transblot. The nitrocellulose membrane was then blocked with 5% w/v non-fat milk in Tris-buffered saline containing 0.1% Tween 20 for 1 h before incubation with mouse monoclonal anti-Cx-43 antibody (C8093 Sigma-Aldrich) (1:1000) and mouse monoclonal anti-myosin light chain (MLC) antibody (M4401 Sigma-Aldrich) (1:500) overnight at 4°C. After washing in Tris-buffered saline containing 0.1% Tween 20, the blot was incubated with secondary antibody IRDye 800CW Goat anti-mouse IgG (1:10 000) (LI-COR Biosciences, Cambridge, UK) at 37°C for 1 h. The immunoblot was then visualized using a LI-COR Odyssey Infrared Imaging Scanner and the densities of bands determined using Odyssey (Application Software 3.0 LI-COR Biosciences).
Due to the high concentration of proteins required for all other antibodies, samples (designated F6–F10 and M6–M10) were prepared in a slightly modified protocol. Samples (F6–F10 and M6–M10) were homogenized on ice in lysis buffer (20 mM Tris, 1 mM EGTA, 320 mM sucrose, 0.1% Triton X100, 1 mM sodium fluoride, 10 mM sodium β-glycerophosphate, pH 7.6) containing protease inhibitor cocktail (Calbiochem) followed by centrifugation at 3000× g for 5 min at 4°C. Supernatant of the samples were then solubilized in 6× solubilization buffer and diluted to 1 mg mL−1 of protein with 1× solubilization buffer. Samples were then heated at 95°C for 5 min followed by centrifugation at 13 000× g for 1 min before loading to the precast gel. The amounts of protein concentration loaded with the respective dilution of antibody used were as followed: for Cx 40, 10 µg of PCAs samples with 20 µg of pig kidney lysate used as positive control were incubated with rabbit polyclonal anti-Cx 40 – aminoterminal end antibody (ab38580 Abcam®, Cambridge, UK) (1:100) and mouse monoclonal anti-GAPDH antibody (G8795 Sigma-Aldrich, Poole, Dorset, UK) (1:40 000). For Cx 37, 15 µg of PCAs samples with 20 µg of pig and rat kidney lysate used as positive controls were incubated with rabbit polyclonal anti-GJA4 antibody (C15878 Assay Biotech, Stratech Scientific Limited, Suffolk, UK) (1:500) and mouse monoclonal anti-β-Actin antibody (A2228 Sigma-Aldrich) (1:40 000). For IKCa, 15 µg of PCAs samples with 10 µg of pig kidney lysate used as positive control were incubated with mouse polyclonal anti-KCNN4 antibody (H00003783-B01P Abnova, Taipei, Taiwan) (1:500) and mouse monoclonal anti-GAPDH antibody (G8795 Sigma-Aldrich) (1:40 000). For all antibodies, the blocking and washing steps used were as described above and the same secondary antibody, IRDye® 800CW Goat anti-mouse IgG (1:10 000) (LI-COR Biosciences) were used for anti-mouse antibody and IRDye 680LT Goat anti-rabbit IgG (1:10 000) (LI-COR Biosciences) for anti-rabbit antibody.
Data analysis
Data are presented as mean percentage relaxation of U46619-induced tone with SEM and n being the number of separate animals. The concentration-response curves were fitted to a sigmoidal curve with a variable slope using four parameters logistic equation in GraphPad Prism (Version 6, GraphPad Software, La Jolla, CA, USA). The maximum percentage relaxation (Rmax) and the negative log of concentration required to produce half the maximal relaxation of the induced tone (pEC50) were calculated from the fitted curves. Data were analysed using two-tailed, paired or unpaired Student's t-test to compare differences between two groups. In three or more groups, one-way anova was used and significant differences between groups were detected by Bonferroni's post hoc test. P-values of less than 0.05 were considered statistically significant.
Materials
All drugs were purchased from Sigma-Aldrich except for apamin and NS309 from Tocris Bioscience (Bristol, UK). Stock solutions of L-NAME, PEG-catalase, carbenoxolone and apamin were made in distilled water. Stock solution of indomethacin was made in absolute ethanol whereas TRAM-34, 18α-GA and NS309 were dissolved in DMSO. Stock solutions of bradykinin or the thromboxane A2 mimetic U46619 (10 mM) were made in water and ethanol respectively. All further dilutions of the stock solutions were made using distilled water except for NS309 which was further diluted with DMSO to 10 mM, 30% DMSO to 1 mM and distilled water to 0.1 mM.
Results
The effects of sex on EDH-type vasorelaxations
In PCAs from male and female animals, bradykinin produced comparable, concentration-dependent vasorelaxant effects, both in terms of the Rmax (Figure 1A) and the pEC50 values (8.50 ± 0.08 in females; 8.38 ± 0.08 in males). In either set of PCAs, the addition of L-NAME and indomethacin reduced the maximum bradykinin-induced vasorelaxation compared with controls and this reduction was greater in male than in female PCAs. The concentration-response of arteries from both male and female pigs was also shifted significantly to the right in the presence of L-NAME and indomethacin, compared with their respective control responses (pEC50 values: 7.65 ± 0.16, females; 7.33 ± 0.27, males). Original traces of recording showing the tissue responses with increasing concentrations of bradykinin are shown in Figure 1B and C.
Figure 1.
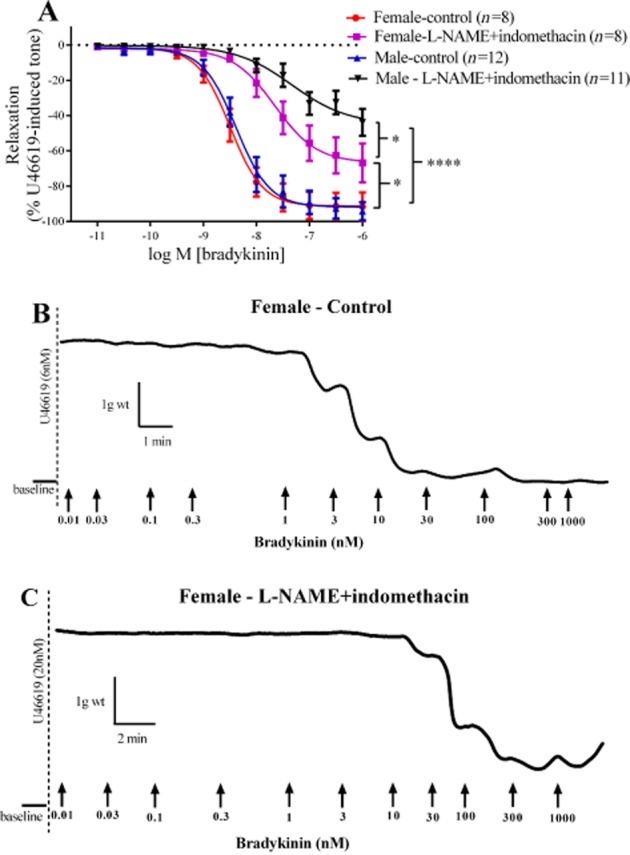
Log concentration-response curves for the vasorelaxant effects of bradykinin in the absence or presence of 300 µM L-NAME and 10 µM indomethacin in male and female PCAs (A). Data are expressed as a percentage change from U46619-induced tone and are mean ± SEM of 8–12 experiments. Original traces of recording showing the responses to increasing concentration of bradykinin (B,C). *P < 0.05, ****P < 0.0001; significantly different as indicated; one-way anova followed by Bonferroni's post hoc test.
The effects of L-NAME, indomethacin and PEG-catalase on bradykinin-induced vasorelaxation in PCAs from male and female pigs
In PCAs from females, treatment with PEG-catalase alone (Figure 2A) significantly reduced the maximum vasorelaxation to bradykinin (with pEC50 = 8.23 ± 0.13), compared with that under control conditions (with pEC50 = 8.46 ± 0.06). However, in the presence of L-NAME and indomethacin (pEC50 = 7.92 ± 0.2), no further inhibition of the Rmax was observed when PEG-catalase was added (pEC50 = 7.63 ± 0.15).
Figure 2.
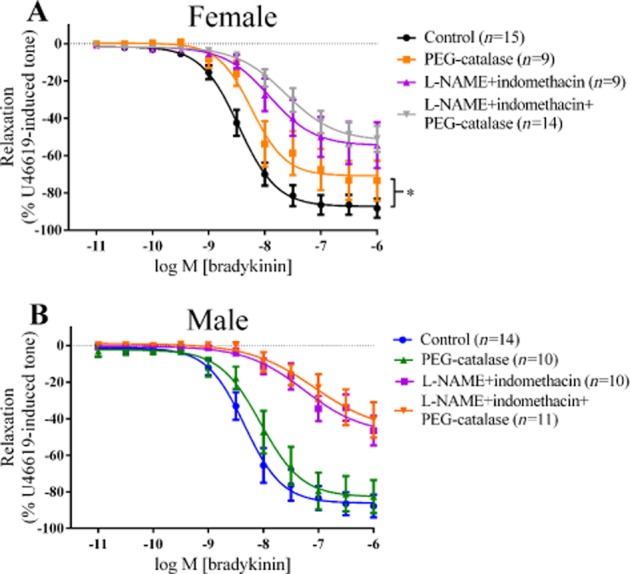
Log concentration-response curves for the vasorelaxant effects of bradykinin in the absence or presence of 300 µM L-NAME, 10 µM indomethacin or 300 U mL−1 PEG-catalase in female (A) and in male (B) PCAs. Data are expressed as a percentage change from U46619-induced tone and are mean ± SEM of 9–15 experiments. *P < 0.05; one-way anova followed by Bonferroni's post hoc test.
Treatment of PCAs from males with PEG-catalase (Figure 2B) did not affect Rmax to bradykinin (pEC50 = 8.05 ± 0.13) compared with control (pEC50 = 8.34 ± 0.09). The presence of L-NAME and indomethacin in male PCAs reduced the vasorelaxant responses to bradykinin (pEC50 = 7.26 ± 0.30) but the additional presence of PEG-catalase did not cause further reductions (pEC50 = 7.07 ± 0.50).
The effects of L-NAME, indomethacin and carbenoxolone on bradykinin-induced vasorelaxation in PCAs from male and female pigs
In PCAs from females, treatment with carbenoxolone alone did not affect vasorelaxation to bradykinin, either as the maximum response or as the pEC50 (Figure 3A). However, in the presence of L-NAME and indomethacin (pEC50 = 7.92 ± 0.20), addition of carbenoxolone further reduced the maximum relaxation to bradykinin (pEC50 = 7.62 ± 0.23).
Figure 3.
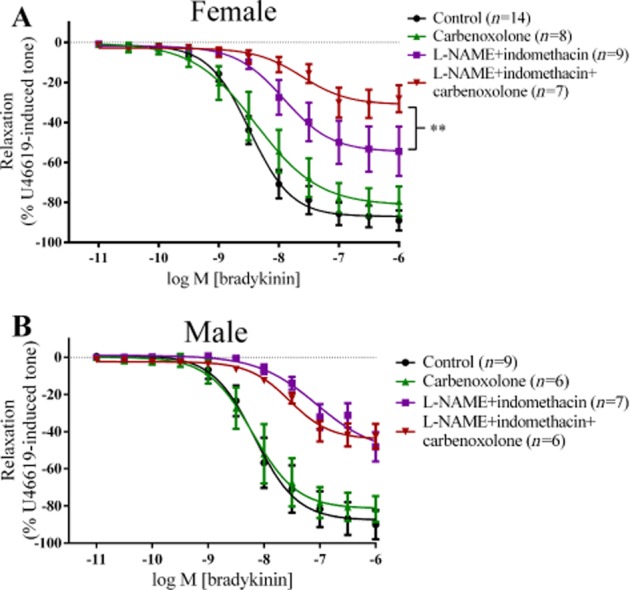
Log concentration-response curves for the vasorelaxant effects of bradykinin in the absence or presence of 300 µM L-NAME, 10 µM indomethacin or 100 µM carbenoxolone in female (A) and in male (B) PCAs. Data are expressed as a percentage change from U46619-induced tone and are mean ± SEM of 6–14 experiments. **P < 0.01, one-way anova followed by Bonferroni's post hoc test.
In PCAs from males, the presence of carbenoxolone alone or in combination with L-NAME and indomethacin did not affect vasorelaxation to bradykinin (Figure 3B).
The effects of L-NAME, indomethacin and 18α-GA on bradykinin-induced vasorelaxation in PCAs from male and female pigs
The addition of 18α-GA to PCAs from female pigs did not affect bradykinin-induced vasorelaxation, compared with the controls (Figure 4A). In the presence of L-NAME and indomethacin, addition of 18α-GA did not affect the Rmax but did shift the curve to the right by 2.2-fold.
Figure 4.
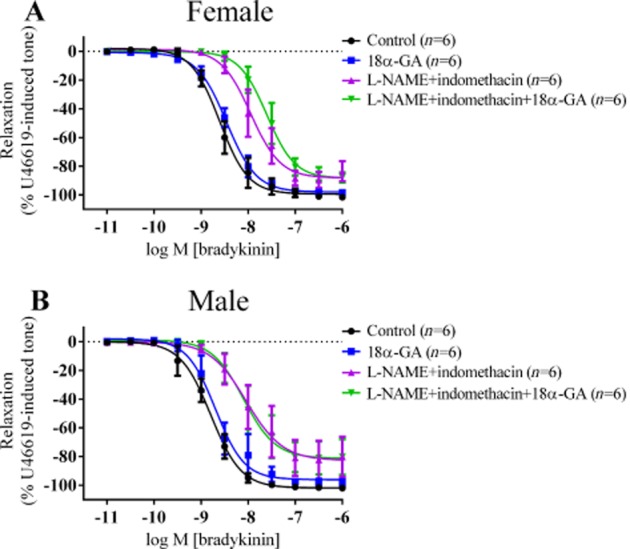
Log concentration-response curves for the vasorelaxant effects of bradykinin in the presence of 300 µM L-NAME, 10 µM indomethacin and 100 µM 18α-glycyrrhetinic acid (18α-GA) in female (A) and in male (B) PCAs. Data are expressed as a percentage change from U46619-induced tone and are mean ± S.E.M of six experiments. In female PCAs, presence of 18α-GA in L-NAME and indomethacin significantly shifted the bradykinin-induced vasorelaxation curve 2.2-fold to the right.
In PCAs from male pigs (Figure 4B), presence of 18α-GA alone had no effect on the bradykinin-induced vasorelaxation compared with the controls. Adding 18α-GA to L-NAME and indomethacin also did not affect the bradykinin-induced vasorelaxation.
The effects of L-NAME, indomethacin, TRAM-34 and/or apamin on bradykinin-induced vasorelaxation in PCAs from male and female pigs
In PCAs from females, treatment with TRAM-34 in the presence of L-NAME and indomethacin (Figure 5A), inhibited the maximum relaxation to bradykinin, without affecting pEC50 values (7.6 ± 0.05, without TRAM-34; 7.5 ± 0.2, with TRAM-34) Similarly, the maximum relaxation to bradykinin was inhibited by apamin in the presence of L-NAME and indomethacin (pEC50 = 7.6 ± 0.2) and the combination of channel blockers produced a greater inhibition of the maximum relaxation in the presence of L-NAME and indomethacin (pEC50 = 7.2 ± 0.2) (Figure 5A).
Figure 5.
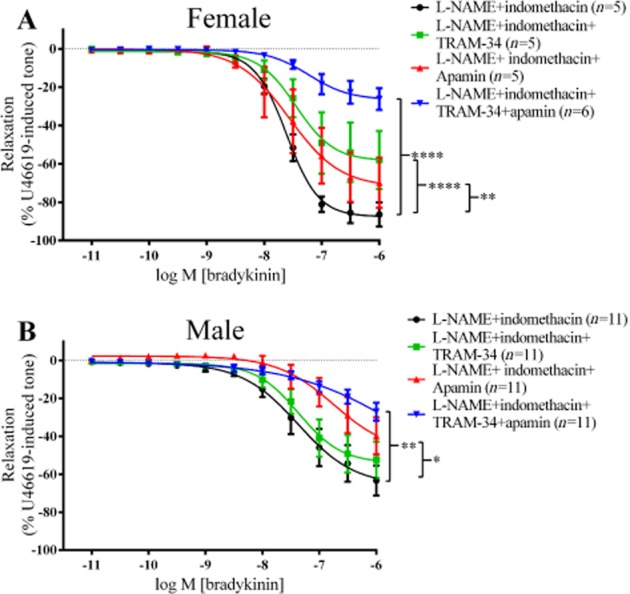
Log concentration-response curves for the vasorelaxant effects of bradykinin in the presence of 300 µM L-NAME, 10 µM indomethacin, 500 nM apamin and/or 10 µM TRAM-34 in female (A) and in male (B). Data are expressed as a percentage change from U46619-induced tone and are mean ± SEM of 5–11 experiments. *P < 0.05, **P < 0.01, ****P < 0.0001; one-way ANOVA followed by Bonferroni's post hoc test.
In PCAs from males, vasorelaxation produced at 1 µM of bradykinin was used for statistical analysis instead of Rmax because the maximum relaxation was not fully defined in some of the groups. Treatment with TRAM-34 in the presence of L-NAME and indomethacin had no effect on the bradykinin-induced vasorelaxation at 1 µM bradykinin compared with L-NAME and indomethacin (Figure 5B). In contrast, addition of apamin alone significantly inhibited the vasorelaxation produced at 1 µM bradykinin, compared with L-NAME and indomethacin. Although adding both TRAM-34 and apamin, in the presence of L-NAME and indomethacin inhibited the vasorelaxation to 1 µM bradykinin, compared with that to L-NAME and indomethacin, this level of inhibition did not differ from that induced by apamin, L-NAME and indomethacin.
The effects of L-NAME, indomethacin and apamin and/or TRAM-34 on NS309-induced vasorelaxation in PCAs from male and female pigs
In PCAs from female (Figure 6A) and male (Figure 6B) pigs, the presence of L-NAME and indomethacin, with or without apamin, had no effect on the Rmax or pEC50 values (5.81 ± 0.05, females; 5.82 ± 0.05, males) of the vasorelaxation induced by the K+ channel opener, NS309. In PCAs from male pigs, in the presence of L-NAME and indomethacin, the addition of TRAM-34 did not affect responses to NS309 (Figure 6C), nor did the combination of TRAM-34 with apamin (Figure 6D). On the other hand, in PCAs from female pigs, the presence of L-NAME, indomethacin and both apamin and TRAM-34 significantly inhibited the NS309-induced vasorelaxation (Figure 6E) at 0.3 µM of NS309, with no differences in Rmax.
Figure 6.
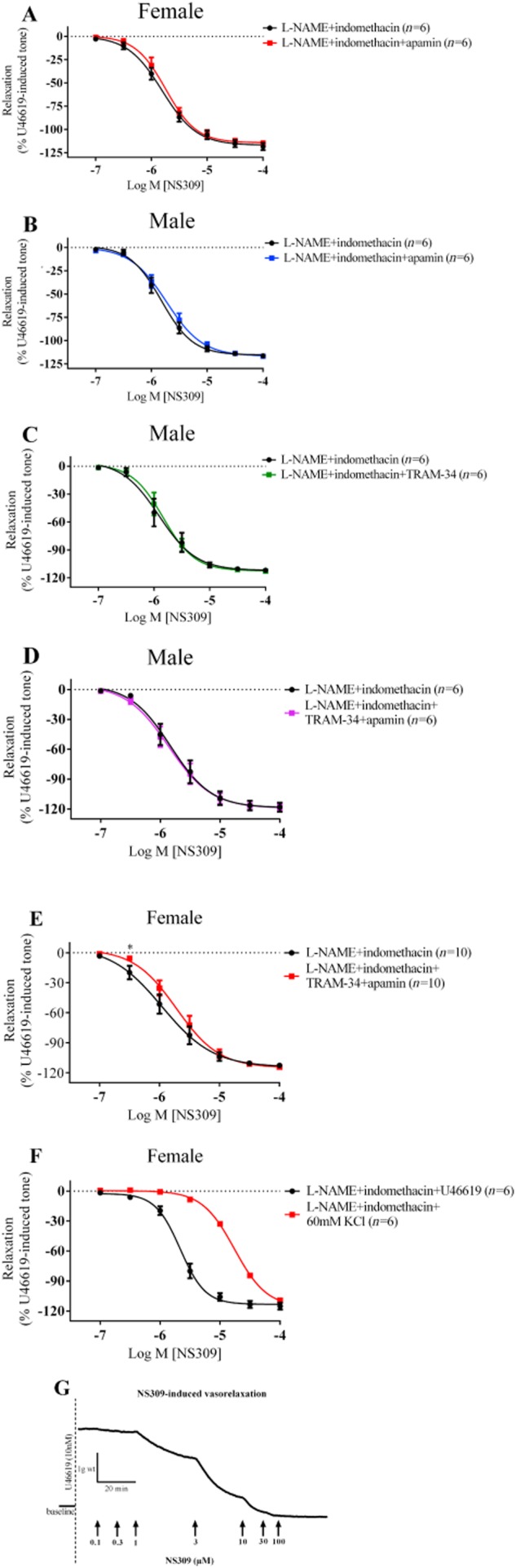
Log concentration-response curves for the vasorelaxant effects of NS309 in the presence of 300 µM L-NAME, 10 µM indomethacin and 500 nM apamin in female (A) and in male (B) PCAs or 10 µM TRAM-34 in male (C) or both apamin and TRAM-34 in male (D) and female (E) PCAs. *P < 0.05, two-tailed, paired Student's t-test. To examine the selectivity of NS309, log concentration-response curves for the vasorelaxant effects of NS309 in the presence of 300 µM L-NAME and 10 µM indomethacin precontracted with either U46619 or 60 mM KCl in female PCAs (F) was constructed where presence of 60 mM KCl significantly shifted the NS309-induced vasorelaxation 8.1-fold to the right (P < 0.001, two-tailed, paired Student's t-test). Data are expressed as a percentage change from U46619-induced tone and are mean ± SEM of five to ten experiments. Original traces of recording showing the responses to increasing concentration of NS309, a selective SKCa and IKCa channels activator (G).
Additional experiments to test the selectivity of NS309 were carried out by precontracting vessels with either 60 mM KCl or U46619 in the presence of L-NAME and indomethacin. As shown in Figure 6F, neither U46619 nor KCl affected the maximum relaxation of NS309, but 60 mM KCl did shift the concentration-response curve 8.1-fold to the right (experimental record in Figure 6G).
Expression of Cx 37, 40 and 43 and IKCa in PCAs from male and female pigs
Western blot analysis demonstrated the presence of Cx 43 (Figure 7A), Cx 40 (Figure 8A) and IKCa (Figure 10A), but not Cx 37 (Figure 9A), proteins in PCAs from male and female pigs. Further quantitative analysis based on the ratio of the protein band intensities to their respective loading control showed no significant differences between PCAs from male and female pigs in Cx 43:MLC (Figure 7B), Cx 40:GAPDH (Figure 8B) and IKCa : GAPDH (Figure 10B) (two-tailed, unpaired Student's t-test). In Figures 8A and 9, the red lower band below 37 kDa is a non-specific band produced by the secondary antibody, IRDye 680LT Goat anti-rabbit IgG (1:10 000) (LI-COR Biosciences). For Cx 40 (Figure 8A), no bands were observed at 40 kDa in the absence of the primary antibody (data not shown).
Figure 7.
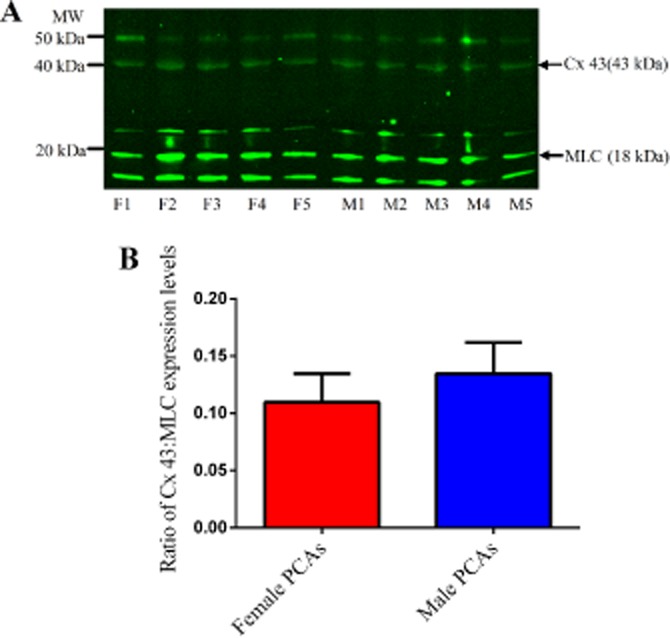
Connexin 43 (43 kDa) and MLC (18 kDa) expression levels in 5 µg of female (F1–F5) and male PCAs (M1–M5) (A). Ratio of the expression levels of connexin 43 to MLC in male and female PCAs based on the intensities of their bands (B). Data are expressed in the ratio of Cx43 to MLC intensities bands and are mean ± SEM of five experiments.
Figure 8.
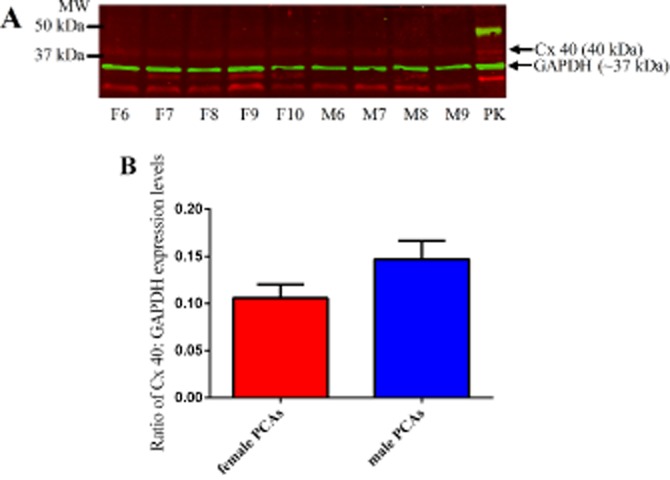
Connexin 40 (40 kDa) and GAPDH (∼37 kDa) expression levels in 10 µg of female (F6–F10) and male PCAs (M6–M9) with 20 µg of pig kidney (PK) lysate as positive control (A). Ratio of the expression levels of connexin 40 to GAPDH in male and female PCAs based on the intensities of their bands (B). Data are expressed in the ratio of Cx40 to GAPDH intensities bands and are mean ± SEM of four to five experiments.
Figure 10.
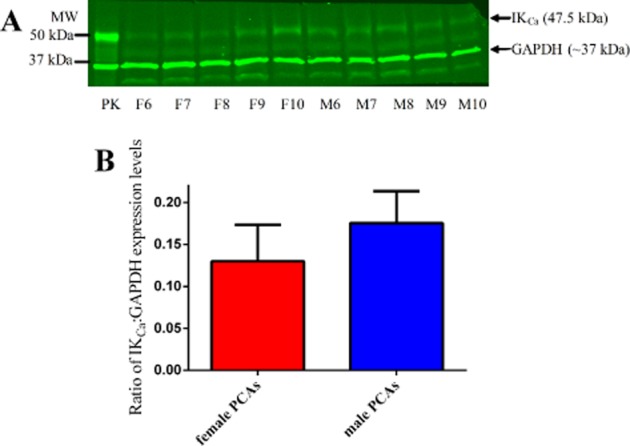
IKCa (47.5 kDa) and GAPDH (∼37 kDa) expression levels in 15 µg of female (F6–F10) and male PCAs (M6–M10) with 10 µg of pig kidney (PK) lysate as positive control (A). Ratio of the expression levels of IKCa to GAPDH in male and female PCAs based on the intensities of their bands (B). Data are expressed in the ratio of IKCa to GAPDH intensities bands and are mean ± SEM of five experiments.
Figure 9.
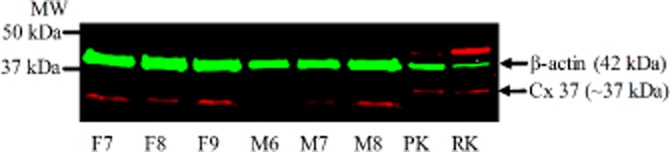
Connexin 37 (∼37 kDa) and β-actin (42 kDa) expression levels in 15 µg of female (F7–F9) and male PCAs (M6–M8) with 20 µg of pig (PK) and rat kidney (RK) lysate as positive controls.
Discussion and conclusions
In the present study, bradykinin-induced vasorelaxation in PCAs from male and female pigs involved a substantial proportion of the response which was resistant to the effects of NO synthase inhibition and COX inhibition and this part of the relaxation was therefore attributed to EDH (McCulloch et al., 1997; Edwards et al., 2010). Here, we demonstrate a significant sex difference in this EDH-mediated response induced by bradykinin. Specifically, the EDH responses were more prominent in female PCAs than in those from males. Similar conclusions have been made in previous studies including isolated mesenteric arteries from rats (McCulloch and Randall, 1998; White et al., 2000), mesenteric arteries from ‘EDH mice’ (eNOS/COX-1 double-knockout mouse) as well as an in vivo study with ‘EDH mice’ (Scotland et al., 2005). However, another study in PCAs reported that the presence of L-NAME and indomethacin did not disclose any significant sex differences in the endothelium-dependent vasorelaxation to bradykinin (Barber and Miller, 1997). This difference could possibly be due to the size of the vessels used. In the present study, small distal PCAs where EDH is believed to be more prominent in endothelium-dependent relaxations (Shimokawa et al., 1996) were used. However, in the study conducted by Barber and Miller (1997), the size of the vessels used was not reported.
The present study also demonstrated that NO played a greater role in PCAs from male pigs compared with female pigs. This might have been due to a higher expression of eNOS in males, compared with females, as a previous study in rat thoracic aorta reported that the gene expression levels of eNOS mRNA is higher in male rats compared with female rats (Kerr et al., 1999). However, expression levels of eNOS alone do not necessarily translate to activity and function of NO because NO availability decreases rapidly as it reacts with superoxide anion (O2–) forming peroxynitrite (Figure 11B) (Kerr et al., 1999).
Figure 11.
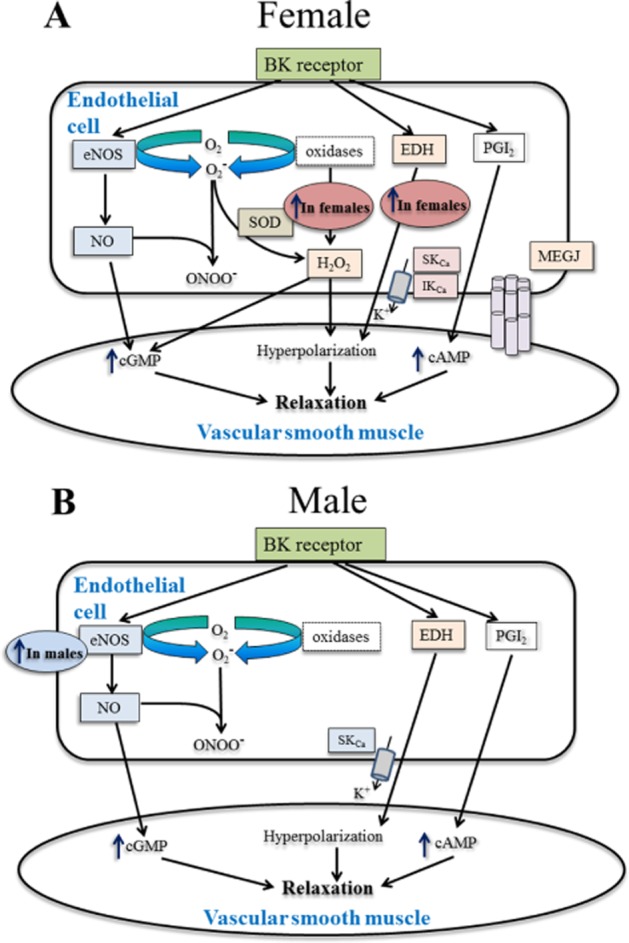
Hypothesized mechanism of action of sex differences in endothelial function underlying bradykinin-induced vasorelaxation in isolated PCAs from female (A) and male (B) pigs. Present study demonstrated a clear sex differences in endothelial function where only in PCAs from female pigs have greater EDH-mediated responses specifically the gap junction communication whereas endogenous H2O2 plays a role in the NO-mediated pathway in female pigs. Figure adapted from Shimokawa (2010).
Another relevant endogenous mediator is H2O2, formed by spontaneous or enzymic dismutation of O2– by superoxide dismutase (SOD) (Figure 11A; Shimokawa, 2010). On its own, H2O2 produced a concentration-dependent vasorelaxation in PCAs (Matoba et al., 2003) and studies have reported that H2O2 is a mediator (Matoba et al., 2000; Shimokawa and Morikawa, 2005; Edwards et al., 2002;2003 Hammond et al., 2011) or modulator (Wheal et al., 2012) of EDH. We therefore assessed the sex-related differences in endothelium-derived H2O2 in PCAs. We found that PEG-catalase alone significantly reduced vasorelaxation to bradykinin in PCAs from females but not males, suggesting that endogenous H2O2 played a significant role only in the responses of female PCAs. Conversely, in the presence of L-NAME and indomethacin, PEG-catalase did not affect the bradykinin-induced vasorelaxation in PCAs from both sexes, indicating that H2O2 does not play a significant role in the EDH-mediated pathway in distal PCAs. These findings differ from a previous study conducted with male PCAs, which concluded that H2O2 was an EDH mediator (Matoba et al., 2003). In their study, porcine coronary microvessels (250–300 µm in diameter) were used and endothelium-dependent vasorelaxations to bradykinin were insensitive to indomethacin and L-NAME, which also differs from the findings in the present study. These differences, or at least that in the absence of NO and PGI2, could be due to the difference in vessel size used. In the present study, experiments were repeated with a higher concentration of PEG-catalase (600 U mL−1) in PCAs from males, but no further inhibition of the bradykinin-induced vasorelaxation was observed (data not shown). Thus a relative lack of catalase is not likely to explain this discrepancy. Another source of endothelium-derived H2O2 is the O2− generated as a by-product from the conversion of L-arginine to NO by eNOS (Figure 1A) (Heinzel et al., 1992). Previous studies have reported that L-NAME caused a reduction in O2− generation (Kerr et al., 1999) and inhibition of H2O2 formation (Heinzel et al., 1992). Therefore, it is possible that formation of endogenous H2O2 in distal PCAs (as used in the present study) was dependent on the eNOS system where formation of H2O2 would also be inhibited in the presence of L-NAME. However, it should be noted that all our experiments were conducted in the presence of both L-NAME and indomethacin, and therefore the contribution of the COX-prostanoid pathway to the formation of H2O2 and its effects cannot be excluded.
On the other hand, in the NO-mediated response, a higher level of NO in PCAs from males could have reacted with O2–, forming peroxynitrite rather than H2O2, as the reaction of O2– with NO is three to four times faster than that with SOD (Wolin, 2009). This might explain why H2O2 did not play a role in the NO-mediated pathway in males. As for the response observed in PCAs from female pigs where endothelium-derived H2O2 was involved in the NO-mediated bradykinin-induced vasorelaxation, we propose that there may be a higher level of SOD present in the endothelial cells in female PCAs, leading to H2O2 formation. A previous study in mitochondria isolated from rat brain and liver showed higher expression and activities of manganese-SOD and glutathione peroxidase in females compared with males, thus providing a protective effect in females during oxidative stress (Borras et al., 2003). However, further studies to determine the SOD levels and activity in PCAs are required. Also, as O2– in the blood vessels can be generated from several different pathways (Wolin, 2009) including NAPDH oxidase, it is possible that there are sex differences in the upstream reactions. Previous studies in age-matched rat aorta (Brandes and Mugge, 1997) and in young healthy human subjects (Ide et al., 2002) concluded that males experience a greater oxidative stress compared with females and this was attributed to the increased production of O2− and reduced activity or availability of O2− scavengers.
Next, we investigated the role of gap junctional communication in male and female arteries as it has been reported that gap junctions play a role in the EDH-mediated response. We examined the effects of two different inhibitors (carbenoxolone and 18α-GA) alone or in combination with L-NAME and indomethacin against bradykinin-induced vasorelaxation. In PCAs from male and female pigs, the presence of carbenoxolone or 18α-GA alone had no effect on vasorelaxation to bradykinin indicating that gap junctional communication did not play a significant role in the NO-mediated response. Conversely, in the EDH pathway, carbenoxolone significantly reduced the maximum relaxation to bradykinin in PCAs from female but not male pigs. The inhibitory effects of both carbenoxolone and 18α-GA in the presence of L-NAME and indomethacin confirmed that gap junction communication played a significant role in the EDH responses induced by bradykinin in PCAs from female pigs but not male pigs. These findings concur with previous studies on isolated myometrial arteries and subcutaneous resistance arteries from pregnant women (Kenny et al., 2002; Lang et al., 2007) while the finding in PCAs from male pigs is in agreement with a previous study using 18α-GA in PCAs from male pigs (Matoba et al., 2003). Gap junctions have been reported to play a role in both NO-dependent and EDH-mediated endothelium-dependent vasorelaxation in mesenteric arteries and thoracic aortae from male rabbits (Chaytor et al., 1998), human mesenteric arteries (Chadha et al., 2011) and PCAs of unspecified sex (Edwards et al., 2000). However, another study conducted on PCAs of unspecified sex using three different types of gap junction inhibitors (18α-GA, 1-heptanol and Gap27) had no effect on the EDH response (Yang et al., 2003). In the NO-dependent responses of the present study, the difference in findings could be due to the difference in the size and/or type of vascular tissue used or species differences (Feletou and Vanhoutte, 2006). The inconsistency of findings for the role of gap junctions in all previous studies could possibly be due to the unspecified sex used (Yang et al., 2003; Chadha et al., 2011).
We have now demonstrated clear sex differences in the role of gap junctional communication in EDH responses. As Cx 43 has previously been implicated in EDH-mediated relaxations (Lang et al., 2007), we investigated whether differential expression of this protein could explain the difference in the sensitivity to gap junction inhibition. However, in the present study, our Western blot analysis for Cx 43 protein in PCAs from male and female pigs did not detect any differences, indicating that expression per se did not contribute to the sex differences observed. Further, Western blot analysis on other subtypes of Cx proteins that have been previously identified in blood vessels (Chaytor et al., 2003; Lang et al., 2007; de Wit and Griffith, 2010) showed no differences in the expression level of Cx 40 proteins and we could not detect Cx 37 proteins in PCAs from male and female pigs. Therefore, the differences in EDH-mediated responses between PCAs from male and female pigs may be due to differences in the operation of the gap junctions, rather than the expression of gap junction proteins. One possibility is that calcium-activated potassium channels (Kca) (Gluais et al., 2005a; Chadha et al., 2011) which are located near the MEGJs differentially influence gap junctional communication, in males and females.
Given the potential differences in KCa-channel activity between sexes, we then examined the effects of apamin, the SKCa blocker and the TRAM-34, an inhibitor of IKCa channels on EDH-mediated responses (Yang et al., 2003; Gluais et al., 2005a; Edwards et al., 2010; Chadha et al., 2011). Here, we demonstrated that in the EDH-mediated response, IKCa channels played a role only in PCAs from female pigs, while the SKCa channels play a role in both sexes. This is in agreement with a previous study conducted on carotid arteries from male guinea pigs where TRAM-34 alone had no effect on endothelium-dependent hyperpolarization, but apamin significantly inhibited acetylcholine-evoked hyperpolarization (Gluais et al., 2005a). Furthermore, another study examining sex differences in EDH-mediated responses reported that apamin had the same effect on the maximum relaxation to acetylcholine in male and female rat mesenteric arteries (White et al., 2000), which is comparable with the present study. Taken together, the present study demonstrates that gap junctional communication and IKCa channels play a role in the EDH-mediated response in PCAs from females, but not males. Interestingly, previous studies using immunohistochemistry with specific antibodies showed that IKCa channels are colocalized with myoendothelial Cx proteins with their function being related to the EDH-mediated activity (Sandow et al., 2006; Chadha et al., 2011). A previous study in male obese rats demonstrated an up-regulation in IKCa and MEGJ expression and activity to compensate for the loss of NO-mediated responses as observed in male age-matched control rats (Chadha et al., 2010). However, Western blot analysis in the present study for IKCa expression level showed no sex-related differences between PCAs.
In order to determine whether there is a difference in the function of IKCa channels between males and females, NS309 was used as a potent and selective SKCa and IKCa channel activator (Leuranguer et al., 2008). NS309 produced a comparable concentration-dependent vasorelaxation in male and female PCAs. Blocking SKCa channels specifically with apamin had no effect on the NS309-induced vasorelaxation in either male or female pigs. Further experiments blocking IKCa channels specifically with TRAM-34 also had no effect on the NS309-induced vasorelaxation. This finding is in line with a previous study measuring the membrane potential of guinea pig carotid arteries where NS309 (10 µM)-induced hyperpolarization was not significantly affected by TRAM-34 or apamin alone in the presence of L-NAME and indomethacin. It is therefore possible that when either SKCa or IKCa channels are blocked separately, there is a compensatory response involving the other KCa channels activated by NS309. However, blocking both SKCa and IKCa channels had little effect on the NS309-induced vasorelaxation, although there was slight inhibition at 0.3 µM in PCAs from female pigs. These data suggest that NS309 is not mediating relaxation through SKCa or IKCa channels in the PCA and therefore cannot be used to determine if there is a difference in the activity of these channels between males and females. Additional experiments to investigate the selectivity of NS309 using high potassium demonstrated that at higher concentrations of NS309 (>3 µM), other vasorelaxation pathways may be involved. This observation is in line with previous studies where loss of selectivity for IKCa and SKCa has been reported at higher concentrations of NS309 (Dalsgaard et al., 2009; Kroigaard et al., 2012). Furthermore, NS309 inhibited voltage-dependent Ca2+ channels (Morimura et al., 2006). Therefore, in the present study, use of NS309 as a selective activator of SKCa and IKCa is questionable and it may not be appropriate to draw any conclusions to support the bradykinin-induced vasorelaxation findings. To our knowledge, NS309 is a more potent and selective SKCa and IKCa activator compared with other activators such as 1-ethyl-2-benzimidazolinone (Leuranguer et al., 2008) or 5,6-dichloro-1-ethyl-1,3-dihydro-2H-benzimidazole-2-one (Morimura et al., 2006).
In conclusion, the present study demonstrates that both NO and EDH-mediated responses contribute significantly towards the endothelium-dependent vasorelaxation induced by bradykinin in male and female isolated distal PCAs. Clear sex differences in endothelial function have been demonstrated where the EDH-mediated responses play a greater role in PCAs from female compared with male pigs. In PCAs from females, endogenous H2O2 played a role in the bradykinin-induced vasorelaxation. Furthermore, gap junctional communication and the IKCa channels appear to be more important in the EDH-mediated pathway in PCAs from females and this could be compensation for the diminished response of a NO-mediated pathway in these PCAs. The sex differences in endothelial function demonstrated in the present study may contribute to a better understanding of the cardiovascular protective effects observed in premenopausal women.
Acknowledgments
We thank Dr. Michael Garle, Dr. Amanda Wheal and Dr. Sue Chan for their assistance and valuable comments on this work, and G Woods & Sons, Clipstone, Nottinghamshire for providing the pig tissues. This work was funded by the School of Life Sciences, University of Nottingham.
Glossary
- 18α-GA
18α-glycyrrhetinic acid
- Cx
connexin
- EDH
endothelium-derived hyperpolarization
- IKCa
intermediate-conductance calcium-activated K+ channel
- MEGJs
myoendothelial gap junctions
- PCA
porcine coronary arterie
- PEG-catalase
polyethelene glycol-catalase
- SKCa
small-conductance calcium-activated K+ channel
- SOD
superoxide dismutase
- U46619
9,11-dideoxy-9a,11a-epoxymethanoprostaglandin F2α
Conflicts of interest
The authors declare no conflicts of interest.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Catterall WA, et al. The Concise Guide to PHARMACOLOGY 2013/14: Ion Channels. Br J Pharmacol. 2013;170:1607–1651. doi: 10.1111/bph.12447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barber DA, Miller VM. Gender differences in endothelium-dependent relaxations do not involve NO in porcine coronary arteries. Am J Physiol. 1997;273(5 Pt 2):H2325–H2332. doi: 10.1152/ajpheart.1997.273.5.H2325. [DOI] [PubMed] [Google Scholar]
- Borras C, Sastre J, Garcia-Sala D, Lloret A, Pallardo FV, Vina J. Mitochondria from females exhibit higher antioxidant gene expression and lower oxidative damage than males. Free Radic Biol Med. 2003;34:546–552. doi: 10.1016/s0891-5849(02)01356-4. [DOI] [PubMed] [Google Scholar]
- Brandes RP, Mugge A. Gender differences in the generation of superoxide anions in the rat aorta. Life Sci. 1997;60:391–396. doi: 10.1016/s0024-3205(96)00663-7. [DOI] [PubMed] [Google Scholar]
- Brondum E, Kold-Petersen H, Simonsen U, Aalkjaer C. NS309 restores EDHF-type relaxation in mesenteric small arteries from type 2 diabetic ZDF rats. Br J Pharmacol. 2010;159:154–165. doi: 10.1111/j.1476-5381.2009.00525.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Busse R, Edwards G, Feletou M, Fleming I, Vanhoutte PM, Weston AH. EDHF: bringing the concepts together. Trends Pharmacol Sci. 2002;23:374–380. doi: 10.1016/s0165-6147(02)02050-3. [DOI] [PubMed] [Google Scholar]
- Campbell WB, Gebremedhin D, Pratt PF, Harder DR. Identification of epoxyeicosatrienoic acids as endothelium-derived hyperpolarizing factors. Circ Res. 1996;78:415–423. doi: 10.1161/01.res.78.3.415. [DOI] [PubMed] [Google Scholar]
- Chadha PS, Haddock RE, Howitt L, Morris MJ, Murphy TV, Grayson TH, et al. Obesity up-regulates intermediate conductance calcium-activated potassium channels and myoendothelial gap junctions to maintain endothelial vasodilator function. J Pharmacol Exp Ther. 2010;335:284–293. doi: 10.1124/jpet.110.167593. [DOI] [PubMed] [Google Scholar]
- Chadha PS, Liu L, Rikard-Bell M, Senadheera S, Howitt L, Bertrand RL, et al. Endothelium-dependent vasodilation in human mesenteric artery is primarily mediated by myoendothelial gap junctions intermediate conductance calcium-activated K + channel and nitric oxide. J Pharmacol Exp Ther. 2011;336:701–708. doi: 10.1124/jpet.110.165795. [DOI] [PubMed] [Google Scholar]
- Chaytor AT, Evans WH, Griffith TM. Central role of heterocellular gap junctional communication in endothelium-dependent relaxations of rabbit arteries. J Physiol. 1998;508(Pt 2):561–573. doi: 10.1111/j.1469-7793.1998.561bq.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaytor AT, Edwards DH, Bakker LM, Griffith TM. Distinct hyperpolarizing and relaxant roles for gap junctions and endothelium-derived H2O2 in NO-independent relaxations of rabbit arteries. Proc Natl Acad Sci U S A. 2003;100:15212–15217. doi: 10.1073/pnas.2435030100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dalsgaard T, Kroigaard C, Bek T, Simonsen U. Role of calcium-activated potassium channels with small conductance in bradykinin-induced vasodilation of porcine retinal arterioles. Invest Ophthalmol Vis Sci. 2009;50:3819–3825. doi: 10.1167/iovs.08-3168. [DOI] [PubMed] [Google Scholar]
- Edwards DH, Li Y, Griffith TM. Hydrogen peroxide potentiates the EDHF phenomenon by promoting endothelial Ca2+ mobilization. Arterioscler Thromb Vasc Biol. 2008;28:1774–1781. doi: 10.1161/ATVBAHA.108.172692. [DOI] [PubMed] [Google Scholar]
- Edwards G, Dora KA, Gardener MJ, Garland CJ, Weston AH. K+ is an endothelium-derived hyperpolarizing factor in rat arteries. Nature. 1998;396:269–272. doi: 10.1038/24388. [DOI] [PubMed] [Google Scholar]
- Edwards G, Thollon C, Gardener MJ, Feletou M, Vilaine J, Vanhoutte PM, et al. Role of gap junctions and EETs in endothelium-dependent hyperpolarization of porcine coronary artery. Br J Pharmacol. 2000;129:1145–1154. doi: 10.1038/sj.bjp.0703188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edwards G, Feletou M, Weston AH. Endothelium-derived hyperpolarising factors and associated pathways: a synopsis. Pflugers Arch. 2010;459:863–879. doi: 10.1007/s00424-010-0817-1. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-derived hyperpolarizing factor: where are we now? Arterioscler Thromb Vasc Biol. 2006;26:1215–1225. doi: 10.1161/01.ATV.0000217611.81085.c5. [DOI] [PubMed] [Google Scholar]
- Feletou M, Vanhoutte PM. Endothelium-dependent hyperpolarization: no longer an f-word! J Cardiovasc Pharmacol. 2013;61:91–92. doi: 10.1097/FJC.0b013e31828197bc. [DOI] [PubMed] [Google Scholar]
- Furchgott RF, Zawadzki JV. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980;288:373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Garry A, Edwards DH, Fallis IF, Jenkins RL, Griffith TM. Ascorbic acid and tetrahydrobiopterin potentiate the EDHF phenomenon by generating hydrogen peroxide. Cardiovasc Res. 2009;84:218–226. doi: 10.1093/cvr/cvp235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluais P, Edwards G, Weston AH, Falck JR, Vanhoutte PM, Feletou M. Role of SK(Ca) and IK(Ca) in endothelium-dependent hyperpolarizations of the guinea-pig isolated carotid artery. Br J Pharmacol. 2005a;144:477–485. doi: 10.1038/sj.bjp.0706003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gluais P, Edwards G, Weston AH, Vanhoutte PM, Feletou M. Hydrogen peroxide and endothelium-dependent hyperpolarization in the guinea-pig carotid artery. Eur J Pharmacol. 2005b;513:219–224. doi: 10.1016/j.ejphar.2005.02.042. [DOI] [PubMed] [Google Scholar]
- Griffith TM. Endothelium-dependent smooth muscle hyperpolarization: do gap junctions provide a unifying hypothesis? Br J Pharmacol. 2004;141:881–903. doi: 10.1038/sj.bjp.0705698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammond S, Mathewson AM, Baker PN, Mayhew TM, Dunn WR. Gap junctions and hydrogen peroxide are involved in endothelium-derived hyperpolarising responses to bradykinin in omental arteries and veins isolated from pregnant women. Eur J Pharmacol. 2011;668:225–232. doi: 10.1016/j.ejphar.2011.06.050. [DOI] [PubMed] [Google Scholar]
- Harris D, Martin PE, Evans WH, Kendall DA, Griffith TM, Randall MD. Role of gap junctions in endothelium-derived hyperpolarizing factor responses and mechanisms of K(+)-relaxation. Eur J Pharmacol. 2000;402:119–128. doi: 10.1016/s0014-2999(00)00512-4. [DOI] [PubMed] [Google Scholar]
- Harris D, McCulloch AI, Kendall DA, Randall MD. Characterization of vasorelaxant responses to anandamide in the rat mesenteric arterial bed. J Physiol. 2002;539(Pt 3):893–902. doi: 10.1113/jphysiol.2001.013489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayabuchi Y, Nakaya Y, Matsuoka S, Kuroda Y. Hydrogen peroxide-induced vascular relaxation in porcine coronary arteries is mediated by Ca2+-activated K + channels. Heart Vessels. 1998;13:9–17. doi: 10.1007/BF02750638. [DOI] [PubMed] [Google Scholar]
- Hedegaard ER, Stankevicius E, Simonsen U, Frobert O. Non-endothelial endothelin counteracts hypoxic vasodilation in porcine large coronary arteries. BMC Physiol. 2011;11:8. doi: 10.1186/1472-6793-11-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinzel B, John M, Klatt P, Bohme E, Mayer B. Ca2+/calmodulin-dependent formation of hydrogen peroxide by brain nitric oxide synthase. Biochem J. 1992;281(Pt 3):627–630. doi: 10.1042/bj2810627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang JH, He GW, Xue HM, Yao XQ, Liu XC, Underwood MJ, et al. TRPC3 channel contributes to nitric oxide release: significance during normoxia and hypoxia-reoxygenation. Cardiovasc Res. 2011;91:472–482. doi: 10.1093/cvr/cvr102. [DOI] [PubMed] [Google Scholar]
- Ide T, Tsutsui H, Ohashi N, Hayashidani S, Suematsu N, Tsuchihashi M, et al. Greater oxidative stress in healthy young men compared with premenopausal women. Arterioscler Thromb Vasc Biol. 2002;22:438–442. doi: 10.1161/hq0302.104515. [DOI] [PubMed] [Google Scholar]
- Kenny LC, Baker PN, Kendall DA, Randall MD, Dunn WR. The role of gap junctions in mediating endothelium-dependent responses to bradykinin in myometrial small arteries isolated from pregnant women. Br J Pharmacol. 2002;136:1085–1088. doi: 10.1038/sj.bjp.0704817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kerr PM, Tam R, Ondrusova K, Mittal R, Narang D, Tran CH, et al. Endothelial feedback and the myoendothelial projection. Microcirculation. 2012;19:416–422. doi: 10.1111/j.1549-8719.2012.00187.x. [DOI] [PubMed] [Google Scholar]
- Kerr S, Brosnan MJ, McIntyre M, Reid JL, Dominiczak AF, Hamilton CA. Superoxide anion production is increased in a model of genetic hypertension: role of the endothelium. Hypertension. 1999;33:1353–1358. doi: 10.1161/01.hyp.33.6.1353. [DOI] [PubMed] [Google Scholar]
- Kroigaard C, Dalsgaard T, Nielsen G, Laursen BE, Pilegaard H, Kohler R, et al. Activation of endothelial and epithelial K(Ca) 2.3 calcium-activated potassium channels by NS309 relaxes human small pulmonary arteries and bronchioles. Br J Pharmacol. 2012;167:37–47. doi: 10.1111/j.1476-5381.2012.01986.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang NN, Luksha L, Newby DE, Kublickiene K. Connexin 43 mediates endothelium-derived hyperpolarizing factor-induced vasodilatation in subcutaneous resistance arteries from healthy pregnant women. Am J Physiol Heart Circ Physiol. 2007;292:H1026–H1032. doi: 10.1152/ajpheart.00797.2006. [DOI] [PubMed] [Google Scholar]
- Leung HS, Leung FP, Yao X, Ko WH, Chen ZY, Vanhoutte PM, et al. Endothelial mediators of the acetylcholine-induced relaxation of the rat femoral artery. Vascul Pharmacol. 2006;44:299–308. doi: 10.1016/j.vph.2006.01.010. [DOI] [PubMed] [Google Scholar]
- Leuranguer V, Gluais P, Vanhoutte PM, Verbeuren TJ, Feletou M. Openers of calcium-activated potassium channels and endothelium-dependent hyperpolarizations in the guinea pig carotid artery. Naunyn Schmiedebergs Arch Pharmacol. 2008;377:101–109. doi: 10.1007/s00210-008-0267-x. [DOI] [PubMed] [Google Scholar]
- Liu MY, Hattori Y, Sato A, Ichikawa R, Zhang XH, Sakuma I. Ovariectomy attenuates hyperpolarization and relaxation mediated by endothelium-derived hyperpolarizing factor in female rat mesenteric artery: a concomitant decrease in connexin-43 expression. J Cardiovasc Pharmacol. 2002;40:938–948. doi: 10.1097/00005344-200212000-00016. [DOI] [PubMed] [Google Scholar]
- Lucchesi PA, Belmadani S, Matrougui K. Hydrogen peroxide acts as both vasodilator and vasoconstrictor in the control of perfused mouse mesenteric resistance arteries. J Hypertens. 2005;23:571–579. doi: 10.1097/01.hjh.0000160214.40855.79. [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Nakashima M, Hirakawa Y, Mukai Y, Hirano K, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in mice. J Clin Invest. 2000;106:1521–1530. doi: 10.1172/JCI10506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Kubota H, Morikawa K, Fujiki T, Kunihiro I, et al. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in human mesenteric arteries. Biochem Biophys Res Commun. 2002;290:909–913. doi: 10.1006/bbrc.2001.6278. [DOI] [PubMed] [Google Scholar]
- Matoba T, Shimokawa H, Morikawa K, Kubota H, Kunihiro I, Urakami-Harasawa L, et al. Electron spin resonance detection of hydrogen peroxide as an endothelium-derived hyperpolarizing factor in porcine coronary microvessels. Arterioscler Thromb Vasc Biol. 2003;23:1224–1230. doi: 10.1161/01.ATV.0000078601.79536.6C. [DOI] [PubMed] [Google Scholar]
- McCulloch AI, Randall MD. Sex differences in the relative contributions of nitric oxide and EDHF to agonist-stimulated endothelium-dependent relaxations in the rat isolated mesenteric arterial bed. Br J Pharmacol. 1998;123:1700–1706. doi: 10.1038/sj.bjp.0701781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCulloch AI, Bottrill FE, Randall MD, Hiley CR. Characterization and modulation of EDHF-mediated relaxations in the rat isolated superior mesenteric arterial bed. Br J Pharmacol. 1997;120:1431–1438. doi: 10.1038/sj.bjp.0701066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moncada S, Gryglewski R, Bunting S, Vane JR. An enzyme isolated from arteries transforms prostaglandin endoperoxides to an unstable substance that inhibits platelet aggregation. Nature. 1976;263:663–665. doi: 10.1038/263663a0. [DOI] [PubMed] [Google Scholar]
- Morimura K, Yamamura H, Ohya S, Imaizumi Y. Voltage-dependent Ca2+-channel block by openers of intermediate and small conductance Ca2+-activated K + channels in urinary bladder smooth muscle cells. J Pharmacol Sci. 2006;100:237–241. doi: 10.1254/jphs.sc0060011. [DOI] [PubMed] [Google Scholar]
- Quignard JF, Feletou M, Thollon C, Vilaine JP, Duhault J, Vanhoutte PM. Potassium ions and endothelium-derived hyperpolarizing factor in guinea-pig carotid and porcine coronary arteries. Br J Pharmacol. 1999;127:27–34. doi: 10.1038/sj.bjp.0702493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sandow SL, Hill CE. Incidence of myoendothelial gap junctions in the proximal and distal mesenteric arteries of the rat is suggestive of a role in endothelium-derived hyperpolarizing factor-mediated responses. Circ Res. 2000;86:341–346. doi: 10.1161/01.res.86.3.341. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Tare M, Coleman HA, Hill CE, Parkington HC. Involvement of myoendothelial gap junctions in the actions of endothelium-derived hyperpolarizing factor. Circ Res. 2002;90:1108–1113. doi: 10.1161/01.res.0000019756.88731.83. [DOI] [PubMed] [Google Scholar]
- Sandow SL, Neylon CB, Chen MX, Garland CJ. Spatial separation of endothelial small- and intermediate-conductance calcium-activated potassium channels (K(Ca)) and connexins: possible relationship to vasodilator function? J Anat. 2006;209:689–698. doi: 10.1111/j.1469-7580.2006.00647.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scotland RS, Madhani M, Chauhan S, Moncada S, Andresen J, Nilsson H, et al. Investigation of vascular responses in endothelial nitric oxide synthase/cyclooxygenase-1 double-knockout mice: key role for endothelium-derived hyperpolarizing factor in the regulation of blood pressure in vivo. Circulation. 2005;111:796–803. doi: 10.1161/01.CIR.0000155238.70797.4E. [DOI] [PubMed] [Google Scholar]
- Shimokawa H. Hydrogen peroxide as an endothelium-derived hyperpolarizing factor. Pflugers Arch. 2010;459:915–922. doi: 10.1007/s00424-010-0790-8. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Morikawa K. Hydrogen peroxide is an endothelium-derived hyperpolarizing factor in animals and humans. J Mol Cell Cardiol. 2005;39:725–732. doi: 10.1016/j.yjmcc.2005.07.007. [DOI] [PubMed] [Google Scholar]
- Shimokawa H, Yasutake H, Fujii K, Owada MK, Nakaike R, Fukumoto Y, et al. The importance of the hyperpolarizing mechanism increases as the vessel size decreases in endothelium-dependent relaxations in rat mesenteric circulation. J Cardiovasc Pharmacol. 1996;28:703–711. doi: 10.1097/00005344-199611000-00014. [DOI] [PubMed] [Google Scholar]
- Sokoya EM, Burns AR, Marrelli SP, Chen J. Myoendothelial gap junction frequency does not account for sex differences in EDHF responses in rat MCA. Microvasc Res. 2007;74:39–44. doi: 10.1016/j.mvr.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tang EH, Vanhoutte PM. Gap junction inhibitors reduce endothelium-dependent contractions in the aorta of spontaneously hypertensive rats. J Pharmacol Exp Ther. 2008;327:148–153. doi: 10.1124/jpet.108.140046. [DOI] [PubMed] [Google Scholar]
- Taylor SG, Weston AH. Endothelium-derived hyperpolarizing factor: a new endogenous inhibitor from the vascular endothelium. Trends Pharmacol Sci. 1988;9:272–274. doi: 10.1016/0165-6147(88)90003-x. [DOI] [PubMed] [Google Scholar]
- Villar IC, Hobbs AJ, Ahluwalia A. Sex differences in vascular function: implication of endothelium-derived hyperpolarizing factor. J Endocrinol. 2008;197:447–462. doi: 10.1677/JOE-08-0070. [DOI] [PubMed] [Google Scholar]
- Wheal AJ, Alexander SP, Randall MD. Hydrogen peroxide as a mediator of vasorelaxation evoked by N-oleoylethanolamine and anandamide in rat small mesenteric arteries. Eur J Pharmacol. 2012;674:384–390. doi: 10.1016/j.ejphar.2011.11.033. [DOI] [PubMed] [Google Scholar]
- White RM, Rivera CO, Davison CA. Nitric oxide-dependent and -independent mechanisms account for gender differences in vasodilation to acetylcholine. J Pharmacol Exp Ther. 2000;292:375–380. [PubMed] [Google Scholar]
- de Wit C, Griffith TM. Connexins and gap junctions in the EDHF phenomenon and conducted vasomotor responses. Pflugers Arch. 2010;459:897–914. doi: 10.1007/s00424-010-0830-4. [DOI] [PubMed] [Google Scholar]
- Wolin MS. Reactive oxygen species and the control of vascular function. Am J Physiol Heart Circ Physiol. 2009;296:H539–H549. doi: 10.1152/ajpheart.01167.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yada T, Shimokawa H, Hiramatsu O, Kajita T, Shigeto F, Goto M, et al. Hydrogen peroxide, an endogenous endothelium-derived hyperpolarizing factor, plays an important role in coronary autoregulation in vivo. Circulation. 2003;107:1040–1045. doi: 10.1161/01.cir.0000050145.25589.65. [DOI] [PubMed] [Google Scholar]
- Yang Q, Ge Z-D, Yang C-Q, Huang Y, He G-W. Bioassay of endothelium-derived hyperpolarizing factor with abolishment of nitric oxide and the role of gap junctions in the porcine coronary circulation. Drug Dev Res. 2003;58:99–110. [Google Scholar]


