Abstract
Background and Purpose
Non-small cell lung cancer (NSCLC) is one of the most commonly diagnosed malignancies in the world. Small-molecule inhibitors of the EGF receptor's tyrosine kinase domain (TKIs), including gefitinib and erlotinib, have been widely used for treating NSCLC. Unfortunately, nearly all patients after initially experiencing a marked improvement while on these drugs, eventually progress to acquire resistance to TKIs. Because there is no effective therapeutic strategy to treat TKI-resistant NSCLC, we evaluated the effects of luteolin, a naturally occurring flavanoid, on T790M mutant NSCLC cells.
Experimental Approach
The effect of luteolin on the viability of NSCLC and normal cell lines was investigated using the Cell Counting Kit-8 (CCK-8) assay. Luteolin-induced apoptosis was assessed by bivariate FITC-annexin V/PI assay, and Western blots were used to measured apoptotic proteins. Co-immunoprecipitation was used to determine the effect of luteolin on the interaction between Hsp90 and mutant EGF receptors. The effect of luteolin on the Akt/mTOR pathway was studied using Western blotting analysis. Its anti-tumour efficacy in vivo was examined in a mouse xenograft model.
Key Results
Luteolin exerted significant anti-tumourigenic effects on the EGF receptor L858R/T790M mutation and erlotinib-resistant NSCLC both at the cellular and animal levels. Mechanistically, luteolin induced degradation of the EGF receptor by inhibiting the association of Hsp90 with the mutant EGF receptor, and, therefore, prevented PI3K/Akt/mTOR signalling, which resulted in NSCLC cell apoptosis.
Conclusion and Implications
Luteolin may be a potential candidate for NSCLC therapy, especially for treatment of patients with acquired erlotinib-resistant NSCLC.
Keywords: luteolin, EGFR, NSCLC, T790M mutation, apoptosis
Introduction
Lung cancer has become the leading cause of death from cancer in China and worldwide (Zhang et al., 2006; Jemal et al., 2008). Non-small cell lung cancer (NSCLC) accounts for approximately 85–90% of all lung cancer cases. This type of cancer is associated with a poor prognosis, as most patients are diagnosed at an advanced stage of the disease when surgery options are limited and are relatively insensitive to chemotherapy and radiation therapy. The EGF (also known as ErbB1) receptor is a member of ErbB family, which is a group of transmembrane receptors with an extracellular ligand-binding domain, a transmembrane domain and an intracellular TK domain that mediates signal transduction (see Alexander et al., 2013). After ligand binding and dimerization, the activated EGF receptor initiates signal transduction through two major pathways: the Ras/Raf/MAPK/ERK and the PI3K/Akt/PTEN/mTOR pathways, which are associated with cell proliferation and survival (Kim et al., 2001; Herbst, 2004; Gerber, 2008). The EGF receptor has been identified as a major oncogene that drives tumourigenesis in many types of cancers including NSCLC (Nicholson et al., 2001; Hirsch et al., 2003; Kalyankrishna and Grandis, 2006). The small-molecule inhibitors of TK (TKIs) that act on the EGF receptor including gefitinib and erlotinib are widely used for the treatment of NSCLC (Balius and Rizzo, 2009). Somatic mutations of the EGF receptor gene were detected in the majority of patients with highly TKI-responsive tumours. The most common EGF receptor mutations observed in NSCLC are small in-frame deletions in exon 19 (△LRE) and the L858R point mutation in exon 21 (Cataldo et al., 2011). However, almost all patients eventually progress to acquire resistance to these TKIs. The DNA sequence of the EGF receptor gene in patients with acquired resistance reveals the presence of a second point mutation, resulting in threonine-to-methionine amino acid change at position 790 (T790M) of the EGF receptor (Kobayashi et al., 2005; Pao et al., 2005). The reason why T790M generates resistance is because threonine 790 is the gatekeeper residue in the EGF receptor and the methionine substitution introduces a bulky amino acid side chain, resulting in a steric hindrance that may interfere with the binding of gefitinib or erlotinib to the EGF receptor (Kobayashi et al., 2005; Kwak et al., 2005; Yun et al., 2008). Although numerous new TKIs have been designed to directly target T790M mutant NSCLC cells (Engelman et al., 2007; Li et al., 2007), most of them are still far from being used clinically because of their relatively weak binding activity to the EGF receptor or severe toxicity (Sos et al., 2010).
Flavonoids are a group of the most abundant polyphenols in our daily diet and display a wide range of pharmacological properties (Middleton et al., 2000). Luteolin (3′,4′,5,7-tetrahydroxyflavone) is one of the flavonoids distributed in various types of vegetables and fruits. Luteolin has been shown to have anti-tumourigenic, anti-inflammatory, antioxidant, anti-cancer and radical scavenging properties (Gábor, 1986; Seelinger et al., 2008a,b). In a previous study we demonstrated that luteolin can act as a potent heat shock protein 90 (Hsp90) inhibitor and induce apoptosis of carcinoma cells (Fu et al., 2012). Another publication also showed a high affinity between luteolin and Hsp90. Hsp90 is an important protein chaperon, which is overexpressed in most tumour cells. It has been reported that the mutant EGF receptor is one of the client proteins of Hsp90 (Shimamura et al., 2005). In the present study, we describe a novel inhibitory effect of luteolin on the proliferation of T790M mutant NSCLC cells and the growth of the NSCLC tumour in mice.
Methods
Chemicals and antibodies
Luteolin (CAS: 491-70-3) and cisplatin were purchased from Sigma-Aldrich (St. Louis, MO, USA). Erlotinib hydrochloride (CAS: 183319-69-9) was from Santa Cruz Biotechnology (Wembley, Middlesex, UK). 17-Dimethylaminoethylamino-17-demethoxygeldanamycin (17-DMAG; CAS: 467214-21-7) was obtained from InvivoGen (San Diego, CA, USA). MG132 and NH4Cl were from Sigma-Aldrich. Cycloheximide (CHX) was obtained from Calbiochem (San Diego, CA, USA). Polyclonal antibodies against PARP, caspase-3, ERK-MAPK, phospho-ERK-MAPK (Thr202/Tyr204), β-actin and rabbit monoclonal antibodies against the EGF receptor, phospho-EGF receptor (Y1068), PI3K (p110α), phospho-PI3K p85 (Tyr458)/p55(Tyr199), Akt, phospho-Akt (Ser473), phospho-p70 S6K (Thr389), phosphor-S6 (Ser235/236), phospho-4EBP1 (Thr37/46) and HER2 were purchased from Cell Signaling Technology (Beverly, MA, USA). Polyclonal antibodies against mTOR, phosphor-mTOR (Ser2448), BAX and Bcl-2 were from Bioworld Biotechnology (Minneapolis, MN, USA). Monoclonal antibody against Hsp90 was obtained from Stressgen Bioreagents (Victoria, BC, Canada). Secondary antibodies coupled to IRDye800 flurophore were purchased from Rockland (Gilbertsville, PA, USA).
Cell culture
NCI-H1975, HCC827, NCI-H820, A549, H9c2, L02 and HEK293 cells were obtained from the Institute of Biochemistry and Cell Biology, the Chinese Academy of Sciences (Shanghai China). These cell lines were passaged for fewer than 6 months after recovery. NCI-H1975, HCC827 and NCI-H820 cell lines were maintained in RPMI 1640 (Invitrogen, Carlsbad, CA, USA) and A549, H9c2, L02, HEK293 in DMEM (Invitrogen) at 37°C in an atmosphere of 5% CO2 humidified environment. Both cell cultures contained 10% (v v-1) FBS (HyClone, Logan, UT, USA) and antibiotics (100 μg·mL−1 streptomycin and 100 U·mL−1 penicillin).
Cell viability assays
Cells were seeded into 96-well plates at a density of 5000–7000 per well and were allowed to adhere for 24 h. Cells were treated with different doses of luteolin or erlotinib for 48 h, and subjected to viability detection by using the Cell Counting Kit-8 (CCK-8; Beyotime, Shanghai, China) according to the manufacturer's specifications. In brief, 10 μL CCK-8 working solution was added to each well and after incubation at 37°C for 2 h. The absorbance of each well at 450 nm was measured using a Synergy2 Multi-Mode Microplate Reader (BIO-TEK, Inc., Winooski, VT, USA).
Co-immunoprecipitation and immunoblot analysis
All cell pellets were rinsed with ice-cold PBS and lysed with the lysis buffer. Lysates were centrifuged (12 500× g) at 4°C for 15 min. Proteins were immunoprecipitated with indicated antibodies. The precleared Protein A/G PLUS-agarose beads (Santa Cruz Biotechnology) were incubated with immunocomplexes and washed with the lysis buffer. The samples were separated by 8–12% SDS–PAGE followed by transfer onto nitrocellulose membranes (Whatman, GE Healthcare, Sanford, ME, USA), and detected by immunoblot analysis. The antibody–antigen complexes were visualized by the LI-COR Odyssey Infrared Imaging System according to the manufacturer's instructions, using IRDye 800 conjugated IgG secondary antibodies (LI-COR Biosciences, Lincoln, NE, USA). Quantification was directly performed on the blot using the LI-COR Odyssey Analysis Software. Aliquots of whole cell lysates were subjected to immunoblotting to confirm appropriate expression of proteins.
Flow-cytometric apoptosis assays
Experiments were performed by using an annexin V-FITC apoptosis detection kit (Roche Molecular Biochemicals, Basel, Switzerland) according to the manufacturer's protocol. Briefly, cells were harvested and washed, and then stained with annexin V and PI in binding buffer at room temperature for 15 min. Samples were detected with the Guava EasyCyte™ System. For each sample, 5000 cells were analysed. Data were analysed by using Guava TUNEL Software, both early apoptotic cells and late apoptotic cells were nominated as apoptotic cells (Guava Technologies, Hayward, CA, USA).
RT-PCR
Total RNA was extracted from cells using the TRIZOL reagent (Invitrogen). RT-PCR was carried out using the M-MLV reverse transcriptase (Invitrogen) with indicated primers. Primers for specific genes were listed below: EGF receptor forward, 5′- CCACCAAATTAGCCTGGACA-3′; EGF receptor reverse, 5′- CGCGACCCTTAGGTATTCTG-3′; GAPDH forward, 5′- CATATGGGGAAGGTGAAGGTCGGAGTC-3′; GAPDH reverse, 5′- GAATTCTTACTCCTTGGAGGCCATGTGG −3′. PCR was performed for 30 cycles in 25 μL reaction mixture at 94°C (30 s), 56°C (30 s) and 72°C (30 s), followed by a 10 min extension at 72°C. Final PCR products were separated on 1% agarose gels and stained with ethidium bromide.
Confocal microscopy
NCI-H1975 cells were seeded onto coverslips (BD Biosciences, San Jose, CA, USA) and then were treated with luteolin (50 μmol·L−1) or 17-DMAG (1 μmol·L−1) for 24 h. Cells were fixed with 4% paraformaldehyde and permeabilized with 0.2% Triton X-100. Monoclonal antibodies to the EGF receptor were applied for 12 h, followed by a 1 h incubation with secondary antibody conjugated with Texas Red (Invitrogen). Stress fibres were identified with FITC-conjugated phalloidin and nuclei were stained with DAPI (Invitrogen). After being washed with PBS, cells were observed under a laser scanning confocal microscope (A1; Nikon, Tokyo, Japan).
Wound-healing assay
NCI-H1975 cells were seeded into a six-well tissue culture dish and allowed to grow to 90% confluency in complete medium, and then injured with a pipette tip. The injured monolayers were washed three times to remove cell debris and incubated in 50 μmol·L−1 luteolin medium for 18 h. Cells were monitored under a microscope equipped with a camera (Nikon).
In vivo tumour growth and histochemical assay
Male nude mice (BALB/c nu/nu) (5–6 weeks old, 18–22 g) were obtained from Shanghai Slac laboratory animal corporation (Shanghai, China) and maintained in pathogen-free conditions. The nude mice were kept under conventional controlled conditions (22°C, 55% humidity and day-night rhythm) and had free access to a standard diet and tap water. Laboratory animal handling and experimental procedures were performed in accordance with the requirements of Provisions and General Recommendation of Chinese Experimental Animals Administration Legislation and were approved by Science and Technology Department of Jiangsu Province. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). For xenograft implantation, we injected NCI-H1975 cells (1 × 106 cells·0.1 mL per mouse) s.c. into the right front axilla of mice. The mice were randomly allocated into five groups and treatment was begun when the tumour volume reached 50–75 mm3. Mice in each group received luteolin (10 and 30 mg·kg−1), erlotinib (100 mg·kg−1), cisplatin (2 mg·kg−1) or vehicle (DMSO) by i.p. injection daily, respectively, for 15 days. Mice weight and tumour volume were measured daily from days 7 to 21. At the end of the experiment, tumour-bearing mice were anaesthetized, and the tumours were weighed after being separated from the surrounding muscles and dermis.
Tumours were fixed in 4% paraformaldehyde for 12 h. Following embedding in paraffin, 5 μm sections were cut. Sections were deparaffinized and rehydrated, and after quenching endogenous peroxidase activity and blocking non-specific binding sites, slides were incubated at 4°C for 12 h with 1:100 dilution of primary antibody directed against CD34, PCNA, EGF receptors and followed by a 30 min incubation with a secondary antibody. For the TUNEL assay, the reaction mixture was prepared by mixing TDT enzyme, buffer and substrate, which contained fluorescein-labelled dNTP. The sections were observed under optical microscope (Nikon, 200×).
Statistical analysis
One-way anova was used to compare the results between two groups. Data are presented as mean ± SD. A P value of <0.05 was regarded as a significant difference. Statistical calculations were performed by Statistical Package for the Social Sciences (SPSS) 13.0 software (SPSS, Chicago, IL, USA).
Results
Luteolin reduces the viability of NCI-H1975 cells
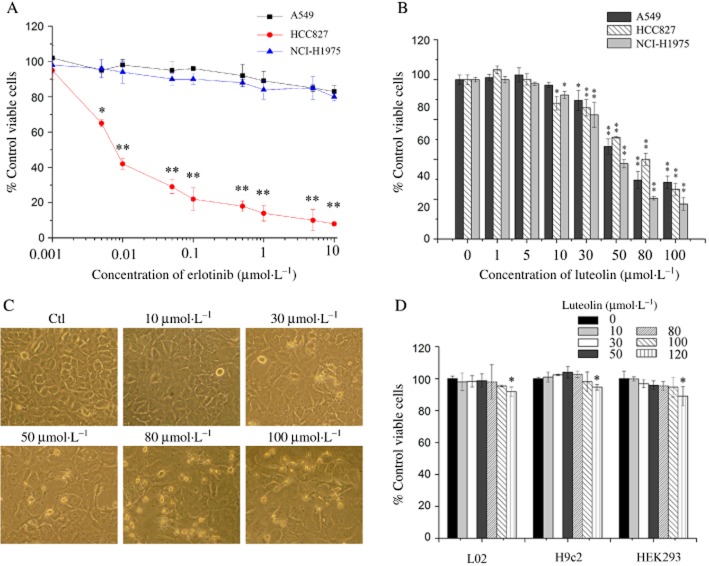
Three kinds of NSCLC cell lines, A549 (non-mutant EGF receptor), HCC827 (E746_A750 del) and NCI-H1975 (L858R/T790M), were used in the experiments. Erlotinib reduced HCC827 cell viability but did not affect the viability of A549 and NCI-H1975 cells (Figure 1A). In order to study the effect of luteolin on NSCLC cell growth, these three kinds of cells were treated with various doses of luteolin for 48 h and then their viabilities were determined by CCK-8 assay. The results showed that luteolin dose-dependently decreased the viabilities of all these three cells (Figure 1B) with IC50 values of 65.28, 70.38 and 55.87 μmol·L−1 respectively. The morphological observations demonstrated that NCI-H1975 cells had abundant cytoplasmic vacuoles after being incubated with luteolin for 48 h and they exhibited protuberant cytoplasmic blebs and progressive shrinkage when the concentration of luteolin was increased. As a result, cells shrank to a round configuration and detached from the flasks (Figure 1C), indicating that luteolin induced NCI-H1975 cell death. Because there is no effective therapeutic strategy for treating NSCLC with acquired resistance to EGF receptor TKIs, we mainly evaluated the effects of luteolin on T790M mutant NSCLC cells.
Figure 1.
Luteolin reduces the viability of NCI-H1975 cells. (A and B) A549, HCC827 and NCI-H1975 cells were treated with a range of concentrations of erlotinib (A) or luteolin (B) for 48 h. Cell viability is shown as relative viability compared to the untreated control. (C) NCI-H1975 cells were treated with luteolin at different concentrations for 48 h. Cell morphology was imaged under a Nikon microscope (200×). (D) L02, H9c2 and HEK293 cells were treated with the indicated concentrations of luteolin for 48 h. Cell viability was expressed as % of surviving cells compared to the control group. Each bar represents the mean ± SD of three independent experiments. *P < 0.05 and **P < 0.01.
It has been found that luteolin does not affect the viability of normal human gingival fibroblasts cells at high concentrations (Yang et al., 2008). Normal keratinocytes remained viable in the presence of at least 100 μmol·L−1 luteolin (Verschooten et al., 2012). To further confirm that luteolin had no toxic effects on other normal cells, the viability of L02, H9c2 and HEK293 cell lines was tested using the CCK-8 assay after a 48 h treatment with 0–120 μmol·L−1 luteolin. The results showed that the viability of these cells was not obviously reduced by luteolin treatment (Figure 1D).
Luteolin triggers apoptosis of NCI-H1975 cells
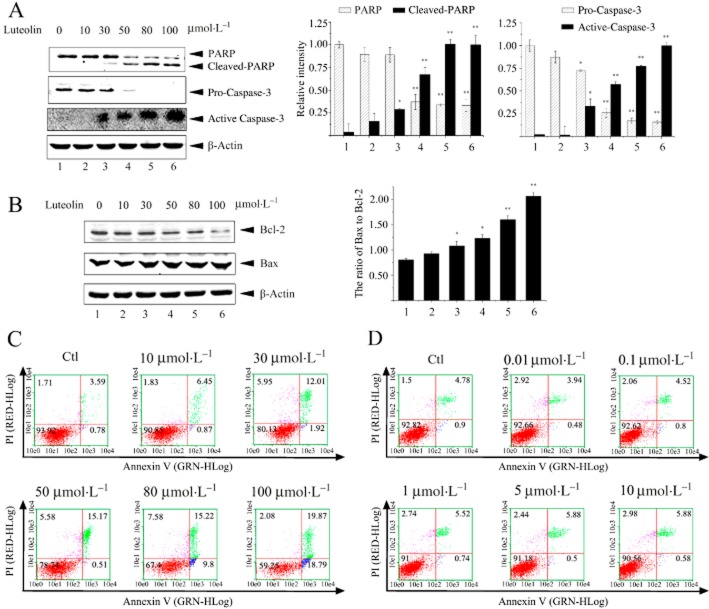
As luteolin induced NCI-H1975 cell death, we further determined whether luteolin caused apoptosis of cells. Caspase-3 is one of the crucial mediators of apoptosis, which frequently catalyses the specific cleavage of some key cellular proteins related to apoptosis, such as PARP, the hallmark of apoptosis (Amé et al., 2004). The results of immunoblotting showed that luteolin dose-dependently reduced the level of pro-caspase-3, and increased cleaved caspase-3 and PARP in NCI-H1975 cells (Figure 2A). We then measured the cellular level of Bcl-2, an anti-apoptotic protein and Bax, a pro-apoptotic protein. As shown in Figure 2B, luteolin dose-dependently raised the ratio of Bax to Bcl-2 by reducing the cellular level of Bcl-2 protein and slightly increasing the concentration of Bax. The bivariate FITC-annexin V/PI assay showed that the number of apoptotic NCI-H1975 cells increased after a 48 h incubation with luteolin. The rate of apoptosis was about 39% when the concentration of luteolin was raised to 100 μmol·L−1. As expected, erlotinib did not induce apoptosis of NCI-H1975 cells (Figure 2D). All these results indicate that luteolin induced NCI-H1975 cell death by promoting their apoptosis.
Figure 2.
Luteolin induces apoptosis of NCI-H1975 cells. (A and B) NCI-H1975 cells were treated with luteolin 0–100 μmol·L−1 for 24 h. Cell lysates were prepared and subjected to immunoblotting with anti-PARP, caspase-3 (A), Bcl-2 and BAX (B) antibodies respectively. β-Actin was used as an internal control. (C and D) NCI-H1975 cells were treated with the indicated concentrations of luteolin (C) or erlotinib (D) for 48 h. The apoptotic cells were assessed by annexin V-FITC/PI detection. The four quadrants denote non-apoptotic live cells (red), early apoptotic stage cells (blue), late apoptotic cells (green) and dead cells (purple). Similar results were obtained from three experiments.
Luteolin down-regulates EGF receptors in NCI-H1975 cells at the protein level
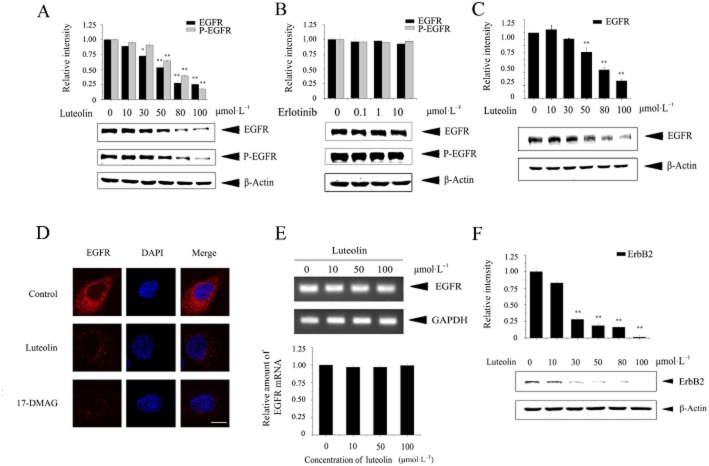
Our previous research indicated that luteolin could inhibit the protein chaperon activity of Hsp90 (Fu et al., 2012). Since the EGF receptor is a client protein of Hsp90, we next determined the effect of luteolin on the EGF receptor level in NCI-H1975 cells. As shown in Figure 3A, luteolin treatment resulted in a dose-dependent reduction in whole EGF receptor protein and in the level of phosphorylated EGF receptor, whereas such changes in EGF receptor levels were not detected in erlotinib-treated cells (Figure 3B). We also determined the effect of luteolin on the EGF receptor level in NCI-H820 (E746_T751del, T790M) cells, which were another kind of erlotinib-resistant and EGF receptor double-mutant NSCLC cell line. Similar results were obtained as with the NCI-H1975 cells; the EGF receptor protein level in NCI-H820 cells was dose-dependently decreased by luteolin treatment (Figure 3C). Further observations obtained by confocal scanning laser microscopy demonstrated a similar reduction in EGF receptor protein in NCI-H1975 cells treated with luteolin or 17-DMAG, one of the Hsp90 inhibitors (Figure 3D). If luteolin down-regulated the EGF receptor protein level by interacting with Hsp90, it would not affect the transcription of the EGF receptor. The RT-PCR results showed that luteolin treatment did not alter EGF receptor mRNA in NCI-H1975 cells (Figure 3E). Luteolin also down-regulated the protein level of ErbB2 (also known as HER2), a member of ErbB family and a client protein of Hsp90 (Figure 3F; Hirsch and Langer, 2004), indicating that luteolin reduced both EGF receptor and ErbB2 protein, which are important in NSCLC development.
Figure 3.
Luteolin down-regulates the mutant EGF receptor protein. (A and B) NCI-H1975 cells were treated with the indicated concentrations of luteolin (A) or erlotinib (B) for 24 h. Cell lysates were prepared and subjected to immunoblotting to measure the protein levels of EGF receptors and phospho-EGF receptors (Y1068) with respective antibodies and equal protein loading was confirmed by β-actin. (C) NCI-H820 cells were treated with the indicated concentrations of luteolin for 24 h. Cell lysates were prepared and subjected to immunoblotting with anti-EGF receptor and β-actin. (D) NCI-H1975 cells were treated with luteolin (50 μmol·L−1) or 17-DMAG (1 μmol·L−1) for 24 h. Cells were then fixed, permeabilized and incubated with anti-EGF receptor monoclonal primary antibody, and then stained with Texas Red conjugated goat anti-mouse IgG. Nuclei were stained with DAPI. Cells were observed under a confocal microscope (red: EGF receptor, blue: nuclei, Scale bar: 10 μm). (E) NCI-H1975 cells were treated with luteolin (0–100 μmol·L−1) for 24 h. Total RNA was isolated and EGF receptor mRNA was detected by RT-PCR. GAPDH was used as a control. (F) ErbB2 (HER2) protein levels were measured by immunoblotting after different concentrations of luteolin treatment for 24 h. Results shown are representative of three experiments.
Luteolin promotes degradation of the EGF receptor through the lysosome pathway
Since inhibition of Hsp90 will result in degradation of its client protein, we evaluated the effect of luteolin on the stability of the EGF receptor. NCI-H1975 cells were treated with luteolin or not in the presence of CHX, a protein synthesis inhibitor, and then were subjected to immunoblotting by using an EGF receptor antibody. The result showed that the EGF receptor level in cells decreased after luteolin treatment, revealing that luteolin reduced the stability of the EGF receptor (Figure 4A).
Figure 4.
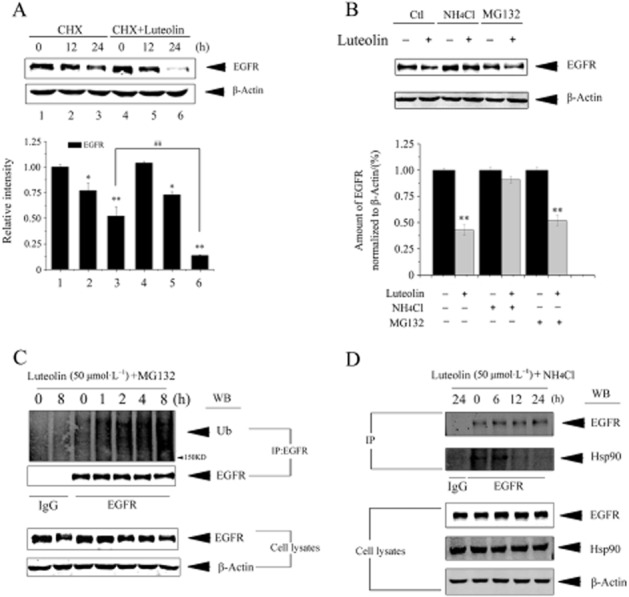
Luteolin promotes lysosome-dependent degradation of the EGF by inhibiting its interaction with Hsp90. (A) NCI-H1975 cells were cultured with 1 μg·mL−1 CHX in the presence or absence of 50 μmol·L−1 luteolin for the indicated time. EGF receptor and β-actin protein levels were assessed by immunoblot analysis. (B) NCI-H1975 cells were co-treated with 50 μmol·L−1 luteolin and NH4Cl (10 mmol·L−1) or MG132 (5 μmol·L−1) for 24 h. EGF receptor protein level was detected by immunoblotting. (C) NCI-H1975 cells were co-treated with 50 μmol·L−1 of luteolin and MG132 for 0–8 h. Cell lysates were subjected to immunoprecipitation with anti-EGF receptor antibody and analysed by immunoblotting with anti-ubiquitination antibody or anti-EGF receptor antibody. Results shown are representative of three experiments. (D) NCI-H1975 cells were treated with 50 μmol·L−1 luteolin for 24 h in presence of NH4Cl to block the lysosomal degradation of the EGF receptor. Cell lysates were subjected to immunoprecipitation with anti-EGF receptor antibody and analysed by immunoblotting with anti-Hsp90 antibody or anti-EGF receptor antibody.
It has been reported that degradation of the EGF receptor is mediated by lysosomal proteases (Haglund et al., 2003; Grøvdal et al., 2004) or proteasome proteases (Longva et al., 2002; Zhang et al., 2005). To investigate the molecular mechanism involved in the luteolin-induced down-regulation of the EGF receptor, the NCI-H1975 cells were treated with a representative lysosomal inhibitor, NH4Cl, or proteasome inhibitor, MG132, before the application of luteolin. We found that NH4Cl but not MG132 significantly blocked the reduction in EGF receptor protein induced by luteolin (Figure 4B). Furthermore, Figure 4C shows that luteolin brought about an ubiquitination of EGF receptor protein. These results strongly suggest that luteolin-induced EGF receptor protein degradation occurred in lysosomes by an ubiquitin-dependent manner. We next conducted an immunoprecipitation experiment to determine whethr luteolin interferes with the binding of EGF receptor to Hsp90 in presence of NH4Cl (blocks EGF receptor lysosomal degradation). As shown in Figure 4D, luteolin treatment decreased the Hsp90/EGF receptor complex but did not alter the Hsp90 level, suggesting that luteolin reduces the stability of the EGF receptor by inhibiting its interaction with Hsp90.
Luteolin suppresses EGF receptor/PI3K/Akt/mTOR signalling in NSCLC cells expressing T790M mutant EGF receptors
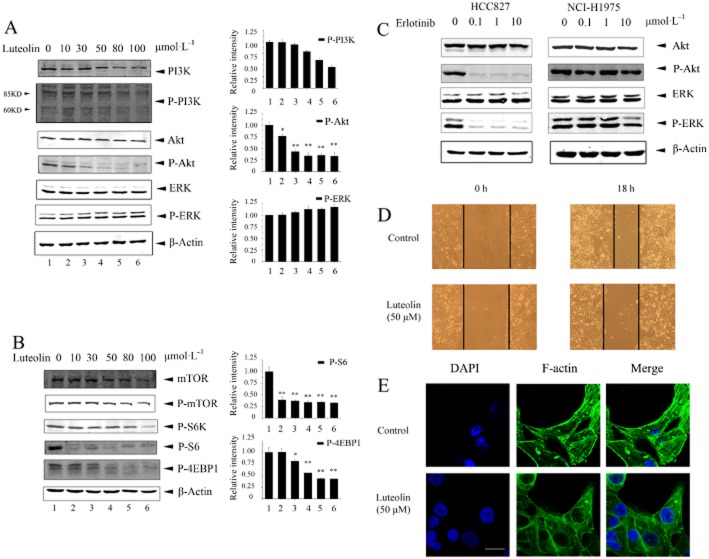
The Ras/Raf/MAPK/ERK and the PI3K/Akt/PTEN/mTOR are two important pathways downstream of EGF receptors (Jorissen et al., 2003; Normanno et al., 2006). To determine whether or not luteolin blocked these two pathways, NCI-H1975 cells were treated with luteolin and then were subjected to the immunoblot assay by using specific antibodies. As shown in Figure 5, luteolin suppressed activation of the PI3K/Akt/PTEN/mTOR pathway (Figure 5A and B), whereas it did not alter the activation of ERK. As a control, erlotinib significantly inhibited ERK1/2 and Akt in HCC827 cells but not in NCI-H1975 cells (Figure 5C).
Figure 5.
Luteolin prevents EGF receptor/PI3K/Akt/mTOR signalling in NCI-H1975 and inhibits cell migration. (A and B) Exponentially growing NCI-H1975 cells were treated with the indicated concentrations of luteolin for 24 h, and lysates were subjected to immunoblotting with the indicated antibodies. (C) HCC827 and NCI-H1975 cells were treated with a range of concentrations of erlotinib for 24 h. Cell lysates were subjected to immunoblotting by using the antibody to Akt, ERK1/2, p-Akt and p-ERK1/2. Equal loading of protein was confirmed by β-actin. (D) Migration of NCI-H1975 cells was evaluated using the scratch wound-healing assay in the absence and presence of 50 μmol·L−1 luteolin. Images of the results were obtained using a Nikon microscope (200×). (E) NCI-H1975 cells were stained with FITC-phalloidin, a substance that specifically detects F-actin. In the control group, stress fibres are present (white arrows). Results shown are representative of three experiments. Scale bar: 10 μm.
Luteolin prevents NCI-H1975 cell migration
Approximately 30–40% of patients with NSCLC die as a result of metastases. To test the effect of luteolin on NCI-H1975 cell migration, a wound-healing assay was employed as described in the Methods. As shown in Figure 5D, the ability of luteolin-treated cells to migrate was reduced compared with control cells. As cytoskeleton dynamics is essential for directional cell motility and migration (Vignjevic and Montagnac, 2008), we treated NCI-H1975 cells with luteolin and observed F-actin under a confocal scanning laser microscope. Without luteolin, the large F-actin bundles were widely dispersed passing through whole cells (Figure 5E), whereas luteolin treatment abolished the formation of these stress fibres, suggesting that luteolin inhibited the migration of NCI-H1975 cells at least in part by disrupting actin assembly and the formation of stress fibres.
Luteolin inhibits the growth of lung adenocarcinoma xenografts in vivo
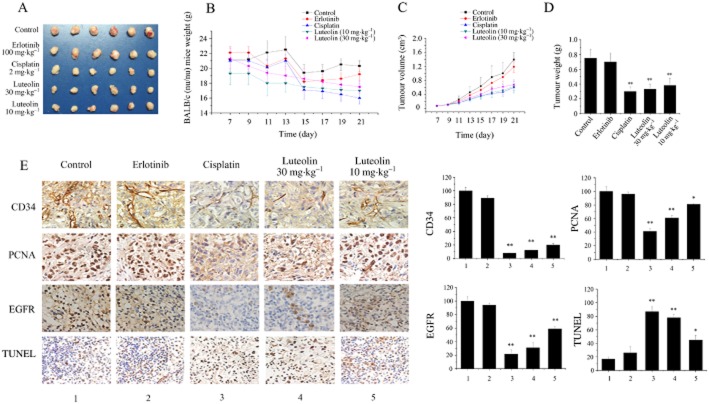
To further evaluate the anti-tumour effects of luteolin, we performed an in vivo study using a nude mouse xenograft model obtained by s.c. inoculation of NCI-H1975 cells. After the establishment of solid tumours, luteolin, erlotinib, cisplatin or vehicle was administered daily respectively. We found that the growth of NCI-H1975 xenograft tumours was inhibited significantly by administration of luteolin and cisplatin to the mice (Figure 6A). As indicated in Figure 6B, there was no loss of body weight or other sign of toxicity manifested following luteolin administration. However, a substantial weight loss (≥20% of the body weight at start of treatment) was observed in the cisplatin-treated mice. On day 21, the volume of the NCI-H1975 xenograft tumours showed reductions of 51.3, 54.9 and 57.1% in mice treated with luteolin (10 or 30 mg·kg−1) or cisplatin (2 mg·kg−1), respectively, compared with control and erlotinib-treated animals (Figure 6C). In addition, luteolin and cisplatin also significantly reduced the weight of the xenograft tumours; the rates of inhibition were 49.4, 56.4 and 59.5% respectively (Figure 6D).
Figure 6.
Luteolin inhibits the growth of lung adenocarcinoma xenografts with exons 19 and 20 mutations. (A) Tumours from xenograft-implanted mice. Thirty mice were randomly divided into five groups (n = 6 mice per group). The treatment groups of mice were administered (i.p.) with luteolin (10 and 30 mg·kg−1), cisplatin (2 mg·kg−1) and erlotinib (100 mg·kg−1) respectively. The negative control group was injected with an equivalent volume of solvent. Treatment was begun 8 days after implantation and continued daily throughout the experiment. At the end of the 15 day treatment, mice were anaesthetized and tumours were taken out. (B) The body weights of mice were measured daily from the beginning of luteolin treatment. (C) Tumour volumes were detected daily by using a calliper. (D) At the end of the experiment, mice were anaesthetized, and the tumours were harvested and weighed. Data represent mean ± SD (n = 6). **P < 0.01. (E) Tumours were subjected to paraffin section and immunohistochemical staining for CD34, PCNA and EGF receptors was performed. Primary antibody specificities are as labelled in each panel. Immunodetectable protein is indicated by a brown stain, while the counterstain colour of nuclei is blue. The bottom panel is the TUNEL result and TUNEL-positive cells have brown staining.
Immunohistochemical detection demonstrated that tumours in mice treated with luteolin and cisplatin had a significant and progressive decrease in CD34 (the microvessel density marker), PCNA (the cell proliferation marker), as well as EGF receptors. The results from the TUNEL assay indicated that there are more apoptotic cells in tumours treated with luteolin than those in tumours treated with solvent.
Discussion
The overexpression of EGF receptors in several epithelial tumours including NSCLC is a predictor of low survival rate, poor prognosis and metastasis. In recent years, targeted therapy with small-molecule EGF receptor TKIs such as gefitinib and erlotinib has receive much attention (Kris et al., 2003; Pérez-Soler et al., 2004; Cataldo et al., 2011). The mutant EGF receptor T790M is the most common mechanism of acquired resistance to gefitinib and erlotinib, and typically emerges within 9–13 months after initiating therapy. Although several different types of agents inhibiting the formation of the mutant T790M EGF receptor are currently in development, negative aspects of these drugs has meant that further applications for them are limited. Luteolin has been shown to possess strong anti-proliferative, anti-inflammatory and antioxidant activity in different human cancer cell lines (Knowles et al., 2000; Seelinger et al., 2008a,b). In the present study, we demonstrated that luteolin reduced the viability of three subtypes of NSCLC cells, including the T790M mutant NSCLC cells, and did not affect the viability of L02, H9c2 and HEK293 cell lines. The in vivo study also showed that luteolin inhibited the growth of TKI-resistant NSCLC xenografts in immunodeficient mice. Unlike cisplatin, luteolin did not affect mice body weight and normal food intake. These results imply that luteolin is relatively safe when used as an anti-cancer agent. But a major barrier to the clinical application of luteolin is its low systemic bioavailability, due to its extensive metabolism by the liver and intestine (Shimoi et al., 1998; Zhou et al., 2008; Chen et al., 2012). However, we may be able to improve the bioavailability of luteolin through structure modification or drug combination. CD34 is considered to be a marker of microvessel density in tumours and luteolin has been shown to suppress the microvessel density in prostate cancer tissue (Pratheeshkumar et al., 2012). PCNA was originally identified as an antigen that is expressed in the nuclei of cells during the DNA synthesis phase of the cell cycle. Immunohistochemical results that showed that luteolin reduced CD34, PCNA and EGF receptors in the tumours and the TUNEL assay revealed that it also increased apoptosis of the tumour cells, which suggest that luteolin has a therapeutic effect on TKI-resistant NSCLC. Cancer cells may be particularly dependent on chaperone proteins to survive in a hypoxic, nutrient-starved microenvironment (Sequist et al., 2010), which makes them particularly sensitive to Hsp90 inhibition. Results from our previous study indicated that luteolin inhibited the interaction of Hsp90 with its client STAT3 and induced apoptosis of HeLa cells (Fu et al., 2012). It has also been reported that inhibition of Hsp90 activity suppresses the mutant EGF receptor-T790M signalling and overcomes kinase inhibitor resistance (Shimamura et al., 2008), and that mutant EGF receptors in NSCLC lines are more sensitive to Hsp90 inhibitors than wild-type EGF receptors (Sawai et al., 2008). In accord with these results, we found that luteolin only slightly decreased EGF receptor protein levels in A549 cells (data not shown) and the viability of A549 cells compared with its effects in NCI-H1975 cells. This suggests that luteolin has only a moderate anti-cancer effect on A549 cells.
In the present study, we demonstrated that luteolin prevented the binding of Hsp90 to mutant EGF receptors, and caused lysosomal protease-dependent degradation of EGF receptors. However, it also induced a marked accumulation of ubiquitinated EGF receptors, which is associated with proteasomal activity. Consistent with this finding is the fact that the down-regulation of EGF receptors is a complex and tightly regulated process (Alwan et al., 2003). When compared with 17-DMAG, a widely studied Hsp90 inhibitor, we found that luteolin acted in a similar manner, down-regulating EGF receptor protein. However, 17-DMAG has not been used as an anti-cancer drug clinically because it is severely toxic at high doses. In contrast, luteolin is a natural antioxidant with a better safety profile and may offer a new therapeutic strategy for treating NSCLC with mutant EGF receptors.
There are two major downstream signalling routes of EGF receptors, which includes the Ras/Raf/MAPK pathway for cell proliferation and PI3K/Akt/mTOR pathway for cell survival. We found that luteolin-induced degradation of mutant EGF receptors mainly influenced the PI3K/Akt/mTOR rather than the Ras/Raf/MAPK/ERK pathway. It reduced the activity of the PI3K/Akt/mTOR, which meant that the apoptosis signalling prevailed. Thus it is not surprising that luteolin weakened the viability of the NCI-H1975 cells and triggered apoptosis of these cells. Since the PI3K/Akt/mTOR pathway has been reported to be activated persistently in EGF receptor-mutant cells (Jorissen et al., 2003; Normanno et al., 2006), EGF receptor degradation will prevent its signalling. The Ras/Raf/MAPK/ERK pathway is also activated by a series of kinases, such as VEGF and PDGF, in addition to the EGF receptor. Uniquely, sorafenib, a small molecular inhibitor of several tyrosine PKs (VEGFR and PDGFR) and Raf kinases, has been found to inhibit the Ras/Raf/MAPK/ERK pathway, while increasing Akt phosphorylation (Gedaly et al., 2010). Several studies have shown that luteolin can activate the ERK MAPK pathway in different kinds of cells (Choi et al., 2008; Tufekci et al., 2011; Kim et al., 2012). Thus it is reasonable to assume that luteolin, by targeting the EGF receptor, slightly increased the activation of Ras/Raf/MAPK/ERK pathway in this double-mutant NSCLC cell. In addition, the present study also showed that luteolin disrupted the formation of stress fibres, suggesting that luteolin could prevent the migration of NCI-H1975 cells.
In conclusion, the present study showed that luteolin, a natural flavonoid, exhibits remarkable anti-tumourigenic activity in EGF receptor L858R/T790M mutant and erlotinib-resistant NSCLC both in vivo and in vitro. Luteolin induced degradation of the mutant EGF receptors by inhibiting their association with Hsp90, which consequently resulted in NSCLC cell apoptosis. Our findings indicate that luteolin has potential as a candidate for NSCLC therapy and may be effective in treating acquired erlotinib-resistant NSCLC.
Acknowledgments
The authors thank Xuegen Wang for helping with the animal experiments.
Glossary
- 17-DMAG
17-dimethylaminoethylamino-17-demethoxygeldanamycin
- CCK-8
Cell Counting Kit-8
- CHX
cycloheximide
- Hsp90
heat shock protein 90
- NSCLC
non-small cell lung cancer
- TKI
tyrosine kinase inhibitor
Conflict of interest
This work was supported by grants from the National Nature Science Foundation of China (Nos. 81072433, 81172798, 31071000 and J1103512), and a project funded by the Priority Academic Program Development of Jiangsu Higher Education Institutions (No. 164320H106).
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: Catalytic receptors. Br J Pharmacol. 2013;170:1676–1705. doi: 10.1111/bph.12449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alwan HA, van Zoelen EJ, van Leeuwen JE. Ligand-induced lysosomal epidermal growth factor receptor (EGFR) degradation is preceded by proteasome-dependent EGFR de-ubiquitination. J Biol Chem. 2003;278:35781–35790. doi: 10.1074/jbc.M301326200. [DOI] [PubMed] [Google Scholar]
- Amé JC, Spenlehauer C, De Murcia G. The PARP superfamily. Bioessays. 2004;26:882–893. doi: 10.1002/bies.20085. [DOI] [PubMed] [Google Scholar]
- Balius TE, Rizzo RC. Quantitative prediction of fold resistance for inhibitors of EGFR. Biochemistry. 2009;48:8435–8448. doi: 10.1021/bi900729a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cataldo VD, Gibbons DL, Pérez-Soler R, Quintás-Cardama A. Treatment of non-small-cell lung cancer with erlotinib or gefitinib. New Engl J Med. 2011;364:947–955. doi: 10.1056/NEJMct0807960. [DOI] [PubMed] [Google Scholar]
- Chen Z, Tu M, Sun S, Kong S, Wang Y, Ye J, et al. The exposure of luteolin is much lower than that of apigenin in oral administration of Flos Chrysanthemi extract to rats. Drug Metab Pharmacokinet. 2012;27:162–168. doi: 10.2133/dmpk.dmpk-11-rg-081. [DOI] [PubMed] [Google Scholar]
- Choi B-M, Lim D-W, Lee J-A, Gao SS, Kwon DY, Kim B-R. Luteolin suppresses cisplatin-induced apoptosis in auditory cells: possible mediation through induction of heme oxygenase-1 expression. J Med Food. 2008;11:230–236. doi: 10.1089/jmf.2007.591. [DOI] [PubMed] [Google Scholar]
- Engelman JA, Zejnullahu K, Gale CM, Lifshits E, Gonzales AJ, Shimamura T, et al. PF00299804, an irreversible pan-ERBB inhibitor, is effective in lung cancer models with EGFR and ERBB2 mutations that are resistant to gefitinib. Cancer Res. 2007;67:11924–11932. doi: 10.1158/0008-5472.CAN-07-1885. [DOI] [PubMed] [Google Scholar]
- Fu J, Chen D, Zhao B, Zhao Z, Zhou J, Xu Y, et al. Luteolin induces carcinoma cell apoptosis through binding Hsp90 to suppress constitutive activation of STAT3. PLoS ONE. 2012;7:e49194. doi: 10.1371/journal.pone.0049194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gábor M. Anti-inflammatory and anti-allergic properties of flavonoids. Prog Clin Biol Res. 1986;213:471–480. [PubMed] [Google Scholar]
- Gedaly R, Angulo P, Hundley J, Daily MF, Chen C, Koch A, et al. PI-103 and sorafenib inhibit hepatocellular carcinoma cell proliferation by blocking Ras/Raf/MAPK and PI3K/AKT/mTOR pathways. Anticancer Res. 2010;30:4951–4958. [PMC free article] [PubMed] [Google Scholar]
- Gerber DE. EGFR inhibition in the treatment of non-small cell lung cancer. Drug Dev Res. 2008;69:359–372. doi: 10.1002/ddr.20268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grøvdal LM, Stang E, Sorkin A, Madshus IH. Direct interaction of Cbl with pTyr 1045 of the EGF receptor (EGFR) is required to sort the EGFR to lysosomes for degradation. Exp Cell Res. 2004;300:388–395. doi: 10.1016/j.yexcr.2004.07.003. [DOI] [PubMed] [Google Scholar]
- Haglund K, Sigismund S, Polo S, Szymkiewicz I, Di Fiore PP, Dikic I. Multiple monoubiquitination of RTKs is sufficient for their endocytosis and degradation. Nat Cell Biol. 2003;5:461–466. doi: 10.1038/ncb983. [DOI] [PubMed] [Google Scholar]
- Herbst RS. Review of epidermal growth factor receptor biology. Int J Radiat Oncol Biol Phys. 2004;59:S21–S26. doi: 10.1016/j.ijrobp.2003.11.041. [DOI] [PubMed] [Google Scholar]
- Hirsch FR, Langer CJ. The role of HER2/neu expression and trastuzumab in non-small cell lung cancer. Semin Oncol. 2004;31:75–82. doi: 10.1053/j.seminoncol.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Hirsch FR, Varella-Garcia M, Bunn PA, Di Maria MV, Veve R, Bremnes RM, et al. Epidermal growth factor receptor in non-small-cell lung carcinomas: correlation between gene copy number and protein expression and impact on prognosis. J Clin Oncol. 2003;21:3798–3807. doi: 10.1200/JCO.2003.11.069. [DOI] [PubMed] [Google Scholar]
- Jemal A, Siegel R, Ward E, Hao Y, Xu J, Murray T, et al. Cancer statistics, 2008. CA Cancer J Clin. 2008;58:71–96. doi: 10.3322/CA.2007.0010. [DOI] [PubMed] [Google Scholar]
- Jorissen RN, Walker F, Pouliot N, Garrett TPJ, Ward CW, Burgess AW. Epidermal growth factor receptor: mechanisms of activation and signalling. Exp Cell Res. 2003;284:31–53. doi: 10.1016/s0014-4827(02)00098-8. [DOI] [PubMed] [Google Scholar]
- Kalyankrishna S, Grandis JR. Epidermal growth factor receptor biology in head and neck cancer. J Clin Oncol. 2006;24:2666–2672. doi: 10.1200/JCO.2005.04.8306. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim ES, Khuri FR, Herbst RS. Epidermal growth factor receptor biology (IMC-C225) Curr Opin Oncol. 2001;13:506–513. doi: 10.1097/00001622-200111000-00014. [DOI] [PubMed] [Google Scholar]
- Kim MJ, Woo JS, Kwon CH, Kim JH, Kim YK, Kim KH. Luteolin induces apoptotic cell death through AIF nuclear translocation mediated by activation of ERK and p38 in human breast cancer cell lines. Cell Biol Int. 2012;36:339–344. doi: 10.1042/CBI20110394. [DOI] [PubMed] [Google Scholar]
- Knowles LM, Zigrossi DA, Tauber RA, Hightower C, Milner JA. Flavonoids suppress androgen-independent human prostate tumor proliferation. Nutr Cancer. 2000;38:116–122. doi: 10.1207/S15327914NC381_16. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Boggon TJ, Dayaram T, Jänne PA, Kocher O, Meyerson M, et al. EGFR mutation and resistance of non-small-cell lung cancer to gefitinib. New Engl J Med. 2005;352:786–792. doi: 10.1056/NEJMoa044238. [DOI] [PubMed] [Google Scholar]
- Kris MG, Natale RB, Herbst RS, Lynch TJ, Jr, Prager D, Belani CP, et al. Efficacy of gefitinib, an inhibitor of the epidermal growth factor receptor tyrosine kinase, in symptomatic patients with non-small cell lung cancer. JAMA. 2003;290:2149–2158. doi: 10.1001/jama.290.16.2149. [DOI] [PubMed] [Google Scholar]
- Kwak EL, Sordella R, Bell DW, Godin-Heymann N, Okimoto RA, Brannigan BW, et al. Irreversible inhibitors of the EGF receptor may circumvent acquired resistance to gefitinib. Proc Natl Acad Sci U S A. 2005;102:7665–7670. doi: 10.1073/pnas.0502860102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li D, Shimamura T, Ji H, Chen L, Haringsma HJ, McNamara K, et al. Bronchial and peripheral murine lung carcinomas induced by T790M-L858R mutant EGFR respond to HKI-272 and rapamycin combination therapy. Cancer Cell. 2007;12:81–93. doi: 10.1016/j.ccr.2007.06.005. [DOI] [PubMed] [Google Scholar]
- Longva KE, Blystad FD, Stang E, Larsen AM, Johannessen LE, Madshus IH. Ubiquitination and proteasomal activity is required for transport of the EGF receptor to inner membranes of multivesicular bodies. J Cell Biol. 2002;156:843–854. doi: 10.1083/jcb.200106056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Middleton E, Kandaswami C, Theoharides TC. The effects of plant flavonoids on mammalian cells: implications for inflammation, heart disease, and cancer. Pharmacol Rev. 2000;52:673–751. [PubMed] [Google Scholar]
- Nicholson R, Gee J, Harper M. EGFR and cancer prognosis. Eur J Cancer. 2001;37:9–15. doi: 10.1016/s0959-8049(01)00231-3. [DOI] [PubMed] [Google Scholar]
- Normanno N, De Luca A, Bianco C, Strizzi L, Mancino M, Maiello MR, et al. Epidermal growth factor receptor (EGFR) signaling in cancer. Gene. 2006;366:2–16. doi: 10.1016/j.gene.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Pao W, Miller VA, Politi KA, Riely GJ, Somwar R, Zakowski MF, et al. Acquired resistance of lung adenocarcinomas to gefitinib or erlotinib is associated with a second mutation in the EGFR kinase domain. PLoS Med. 2005;2:e73. doi: 10.1371/journal.pmed.0020073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pérez-Soler R, Chachoua A, Hammond LA, Rowinsky EK, Huberman M, Karp D, et al. Determinants of tumor response and survival with erlotinib in patients with non-small-cell lung cancer. J Clin Oncol. 2004;22:3238–3247. doi: 10.1200/JCO.2004.11.057. [DOI] [PubMed] [Google Scholar]
- Pratheeshkumar P, Son Y-O, Budhraja A, Wang X, Ding S, Wang L, et al. Luteolin inhibits human prostate tumor growth by suppressing vascular endothelial growth factor receptor 2-mediated angiogenesis. PLoS ONE. 2012;7:e52279. doi: 10.1371/journal.pone.0052279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawai A, Chandarlapaty S, Greulich H, Gonen M, Ye Q, Arteaga CL, et al. Inhibition of Hsp90 down-regulates mutant epidermal growth factor receptor (EGFR) expression and sensitizes EGFR mutant tumors to paclitaxel. Cancer Res. 2008;68:589–596. doi: 10.1158/0008-5472.CAN-07-1570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seelinger G, Merfort I, Schempp CM. Anti-oxidant, anti-inflammatory and anti-allergic activities of luteolin. Planta Med. 2008a;74:1667–1677. doi: 10.1055/s-0028-1088314. [DOI] [PubMed] [Google Scholar]
- Seelinger G, Merfort I, Wölfle U, Schempp CM. Anti-carcinogenic effects of the flavonoid luteolin. Molecules. 2008b;13:2628–2651. doi: 10.3390/molecules13102628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sequist LV, Gettinger S, Senzer NN, Martins RG, Jänne PA, Lilenbaum R, et al. Activity of IPI-504, a novel heat-shock protein 90 inhibitor, in patients with molecularly defined non-small-cell lung cancer. J Clin Oncol. 2010;28:4953–4960. doi: 10.1200/JCO.2010.30.8338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimamura T, Lowell AM, Engelman JA, Shapiro GI. Epidermal growth factor receptors harboring kinase domain mutations associate with the heat shock protein 90 chaperone and are destabilized following exposure to geldanamycins. Cancer Res. 2005;65:6401–6408. doi: 10.1158/0008-5472.CAN-05-0933. [DOI] [PubMed] [Google Scholar]
- Shimamura T, Li D, Ji H, Haringsma HJ, Liniker E, Borgman CL, et al. Hsp90 inhibition suppresses mutant EGFR-T790M signaling and overcomes kinase inhibitor resistance. Cancer Res. 2008;68:5827–5838. doi: 10.1158/0008-5472.CAN-07-5428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimoi K, Okada H, Furugori M, Goda T, Takase S, Suzuki M, et al. Intestinal absorption of luteolin and luteolin 7-O-β-glucoside in rats and humans. FEBS Lett. 1998;438:220–224. doi: 10.1016/s0014-5793(98)01304-0. [DOI] [PubMed] [Google Scholar]
- Sos ML, Rode HB, Heynck S, Peifer M, Fischer F, Klüter S, et al. Chemogenomic profiling provides insights into the limited activity of irreversible EGFR inhibitors in tumor cells expressing the T790M EGFR resistance mutation. Cancer Res. 2010;70:868–874. doi: 10.1158/0008-5472.CAN-09-3106. [DOI] [PubMed] [Google Scholar]
- Tufekci KU, Civi Bayin E, Genc S, Genc K. The Nrf2/ARE pathway: a promising target to counteract mitochondrial dysfunction in Parkinson's disease. Parkinsons Dis. 2011;2011:314082. doi: 10.4061/2011/314082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verschooten L, Barrette K, Van Kelst S, Romero NR, Proby C, De Vos R, et al. Autophagy inhibitor chloroquine enhanced the cell death inducing effect of the flavonoid luteolin in metastatic squamous cell carcinoma cells. PLoS ONE. 2012;7:e48264. doi: 10.1371/journal.pone.0048264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vignjevic D, Montagnac G. Reorganisation of the dendritic actin network during cancer cell migration and invasion. Semin Cancer Biol. 2008;18:12–22. doi: 10.1016/j.semcancer.2007.08.001. [DOI] [PubMed] [Google Scholar]
- Yang SF, Yang WE, Chang HR, Chu SC, Hsieh YS. Luteolin induces apoptosis in oral squamous cancer cells. J Dent Res. 2008;87:401–406. doi: 10.1177/154405910808700413. [DOI] [PubMed] [Google Scholar]
- Yun CH, Mengwasser KE, Toms AV, Woo MS, Greulich H, Wong KK, et al. The T790M mutation in EGFR kinase causes drug resistance by increasing the affinity for ATP. Proc Natl Acad Sci U S A. 2008;105:2070–2075. doi: 10.1073/pnas.0709662105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B, Srirangam A, Potter DA, Roman A. HPV16 E5 protein disrupts the c-Cbl–EGFR interaction and EGFR ubiquitination in human foreskin keratinocytes. Oncogene. 2005;24:2585–2588. doi: 10.1038/sj.onc.1208453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Chen W, Kong L, Li L, Lu F, Li G, et al. An analysis of cancer incidence and mortality from 30 cancer registries in China, 1998–2002. Bull Chin Cancer. 2006;15:430–448. [Google Scholar]
- Zhou P, Li LP, Luo SQ, Jiang HD, Zeng S. Intestinal absorption of luteolin from peanut hull extract is more efficient than that from individual pure luteolin. J Agric Food Chem. 2008;56:296–300. doi: 10.1021/jf072612+. [DOI] [PubMed] [Google Scholar]