Abstract
Background and Purpose
Marijuana smoking is widespread in many countries, and the use of smoked synthetic cannabinoids is increasing. Smoking a marijuana joint leads to bronchodilation in both healthy subjects and asthmatics. The effects of Δ9-tetrahydrocannabinol and synthetic cannabinoids on human bronchus reactivity have not previously been investigated. Here, we sought to assess the effects of natural and synthetic cannabinoids on cholinergic bronchial contraction.
Experimental Approach
Human bronchi isolated from 88 patients were suspended in an organ bath and contracted by electrical field stimulation (EFS) in the presence of the phytocannabinoid Δ9-tetrahydrocannabinol, the endogenous 2-arachidonoylglycerol, the synthetic dual CB1 and CB2 receptor agonists WIN55,212-2 and CP55,940, the synthetic, CB2-receptor-selective agonist JWH-133 or the selective GPR55 agonist O-1602. The receptors involved in the response were characterized by using selective CB1 and CB2 receptor antagonists (SR141716 and SR144528 respectively).
Key Results
Δ9-tetrahydrocannabinol, WIN55,212-2 and CP55,940 induced concentration-dependent inhibition of cholinergic contractions, with maximum inhibitions of 39, 76 and 77% respectively. JWH-133 only had an effect at high concentrations. 2-Arachidonoylglycerol and O-1602 were devoid of any effect. Only CB1 receptors were involved in the response because the effects of cannabinoids were antagonized by SR141716, but not by SR144528. The cannabinoids did not alter basal tone or contractions induced by exogenous Ach.
Conclusions and Implications
Activation of prejunctional CB1 receptors mediates the inhibition of EFS-evoked cholinergic contraction in human bronchus. This mechanism may explain the acute bronchodilation produced by marijuana smoking.
Keywords: receptors, cannabinoid, bronchi, humans, muscle contraction, cholinergic fibres
Introduction
Although the marijuana plant has been consumed for centuries, exposure to synthetic cannabinoids (sometimes referred to as ‘spice’) has increased substantially over the past five years (Winstock and Barratt, 2013). Marijuana smoking appears to be increasingly prevalent among young people (Miech and Koester, 2012; Kuehn, 2013); the US National Institute on Drug Abuse's latest ‘Monitoring the Future’ survey of teen drug use showed that the consumption of synthetic cannabinoids was alarmingly high, with 11% of 12th graders reporting past-year use (Kuehn, 2013). The synthetic cannabinoid market is growing quickly as novel recreational substances are being synthesized on a regular basis. The cannabinoid family includes about 35 substances, some of which (including JWH-018, CP 47,497 and HU-210) are now prohibited in a number of countries (including the USA, New Zealand, Australia and many European countries). As is the case for marijuana, smoking is the most common route of entry for these compounds [accounting for about 90% of reported use (Forrester et al., 2012)]. The drugs are added to relatively inert, smokable plant matter. In terms of central effects, synthetic cannabinoids have both a quicker time to peak onset of effect and a shorter duration of action than marijuana (Winstock and Barratt, 2013). Although the effects of marijuana smoking on the lung were reviewed very recently, the effects of synthetic cannabinoids on lung function have not been characterized (Tashkin, 2013).
Smoking marijuana leads to acute bronchodilation for at least an hour in both healthy, regular marijuana smokers and marijuana-naïve asthmatics. This bronchodilation is probably due to Δ9-tetrahydrocannabinol (Δ9-THC) (Vachon et al., 1973) as the latter compound also induces a dose-dependent bronchodilator response after oral administration (Vachon et al., 1973; Tashkin et al., 1974; Abboud and Sanders, 1976; Gong et al., 1984). In stable asthmatic subjects, inhalation of aerosolized Δ9-THC improves respiratory function (Williams et al., 1976), and acute marijuana smoking also leads to reversal of bronchoconstriction provoked by exercise or methacholine inhalation (Tashkin et al., 1975). Cannabinoid receptor agonists act through at least two distinct types of receptors (the cannabinoid CB1 and CB2 receptors) (Pertwee et al., 2010; receptor nomenclature follows Alexander et al., 2013). Because Δ9-THC is devoid of a direct effect on human bronchial smooth muscle (Shapiro and Tashkin, 1976; Orzelek-O'Neil et al., 1980), one can hypothesize that the compound's bronchodilatory effects are exerted indirectly. In the airways, the dominant autonomic innervation is provided by the parasympathetic nervous system, which induces bronchoconstriction via efferent, cholinergic pathways that travel through the vagus nerve and then synapse in the parasympathetic ganglia of the airways (Racke and Matthiesen, 2004). The observation of cannabinoid receptors on airway nerves (Calignano et al., 2000) and the fact that cannabinoids inhibit electrical field stimulation (EFS)-induced cholinergic contraction in smooth muscle preparations from the guinea pig ileum (Izzo et al., 1998) suggest that cannabinoid receptors may be involved in the contraction of human airways mediated by cholinergic nerves.
The objectives of the present study were thus to (i) establish whether cannabinoids can alter bronchial reactivity by modulating contractions mediated by cholinergic nerves; (ii) identify the receptors involved in this response; and (iii) compare the effects of the marijuana cannabinoid Δ9-THC with those of synthetic cannabinoids now used as recreational drugs. We found that activation of prejunctional cannabinoid CB1 receptors with natural or synthetic agonists mediated the inhibition of EFS-evoked cholinergic contractions in human bronchus.
Methods
Human bronchus samples
The use of resected lung tissues for research purposes was approved by the local independent ethics committee (Comité de Protection des Personnes Ile de France VIII, Boulogne Billancourt, France). All patients provided their written informed consent to research use of their samples. Human lung bronchi were obtained from macroscopically normal tissues from 88 patients (63 men and 25 women; age range: 45–84; mean ± SD age: 65 ± 1) undergoing surgical resection for lung carcinoma at Foch Hospital (Suresnes, France) or the Val d'Or Clinic (St Cloud, France).
Reverse transcriptase–quantitative polymerase chain reaction (RT-qPCR) analysis
The RT-qPCR experiments were performed as described previously, with some modifications (Buenestado et al., 2012). Bronchial rings were crushed and homogenized in TRIzol® reagent immediately after dissection, using a TissueLyser LT ball mill (Qiagen, Courtaboeuf, France). Total RNA was extracted from bronchus homogenates using TRIzol. The amount of RNA extracted was estimated by spectrophotometry at 260 nm (Biowave DNA; Biochrom, Cambridge, UK) and the quality of the preparation was assessed in a microfluidic electrophoresis system (RNA Standard Sensitivity kits for Experion®; Bio-Rad, Marnes-la-Coquette, France). After treatment with DNase I (Life Technologies, Saint Aubin, France), 1 μg of total RNA was reverse-transcribed (SuperScript® III First-Strand SuperMix kit; Life Technologies). The resulting cDNA was then used for RT-qPCR experiments with TaqMan® chemistry (Life Technologies). After initial denaturation at 95°C for 10 min, 20 ng of cDNA was amplified (using Gene Expression Master Mix; Life Technologies) in 40 annealing/extension cycles (95°C for 15 s and 60°C for 1 min) in a StepOnePlus thermocycler (Life Technologies). The sample's fluorescence was measured after each cycle and the threshold cycle (Ct) of the real-time PCR was defined as the point at which a fluorescence signal corresponding to the amplification of a PCR product was detectable. The reaction volume was 10 μL. The presence of CNR1, CNR2 and GPR55 gene transcripts in the bronchial tissue was analysed with a specific TaqMan array based upon pre-designed reagents (Assay-on-Demand®; Life Technologies). To validate the extraction of intact cellular mRNA and to standardize the quantitative data, three reference genes [those for hypoxanthine phosphoribosyltransferase (HPRT1), glyceraldehyde-3-phosphate dehydrogenase (GAPDH) and β-glucuronidase (GUSB)] were amplified simultaneously.
Preparation of tissues for organ bath studies
The bronchi were dissected away from adhering lung parenchyma and vessels and cut into rings of identical length and diameter, as described previously (Grassin-Delyle et al., 2010). Bronchial segments with an inner diameter of between 1 and 4 mm were selected. A total of 656 bronchial rings were prepared and used in the present study. On the day before the experiment, the human bronchial segments were stored at 4°C in Krebs–Henseleit solution. On the day of the experiment, the segments were placed in an isolated organ bath filled with 5 mL of Krebs–Henseleit solution, oxygenated with 95%/5% O2/CO2 and thermostated at 37°C. Tension was measured isometrically with a strain gauge (UF1; Piodem, Canterbury, UK) connected to an amplifier (EMKA Technologies, Paris, France). Data were acquired, processed and analysed with a computerized system running IOX v1.56.8 and Datanalyst v1.58 software (EMKA Technologies).
Effect of cannabinoids on basal tone and on contraction in response to exogenous ACh
The preparations were suspended with an initial load of 3 g and equilibrated for 60–90 min. The Krebs solution in the bath was changed every 15–20 min. At the end of the equilibration period, the resting load was stable at 2–4 g. Bronchi were first contracted maximally with ACh (3 mM), washed and then equilibrated for 60 min prior to initiation of the experimental procedures. To assess the role of cannabinoid receptors in the regulation of basal tone, increasing concentrations of each cannabinoid receptor agonist were added to the organ bath every 15 min. To investigate the agonists' effects on contraction in response to exogenous ACh, an initial cumulative concentration–response curve was obtained for ACh concentrations ranging from 10 nM to 3 mM. After extensive washing and equilibration for 60 min, rings were incubated with cannabinoid receptor agonists or vehicle for 30 min, prior to measurement of a second cumulative concentration–response curve for ACh.
Electrical field stimulation
EFS experiments were performed as described previously (Naline et al., 2007). Briefly, EFS was produced in organ baths fitted with two platinum plate electrodes (1 cm2) placed alongside the tissue (10 mm apart) and connected to a stimulator (EMKA Technologies). Biphasic, square-wave pulses at a constant current of 320 mA and with a pulse duration of 1 ms were delivered for 10 s at a frequency of 5 Hz. Eight to sixteen bronchial rings were simultaneously tested, with at least one control preparation per series of eight rings. Maximal contraction in response to 3 mM ACh was assessed before the start of the EFS experiments. The control preparations were subjected to EFS as a check on the stability of the system's response during the experimental session. To assess each preparation's baseline response, a first train of EFS was applied twice at 10 min intervals. Compounds or vehicles were then added to the bath 30 min before delivery of a second train of stimulations (every 10 min for 1 h and then every 20 min for 4 h). In experiments with cannabinoid receptor antagonists, the antagonist was added 30 min before the agonist. The cholinergic nature of the contraction was assessed in a series of experiments in which bronchi were treated with atropine (from 10 nM to 10 μM), TTX (from 10 nM to 1 μM) or hexamethonium (from 1 to 100 μM).
Cannabinoid receptor agonists and antagonists
We assessed the effects of the endogenous cannabinoid 2-AG, the plant-derived cannabinoid Δ9-THC and the synthetic compounds CP55,940, WIN55,212-2, WIN55,212-3 and JWH-133. The relative affinities of each agonist for the CB1 and CB2 receptors are given in Table 1. Δ9-THC, 2-AG, CP55,940 and WIN55,212-2 are non-selective CB1 and CB2 receptor agonists, whereas JWH-133 is selective for CB2 receptors (Huffman et al., 1999) and WIN55,212-3 is the inactive enantiomer of WIN55,212-2. Δ9-THC is a cannabinoid receptor partial agonist (Pertwee et al., 2010). The orphan receptor GPR55 has been described as a target for cannabinoid receptor ligands (anandamide, Δ9-THC, CP55,940) (Ryberg et al., 2007). We also tested the effects of O-1602 (a selective GPR55 agonist). To unambiguously determine which cannabinoid receptor subtype was involved, the effects of the CB1-selective antagonist SR141716 [pA2 for CB1 receptors: 7.9 (Rinaldi-Carmona et al., 1994)] and the CB2-selective antagonist SR144528 [pA2 for CB2 receptors: 6.3 (Rinaldi-Carmona et al., 1998)] on EFS-induced contraction were studied in the presence and absence of the above-mentioned cannabinoid receptor agonists.
Table 1.
The ranges of CB1 and CB2 receptor Ki values (nM) for selected cannabinoid receptor agonists and antagonists (Pertwee et al., 2010)
| CB1 | CB2 | |
|---|---|---|
| Agonists | ||
| 2-Arachidonoylglycerol (2-AG) | 58.3–472 | 145–1400 |
| Delta-9-tetrahydrocannabinol (Δ9-THC) | 5.05–80.3 | 3.13–75.3 |
| WIN55,212-2 | 1.89–123 | 0.28–16.2 |
| CP55,940 | 0.5–5.0 | 0.69–2.8 |
| JWH-133 | 677 | 3.4 |
| Antagonists | ||
| SR141716 | 1.8–12.3 | 514–13 200 |
| SR144528 | 50.3–>10 000 | 0.28–5.6 |
Data analysis
Values in the text and figures are expressed as the arithmetic mean ± SEM from experiments on bronchi from n individual donors. For the effects on basal tone, values were expressed as changes in tension (g) in comparison with the basal tone. For contraction in response to exogenous ACh, the maximal contraction (Emax) and potency (pEC50, defined as the negative logarithm of the molar concentration of agonist producing 50% of Emax) obtained from the second concentration–response curves with vehicle or agonist were analysed and compared. For EFS experiments, values were expressed as the percentage inhibition of the baseline contraction in response to the first train of stimulations. The onset of action was defined as the time needed for a given concentration of agonist to inhibit an EFS-induced cholinergic contraction by 20%.
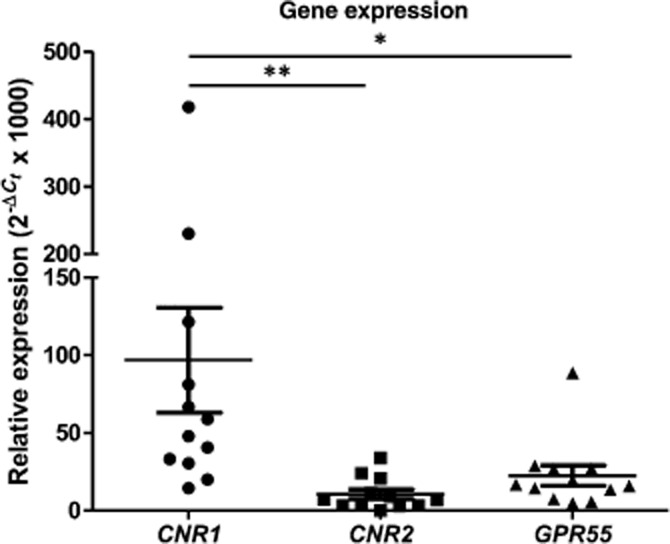
The quantitative data obtained from the RT-qPCR experiments were expressed as relative expression (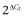 ) (Livak and Schmittgen, 2001), where ΔCt is the difference between the target gene Ct and the mean Ct of the reference genes.
) (Livak and Schmittgen, 2001), where ΔCt is the difference between the target gene Ct and the mean Ct of the reference genes.
Statistical analyses were performed with NCSS software for Windows (version 2007; NCSS LLC, Kaysville, UT, USA) by applying a two-way, repeated-measures anova for paired data and then a Tukey–Kramer multiple comparison test. The threshold for statistical significance was set to P < 0.05.
Materials
ACh hydrochloride, indomethacin, montelukast, atropine, tetrodotoxin (TTX), hexamethonium and JWH-133 were purchased from Sigma-Aldrich (Saint-Quentin-Fallavier, France); WIN55,212-2, WIN55,212-3, 2-arachidonoylglycerol (2-AG), CP55,940 and O-1602 were obtained from Tocris (Bristol, UK); and Δ9-THC was purchased from LGC Standards (Molsheim, France). SR141716 and SR144528 were synthesized by Sanofi-Aventis (Montpellier, France). Stock solutions of indomethacin and montelukast (both 1 mM) were prepared in ethanol, whereas stock solutions of Δ9-THC, 2-AG, WIN55,212-2, WIN55,212-3, CP55,940, O-1602, JWH-133, SR141716 and SR144528 (all 10 mM) were prepared in dimethyl sulfoxide. Subsequent dilutions were performed with Krebs–Henseleit solution (NaCl 119 mM, 5.4 mM KCl, 2.5 mM CaCl2, 1.2 mM KH2PO4, 1.2 mM MgSO4, 25 mM NaHCO3 and 11.7 mM glucose) and stock solutions were kept at −20°C prior to use. The maximum final concentrations of organic solvent (vehicle) in the organ bath did not alter bronchial contractility.
Results
Cannabinoid receptor gene expression in human bronchi
Bronchi from 12 patients were screened for expression of the genes coding for the CB1, CB2 and GPR55 receptors (CNR1, CNR2 and GPR55 respectively) (Figure 1). Although all three transcripts were found in the bronchi, the CB1 receptor transcript was significantly more abundant than those of the CB2 and GPR55 receptors.
Figure 1.
Relative expression ( × 1000) of CNR1, CNR2 and GPR55 gene transcripts in human bronchi (n = 12). HPRT1, GAPDH and GUSB were used as housekeeping genes for the normalization of data (Livak and Schmittgen, 2001). Data are shown for each individual and as mean ± SEM. *P < 0.05; **P < 0.01.
× 1000) of CNR1, CNR2 and GPR55 gene transcripts in human bronchi (n = 12). HPRT1, GAPDH and GUSB were used as housekeeping genes for the normalization of data (Livak and Schmittgen, 2001). Data are shown for each individual and as mean ± SEM. *P < 0.05; **P < 0.01.
The cholinergic nature of the EFS-induced contraction
Control stimulations in 142 bronchial rings caused a mean increase in tension of 1.1 ± 0.1 g over basal tone, which represents 28% of the maximal contraction obtained with 3 mM exogenous ACh. Both atropine (n = 3) and TTX (n = 5) inhibited EFS-induced contraction at concentrations equal to or greater than 0.01 and 0.1 μM respectively (Figure 2). The ganglion-blocker hexamethonium (n = 5) was devoid of the effect below and at the highest concentration tested (100 μM).
Figure 2.
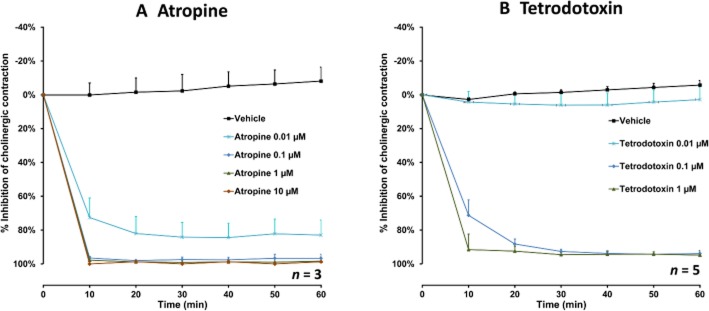
The effect of atropine (0.01–10 μM, n = 3) (A) and TTX (0.01–1 μM, n = 5) (B) on EFS-induced cholinergic contraction in human bronchi. Data are expressed as mean ± SEM percentage inhibition of cholinergic contraction with bronchi from n different patients.
Effects of Δ9-THC and the endogenous cannabinoid receptor agonist 2-AG on bronchial reactivity
Δ9-THC inhibited EFS-induced cholinergic contraction, as shown in a representative trace in Figure 3. The inhibition was concentration-dependent, with a mean maximum inhibitory effect of 39% at a concentration of 30 μM after 4 h of stimulation (Figure 4). However, a submaximal effect was obtained after just 1 h (25%). A two-way anova revealed an effect of both concentration (P < 0.05) and time (P < 0.001) (n = 7). In contrast, 2-AG (up to a concentration of 30 μM) did not affect EFS-induced contraction (n = 5). Furthermore, neither Δ9-THC nor 2-AG (each at 10 μM) affected basal tone or contraction in response to exogenous ACh (n = 5) (Table 2).
Figure 3.
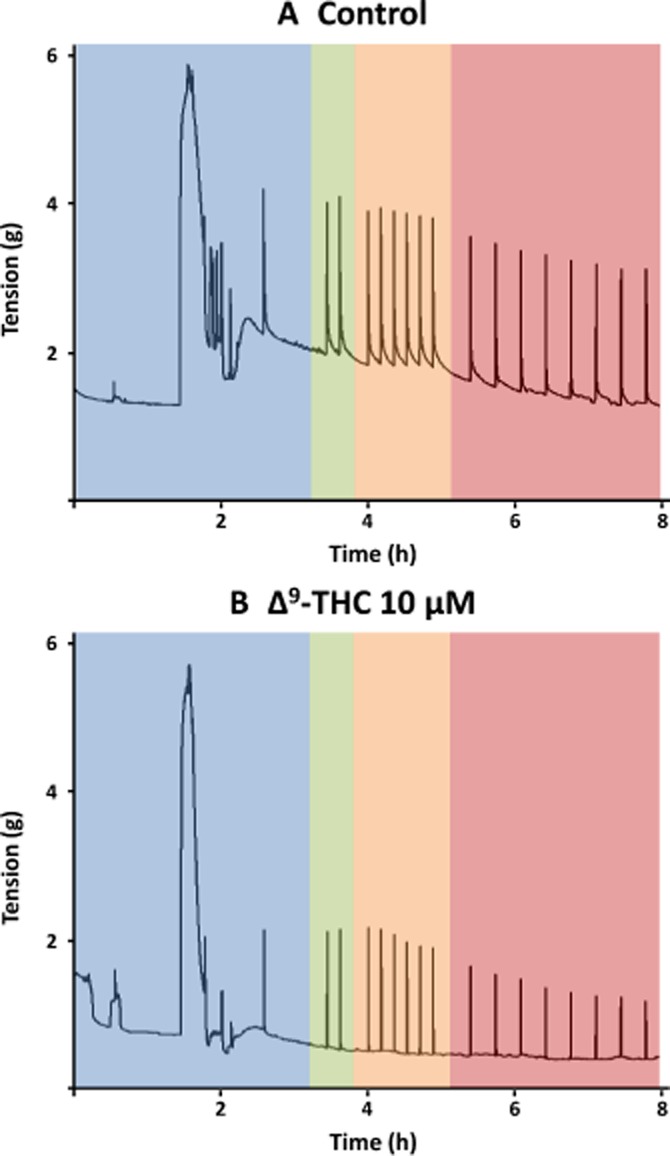
A representative trace of EFS-induced cholinergic contraction in human bronchi in the presence of vehicle only (A) or 10 μM delta-9-tetrahydrocannabinol (Δ9-THC) (B). Bronchial rings were suspended and equilibrated in the organ bath. Maximal contraction was obtained by the addition of 3 mM ACh, the rings were washed three times and an initial stimulation was delivered in order to assess the ring's reactivity (blue area). After another stabilization period, the first of two trains of EFS was applied (with a 10 min interval) to assess each preparation's baseline response prior to addition of vehicle or Δ9-THC to the bath (green area). Lastly, trains of stimulation were delivered every 10 min for 1 h (orange area) and then every 20 min for 4 h (red area).
Figure 4.
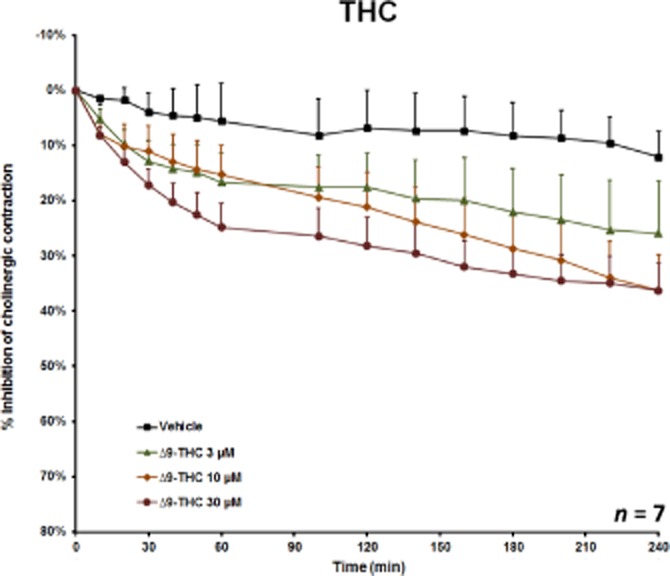
The effect of 3–30 μM delta-9-tetrahydrocannabinol (Δ9-THC) on EFS-induced cholinergic contraction in human bronchi. Data are shown as mean ± SEM percentage inhibition of cholinergic contraction obtained with bronchi from seven different patients.
Table 2.
The effects of the cannabinoid receptor agonists on contraction of human bronchial rings in response to exogenous ACh(n = 5–6)
| Vehicle only | Treated with agonist | P first versus second | ||||
|---|---|---|---|---|---|---|
| Emax (%) | pEC50 | Emax (%) | pEC50 | Emax (%) | pEC50 | |
| 2-AG | 97.8 ± 2.1 | 5.3 ± 0.1 | 99.5 ± 2.4 | 5.2 ± 0.1 | 0.62 | 0.26 |
| Δ9-THC | 97.9 ± 2.1 | 5.3 ± 0.1 | 99.0 ± 2.5 | 5.5 ± 0.1 | 0.73 | 0.32 |
| WIN55,212-2 | 96.5 ± 4.0 | 4.8 ± 0.1 | 97.7 ± 3.1 | 5.1 ± 0.1 | 0.82 | 0.24 |
| CP55,940 | 95.1 ± 2.2 | 5.5 ± 0.1 | 96.9 ± 1.9 | 5.5 ± 0.1 | 0.57 | 0.94 |
| JWH-133 | 96.4 ± 2.9 | 4.8 ± 0.1 | 98.6 ± 1.7 | 5.1 ± 0.1 | 0.52 | 0.06 |
A concentration–response curve for ACh was obtained before and after a 30 min incubation with vehicle or 10 μM of agonist. The response was modelled with non-linear regression; the respective Emax and pEC50 of the second concentration–response curves with vehicle or agonist were compared in an extra sum-of-squares F-test. None of the P values (last column) reached significance (<0.05).
Effect of synthetic cannabinoid receptor agonists on bronchial reactivity
The non-selective cannabinoid agonists WIN55,212-2 (Figure 5A) and CP55,940 (Figure 5B) inhibited EFS-induced contraction in a concentration-dependent manner, with mean maximum effects of 76% for 10 μM WIN55,212-2 and 77% for 1 μM CP55,940 after 4 h of stimulation. Again, a submaximal effect (62 and 60%, respectively) was obtained after 60 min. In contrast to CP55,940, WIN55,212-2 is reportedly devoid of any effect on the orphan receptor GPR55 (Ryberg et al., 2007). In the present study, the selective GPR55 agonist O-1602 had no effect on EFS-induced contraction (n = 5). Hence, these two findings conclusively rule out any involvement of the orphan receptor GPR55 in cannabinoid-induced inhibition of EFS-induced contraction. Significant inhibition of EFS-induced contraction was observed only for the highest concentrations of the CB2-receptor-selective agonist JWH-133 (3 and 10 μM; n = 5–8, P < 0.05) (Figure 5C); maximum inhibition (32%) was observed for 10 μM after 4 h of stimulation. None of these agonists (up to 10 μM) affected basal tone or contraction in response to exogenous ACh (n = 5) (Table 2).
Figure 5.
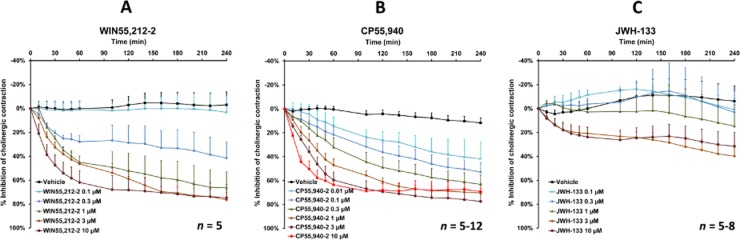
The effect of the synthetic cannabinoid receptor agonists WIN55,212-2 (0.1–10 μM, n = 5) (A), CP55,940 (0.01–10 μM, n = 5–12) (B) and JWH-133 (0.1–10 μM, n = 5–8) (C) on EFS-induced cholinergic contraction in human bronchi. Data are shown as mean ± SEM percentage inhibition of cholinergic contraction obtained with bronchi from n different patients.
Time to onset of effect of cannabinoid receptor agonists
The mean ± SD [range] time needed to achieve 20% inhibition of the EFS-induced cholinergic contraction with 10 μM of the different agonists was 9.1 ± 1.1 min [7–12] for CP55,940 (n = 5), 12.3 ± 3.2 min [6–20] for WIN55,212-2 (n = 5); 69.3 ± 20.5 min [8–160] for Δ9-THC (n = 7) and 167.0 ± 76.0 min [7–540] for JWH-133 (n = 8). The weaker effect of the two latter compounds probably explains the longer time to onset of action (as defined as 20% inhibition).
Characterization of the cannabinoid receptors involved in the inhibition of EFS-induced contraction
WIN55,212-3 (an enantiomer of WIN55,212-2 that has no effect on cannabinoid receptors) was devoid of any effect (n = 6); this observation argues in favour of the involvement of cannabinoid receptors in the inhibitory effect of WIN55,212-2. The results for JWH-133 (whose affinity for the CB2 receptor is about 200 times that for the CB1 receptor) (Table 1) (Huffman et al., 1999) suggest that CB1 receptors have a prominent role in the inhibition of EFS-induced contraction. With a view to further characterizing the cannabinoid receptor subtype, the effects of agonists on the EFS-induced cholinergic contraction were then investigated in the presence or absence of SR141716 and SR144528 (selective CB1 and CB2 antagonists respectively). At a concentration of 10 μM, neither of the antagonists had an effect on basal tone, the concentration–response curves for exogenous ACh or EFS-induced contraction (Table 3). In a first series of experiments, reversal of the inhibitory effects of WIN55,212-2 and JWH-133 was probed in the presence of SR141716 or SR144528. The CB1 receptor antagonist SR141716 (at concentrations of 0.01–3 μM) inhibited the response to 3 μM WIN55,212-2 in a concentration-dependent manner (Figure 6A), whereas the CB2 receptor antagonist SR144528 had no effect (at concentrations up to 10 μM) (Figure 6B). Similarly, the response to 10 μM JWH-133 was fully inhibited by SR141716 (at 0.1–3 μM), whereas SR144528 had no influence (at concentrations up to 10 μM). Furthermore, 3 μM SR141716 also reversed the response to Δ9-THC and CP55,940 (Figure 7). The mean maximum inhibition after 120 min was 82% for WIN55,212-2, 80% for Δ9-THC and 30% for CP55,940. SR141716's weaker reversal of the inhibitory effect of CP55,940 can be explained by the 5- to 200-fold higher binding affinity of CP55,940 for CB1 receptors.
Table 3.
The effects of the selective CB1 and CB2 receptor antagonists SR141716 and SR144528 on basal tone, contraction in response to exogenous ACh and EFS-induced contraction
| SR141716 10 μM | SR144528 10 μM | |||||
|---|---|---|---|---|---|---|
| Effect | n | P | Effect | n | P | |
| Change in basal tone (g) | 0.08 ± 0.06 | 6 | 0.14 | 0.6 ± 0.2 | 6 | 0.052 |
| Mean change of pEC50 in concentration–response curves for exogenous ACh | 0.09 | 5 | 0.73 | 0.09 | 5 | 0.67 |
| Maximum change in EFS-induced contraction (% vs. control) | 13 ± 5 | 6 | 0.25 | 10 ± 5 | 6 | 0.10 |
For the effect on basal tone, tension was measured in bronchial rings before and after exposure to 10 μM of each antagonist; the difference in tension was analysed in a paired t-test. For the effect on exogenous ACh, a concentration–response curve for ACh was obtained before and after a 30 min incubation with vehicle or 10 μM of agonist; the response was modelled with non-linear regression and the Emax and pEC50 of the second concentration–response curves with vehicle or agonist were compared with extra sum-of-squares F-test. For the effect on EFS-induced contraction, bronchial rings from the same patients were subjected to EFS either with vehicle only or with 10 μM of each antagonist, the difference between contraction in the control condition and contraction with the antagonist was calculated for each time point. Only the time point with the greatest difference is presented in the table. For EFS-induced contraction, statistical analysis was performed with a two-way, repeated-measures anova, followed by a Tukey–Kramer multiple comparison test.
Figure 6.
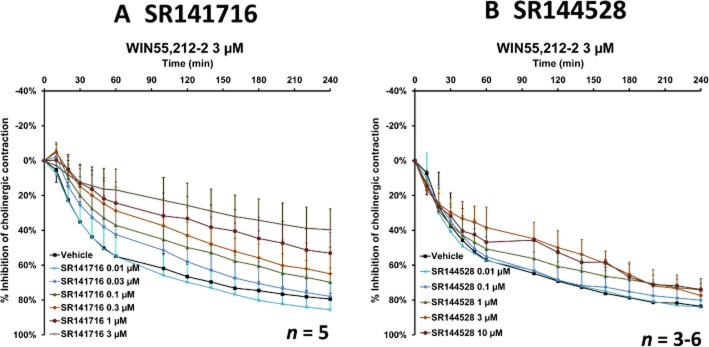
The effect of the CB1 receptor antagonist SR141716 (A) and the CB2 receptor antagonist SR144528 (B) on inhibition of EFS-induced cholinergic contraction in human bronchi by 3 μM WIN55,212-2. Data are shown as mean ± SEM percentage inhibition of cholinergic contraction obtained with bronchi from three to six different patients.
Figure 7.
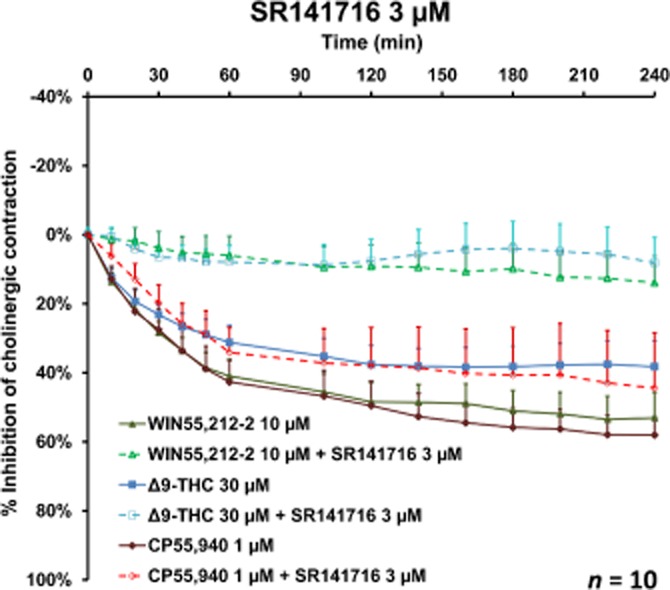
The effect of the CB1 receptor antagonist SR141716 (3 μM) on inhibition of EFS-induced cholinergic contraction in human bronchi by Δ9-THC, WIN55,212-2 and CP55,940. Data are shown as mean ± SEM value percentage inhibition of cholinergic contraction obtained with bronchi from 10 different patients.
Discussion
The present study is the first to show that prejunctional cannabinoid CB1 receptors inhibit cholinergic-mediated contraction in human bronchi. In both human and animal airways, the rapid contraction induced by EFS is due to ACh release from post-ganglionic nerves (Back et al., 2006; Schleputz et al., 2012). The cholinergic nature of the contraction and the involvement of muscarinic receptors were demonstrated by the inhibition of the EFS-induced contraction by atropine and TTX but not by the ganglion-blocker hexamethonium, in agreement with previous findings (Takahashi et al., 1994; Ellis and Conanan, 1996; Fernandes et al., 1999). Cannabinoid CB1 receptors are concentrated in the CNS but are also found in some peripheral tissues. In contrast, cannabinoid CB2 receptors are primarily found in peripheral tissues, such as immune cells, but can also be detected in the CNS (Onaivi, 2011). In guinea pig and rat airways, the endogenous cannabinoid anandamide and the synthetic agonists WIN55,212-2, CP55,940 and JWH-133 inhibited the EFS-induced release of ACh (Spicuzza et al., 2000) and inhibited EFS-induced contraction (Yousif and Oriowo, 1999; Yoshihara et al., 2004). The fact that these inhibitions were not affected by the CB1 receptor antagonist SR141716A but were dampened by a CB2 receptor antagonist (Yoshihara et al., 2004) suggests the involvement of prejunctional CB2 receptors in the guinea pig and rat. In contrast, our present results conclusively demonstrate the involvement of CB1 receptors in the EFS-mediated contraction of human bronchi because (i) the selective CB1 receptor antagonist SR141716A was found to reverse the inhibitory effect of dual CB1 and CB2 receptor agonists (Δ9-THC, WIN55,212-2 and CP55,940) and (ii) the selective CB2 receptor antagonist SR144528 was inactive. The involvement of the orphan receptor GPR55 in the cannabinoids' inhibitory effects was also ruled out by the absence of an effect of O-1602. Antagonist affinity is a key factor when assessing receptor selectivity. The SR141716 concentration that reversed the agonist-induced inhibition was similar to that used in other publications and was close to its pA2 for the CB1 receptor [pA2 = 7.9 (Rinaldi-Carmona et al., 1994)]; this conclusively demonstrates the involvement of CB1 receptors. Furthermore, JWH-133 (which binds 200 times more strongly to the CB2 receptor than to the CB1 receptor) only exerted an inhibitory effect on EFS-induced contraction at concentrations above 3 μM. This effect was inhibited by SR141716 but not by SR144528 – suggesting that CB1 receptors are activated by JWH-133 at its highest concentrations.
In human bronchi, we found that CB1 receptor gene transcripts were more abundant than CB2 receptor gene transcripts. In rat lungs, electron microscopy experiments have revealed that CB1 receptors are located on nerve fibres situated near smooth muscle cells (Calignano et al., 2000) but not on the smooth muscle cells themselves. In the present study, the absence of functional CB1 or CB2 receptors on human bronchial smooth muscles was suggested by the cannabinoid agonists' lack of direct effects on basal tone or ACh-induced contraction. This observation further suggests that prejunctional CB1 receptors are involved in the phenomena observed here.
In the rat lung, activation of CB1 receptors with anandamide inhibits the bronchospasm and cough evoked by capsaicin (Calignano et al., 2000) and suggests that this cannabinoid receptor subtype may be also involved in the inhibition of neuropeptide release by C-fibres in the rat. In contrast, in vivo observations in a conscious guinea pig model of cough (Patel et al., 2003) and in vitro observations on sensory C- and Aδ-fibres in isolated vagus nerves from humans and guinea pigs (Belvisi et al., 2008) have suggested that the CB2 receptor is involved in the modulation of neuropeptide release by sensory nerves in the human and guinea pig airways.
In cardiovascular and gastrointestinal preparations, CB1 receptors are able to modulate pre- and postjunctional neurotransmission (Niederhoffer and Szabo, 1999; Niederhoffer et al., 2003; Hinds et al., 2006). Δ9-THC inhibits the release of ACh from the myenteric plexus in guinea pigs (Coutts and Pertwee, 1997). The most prominent intestinal effect of cannabinoids is the CB1-mediated presynaptic inhibition of ACh release (Heinemann et al., 1999; Storr et al., 2004; Sibaev et al., 2009). In strips of human colon, activation of the CB1 receptors located on cholinergic motor neurons inhibits EFS-induced ACh release and neurogenic circular muscle contraction (Hinds et al., 2006). Taken as a whole, these results suggest the existence of species-, tissue- and nerve-related differences in the expression of cannabinoid receptor subtypes on peripheral nerve fibres.
Δ9-THC is the active ingredient in Cannabis sativa and is mainly responsible for the psychoactive and respiratory effects of smoked marijuana. It displays moderate affinity and low relative intrinsic activity (partial agonism) for cannabinoid receptors (Pertwee et al., 2010). There are still very few data on the tissue concentrations of Δ9-THC in vivo. The lung was found to be the organ that contained the highest Δ9-THC concentration in porcine models, with a lung/blood concentration ratio of about 80 (Brunet et al., 2006; 2010). The inhalation of 34 mg of Δ9-THC in humans yields blood concentrations that peak at 0.85 μM (Huestis et al., 1992). On the basis of the lung/blood concentration ratio in the pig, lung concentrations in humans might peak at 67 μM – twice the highest organ bath concentration studied in the present study. These observations suggest that our findings have clinical relevance and may explain the bronchodilation following inhalation of smoke from herbal cannabis (Tashkin et al., 1974).
We also chose to investigate the effects of the endogenous cannabinoid 2-AG, which is about 170 times more abundant than anandamide (the other endogenous agonist) (Stella et al., 1997). The affinity of 2-AG for the CB1 and CB2 receptors is relatively low but is greater than that of anandamide. On the basis of relative affinities for the cannabinoid receptor subtypes, we also investigated synthetic agonists from the three least related chemical families (i.e. CP, WIN and JWH derivatives), which are reportedly used as recreational drugs. WIN55,212-2 has moderate affinity for the CB1 and CB2 receptor subtypes, CP55,940 has high affinity for both subtypes and JWH-133 may be considered as a selective CB2 receptor agonist (Table 1). Other agonists found in herbal mixtures or human tissue samples (such as JWH-018, JWH-073, JWH-210, JWH-250 and CP47,457) (Logan et al., 2012; Hermanns-Clausen et al., 2013) have Ki values for CB1 receptors of between 0.5 and 9 nM (Huffman et al., 2005a,b), which are equal to or greater than that of CP55,940 (the most potent agonist used in the present study). With regard to the EFS-mediated contraction of human bronchus, (i) the low-affinity endocannabinoid 2-AG was devoid of any effect up to 30 μM and (ii) the partial agonist Δ9-THC had half the inhibitory effect of the synthetic compounds CP55,940 and WIN55,212-2 [which display higher relative intrinsic activity at cannabinoid receptors (Pertwee et al., 2010)]. The greater efficacy (at least as measured in vitro) and more rapid onset of action may result in more intense, faster bronchodilation after the consumption of high-affinity, synthetic cannabinoids.
Chronic, high-level marijuana smoking may produce partial tachyphylaxis and can even lead to mild airway obstruction (probably due to the chronic inflammatory changes associated with regular marijuana smoking) (Tashkin et al., 1976; Tashkin, 2013). However, a recent large-scale, 20 year follow-up study showed that low-level, chronic marijuana use was associated with an increase in forced expiratory volume and forced vital capacity (Pletcher et al., 2012); this agrees with other literature data showing that chronic marijuana consumption via a vaporizer (which reduces the amount of tobacco-derived airway irritants) also improves respiratory function (Van Dam and Earleywine, 2010).
In conclusion, our present results show that the activation of prejunctional CB1 receptors inhibits cholinergic contraction in human bronchi. The cannabinoids' inhibitory effects on the cholinergic-mediated contraction may explain the acute bronchodilation produced by marijuana smoking. One can expect to observe bronchodilation in consumers of synthetic, recreational cannabinoids. However, the clinical effects of acute and chronic exposure to these compounds remain to be characterized.
Acknowledgments
The authors wish to acknowledge David Fraser (Biotech Communication, Damery, France) for providing copy editing services and Jean-François Dreyfus (Hôpital Foch, Suresnes, France), for statistical advice.
Glossary
- 2-AG
2-arachidonoylglycerol
- Δ9-THC
Δ9-tetrahydrocannabinol
- EFS
electrical field stimulation
- TTX
tetrodotoxin
Conflict of interest
None.
References
- Abboud RT, Sanders HD. Effect of oral administration of delta-tetrahydrocannabinol on airway mechanics in normal and asthmatic subjects. Chest. 1976;70:480–485. doi: 10.1378/chest.70.4.480. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL. Spedding M, et al. The Concise Guide to PHARMACOLOGY 2013/14: G Protein-Coupled Receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Back M, Costantino M, Brink C, Norel X. Effect of cold storage on cholinergic responses induced by electrical field stimulation in human bronchi. Pulm Pharmacol Ther. 2006;19:297–302. doi: 10.1016/j.pupt.2005.02.014. [DOI] [PubMed] [Google Scholar]
- Belvisi MG, Patel HJ, Freund-Michel V, Hele DJ, Crispino N, Birrell MA. Inhibitory activity of the novel CB2 receptor agonist, GW833972A, on guinea-pig and human sensory nerve function in the airways. Br J Pharmacol. 2008;155:547–557. doi: 10.1038/bjp.2008.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brunet B, Doucet C, Venisse N, Hauet T, Hebrard W, Papet Y, et al. Validation of large white pig as an animal model for the study of cannabinoids metabolism: application to the study of THC distribution in tissues. Forensic Sci Int. 2006;161:169–174. doi: 10.1016/j.forsciint.2006.04.018. [DOI] [PubMed] [Google Scholar]
- Brunet B, Hauet T, Hebrard W, Papet Y, Mauco G, Mura P. Postmortem redistribution of THC in the pig. Int J Legal Med. 2010;124:543–549. doi: 10.1007/s00414-009-0403-2. [DOI] [PubMed] [Google Scholar]
- Buenestado A, Grassin-Delyle S, Guitard F, Naline E, Faisy C, Israel-Biet D, et al. Roflumilast inhibits the release of chemokines and TNF-alpha from human lung macrophages stimulated with lipopolysaccharide. Br J Pharmacol. 2012;165:1877–1890. doi: 10.1111/j.1476-5381.2011.01667.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calignano A, Katona I, Desarnaud F, Giuffrida A, La Rana G, Mackie K, et al. Bidirectional control of airway responsiveness by endogenous cannabinoids. Nature. 2000;408:96–101. doi: 10.1038/35040576. [DOI] [PubMed] [Google Scholar]
- Coutts AA, Pertwee RG. Inhibition by cannabinoid receptor agonists of acetylcholine release from the guinea-pig myenteric plexus. Br J Pharmacol. 1997;121:1557–1566. doi: 10.1038/sj.bjp.0701301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellis JL, Conanan ND. Prejunctional inhibition of cholinergic responses by prostaglandin E2 in human bronchi. Am J Respir Crit Care Med. 1996;154:244–246. doi: 10.1164/ajrccm.154.1.8680688. [DOI] [PubMed] [Google Scholar]
- Fernandes LB, Henry PJ, Goldie RG. Endothelin-1 potentiates cholinergic nerve-mediated contraction in human isolated bronchus. Eur Respir J. 1999;14:439–442. doi: 10.1034/j.1399-3003.1999.14b33.x. [DOI] [PubMed] [Google Scholar]
- Forrester MB, Kleinschmidt K, Schwarz E, Young A. Synthetic cannabinoid and marijuana exposures reported to poison centers. Hum Exp Toxicol. 2012;31:1006–1011. doi: 10.1177/0960327111421945. [DOI] [PubMed] [Google Scholar]
- Gong H, Jr, Tashkin DP, Simmons MS, Calvarese B, Shapiro BJ. Acute and subacute bronchial effects of oral cannabinoids. Clin Pharmacol Ther. 1984;35:26–32. doi: 10.1038/clpt.1984.4. [DOI] [PubMed] [Google Scholar]
- Grassin-Delyle S, Naline E, Buenestado A, Risse PA, Sage E, Advenier C, et al. Expression and function of human hemokinin-1 in human and guinea pig airways. Respir Res. 2010;11:139. doi: 10.1186/1465-9921-11-139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann A, Shahbazian A, Holzer P. Cannabinoid inhibition of guinea-pig intestinal peristalsis via inhibition of excitatory and activation of inhibitory neural pathways. Neuropharmacology. 1999;38:1289–1297. doi: 10.1016/s0028-3908(99)00056-8. [DOI] [PubMed] [Google Scholar]
- Hermanns-Clausen M, Kneisel S, Szabo B, Auwarter V. Acute toxicity due to the confirmed consumption of synthetic cannabinoids: clinical and laboratory findings. Addiction. 2013;108:534–544. doi: 10.1111/j.1360-0443.2012.04078.x. [DOI] [PubMed] [Google Scholar]
- Hinds NM, Ullrich K, Smid SD. Cannabinoid 1 (CB1) receptors coupled to cholinergic motorneurones inhibit neurogenic circular muscle contractility in the human colon. Br J Pharmacol. 2006;148:191–199. doi: 10.1038/sj.bjp.0706710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huestis MA, Henningfield JE, Cone EJ. Blood cannabinoids. I. Absorption of THC and formation of 11-OH-THC and THCCOOH during and after smoking marijuana. J Anal Toxicol. 1992;16:276–282. doi: 10.1093/jat/16.5.276. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Liddle J, Yu S, Aung MM, Abood ME, Wiley JL, et al. 3-(1′,1′-Dimethylbutyl)-1-deoxy-delta8-THC and related compounds: synthesis of selective ligands for the CB2 receptor. Bioorg Med Chem. 1999;7:2905–2914. doi: 10.1016/s0968-0896(99)00219-9. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Szklennik PV, Almond A, Bushell K, Selley DE, He H, et al. 1-Pentyl-3-phenylacetylindoles, a new class of cannabimimetic indoles. Bioorg Med Chem Lett. 2005a;15:4110–4113. doi: 10.1016/j.bmcl.2005.06.008. [DOI] [PubMed] [Google Scholar]
- Huffman JW, Zengin G, Wu MJ, Lu J, Hynd G, Bushell K, et al. Structure-activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg Med Chem. 2005b;13:89–112. doi: 10.1016/j.bmc.2004.09.050. [DOI] [PubMed] [Google Scholar]
- Izzo AA, Mascolo N, Borrelli F, Capasso F. Excitatory transmission to the circular muscle of the guinea-pig ileum: evidence for the involvement of cannabinoid CB1 receptors. Br J Pharmacol. 1998;124:1363–1368. doi: 10.1038/sj.bjp.0701964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehn BM. Teen perceptions of marijuana risks shift: use of alcohol, illicit drugs, and tobacco declines. JAMA. 2013;309:429–430. doi: 10.1001/jama.2012.211240. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Logan BK, Reinhold LE, Xu A, Diamond FX. Identification of synthetic cannabinoids in herbal incense blends in the United States. J Forensic Sci. 2012;57:1168–1180. doi: 10.1111/j.1556-4029.2012.02207.x. [DOI] [PubMed] [Google Scholar]
- Miech R, Koester S. Trends in U.S., past-year marijuana use from 1985 to 2009: an age-period-cohort analysis. Drug Alcohol Depend. 2012;124:259–267. doi: 10.1016/j.drugalcdep.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naline E, Trifilieff A, Fairhurst RA, Advenier C, Molimard M. Effect of indacaterol, a novel long-acting beta2-agonist, on isolated human bronchi. Eur Respir J. 2007;29:575–581. doi: 10.1183/09031936.00032806. [DOI] [PubMed] [Google Scholar]
- Niederhoffer N, Szabo B. Effect of the cannabinoid receptor agonist WIN55212-2 on sympathetic cardiovascular regulation. Br J Pharmacol. 1999;126:457–466. doi: 10.1038/sj.bjp.0702337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niederhoffer N, Schmid K, Szabo B. The peripheral sympathetic nervous system is the major target of cannabinoids in eliciting cardiovascular depression. Naunyn Schmiedebergs Arch Pharmacol. 2003;367:434–443. doi: 10.1007/s00210-003-0755-y. [DOI] [PubMed] [Google Scholar]
- Onaivi ES. Commentary: functional neuronal CB2 cannabinoid receptors in the CNS. Curr Neuropharmacol. 2011;9:205–208. doi: 10.2174/157015911795017416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orzelek-O'Neil RM, Goodman FR, Forney RB. Delta-9-tetrahydrocannabinol on isolated human bronchioles. Arch Int Pharmacodyn Ther. 1980;246:71–83. [PubMed] [Google Scholar]
- Patel HJ, Birrell MA, Crispino N, Hele DJ, Venkatesan P, Barnes PJ, et al. Inhibition of guinea-pig and human sensory nerve activity and the cough reflex in guinea-pigs by cannabinoid (CB2) receptor activation. Br J Pharmacol. 2003;140:261–268. doi: 10.1038/sj.bjp.0705435. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pertwee RG, Howlett AC, Abood ME, Alexander SP, Di Marzo V, Elphick MR, et al. International Union of Basic and Clinical Pharmacology. LXXIX. Cannabinoid receptors and their ligands: beyond CB1 and CB2. Pharmacol Rev. 2010;62:588–631. doi: 10.1124/pr.110.003004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pletcher MJ, Vittinghoff E, Kalhan R, Richman J, Safford M, Sidney S, et al. Association between marijuana exposure and pulmonary function over 20 years. JAMA. 2012;307:173–181. doi: 10.1001/jama.2011.1961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Racke K, Matthiesen S. The airway cholinergic system: physiology and pharmacology. Pulm Pharmacol Ther. 2004;17:181–198. doi: 10.1016/j.pupt.2004.03.001. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Heaulme M, Shire D, Calandra B, Congy C, et al. SR141716A, a potent and selective antagonist of the brain cannabinoid receptor. FEBS Lett. 1994;350:240–244. doi: 10.1016/0014-5793(94)00773-x. [DOI] [PubMed] [Google Scholar]
- Rinaldi-Carmona M, Barth F, Millan J, Derocq JM, Casellas P, Congy C, et al. SR 144528, the first potent and selective antagonist of the CB2 cannabinoid receptor. J Pharmacol Exp Ther. 1998;284:644–650. [PubMed] [Google Scholar]
- Ryberg E, Larsson N, Sjogren S, Hjorth S, Hermansson NO, Leonova J, et al. The orphan receptor GPR55 is a novel cannabinoid receptor. Br J Pharmacol. 2007;152:1092–1101. doi: 10.1038/sj.bjp.0707460. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schleputz M, Rieg AD, Seehase S, Spillner J, Perez-Bouza A, Braunschweig T, et al. Neurally mediated airway constriction in human and other species: a comparative study using precision-cut lung slices (PCLS) PLoS ONE. 2012;7:e47344. doi: 10.1371/journal.pone.0047344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shapiro BJ, Tashkin DP. The Pharmacology of Marijuana. Raven edn. New York: Raven Press; 1976. [Google Scholar]
- Sibaev A, Yuce B, Kemmer M, Van Nassauw L, Broedl U, Allescher HD, et al. Cannabinoid-1 (CB1) receptors regulate colonic propulsion by acting at motor neurons within the ascending motor pathways in mouse colon. Am J Physiol Gastrointest Liver Physiol. 2009;296:G119–G128. doi: 10.1152/ajpgi.90274.2008. [DOI] [PubMed] [Google Scholar]
- Spicuzza L, Haddad EB, Birrell M, Ling A, Clarke D, Venkatesan P, et al. Characterization of the effects of cannabinoids on guinea-pig tracheal smooth muscle tone: role in the modulation of acetylcholine release from parasympathetic nerves. Br J Pharmacol. 2000;130:1720–1726. doi: 10.1038/sj.bjp.0703497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stella N, Schweitzer P, Piomelli D. A second endogenous cannabinoid that modulates long-term potentiation. Nature. 1997;388:773–778. doi: 10.1038/42015. [DOI] [PubMed] [Google Scholar]
- Storr M, Sibaev A, Marsicano G, Lutz B, Schusdziarra V, Timmermans JP, et al. Cannabinoid receptor type 1 modulates excitatory and inhibitory neurotransmission in mouse colon. Am J Physiol Gastrointest Liver Physiol. 2004;286:G110–G117. doi: 10.1152/ajpgi.00148.2003. [DOI] [PubMed] [Google Scholar]
- Takahashi T, Belvisi MG, Patel H, Ward JK, Tadjkarimi S, Yacoub MH, et al. Effect of Ba 679 BR, a novel long-acting anticholinergic agent, on cholinergic neurotransmission in guinea pig and human airways. Am J Respir Crit Care Med. 1994;150(6):1640–1645. doi: 10.1164/ajrccm.150.6.7952627. Pt 1) [DOI] [PubMed] [Google Scholar]
- Tashkin DP. Effects of marijuana smoking on the lung. Ann Am Thorac Soc. 2013;10:239–247. doi: 10.1513/AnnalsATS.201212-127FR. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Shapiro BJ, Frank IM. Acute effects of smoked marijuana and oral delta9-tetrahydrocannabinol on specific airway conductance in asthmatic subjects. Am Rev Respir Dis. 1974;109:420–428. doi: 10.1164/arrd.1974.109.4.420. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Shapiro BJ, Lee YE, Harper CE. Effects of smoked marijuana in experimentally induced asthma. Am Rev Respir Dis. 1975;112:377–386. doi: 10.1164/arrd.1975.112.3.377. [DOI] [PubMed] [Google Scholar]
- Tashkin DP, Shapiro BJ, Lee YE, Harper CE. Subacute effects of heavy marihuana smoking on pulmonary function in healthy men. N Engl J Med. 1976;294:125–129. doi: 10.1056/NEJM197601152940302. [DOI] [PubMed] [Google Scholar]
- Vachon L, FitzGerald MX, Solliday NH, Gould IA, Gaensler EA. Single-dose effects of marihuana smoke. Bronchial dynamics and respiratory-center sensitivity in normal subjects. N Engl J Med. 1973;288:985–989. doi: 10.1056/NEJM197305102881902. [DOI] [PubMed] [Google Scholar]
- Van Dam NT, Earleywine M. Pulmonary function in cannabis users: support for a clinical trial of the vaporizer. Int J Drug Policy. 2010;21:511–513. doi: 10.1016/j.drugpo.2010.04.001. [DOI] [PubMed] [Google Scholar]
- Williams SJ, Hartley JP, Graham JD. Bronchodilator effect of delta1-tetrahydrocannabinol administered by aerosol of asthmatic patients. Thorax. 1976;31:720–723. doi: 10.1136/thx.31.6.720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winstock AR, Barratt MJ. Synthetic cannabis: a comparison of patterns of use and effect profile with natural cannabis in a large global sample. Drug Alcohol Depend. 2013;131:106–111. doi: 10.1016/j.drugalcdep.2012.12.011. [DOI] [PubMed] [Google Scholar]
- Yoshihara S, Morimoto H, Yamada Y, Abe T, Arisaka O. Cannabinoid receptor agonists inhibit sensory nerve activation in guinea pig airways. Am J Respir Crit Care Med. 2004;170:941–946. doi: 10.1164/rccm.200306-775OC. [DOI] [PubMed] [Google Scholar]
- Yousif MH, Oriowo MA. Inhibitory effects of cannabinoid receptor ligands on electrically-evoked responses in rat isolated tracheal ring segments. Pharmacol Res. 1999;40:415–421. doi: 10.1006/phrs.1999.0532. [DOI] [PubMed] [Google Scholar]


