Abstract
Background and Purpose
Intercellular communication via gap junctions, comprised of connexin (Cx) proteins, allow for communication between astrocytes, which in turn is crucial for maintaining CNS homeostasis. The expression of Cx43 is decreased in post-mortem brains from patients with major depression. A potentially novel mechanism of tricyclic antidepressants is to increase the expression and functioning of gap junctions in astrocytes.
Experimental Approach
The effect of amitriptyline on the expression of Cx43 and gap junction intercellular communication (GJIC) in rat primary cultured cortical astrocytes was investigated. We also investigated the role of p38 MAPK intracellular signalling pathway in the amitriptyline-induced expression of Cx43 and GJIC.
Key Results
Treatment with amitriptyline for 48 h significantly up-regulated Cx43 mRNA, protein and GJIC. The up-regulation of Cx43 was not monoamine-related since noradrenaline, 5-HT and dopamine did not induce Cx43 expression and pretreatment with α- and β-adrenoceptor antagonists had no effect. Intracellular signalling involved p38 MAPK, as amitriptyline significantly increased p38 MAPK phosphorylation and Cx43 expression and GJIC were significantly blocked by the p38 inhibitor SB 202190. Furthermore, amitriptyline-induced Cx43 expression and GJIC were markedly reduced by transcription factor AP-1 inhibitors (curcumin and tanshinone IIA). The translocation of c-Fos from the cytosol and the nucleus of cortical astrocytes was increased by amitriptyline, and this response was dependent on p38 activity.
Conclusion and Implication
These findings indicate a novel mechanism of action of amitriptyline through cortical astrocytes, and further suggest that targeting this mechanism could lead to the development of a new class of antidepressants.
Keywords: amitriptyline, antidepressants, AP-1, astrocyte, connexin43, gap junction, major depressive disorder, p38
Introduction
Emerging evidence has demonstrated that glial cells have key roles in the pathophysiology of major depressive disorder (Rajkowska and Miguel-Hidalgo, 2007; Banasr and Duman, 2008). Recent studies have shown that the density and number of glial cells were decreased in several cortical areas of post-mortem brains of patients with major depressive disorder (Ongür et al., 1998; Rajkowska et al., 1999; Müller et al., 2001). The decrease in glial density was associated in particular with a reduction in glial fibrillary acidic protein, a marker for astrocytes, suggesting that astroglial activity might be a crucial component in the pathogenesis of major depressive disorder.
Recent reports have emphasized the importance of intercellular communication in maintaining the function of the CNS in terms of homeostatic and metabolic support (Giaume et al., 2010; Pannasch and Rouach, 2013). In particular, gap junctions, channels that connect cells to neighbouring cells, provide for direct intercellular communication (Theis et al., 2005). A gap junction consists of two connexons, each of which is composed of six connexin (Cx) proteins. Eleven Cxs have been identified in various cell types in the CNS, and of these, Cx43 is the most ubiquitously expressed and predominantly found in astrocytes (Giaume and Theis, 2010; Rash et al., 2012). The disturbance of cell communication via gap junctions could lead to impaired synaptic transmission in the CNS (Pannasch et al., 2011; Pannasch and Rouach, 2013). It has been demonstrated that the alteration of Cx43 expression in astrocytes is closely related to the initiation of various neuropathological disorders, including epilepsy, ischaemia, Parkinson's disease and Huntington's disease (Vis et al., 1998; Fonseca et al., 2002; Nakase et al., 2006; Kawasaki et al., 2009). In addition, a recent study indicates that the disturbance of cell communication through the downregulation of Cx43-gap junction in the rat prefrontal cortex induces depressive-like behaviour (Sun et al., 2012). The expression level of Cx43 mRNA was reduced in post-mortem brains from patients with major depression disorder compared with those from subjects without a history of psychiatric illness (Bernard et al., 2011). Thus, the modulation of Cx43-gap junction function could be an effective approach to treating major depressive disorder.
The generally accepted mechanism of the effect of antidepressants in ameliorating affective disorders is through increasing synaptic levels of monoamines, such as NA and 5-HT, by inhibiting their re-uptake by monoaminergic neurons. However, recent studies indicate the possibility that antidepressants could have a number of pharmacological actions distinct from inhibition of monoamine transporters, which, upon elucidation, could lead to therapeutics for major depressive disorder with novel mechanisms. For example, it was found that treatment with the prototypical tricyclic antidepressant amitriptyline induces the production of neurotrophic/growth factors, both in vivo and in vitro, and these increases could be associated with the efficacy of antidepressant drugs for major depressive disorder (Mallei et al., 2002; Hisaoka et al., 2011; Kajitani et al., 2012). Furthermore, it has been recently observed that chronic treatment with either fluoxetine or duloxetine, antidepressants that selectively inhibit the re-uptake of 5-HT and both NA/5-HT, respectively, reversed the reduction of Cx43-gap junction function due to chronic unpredictable stress, a well-characterized rodent model of depression (Sun et al., 2012). Although improvements in intracellular communication could be a significant source of the therapeutic effect of antidepressants on major depressive disorder, the mechanisms underlying the regulation of Cx43-gap junction function by antidepressants in astrocytes is largely unknown. Thus, to clarify this point, in the current study we investigated the effect of amitriptyline on the expression of Cx43 and the subsequent gap junction intercellular communication (GJIC) in rat cultured cortical astrocytes. Moreover, the specific intracellular signalling cascade involved in the expression of Cx43 in cortical astrocytes following amitriptyline treatment was also examined.
The receptor nomenclature used in this manuscript accords with BJP's Guide to Pharmacology (Alexander et al., 2013).
Methods
Cell culture
The preparation of cultured cortical astrocytes has been described previously (Sugimoto et al., 2011; Kajitani et al., 2012). All experimental procedures and animal handling were performed according to both the Guiding Principles for the Care and Use of Laboratory Animals approval by the Japanese Pharmacological Society and the guidelines of Hiroshima University (Hiroshima, Japan). All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010). In brief, the cerebral cortex isolated from neonatal Wistar rats were minced, and then incubated with trypsin and DNase I. Dissociated cells were suspended in DMEM supplemented with 10% fetal calf serum (FCS) and penicillin/streptomycin (100 units·mL−1 and 100 μg·mL−1 respectively). Thereafter, cell suspensions were plated in 75 cm2 tissue culture flasks (7.5–10 × 106 cells per flask) precoated with poly-L-lysine (10 μg·mL−1). Cells were maintained in a 10% CO2 incubator at 37°C. After 8–12 days, the growth flasks containing mixed glial cells were shaken by a rotary shaker at 100 rpm for 15 h, and washed with PBS to remove neuronal and microglial cells. Adherent cells were trypsinized (0.25%) and again seeded to new flasks. After reached confluence (about 10 days), the cells were vigorously shaken by hand. Adherent cells were trypsinized (0.25%) and plated onto 35 mm diameter dishes (0.9 × 106 cells). After 3 days, the medium was replaced with DMEM without FCS and antibiotics. After an additional 24 h of incubation, cells were used for experiments. Most, if not all, of the cells obtained using the current method were astrocytes, as described in our previous report.
Western blot analysis
Cells were solubilized in radioimmunoprecipitation assay buffer with inhibitors (100 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1 mM EDTA, 1% Triton X-100, 1% sodium deoxycholate, 0.1% SDS, 20 μg·mL−1 aprotinin, 20 μg·mL−1 leupeptin, 1 mM PMSF and phosphatase inhibitor cocktail 2 (Nacalai Tesque, Kyoto, Japan). The lysates were centrifuged at 14 000× g for 10 min at 4°C and the supernatant was added to Laemli's buffer and boiled for 5 min. Equal amounts of protein were separated by 10% SDS-PAGE and blotted onto nitrocellulose or PVDF membranes. Non-specific binding was reduced with blocking buffer, and the membranes were subsequently incubated with a purified polyclonal antibody against rat Cx43, phospho-p38, and total-p38 (1:1000, Cell Signaling Technology, Beverly, MA, USA), c-Fos (1:1000, Santa Cruz Biotechnology, Santa Cruz, CA, USA), Oct-1 (1:1000, Santa Cruz Biotechnology), heat shock protein 90 (HSP90; 1:1000, Santa Cruz Biotechnology) or monoclonal antibody against β-actin (1:10 000; Sigma Chemical Co., St. Louis, MO, USA) overnight at 4°C. After being washed, the membranes were incubated with a horseradish peroxidase-conjugated secondary antibody (Santa Cruz Biotechnology) for 1 h at room temperature. Then, membranes were rinsed and incubated with Luminescence reagent (Thermo Fisher Scientific, Rockford, IL, USA). Finally, the membranes were exposed to X-ray film. For quantification of signals, the densities of specific bands were measured with Science Lab Image Gauge (Fuji Film, Tokyo, Japan).
Preparation of nuclear and cytosolic protein
The separation of nuclear and cytosolic protein fraction in cultured cortical astrocytes was performed with an NE-PER Cytoplasmic and Nuclear Protein extraction kit (Thermo Fisher Scientific) according to the manufacturer's protocol.
Real-time PCR analysis
cDNA synthesized using 1 μg of total RNA from each sample was subjected to real-time PCR assay with specific primers and EXPRESS SYBR® GreenER™ qPCR SuperMix (Invitrogen, Carlsbad, CA, USA). The sequences of the primers were as follows: Cx43, 5′-CGTGGAGATGCACCTGAA-3′ (forward) and 5′-CCACTGGATGAGCAGGAA-3′ (reverse), Cx26, 5′-GGAGATGAGCAAGCCGAT-3′ (forward) and 5′-TAGGCCACGTGCATAGCT-3′ (reverse), Cx30, 5′-CGTCTGCTACGACCACTT-3′ (forward) and 5′-GCACCTTCTGCCGTTTGA-3′ (reverse), glyceraldehydes-3-phosphate dehydrogenase (GAPDH), 5′-AGCCCAGAACATCATCCCTG-3′ (forward) and 5′-CACCACCTTCTTGATGTCATC-3′ (reverse). Real-time PCR assays were conducted using a DNA engine Opticon 2 real-time PCR detection system (Bio-Rad, Hercules, CA, USA). The three-step amplification protocol consisted of 3 min at 95°C followed by 40 cycles of 95°C for 15 s, 60°C for 30 s and 72°C for 30 s. RNA quantities of target genes were calculated using the Ct method. The Ct values of Cx43, Cx26 and Cx30 amplification were normalized to those obtained with the amplification of GAPDH.
Scrape loading/dye transfer assay (SLDT)
The SLDT assay is based on monitoring the transfer of the fluorescent dye Lucifer yellow from one cell to adjacent cells, and was performed as previously described (Zhang et al., 2013). Briefly, astrocytes were incubated in HEPES-buffered salt solution for 10 min containing the following (in mM): 140 NaCl, 5.5 KCl, 1.8 CaCl2, 1 MgCl2, 10 glucose, 10 HEPES, pH 7.35. Cells were then washed with Ca2+-free HEPES solution for 1 min, and scrape loading was achieved with a razor blade in the same Ca2+-free solution containing Lucifer yellow (1 mg·mL−1). After 1 min, cells were washed several times with the HEPES-buffered salt solution. After loading for 8 min, fluorescent images were captured with an inverted fluorescent microscope equipped for epifluorescence (Olympus, Tokyo, Japan) and images were analysed in Image J (National Institute of Health, Bethesda, MD, USA). For each trial, quantification of the change in GJIC induced by different treatments was performed by determining the fluorescence area and intensity in consecutive fields over time.
Statistical analysis
Data are expressed as the mean ± SEM of at least three independent determinations. Differences between means were determined using anova with a pairwise comparison by the Tukey–Kramer method. When the number of experiments was three, differences between groups were analysed using the Scheffe method. Differences were considered to be significant when the P-value was less than 0.05.
Materials
Amitriptyline, clomipramine, diazepam, diphenhydramine, fluvoxamine and trihexyphenidyl were obtained from Wako Pure Chemicals Industries (Osaka, Japan). Curcumin, haloperidol, 5-HT, Lucifer yellow, NA and propranolol were purchased from Sigma. SB 202190, SP600125, and U0126 were obtained from Tocris Cookson (Bristol, UK). DA was purchased from Nacalai Tesque. Prazosin was obtained from Tokyo Chemical Industry (Tokyo, Japan). Yohimbine was from Katayama Chemical Industry (Osaka, Japan). Tanshinone IIA was from Enzo Life Sciences (Plymouth Meeting, PA, USA). Cocaine was from Sankyo (Tokyo, Japan).
Results
Amitriptyline induced the up-regulation of Cx43 expression and GJIC in cortical astrocytes
To examine the effect of amitriptyline on Cx43 expression in cortical astrocytes, changes in mRNA and protein levels of Cx43 were quantified. As shown in Figure 1A, treatment with amitriptyline (25 μM) led to a significant increase in Cx43 mRNA at 24, 36 and 48 h post-treatment. In contrast, short-term incubation (1–12 h) with amitriptyline had no effect. In the current study, three immunopositive bands (P0, P1 and P2) were detected at approximately 39–44 kDa by Western blot using a Cx43 antibody (Figure 1B), which was also described in a previous report (Zhang et al., 2013). ‘P0’ indicates the non-phosphorylated Cx43, and ‘P1’ and ‘P2’ corresponded to phosphorylated Cx43. In this study, the sum of the three Cx43 immunopositive bands (P0 + P1 + P2) was regarded as total Cx43 expression. Whereas 24 h of treatment with 25 μM of amitriptyline induced an increase of Cx43 protein, treatment for 48 h led to a significant expression of Cx43 protein with 10 μM as well as 25 μM of amitriptyline (Figure 1B). By contrast, treatment with either 1 or 5 μM of amitriptyline for 48 h did not affect Cx43 protein expression (Figure 1C). To elucidate whether the up-regulation of Cx43 expression reflects gap junction permeability, the effect of amitriptyline on GJIC was investigated. As shown in Figure 1D, the transfer of Lucifer yellow through GJIC was significantly increased after treatment with amitriptyline (25 μM, 48 h) compared with that of vehicle. To demonstrate that the effect of amitriptyline on Cx expression was limited to Cx43, the effect of amitriptyline on Cx26 and Cx30, which are also expressed in astrocytes, was examined. As shown in Figure 2, treatment with amitriptyline (25 μM) up to 48 h did not significantly induce mRNA expression of either Cx26 or Cx30. In fact, short-term incubation (1–12 h) with amitriptyline tended to decrease the levels of Cx26 and Cx30 mRNA compared to control levels.
Figure 1.
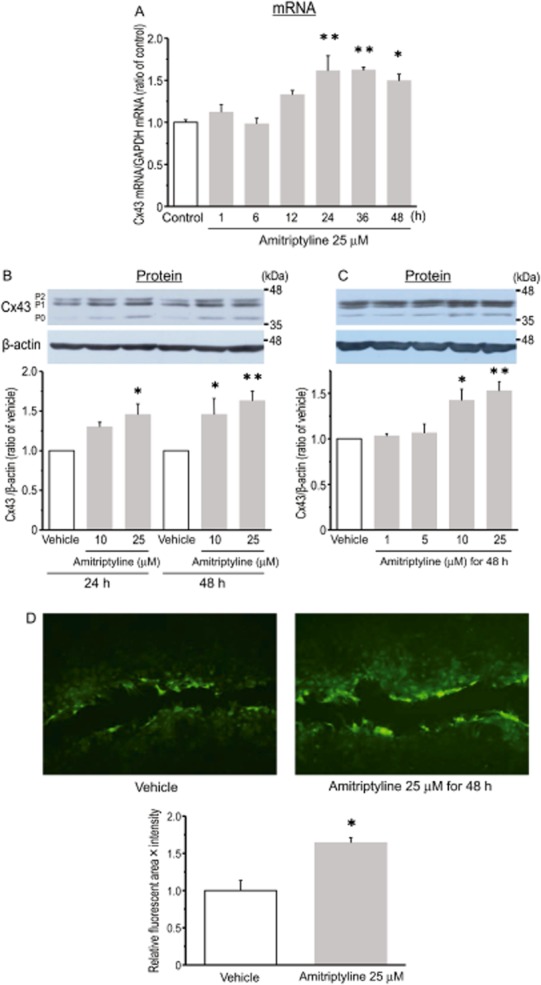
Treatment of rat cultured cortical astrocytes with amitriptyline led to a significant increase in Cx43 expression and gap junction permeability. Effect of amitriptyline on Cx43 mRNA expression (A). Cells were treated with amitriptyline (25 μM) for the periods indicated (h). The expression of Cx43 mRNA was normalized to that of GAPDH mRNA, used as an internal control. Data are expressed as a ratio of the levels of control (time = 0). Data represent the mean ± SEM (bars) for three to seven independent experiments. *P < 0.05, **P < 0.01 versus levels at time = 0. Effect of amitriptyline on Cx43 protein expression (B, C). Cells were treated with amitriptyline (10 or 25 μM) for either 24 or 48 h. In addition, the concentration-dependent effects of amitriptyline (1–25 μM) for 48 h are shown (C). The expression of Cx43 protein was normalized as a ratio to the level of vehicle-treated astrocytes. Data represent the mean ± SEM (bars) for three to seven independent experiments. *P < 0.05, **P < 0.01 versus vehicle treatment. Effect of amitriptyline on GJIC function in cultured cortical astrocytes (D). After treatment with 25 μM of amitriptyline for 48 h, cells were cut with a razor blade in the presence of Lucifer yellow. Upper photographs show representative spreading of dye taken as an indicator of intercellular junction coupling. The graph shows the quantification of the extent of fluorescence (area × intensity). Values are expressed as a ratio to vehicle-treated astrocytes. Data represent the mean ± SEM (bars) for at least three independent experiments. *P < 0.05 versus vehicle treatment.
Figure 2.

Effect of amitriptyline on the expression of Cx26 and Cx30 mRNA in cultured cortical astrocytes. Cells were treated with 25 μM of amitriptyline for the periods indicated (h). Data represent the mean ± SEM (bars) for three to nine independent experiments.
Whether different classes of antidepressants could induce Cx43 protein expression was investigated. As shown in Figure 3A, treatment with either clomipramine (10 μM; a tricyclic antidepressant, a 5-HT/NA re-uptake inhibitor) or fluvoxamine (25 μM; a selective 5-HT re-uptake inhibitor) significantly induced the expression of Cx43 protein, in which the degrees of up-regulation were almost similar compared to that of amitriptyline. In contrast, neither milnacipran (25 μM; a 5-HT/NA re-uptake inhibitor) nor cocaine (100 μM; a non-selective monoamine transporter inhibitor) increased the expression of Cx43 protein (Figure 3A). Furthermore, the effects of other psychotropic drugs such as diazepam (25 μM; a benzodiazepine), trihexyphenidyl (25 μM; an anticholinergic driug), haloperidol (25 μM; an antipsychotic D2-DA receptor antagonist) and diphenhydramine (25 μM; an antihistaminergic drug) on Cx43 protein expression were examined. As shown in Figure 3B, these non-antidepressant drugs had no effect on the expression of Cx43 protein.
Figure 3.
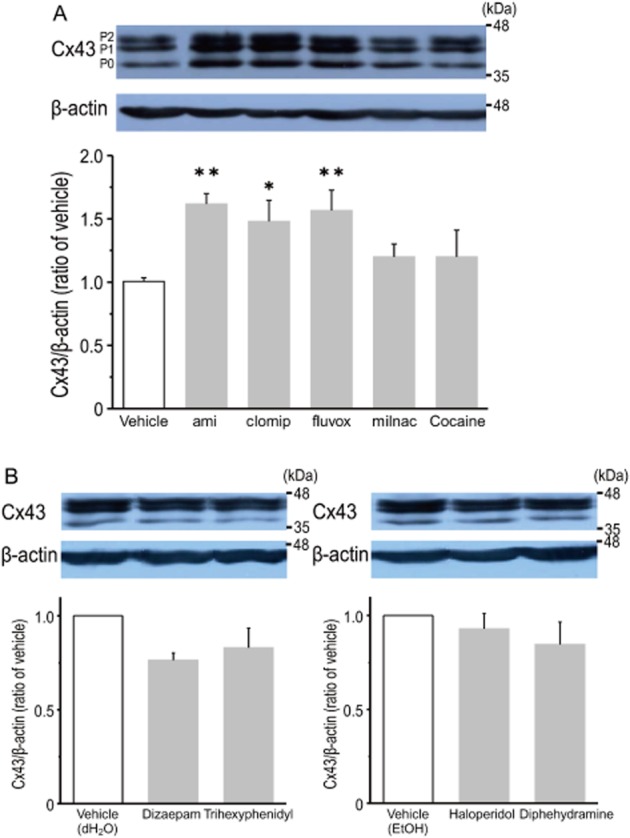
Effects of various psychotropic drugs on the expression of Cx43 protein in cultured cortical astrocytes. Effects of monoamine re-uptake blockers on the expression of Cx43 protein (A). Cells were treated with either clomipramine (clomip, 10 μM), fluvoxamine (fluvox, 25 μM), milnacipran (milnac, 25 μM) or cocaine (100 μM) for 48 h. Amitriptyline (ami, 25 μM) was used as a positive control. Data represent the mean ± SEM (bars) for four to six independent experiments. *P < 0.05, **P < 0.01 versus vehicle treatment. Effects of non-antidepressant drugs on the expression of Cx43 protein in cultured cortical astrocytes (B). Cells were treated with either diazepam (25 μM), trihexyphenidyl (25 μM), haloperidol (25 μM) or dephenhydramine (25 μM) for 48 h. Distilled H2O (dH2O) or EtOH were used as vehicle. Data represent the mean ± SEM (bars) for three independent experiments.
Amitriptyline-induced Cx43 expression is independent of the monoaminergic system in cortical astrocytes
In the current experimental set-up, it is possible that treatment with amitriptyline might lead to the accumulation of monoamines, which are spontaneously released from cells, including astrocytes, in the extracellular space by the inhibition of monoamine transporters. Furthermore, it has been previously shown that stimulation of adrenoceptors induce the expression of Cx43 in cultured rat cardiomyocytes (Salameh et al., 2008; 2009). Thus, activation of adrenoceptors could contribute to the amitriptyline-induced expression of Cx43 protein in cortical astrocytes. However, as shown in Figure 4A, pretreatment with prazosin (10 μM; α1-adrenoceptor antagonist), yohimbine (10 μM; α2-adrenoceptor antagonist) and propranolol (10 μM; β-adrenoceptor antagonist) had no effect on the amitriptyline-induced expression of Cx43 protein. In addition, treatment with a combination of these antagonists did not block the effect of amitriptyline. The concentrations of these antagonists used were based on the previous studies that showed significant block of their respective agonists (Morioka et al., 2009; Kajitani et al., 2012). In addition, stimulation of cortical astrocytes with either NA, 5-HT or DA (10 μM each) for 48 h had no influence on Cx43 protein expression (Figure 4B).
Figure 4.
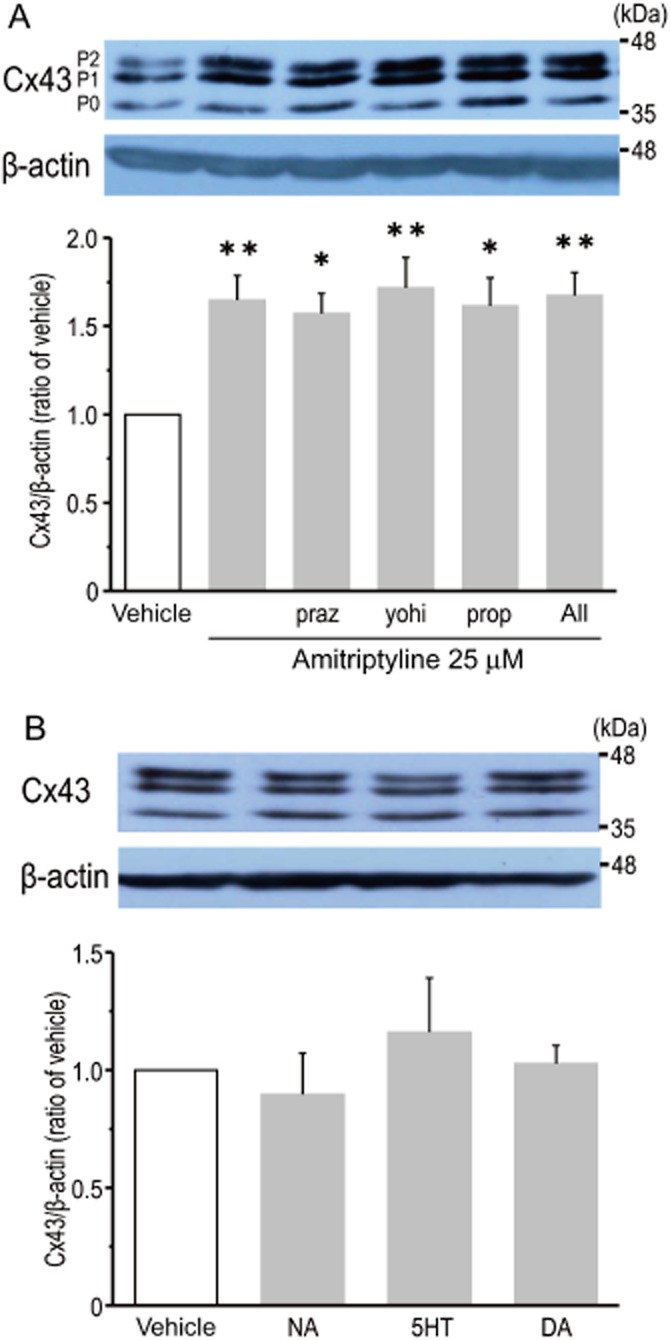
A monoamine-dependent pathway is not crucial in the amitriptyline-induced expression of Cx43 protein in cultured cortical astrocytes. Effects of adrenoceptor antagonists on amitriptyline-induced Cx43 protein expression (A). After treatment with either prazocin (praz, 10 μM), yohimbine (yohi, 10 μM), propranolol (prop, 10 μM) or a combination of the three antagonists for 30 min, cells were stimulated with 25 μM amitriptyline for 48 h. Data represent the mean ± SEM (bars) for seven to nine independent experiments. *P < 0.05, **P < 0.01 versus vehicle treatment. Effects of monoamines on the expression of Cx43 protein (B). Cells were treated with NA (10 μM), 5-HT (5-HT, 10 μM) or DA (10 μM) for 48 h. Data represent the mean ± SEM (bars) for three to four independent experiments.
The activation of p38 is associated with amitriptyline-induced Cx43 expression and GJIC
Studies have indicated that treatment with antidepressants induces the activation of MAPKs in a range of cell types (Mercier et al., 2004; Hisaoka et al., 2007; Lin et al., 2012). Thus, it is possible that MAPKs are involved in the amitriptyline-induced expression of Cx43. The amitriptyline-induced increase of Cx43 mRNA (24 and 48 h of treatment, Figure 5A) and Cx43 protein (48 h of treatment, Figure 5B) was significantly blocked by pretreatment with SB 202190, a p38 inhibitor (Figure 5A and B). On the other hand, treatment with either U0126 or SP600125, inhibitors of MAPK kinase and JNK, respectively, had no effect on the amitriptyline-induced response (Figure 5A and B). As a negative control, treatment with these inhibitors alone, without amitriptyline, had no effect on the expression of Cx43 mRNA and protein. In addition, the involvement of p38 activity in the up-regulation of GJIC by amitriptyline was examined. As shown in Figure 5C, treatment with SB 202190 significantly prevented the amitriptyline-induced up-regulation of gap junction function in cultured cortical astrocytes. Furthermore, it was found that treatment of cortical astrocytes with amitriptyline significantly increased the phosphorylation of p38 (Figure 6). Significant phosphorylation appeared at 1 h of incubation, and was maintained for at least 24 h. The level of p38 phosphorylation after 48 h of treatment tended to be higher than that of vehicle treatment, although this increase was not statistically significant.
Figure 5.
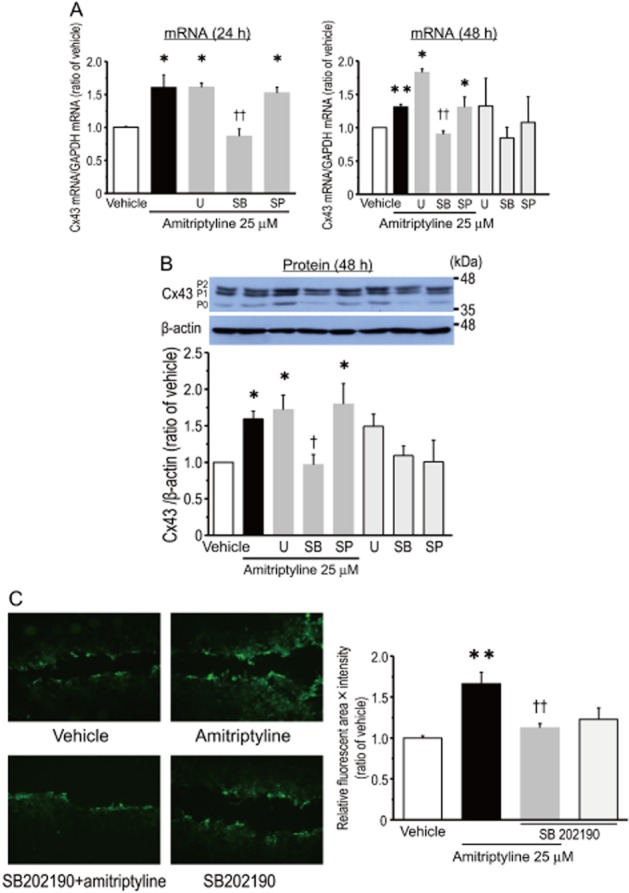
p38 MAPK is involved in the amitriptyline-induced expression of Cx43 and gap junction permeability in cultured cortical astrocytes. Effects of MAPK inhibitors on the amitriptyline-induced expression of Cx43 mRNA (A) and Cx43 protein (B). After treatment with either U0126 (U, 10 μM), SB 202190 (SB, 10 μM) or SP600125 (SP, 10 μM) for 30 min, cells were stimulated with 25 μM amitriptyline for 24 h (A) or 48 h (A, B). Data represent the mean ± SEM (bars) for four to seven independent experiments. *P < 0.05, **P < 0.01 versus vehicle treatment. †P < 0.05, ††P < 0.01 versus amitriptyline treatment alone. Effect of SB 202190 on amitriptyline-induced GJIC in cultured cortical astrocytes (C). After treatment with SB 202190 (10 μM) for 30 min, cells were stimulated with 25 μM amitriptyline for 48 h. Upper photographs show representative spreading of dye taken as an indicator of intercellular junction coupling. The graph shows quantification of the extent of fluorescence (area × intensity). Values are expressed as a ratio to vehicle-treated astrocytes. Data represent the mean ± SEM (bars) for three to five independent experiments. **P < 0.01 versus vehicle treatment. ††P < 0.01 versus amitriptyline treatment alone.
Figure 6.
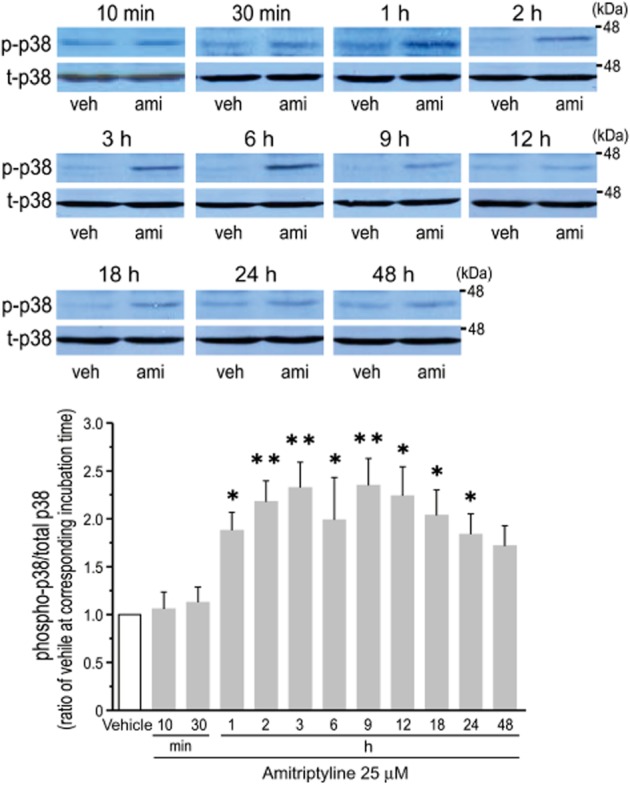
Effect of amitriptyline on the phosphorylation of p38 in cultured cortical astrocytes. Cells were stimulated with either vehicle (veh) or amitriptyline (ami, 25 μM) for the periods indicated (h). Upper panels show a representative blot of phosphorylated p38 (p-p38) and total p-38 (t-p38). Bar graph shows quantification from each blot. Each level of p38 phosphorylation was normalized to total p38, and expressed as a ratio to vehicle-treated levels at each time point. Data represent the mean ± SEM (bars) for 5 to 14 independent experiments. *P < 0.05, **P < 0.01 versus vehicle treatment.
c-Fos/AP-1 has a crucial role in the amitriptyline-induced expression of Cx43 in cortical astrocytes
It has been suggested that the transcription factor activator protein-1 (AP-1) is involved in MAPKs-mediated signalling, and contributes to the expression of Cx43 (Cortez et al., 2007; Guan et al., 2008; Salameh et al., 2009). Thus, the involvement of AP-1 in the amitriptyline-induced expression of Cx43 was examined using a pharmacological approach. Pretreatment of cortical astrocytes with either curcumin or tanshinone IIA, which inhibit AP-1 activity (Müller et al., 2009; Lin et al., 2011), significantly blocked the amitriptyline-induced expression of Cx43 mRNA and Cx43 protein (Figure 7A and B). Treatment with inhibitors alone, without amitriptyline, had no effect on the expression of both Cx43 mRNA and Cx43 protein. In addition, since both curcumin and tanshinone IIA also have inhibitory effects on NF-κB activity, the effect of BAY-11-7082, which selectively inhibits NF-κB activity, on amitriptyline-induced Cx43 protein expression was investigated (Wang et al., 2010; Morinelli et al., 2013). Pretreatment with BAY-11-7082 (3 μM) had no effect on the amitriptyline-induced response (Figure 7C). Next, the effect of tanshinone IIA on the amitriptyline-induced increase in GJIC was evaluated. As shown in Figure 8, pretreatment with tanshinone IIA significantly blocked the amitriptyline-induced up-regulation of gap junction function. Furthermore, the effect of amitriptyline on the translocation of c-Fos from cytosol to nucleus was examined. Treatment of cortical astrocytes with amitriptyline for 24 h markedly increased the nuclear content of c-Fos, and at the same time significantly decreased the cytosolic content of c-Fos, compared with vehicle treatment (Figure 9). These responses were significantly prevented by pretreatment with SB 202190 (Figure 9). Treatment with SB 202190 alone had no effect on the translocation of c-Fos.
Figure 7.
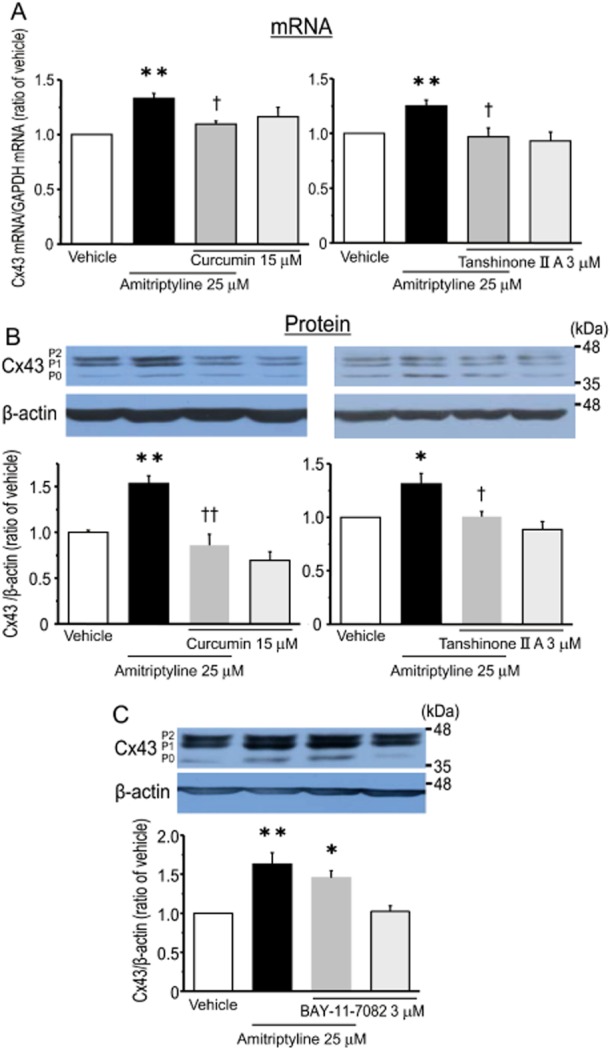
AP-1 is a key factor in the amitriptyline-induced expression of Cx43 in cultured cortical astrocytes. Effect of AP-1 inhibitors (curcumin and tanshinone IIA) on the amitriptyline-induced expression of Cx43 mRNA (A) and Cx43 protein (B). After treatment with either curcumin (15 μM) or tanshinone IIA (3 μM) for 30 min, cells were stimulated with 25 μM amitriptyline for 48 h. Data represent the mean ± SEM (bars) for four to nine independent experiments. *P < 0.05, **P < 0.01 versus vehicle treatment. †P < 0.05, ††P < 0.01 versus amitriptyline treatment alone. Effects of NF-κB inhibitor BAY-11-7082 on amitriptyline-induced expression of Cx43 protein (C). After treatment with BAY-11-7082 (3 μM) for 30 min, cells were stimulated with amitriptyline (25 μM) for 48 h. Data represent the mean ± SEM (bars) for four independent experiments. *P < 0.05, **P < 0.01 versus vehicle treatment.
Figure 8.

Effect of tanshinone IIA on amitriptyline-induced GJIC in cultured cortical astrocytes. After treatment with tanshinone IIA (3 μM) for 30 min, cells were stimulated with amitriptyline (25 μM) for 48 h. Upper photographs show representative spreading of dye taken as an indicator of intercellular junction coupling. The graph shows the quantification of the extent of fluorescence (area × intensity). Values are expressed as a ratio to vehicle-treated astrocytes. Data represent the mean ± SEM (bars) for three to five independent experiments. **P < 0.01 versus vehicle treatment. ††P < 0.01 versus amitriptyline treatment alone.
Figure 9.
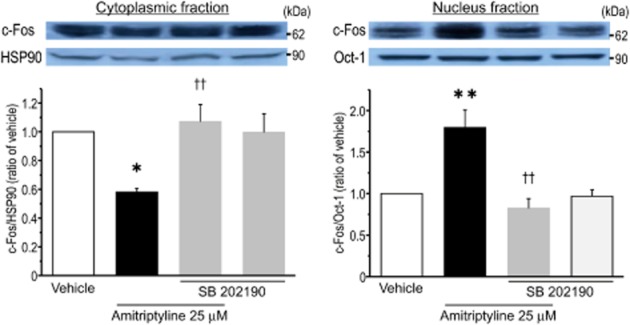
Treatment of cortical astrocytes with amitriptyline led to a significant translocation of c-Fos from the cytosol to the nucleus through p38 activity. After treatment with SB 202190 (3 μM) for 30 min, cells were then stimulated with amitriptyline (25 μM) for 24 h. Cytoplasmic (left) and nuclear (right) fractions were prepared by using a commercially available kit as described in Methods. Lysates were then subjected to Western blotting to quantify c-Fos levels. Upper panels show representative blots of c-Fos, HSP90 and Oct-1. Bar graphs show quantification from each blot. Each level of c-Fos was normalized for either HSP90 or Oct-1 for cytoplasmic (left) or nuclear (right) fraction, respectively, and expressed as a ratio to vehicle-treated levels of each fraction. Data represent the mean ± SEM (bars) for five independent experiments. *P < 0.05, **P < 0.01 versus vehicle treatment. ††P < 0.01 versus amitriptyline treatment alone.
Discussion
The findings of the current study indicate the potential of amitriptyline to up-regulate Cx43-gap junction function in cortical astrocytes, thereby enhancing intercellular communication. Treatment of cortical astrocytes with amitriptyline for more than 24 h led to a significant increase of Cx43 expression at transcriptional and translational levels. Furthermore, these increases were regulated via the activation of a specific intracellular signalling cascade, p38 MAPK and the subsequent increase in AP-1 activity by translocation of c-Fos from the cytosol to the nucleus. Thus, via the pathway elaborated in the current study, the modulation of Cx43/gap junction function in astrocytes could underlie amitriptyline's therapeutic effect on major depressive disorder.
It has been previously demonstrated that astrocytes express a number of Cxs, Cx26 and Cx30, in addition to Cx43 (Giaume et al., 1991; Nagy et al., 2001; Gosejacob et al., 2011), although Cx26 shows a regional expression pattern that is different from other Cxs and there is no clear consensus regarding its contributions to astrocytic gap junctions (Filippov et al., 2003). Bernard et al. have shown significant reductions of both Cx43 and Cx30 mRNA expression in the locus coeruleus from post-mortem brains from patients with major depressive disorder (Bernard et al., 2011). However, in the current study, amitriptyline did not induce the expression of Cx26 and Cx30 in cortical astrocytes – if anything, treatment with amitriptyline for 1–12 h tended to decrease the expression of these Cxs. By contrast, the Cx43 subtype is pivotal in organizing gap junctions in astrocytes (Giaume et al., 1991), and Cx43-deficient astrocytes in culture display a strong loss of dye transfer among cells (Theis et al., 2004). Thus, a change in Cx43 expression by amitriptyline directly influences astrocytic gap junction permeability. In fact, the current study demonstrated enhanced gap junction permeability concomitant with the up-regulation of Cx43 expression following treatment with amitriptyline.
It has been suggested that amitriptyline could have distinct pharmacological actions, independent of the inhibition of monoamine transporters (Perisic et al., 2010; Huang et al., 2012; Kajitani et al., 2012). In the current study, the up-regulation of Cx43 protein expression was found after treatment with clomipramine and fluvoxamine in addition to amitriptyline. However, not all monoamine transporter blocking drugs showed efficacy in the current study. Cocaine, in particular, a potent inhibitor of monoamine transporters (Hashimoto et al., 2005), had no effect. These observations indicate a differential pharmacological action, distinct from the inhibition of monoamine transporters and the accumulation of monoamines in extracellular space, of some antidepressants is involved in the up-regulation of Cx43. This hypothesis is supported by the fact that treatment of astrocytes with either NA, 5-HT or DA had no significant effect on Cx43 expression. By contrast, some studies have demonstrated that the α1- and β-adrenoceptors could contribute to the regulation of Cx43 expression in cultured cardiomyocytes (Salameh et al., 2008; 2009). Nonetheless, these receptors are not involved in the regulation of Cx43 expression in astrocytes because the amitriptyline-induced expression of Cx43 was not affected by treatment with antagonists for α1-, α2- and β-adrenoceptors. In addition, a previous report has shown that NA, 5-HT and DA were not detectable in culture medium of cortical astrocytes after amitriptyline treatment for 24 h (Kajitani et al., 2012). Therefore, the current findings strongly indicate that amitriptyline could regulate the expression of Cx43 via a mechanism that is independent of the monoaminergic system.
The intracellular signalling pathway induced by amitriptyline that leads to increased intercellular communication, via increased expression and function of Cx43, has yet to be elucidated. Several studies have suggested that the activation of MAPKs is associated with the monoamine-independent action of antidepressants (Mercier et al., 2004; Duman et al., 2007). Previous reports demonstrated that amitriptyline could induce the phosphorylation of ERK, p38 and JNK in C6 glioma cells (Chung et al., 2007; Hisaoka et al., 2007), which possess astrocyte-like characteristics. It has been previously shown that matrix metalloproteinases-mediated fibroblast growth factor receptor signalling could be involved in the amitriptyline-induced ERK phosphorylation in C6 cells (Hisaoka et al., 2011). Previous reports have indicated that amitriptyline could have either stimulatory or inhibitory effects on the phosphorylation of p38 in various cell types (Lirk et al., 2006; Tai et al., 2009; Galeotti and Ghelardini, 2012). Furthermore, β-adrenoceptor-stimulated up-regulation of Cx43 is mediated by three types of MAPK in rat cultured cardiomyocytes (Tacheau et al., 2008; Salameh et al., 2009). It has also been found that JNK signalling is involved in the reduction in Cx43 expression in rat cultured spinal astrocytes treated with TNF-α and interferon (Zhang et al., 2013). The wide ranging results found in the literature could be the result of using different stimulating agents in various tissue types. In the current study, treatment of cortical astrocytes with amitriptyline clearly led to a potent and long-lasting (1–24 h) enhancement of p38 phosphorylation. The current study also found, using a pharmacological approach, that neither ERK nor JNK is important in the amitriptyline-induced expression of Cx43, whereas p38 is, in fact, crucial. However, the precise molecular mechanism by which amitriptyline regulates phosphorylation of p38 is not completely understood and is an important subject for further investigation.
The current study demonstrated that amitriptyline regulated the expression of Cx43 at the transcriptional level, so this effect should be accompanied by the activation of transcription factors. Thus, the current study focused on the involvement of c-Fos/AP-1 since it has been demonstrated that there are several consensus AP-1 binding sites in the Cx43 promoter region (Yu et al., 1994; Geimonen et al., 1996) and AP-1 is an essential player in the regulation of Cx43 expression in various cell types (Tacheau et al., 2008; Salameh et al., 2009). In addition, systemic chronic administration of amitriptyline induced a persistent and enhanced level of AP-1 activity in rat frontal cortex (Okamoto et al., 2003). However, the involvement of AP-1 activity in astrocytic Cx43 expression has yet to be elaborated. Using AP-1 inhibitors curcumin and tanshinone IIA, the current data demonstrate a significant involvement of AP-1 in the amitriptyline-induced expression of Cx43. Although it has been reported that these inhibitors could also block NF-κB activity, and NF-κB-dependent signalling is associated with the positive regulation of Cx43 expression, incubation with the NF-κB inhibitor BAY-11-7082 had no effect on the astrocytes' response to amitriptyline. Thus, up-regulation of Cx43 expression by amitriptyline is dependent on AP-1 activity. The current study reveals that translocation of c-Fos to the nucleus was significantly increased after treatment with amitriptyline, and this response was sensitive to SB 202190 treatment, indicating a p38-sensitive AP-1 translocation requirement for Cx43 transcription. It has been shown that p38 activity is involved in the phosphorylation and stabilization of c-Fos (Iwatsuki et al., 2011). In addition, the phosphorylation of c-Fos by p38 is necessary for translocation from the cytosol to the nucleus following exposure of HEK293 cells co-transfected with the expression vectors for both c-Fos and p38 to UV light (Tanos et al., 2005). Therefore, in the current study, the translocation of c-Fos might be directly mediated by p38, which in turn, could contribute to the transcriptional regulation of Cx43 in cortical astrocytes. It has been suggested that c-Jun, which is another component of AP-1, is phosphorylated by JNK following activation of AP-1. In fact, in a human leukaemic cell line, it was reported that the expression of Cx43 is mediated by a JNK/c-Jun/AP-1 pathway (Gao et al., 2007). However, c-Jun phosphorylation might not be involved in the current study, because preliminary experiments with astrocytes revealed that c-Jun is phosphorylated via a mechanism that is independent of p38 activity, which in turn could be dependent on JNK activity (data not shown).
It has been demonstrated that antidepressants accumulate in the brain at concentrations several-fold higher than that in blood because of their highly lipophilic properties. In fact, it has previously shown that the brain concentration of amitriptyline is approximately 10 to 35 times higher than the plasma concentration of amitriptyline (Glotzbach and Preskorn, 1982; Miyake et al., 1990). The therapeutic plasma concentration of amitriptyline ranges from approximately 0.36 to 0.9 μM (O'Donnell and Shelton, 2011). Therefore, brain tissue concentrations could be, for example, between 3.6 and 31.5 μM. The minimum concentration of amitriptyline that induced the expression of Cx43, 10 μM, is within the estimated concentration in the brain that is attained after treatment at clinically relevant doses.
The results of the current study support a novel mechanism for the therapeutic effect of antidepressants: the up-regulation of GJIC between brain astrocytes. Indeed, it has been shown that intercellular communication between astrocytes is critical in maintaining CNS function, and a decrease in GJIC could be a crucial component in the induction of affective disorders such as major depressive disorder (Bernard et al., 2011; Sun et al., 2012; Rajkowska and Stockmeier, 2013). Thus, amitriptyline could improve depressive symptoms by increasing GJIC between astrocytes through an increase in Cx43 expression. The current findings suggest a potentially novel therapeutic approach, which is monoamine-independent and targets astrocytes rather than neurons, in the amelioration of major depressive disorder. Further confirmation is needed, however, to support a role for astrocytic GJIC in depression; in vivo pharmacological experiments would be needed to confirm that modulation of GJIC is in fact a key mechanism of amitriptyline's therapeutic effect.
Acknowledgments
This work was supported in part by Grants-in-Aid for young Scientists (B) from the Japan Society for the Promotion of Science (grant number: 24790253), and by grants from the Japanese Research Smoking Association and the Takeda Science Foundation. We wish to thank the Analysis Center of Life Science, Hiroshima University for the use of their facilities. We also thank Dr. Aldric T. Hama for his careful editing of the manuscript.
Glossary
- AP-1
activator protein-1
- Cx
connexin
- GJIC
gap junction intercellular communication
- SLDT
scrape loading/dye transfer
Conflict of interest
The authors declare no conflict of interest.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G-Protein Coupled Receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banasr M, Duman RS. Glial loss in the prefrontal cortex is sufficient to induce depressive-like behaviors. Biol Psychiatry. 2008;64:863–870. doi: 10.1016/j.biopsych.2008.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard R, Kerman IA, Thompson RC, Jones EG, Bunney WE, Barchas JD, et al. Altered expression of glutamate signaling, growth factor, and glia genes in the locus coeruleus of patients with major depression. Mol Psychiatry. 2011;16:634–646. doi: 10.1038/mp.2010.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung EY, Shin SY, Lee YH. Amitriptyline induces early growth response-1 gene expression via ERK and JNK mitogen-activated protein kinase pathways in rat C6 glial cells. Neurosci Lett. 2007;422:43–48. doi: 10.1016/j.neulet.2007.05.057. [DOI] [PubMed] [Google Scholar]
- Cortez DM, Feldman MD, Mummidi S, Valente AJ, Steffensen B, Vincenti M, et al. IL-17 stimulates MMP-1 expression in primary human cardiac fibroblasts via p38 MAPK- and ERK1/2-dependent C/EBP-beta, NF-kappaB, and AP-1 activation. Am J Physiol Heart Circ Physiol. 2007;293:H3356–H3365. doi: 10.1152/ajpheart.00928.2007. [DOI] [PubMed] [Google Scholar]
- Duman CH, Schlesinger L, Kodama M, Russell DS, Duman RS. A role for MAP kinase signaling in behavioral models of depression and antidepressant treatment. Biol Psychiatry. 2007;61:661–670. doi: 10.1016/j.biopsych.2006.05.047. [DOI] [PubMed] [Google Scholar]
- Filippov MA, Hormuzdi SG, Fuchs EC, Monyer H. A reporter allele for investigating connexin 26 gene expression in the mouse brain. Eur J Neurosci. 2003;18:3183–3192. doi: 10.1111/j.1460-9568.2003.03042.x. [DOI] [PubMed] [Google Scholar]
- Fonseca CG, Green CR, Nicholson LF. Upregulation in astrocytic connexin 43 gap junction levels may exacerbate generalized seizures in mesial temporal lobe epilepsy. Brain Res. 2002;929:105–116. doi: 10.1016/s0006-8993(01)03289-9. [DOI] [PubMed] [Google Scholar]
- Galeotti N, Ghelardini C. Selective modulation of the PKCε/p38MAP kinase signalling pathway for the antidepressant-like activity of amitriptyline. Neuropharmacology. 2012;62:289–296. doi: 10.1016/j.neuropharm.2011.07.020. [DOI] [PubMed] [Google Scholar]
- Gao FH, Wang Q, Wu YL, Li X, Zhao KW, Chen GQ. c-Jun N-terminal kinase mediates AML1-ETO protein-induced connexin-43 expression. Biochem Biophys Res Commun. 2007;356:505–511. doi: 10.1016/j.bbrc.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Geimonen E, Jiang W, Ali M, Fishman GI, Garfield RE, Andersen J. Activation of protein kinase C in human uterine smooth muscle induces connexin-43 gene transcription through an AP-1 site in the promoter sequence. J Biol Chem. 1996;271:23667–23674. doi: 10.1074/jbc.271.39.23667. [DOI] [PubMed] [Google Scholar]
- Giaume C, Theis M. Pharmacological and genetic approaches to study connexin-mediated channels in glial cells of the central nervous system. Brain Res Rev. 2010;63:160–176. doi: 10.1016/j.brainresrev.2009.11.005. [DOI] [PubMed] [Google Scholar]
- Giaume C, Fromaget C, el Aoumari A, Cordier J, Glowinski J, Gros D. Gap junctions in cultured astrocytes: single-channel currents and characterization of channel-forming protein. Neuron. 1991;6:133–143. doi: 10.1016/0896-6273(91)90128-m. [DOI] [PubMed] [Google Scholar]
- Giaume C, Koulakoff A, Roux L, Holcman D, Rouach N. Astroglial networks: a step further in neuroglial and gliovascular interactions. Nat Rev Neurosci. 2010;11:87–99. doi: 10.1038/nrn2757. [DOI] [PubMed] [Google Scholar]
- Glotzbach RK, Preskorn SH. Brain concentrations of tricyclic antidepressants: single-dose kinetics and relationship to plasma concentrations in chronically dosed rats. Psychopharmacology (Berl) 1982;78:25–27. doi: 10.1007/BF00470582. [DOI] [PubMed] [Google Scholar]
- Gosejacob D, Dublin P, Bedner P, Hüttmann K, Zhang J, Tress O, et al. Role of astroglial connexin30 in hippocampal gap junction coupling. Glia. 2011;59:511–519. doi: 10.1002/glia.21120. [DOI] [PubMed] [Google Scholar]
- Guan J, Wu X, Arons E, Christou H. The p38 mitogen-activated protein kinase pathway is involved in the regulation of heme oxygenase-1 by acidic extracellular pH in aortic smooth muscle cells. J Cell Biochem. 2008;105:1298–1306. doi: 10.1002/jcb.21930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hashimoto W, Kitayama S, Kumagai K, Morioka N, Morita K, Dohi T. Transport of dopamine and levodopa and their interaction in COS-7 cells heterologously expressing monoamine neurotransmitter transporters and in monoaminergic cell lines PC12 and SK-N-SH. Life Sci. 2005;76:1603–1612. doi: 10.1016/j.lfs.2004.08.029. [DOI] [PubMed] [Google Scholar]
- Hisaoka K, Takebayashi M, Tsuchioka M, Maeda N, Nakata Y, Yamawaki S. Antidepressants increase glial cell line-derived neurotrophic factor production through monoamine-independent activation of protein tyrosine kinase and extracellular signal-regulated kinase in glial cells. J Pharmacol Exp Ther. 2007;321:148–157. doi: 10.1124/jpet.106.116558. [DOI] [PubMed] [Google Scholar]
- Hisaoka K, Tsuchioka M, Yano R, Maeda N, Kajitani N, Morioka N, et al. Tricyclic antidepressant amitriptyline activates fibroblast growth factor receptor signaling in glial cells: involvement in glial cell line-derived neurotrophic factor production. J Biol Chem. 2011;286:21118–21128. doi: 10.1074/jbc.M111.224683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang YN, Tsai RY, Lin SL, Chien CC, Cherng CH, Wu CT, et al. Amitriptyline attenuates astrocyte activation and morphine tolerance in rats: role of the PSD-95/NR1/nNOS/PKCγ signaling pathway. Behav Brain Res. 2012;229:401–411. doi: 10.1016/j.bbr.2012.01.044. [DOI] [PubMed] [Google Scholar]
- Iwatsuki M, Inageda K, Matsuoka M. Cadmium induces phosphorylation and stabilization of c-Fos in HK-2 renal proximal tubular cells. Toxicol Appl Pharmacol. 2011;251:209–216. doi: 10.1016/j.taap.2010.12.015. [DOI] [PubMed] [Google Scholar]
- Kajitani N, Hisaoka-Nakashima K, Morioka N, Okada-Tsuchioka M, Kaneko M, Kasai M, et al. Antidepressant acts on astrocytes leading to an increase in the expression of neurotrophic/growth factors: differential regulation of FGF-2 by noradrenaline. PLoS ONE. 2012;7:e51197. doi: 10.1371/journal.pone.0051197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki A, Hayashi T, Nakachi K, Trosko JE, Sugihara K, Kotake Y, et al. Modulation of connexin 43 in rotenone-induced model of Parkinson's disease. Neuroscience. 2009;160:61–68. doi: 10.1016/j.neuroscience.2009.01.080. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: Reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin HY, Tang CH, Chen JH, Chuang JY, Huang SM, Tan TW, et al. Peptidoglycan induces interleukin-6 expression through the TLR2 receptor, JNK, c-Jun, and AP-1 pathways in microglia. J Cell Physiol. 2011;226:1573–1582. doi: 10.1002/jcp.22489. [DOI] [PubMed] [Google Scholar]
- Lin HY, Yeh WL, Huang BR, Lin C, Lai CH, Lin H, et al. Desipramine protects neuronal cell death and induces heme oxygenase-1 expression in Mes23.5 dopaminergic neurons. PLoS ONE. 2012;7:e50138. doi: 10.1371/journal.pone.0050138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lirk P, Haller I, Myers RR, Klimaschewski L, Kau YC, Hung YC, et al. Mitigation of direct neurotoxic effects of lidocaine and amitriptyline by inhibition of p38 mitogen-activated protein kinase in vitro and in vivo. Anesthesiology. 2006;104:1266–1273. doi: 10.1097/00000542-200606000-00023. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallei A, Shi B, Mocchetti I. Antidepressant treatments induce the expression of basic fibroblast growth factor in cortical and hippocampal neurons. Mol Pharmacol. 2002;61:1017–1024. doi: 10.1124/mol.61.5.1017. [DOI] [PubMed] [Google Scholar]
- Mercier G, Lennon AM, Renouf B, Dessouroux A, Ramaugé M, Courtin F, et al. MAP kinase activation by fluoxetine and its relation to gene expression in cultured rat astrocytes. J Mol Neurosci. 2004;24:207–216. doi: 10.1385/JMN:24:2:207. [DOI] [PubMed] [Google Scholar]
- Miyake K, Fukuchi H, Kitaura T, Kimura M, Sarai K, Nakahara T. Pharmacokinetics of amitriptyline and its demethylated metabolite in serum and specific brain regions of rats after acute and chronic administration of amitriptyline. J Pharm Sci. 1990;79:288–291. doi: 10.1002/jps.2600790403. [DOI] [PubMed] [Google Scholar]
- Morinelli TA, Lee MH, Kendall RT, Luttrell LM, Walker LP, Ullian ME. Angiotensin II activates NF-κB through AT1A receptor recruitment of β-arrestin in cultured rat vascular smooth muscle cells. Am J Physiol Cell Physiol. 2013;304:C1176–C1186. doi: 10.1152/ajpcell.00235.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morioka N, Tanabe H, Inoue A, Dohi T, Nakata Y. Noradrenaline reduces the ATP-stimulated phosphorylation of p38 MAP kinase via beta-adrenergic receptors-cAMP-protein kinase A-dependent mechanism in cultured rat spinal microglia. Neurochem Int. 2009;55:226–234. doi: 10.1016/j.neuint.2009.03.004. [DOI] [PubMed] [Google Scholar]
- Müller B, Prante C, Kleesiek K, Götting C. Identification and characterization of the human xylosyltransferase I gene promoter region. J Biol Chem. 2009;284:30775–30782. doi: 10.1074/jbc.M109.016592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Müller MB, Lucassen PJ, Yassouridis A, Hoogendijk WJ, Holsboer F, Swaab DF. Neither major depression nor glucocorticoid treatment affects the cellular integrity of the human hippocampus. Eur J Neurosci. 2001;14:1603–1612. doi: 10.1046/j.0953-816x.2001.01784.x. [DOI] [PubMed] [Google Scholar]
- Nagy JI, Li X, Rempel J, Stelmack G, Patel D, Staines WA, et al. Connexin26 in adult rodent central nervous system: demonstration at astrocytic gap junctions and colocalization with connexin30 and connexin43. J Comp Neurol. 2001;441:302–323. doi: 10.1002/cne.1414. [DOI] [PubMed] [Google Scholar]
- Nakase T, Yoshida Y, Nagata K. Enhanced connexin 43 immunoreactivity in penumbral areas in the human brain following ischemia. Glia. 2006;54:369–375. doi: 10.1002/glia.20399. [DOI] [PubMed] [Google Scholar]
- O'Donnell JM, Shelton RC. Drug Therapy of Depression and Anxiety Disorders. 12 edn. New York: McGraw-Hill Book Companies; 2011. [Google Scholar]
- Okamoto H, Shino Y, Hashimoto K, Kumakiri C, Shimizu E, Shirasawa H, et al. Dynamic changes in AP-1 transcription factor DNA binding activity in rat brain following administration of antidepressant amitriptyline and brain-derived neurotrophic factor. Neuropharmacology. 2003;45:251–259. doi: 10.1016/s0028-3908(03)00148-5. [DOI] [PubMed] [Google Scholar]
- Ongür D, Drevets WC, Price JL. Glial reduction in the subgenual prefrontal cortex in mood disorders. Proc Natl Acad Sci U S A. 1998;95:13290–13295. doi: 10.1073/pnas.95.22.13290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pannasch U, Rouach N. Emerging role for astroglial networks in information processing: from synapse to behavior. Trends Neurosci. 2013;36:405–417. doi: 10.1016/j.tins.2013.04.004. [DOI] [PubMed] [Google Scholar]
- Pannasch U, Vargová L, Reingruber J, Ezan P, Holcman D, Giaume C, et al. Astroglial networks scale synaptic activity and plasticity. Proc Natl Acad Sci U S A. 2011;108:8467–8472. doi: 10.1073/pnas.1016650108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perisic T, Zimmermann N, Kirmeier T, Asmus M, Tuorto F, Uhr M, et al. Valproate and amitriptyline exert common and divergent influences on global and gene promoter-specific chromatin modifications in rat primary astrocytes. Neuropsychopharmacology. 2010;35:792–805. doi: 10.1038/npp.2009.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ. Gliogenesis and glial pathology in depression. CNS Neurol Disord Drug Targets. 2007;6:219–233. doi: 10.2174/187152707780619326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Stockmeier CA. Astrocyte pathology in major depressive disorder: insights from human postmortem brain tissue. Curr Drug Targets. 2013;14:1225–1236. doi: 10.2174/13894501113149990156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkowska G, Miguel-Hidalgo JJ, Wei J, Dilley G, Pittman SD, Meltzer HY, et al. Morphometric evidence for neuronal and glial prefrontal cell pathology in major depression. Biol Psychiatry. 1999;45:1085–1098. doi: 10.1016/s0006-3223(99)00041-4. [DOI] [PubMed] [Google Scholar]
- Rash JE, Kamasawa N, Davidson KG, Yasumura T, Pereda AE, Nagy JI. Connexin composition in apposed gap junction hemiplaques revealed by matched double-replica freeze-fracture replica immunogold labeling. J Membr Biol. 2012;245:333–344. doi: 10.1007/s00232-012-9454-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salameh A, Krautblatter S, Baessler S, Karl S, Rojas Gomez D, Dhein S, et al. Signal transduction and transcriptional control of cardiac connexin43 up-regulation after alpha 1-adrenoceptor stimulation. J Pharmacol Exp Ther. 2008;326:315–322. doi: 10.1124/jpet.108.136663. [DOI] [PubMed] [Google Scholar]
- Salameh A, Krautblatter S, Karl S, Blanke K, Gomez DR, Dhein S, et al. The signal transduction cascade regulating the expression of the gap junction protein connexin43 by beta-adrenoceptors. Br J Pharmacol. 2009;158:198–208. doi: 10.1111/j.1476-5381.2009.00344.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto T, Morioka N, Sato K, Hisaoka K, Nakata Y. Noradrenergic regulation of period1 expression in spinal astrocytes is involved in protein kinase A, c-Jun N-terminal kinase and extracellular signal-regulated kinase activation mediated by α1- and β2-adrenoceptors. Neuroscience. 2011;185:1–13. doi: 10.1016/j.neuroscience.2011.04.024. [DOI] [PubMed] [Google Scholar]
- Sun JD, Liu Y, Yuan YH, Li J, Chen NH. Gap junction dysfunction in the prefrontal cortex induces depressive-like behaviors in rats. Neuropsychopharmacology. 2012;37:1305–1320. doi: 10.1038/npp.2011.319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tacheau C, Fontaine J, Loy J, Mauviel A, Verrecchia F. TGF-beta induces connexin43 gene expression in normal murine mammary gland epithelial cells via activation of p38 and PI3K/AKT signaling pathways. J Cell Physiol. 2008;217:759–768. doi: 10.1002/jcp.21551. [DOI] [PubMed] [Google Scholar]
- Tai YH, Tsai RY, Lin SL, Yeh CC, Wang JJ, Tao PL, et al. Amitriptyline suppresses neuroinflammation-dependent interleukin-10-p38 mitogen-activated protein kinase-heme oxygenase-1 signaling pathway in chronic morphine-infused rats. Anesthesiology. 2009;110:1379–1389. doi: 10.1097/ALN.0b013e31819fccd5. [DOI] [PubMed] [Google Scholar]
- Tanos T, Marinissen MJ, Leskow FC, Hochbaum D, Martinetto H, Gutkind JS, et al. Phosphorylation of c-Fos by members of the p38 MAPK family. Role in the AP-1 response to UV light. J Biol Chem. 2005;280:18842–18852. doi: 10.1074/jbc.M500620200. [DOI] [PubMed] [Google Scholar]
- Theis M, Speidel D, Willecke K. Astrocyte cultures from conditional connexin43-deficient mice. Glia. 2004;46:130–141. doi: 10.1002/glia.10350. [DOI] [PubMed] [Google Scholar]
- Theis M, Söhl G, Eiberger J, Willecke K. Emerging complexities in identity and function of glial connexins. Trends Neurosci. 2005;28:188–195. doi: 10.1016/j.tins.2005.02.006. [DOI] [PubMed] [Google Scholar]
- Vis JC, Nicholson LF, Faull RL, Evans WH, Severs NJ, Green CR. Connexin expression in Huntington's diseased human brain. Cell Biol Int. 1998;22:837–847. doi: 10.1006/cbir.1998.0388. [DOI] [PubMed] [Google Scholar]
- Wang L, Zhang X, Liu L, Cui L, Yang R, Li M, et al. Tanshinone II A down-regulates HMGB1, RAGE, TLR4, NF-kappaB expression, ameliorates BBB permeability and endothelial cell function, and protects rat brains against focal ischemia. Brain Res. 2010;1321:143–151. doi: 10.1016/j.brainres.2009.12.046. [DOI] [PubMed] [Google Scholar]
- Yu W, Dahl G, Werner R. The connexin43 gene is responsive to oestrogen. Proc Biol Sci. 1994;255:125–132. doi: 10.1098/rspb.1994.0018. [DOI] [PubMed] [Google Scholar]
- Zhang FF, Morioka N, Nakashima-Hisaoka K, Nakata Y. Spinal astrocytes stimulated by tumor necrosis factor-α and/or interferon-γ attenuate connexin 43-gap junction via c-jun terminal kinase activity. J Neurosci Res. 2013;91:745–756. doi: 10.1002/jnr.23213. [DOI] [PubMed] [Google Scholar]


