Abstract
Background and Purpose
There is current interest in oxytocin (OT) as a possible therapeutic in psychiatric disorders. However, the usefulness of OT may be constrained by peripheral autonomic effects, which may involve an action at both OT and vasopressin V1A receptors. Here, we characterized the cardiovascular and thermoregulatory effects of OT, vasopressin (AVP) and the non-peptide OT receptor agonist WAY 267,464 in rats, and assessed the relative involvement of the OT and V1A receptors in these effects.
Experimental Approach
Biotelemetry in freely moving male Wistar rats was used to examine body temperature and heart rate after OT (0.01 – 1 mg kg−1; i.p.), AVP (0.001 – 0.1 mg kg−1; i.p.) or WAY 267,464 (10 and 100 mg kg−1; i.p.). The actions of the OT receptor antagonist Compound 25 (C25, 5 and 10 mg kg−1) and V1A receptor antagonist SR49059 (1 and 10 mg kg−1) were studied, as well as possible V1A receptor antagonist effects of WAY 267,464.
Key Results
OT and AVP dose-dependently reduced body temperature and heart rate. WAY 267,464 had similar, but more modest, effects. SR49059, but not C25, prevented the hypothermia and bradycardia induced by OT and AVP. WAY 267,464 (100 mg·kg−1) prevented the effects of OT, and to some extent AVP.
Conclusions and Implications
Peripherally administered OT and AVP have profound cardiovascular and thermoregulatory effects that appear to principally involve the V1A receptor rather than the OT receptor. Additionally, WAY 267,464 is not a simple OT receptor agonist, as it has functionally relevant V1A antagonist actions.
Keywords: oxytocin; vasopressin; WAY 267,464; Compound 25; SR49059; vasopressin V1A receptor; body temperature; heart rate; biotelemetry
Introduction
Oxytocin (OT) and vasopressin (AVP) are two structurally related neuropeptides with a well-documented role in social behaviour, learning and memory, emotion, and motivation (for review see Benarroch, 2013). Both neuropeptides also have major physiological functions with OT involved in milk ejection, uterine contraction (Borrow and Cameron, 2011), energy balance (Olszewski et al., 2010) and cardiovascular homeostasis (Gutkowska and Jankowski, 2012), and AVP playing important roles in regulating water retention and blood pressure (Frank and Landgraf, 2008). There is current widespread interest in the use of peripherally administered OT in treating human psychopathology characterized by impaired social behaviour, including autism (Domes et al., 2013), addictions (McGregor and Bowen, 2012) and schizophrenia (Feifel et al., 2012).
OT and AVP, like most peptides, are large molecules with physiochemical properties that impede entry into the brain (Mens et al., 1983). While there is emerging evidence of brain penetration of OT in rodents (Neumann et al., 2013), only a small proportion appears to pass, necessitating substantial peripheral doses to obtain clear behavioural effects (Uvnäs-Moberg et al., 1994; Klenerova et al., 2009; Hicks et al., 2012). Such high peripheral doses may result in poor selectivity for their cognate receptors as well as significant cardiovascular and other off-target effects that may influence behaviour (Ring, 2011; Carson et al., 2013). For example, OT influences body temperature (Lundeberg et al., 1994; Ring et al., 2006), and can cause a strong bradycardic response (Costa-e-Sousa et al., 2005). Similarly, peripherally delivered AVP can cause dose-dependent hypothermia (Okuno et al., 1965) and cardiovascular effects (Chernoff and Grabowski, 1971).
OT has affinity for both OT and AVP receptors and demonstrates surprisingly strong binding and functional activity at the AVP V1A receptor (receptor nomenclature conforms to Alexander et al., 2013) (Manning et al., 2012). Indeed, OT-induced proconvulsive effects (Loyens et al., 2011), smooth muscle contractions (Gupta et al., 2009), analgesia (Schorscher-Petcu et al., 2010) and even social behaviour (Sala et al., 2011; Ramos et al., 2013), may be primarily mediated by the V1A receptor rather than the OT receptor. However, the extent to which the V1A receptor mediates thermoregulatory and cardiac effects of OT relative to the OT receptor requires further investigation.
The poor brain penetration of OT has stimulated interest in the development of ‘small-molecule’ non-peptide ligands that selectively target the OT and/or V1A receptor, with improved oral bioavailability and brain penetration relative to OT (Manning et al., 2012). The first-generation non-peptide OT receptor agonist WAY 267,464, similar to OT, produces anxiolytic- and antipsychotic-like effects in rodents (Ring et al., 2010). We recently showed that WAY 267,464 and OT caused similar, although slightly divergent patterns of c-Fos expression in rat brain, and subtly different behavioural effects in rodents (Hicks et al., 2012). In vitro receptor binding and functional assays indicated unanticipated antagonist properties of WAY 267,464 at the V1A receptor, in addition to primary OT receptor agonist effects. This would presumably distinguish WAY 267,464 from OT itself, which is both a V1A and OT receptor agonist (Hicks et al., 2012).
Here, we used biotelemetry to characterize the effects of peripherally administered OT, AVP and WAY 267,464 on body temperature and heart rate in freely moving rats. To determine the exact role of the OT and V1A receptors in the observed changes in body temperature and heart rate, we examined the antagonist effects of the selective non-peptide OT receptor antagonist Compound 25 (C25) (Brown et al., 2010; Ramos et al., 2013), and the selective non-peptide V1A receptor antagonist SR49059 (Serradeil-Le Gal et al., 1993). Finally, given the possibility that WAY 267,464 may have V1A receptor antagonist properties, we examined whether this compound might also antagonize some of the observed effects of OT and AVP.
Methods
Drugs and drug preparation
OT and AVP were purchased from AusPep, Ltd. (Parkville, VIC, Australia). The V1A receptor antagonist SR49059 ((S)-1-[(2R,3S)-5-chloro-3-(2-chloro-phenyl)-1-(3,4-dimethoxy-benzenesulfonyl)-3-hydroxy-2,3-dihydro-1H-indole-2-carbonyl]-pyrrolidine-2-carboxylic acid amide) was obtained from Axon Medchem BV (Groningen, The Netherlands). C25 (5-(3-(3-(2-chloro-4-fluorophenoxy)azetidin-1-yl)-5-(methoxymethyl)-4H-1,2,4-triazol-4-yl)-2-methoxypyridine) and WAY 267,464 (4-(3,5-dihydroxybenzyl)-N-(2-methyl-4-(1-methyl-1,4,5,10-tetrahydrobenzo[β]pyrrolo[2,3-e][1,4]diazepine-5-carbonyl)benzyl)piperazine-1-carboxamide) were synthesized according to the procedures of Brown et al. (2010) and Hudson et al. (2005), respectively, and were considered to be of >95% purity according to proton, carbon nuclear magnetic resonance spectroscopy and mass spectrometry. All drugs were dissolved in a 15% DMSO, 2% Tween-80 and 83% physiological saline vehicle (Hicks et al., 2012) and administered to rats via i.p. injection at a volume of 2 mL·kg−1, or 4 mL·kg−1 for WAY 267,464.
Animals and housing
All experiments were performed in male Wistar rats (total n = 27; Animal Resources Centre, Perth, Australia) weighing 259–309 g at the start of testing. For 7 days before surgery, rats were housed in groups of three to four in large plastic tubs (640 × 400 × 220 mm) with corn cob bedding in a temperature-controlled colony room (23 ± 0.5°C) maintained on a reverse 12 h light/dark cycle (lights off at 09:00 h). Rats were provided with environmental enrichment that consisted of a perspex box, a chew stick and nesting material. After surgery, rats were single housed in translucent Plexiglas tubs (420 × 260 × 180 mm) to allow for recording of individual animals using the biotelemetry system. These individual tubs had corn cob bedding and a chew stick and nesting material for environmental enrichment, and were kept on racks in a separate test room that was maintained under identical temperature and light conditions to the main colony room. Food and water were available ad libitum during all experiments. All experimental procedures were conducted under the approval of the University of Sydney Animal Ethics Committee in accordance with the Australian Code of Practice for the Care and Use of Animals for Scientific Purposes (7th Edition, 2004), and are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath et al., 2010).
Surgery
Rats were anaesthetized with isoflurane gas (induction: 3% isoflurane, 3 L·min−1 O2; maintenance: 2% isoflurane, 3 L·min−1 O2), placed on a heating pad and the abdominal area was shaved and cleaned with ethanol. After testing of withdrawal reflexes to ensure adequate depth of anaesthesia, a rostro-caudal incision of approximately 2 cm was made along the midline of the abdomen, and the radiotelemetry transmitter was inserted into the peritoneal cavity. Transmitters were secured to the abdominal wall with non-absorbable sutures during closure of the peritoneal cavity. The biopotential leads from the transmitter were arranged in an Einthoven bipolar-lead II configuration with one lead placed near the right foreleg, and the other at the left hind leg. Animals were administered an analgesic (Ilium Flunixil, Troy Laboratories, Glendenning, Australia; 0.02 mg·kg−1, i.p.), an antibiotic (Ilium Oxytet-200 L.A., Troy Laboratories; 0.1 mg·kg−1, i.p.) and sterile physiological saline (0.9%; 2.5 mL·kg−1, i.p.) postoperatively for 3 days, and allowed to recover for 7 days before testing commenced.
Measurement of body temperature and heart rate
Surgically implanted radiotelemetry transmitters [TA11CTA-F40, Data Sciences International (DSI), St. Paul, MN, USA] were used to measure the body temperature (°C) and ECG of freely moving rats. Transmitters were turned on by means of a magnet held close to the animal's abdomen that activated a magnetic switch within the transmitter. Radio signals from the transmitters were detected by a total of eight RPC-1 receivers connected to two Data Exchange Matrices (Dataquest A.R.T., DSI). The temperature and ECG for each implanted transmitter was calibrated according to the manufacturer's configuration settings. A personal computer (Dell, Precision T3500) inside the room running the Dataquest A.R.T. software (version 4.3, DSI) was used for configuration, control, acquisition and storage of body temperature and ECG data. Heart rate (beats min−1) was derived from the ECG data.
Experimental procedures
All experiments were conducted at an ambient temperature of 23°C (±0.5). For each experiment described later, body temperature and heart rate were averaged over 5 min intervals from 60 min before, to 180 min after drug treatment. This post-injection period was sufficiently long for the majority of the observed drug effects to return to baseline levels. Rats remained in their individual home cages throughout the experiments, with the exception of when they were briefly removed to be injected with their allocated treatment. All experiments were performed during the dark phase and commenced 3 h after lights were switched off. Rats show a major activity burst immediately after light offset (Sei et al., 1997), so this time point was chosen to ensure the animal's body temperature and heart rate had stabilized following the transition into the dark phase.
Each drug treatment was separated by a 48 h washout period to ensure adequate drug clearance, and each rat received a maximum of six specific drug treatments (excluding vehicle treatment). To minimize any changes in body temperature and heart rate resulting from handling or injection stress, rats were handled for 5 min each for 5 days prior to testing, and were given a dummy injection of vehicle (2 mL·kg−1) on the last 2 days of the handling period.
OT dose–response (Experiment 1)
An initial exploratory experiment characterized the dose-dependent effects of OT on body temperature and heart rate after peripheral administration. Three experimentally naïve rats were each tested with vehicle before three doses of OT (0.01, 0.1 and 1 mg·kg−1) were given in ascending order.
C25 and OT (Experiments 2A and 2B)
To determine whether the body temperature and heart rate effects of OT are mediated by the OT receptor, four experimentally naïve rats were administered OT with or without the selective OT receptor antagonist C25 at two dose levels (5 and 10 mg·kg−1). Drug combinations included (i) vehicle + vehicle; (ii) C25 (5 mg·kg−1) + OT (1 mg·kg−1); (iii) C25 (10 mg·kg−1) + OT (1 mg·kg−1); and (iv) vehicle + OT (1 mg·kg−1), and were administered in this sequence. Experiment 2B was performed 1 week after the conclusion of Experiment 2A to determine whether C25 by itself has intrinsic effects on body temperature and heart rate. The same cohort of rats (n = 4) from Experiment 2A were administered (i) vehicle + vehicle; (ii) C25 (5 mg·kg−1) + vehicle; and (iii) C25 (10 mg·kg−1) + vehicle, in this sequence. The first and second injections were separated by 15 min in both experiments.
SR49059 and OT (Experiments 3A and 3B)
This experiment employed the selective V1A receptor antagonist SR49059 to examine the extent to which OT acts on the V1A receptor to affect body temperature and heart rate. SR49059 was administered at a dose of 1 mg·kg−1, which produces significant inhibitory effects in a rat model of AVP-induced hypertension (Serradeil-Le Gal et al., 1993) and prevents OT-induced prosocial effects (Ramos et al., 2013). A maximal dose of 10 mg·kg−1 was also tested. Four experimentally naïve rats were given the following treatments in the order of: (i) vehicle + vehicle; (ii) SR49059 (1 mg·kg−1) + OT (1 mg·kg−1); (iii) SR49059 (10 mg·kg−1) + OT (1 mg·kg−1); and (iv) vehicle + OT (1 mg·kg−1). Experiment 3B, performed 1 week after the conclusion of Experiment 3A, determined any intrinsic effects of SR49059 alone at 1 and 10 mg·kg−1 on body temperature and heart rate. The same cohort of rats (n = 4) from Experiment 3A were administered (i) vehicle + vehicle; (ii) SR49059 (1 mg·kg−1) + vehicle; and (iii) SR49059 (10 mg·kg−1) + vehicle, in this sequence. The first and second injections were separated by 15 min in both experiments.
AVP dose–response (Experiment 4)
Experiment 4 explored the dose-dependent effects of peripheral AVP on body temperature and heart rate. Four experimentally naïve rats were treated with vehicle and then three doses of AVP (0.001, 0.01 and 0.1 mg·kg−1) in ascending order.
SR49059 and AVP (Experiment 5)
Four experimentally naïve rats were given the following treatments in the order of: (i) vehicle + vehicle; (ii) SR49059 (1 mg·kg−1) + AVP (0.1 mg·kg−1); (iii) SR49059 (10 mg·kg−1) + AVP (0.1 mg·kg−1); and (iv) vehicle + AVP (0.1 mg·kg−1). The first and second injections were separated by 15 min.
WAY 267,464 dose–response (Experiment 6)
Experiment 6 examined whether WAY 267,464 has OT-like effects on body temperature and heart rate, given their previously observed similarities (Ring et al., 2010; Hicks et al., 2012). Four experimentally naïve rats were first tested with vehicle followed by consecutive tests with WAY 267,464 (10 and 100 mg·kg−1) in ascending order, with these doses based on our previous study (Hicks et al., 2012).
C25 and WAY 267,464 (Experiment 7)
Experiment 7 determined whether WAY 267,464 acts on the OT receptor to affect body temperature and heart rate. One week after Experiment 6, the same cohort of rats in Experiment 6 (n = 4) received in the following order: (i) vehicle + vehicle; (ii) C25 (10 mg·kg−1) + WAY 267,464 (100 mg·kg−1); and (iii) vehicle + WAY 267,464 (100 mg·kg−1). The first and second injections were separated by 15 min. One rat was removed from the analysis because of a transmitter failure.
WAY 267,464 and OT (Experiment 8)
Our recent research has raised the possibility that WAY 267,464 might have a V1A receptor antagonist action (Hicks et al., 2012). Accordingly, we assessed whether the hypothermia and bradycardia produced by OT might be prevented by pretreatment with WAY 267,464. Four experimentally naïve rats were given in the following order: (i) vehicle + vehicle; (ii) WAY 267,464 (10 mg·kg−1) + OT (1 mg·kg−1); (iii) WAY 267,464 (100 mg·kg−1) + OT (1 mg·kg−1); and (iv) vehicle + OT (1 mg·kg−1). The first and second injections were separated by 20 min.
WAY 267,464 and AVP (Experiment 9)
In the final experiment, WAY 267,464 was given in combination with AVP as a further test of its putative V1A receptor antagonist action. One week after the AVP dose–response experiment (Experiment 4), the same group of rats used in Experiment 4 (n = 4) received treatments as follows: (i) vehicle + vehicle; (ii) WAY 267,464 (10 mg·kg−1) + AVP (0.1 mg·kg−1); (iii) WAY 267,464 (100 mg·kg−1) + AVP (0.1 mg·kg−1); and (iv) vehicle + AVP (0.1 mg·kg−1). The first and second injections were separated by 20 min.
Statistical analysis
For Experiments 1, 4 and 6 (the dose–response studies of OT, AVP and WAY 267,464), orthogonal polynomial contrasts were used to examine changes in body temperature and heart rate across ascending doses of each drug treatment averaged over the 180 min post-injection period. For the remaining experiments, planned contrasts (two-way repeated measures anova) examined treatment × time effects on body temperature and heart rate with each treatment compared with the relevant control condition. Any baseline (pretreatment) differences in average body temperature and heart rate between treatment conditions were identified during the 60 min before drug treatment using a priori contrasts (one-way repeated measures anova) and are noted in the results section. The Bonferroni correction was used to adjust for non-orthogonal contrasts, and problems with sphericity of the data (Mauchly's test) were addressed using the Greenhouse–Geisser correction. Corrected degrees of freedom are reported where relevant. All analyses were conducted using Statistical Package for the Social Sciences (SPSS) version 19 (SPSS Inc., IBM, Chicago, IL, USA) with significance set at 0.05 or where corrected using Bonferroni (P < 0.05/2 = 0.025 or P < 0.05/3 = 0.017).
Results
Table 1 shows the average body temperature and heart rate during the 60 min baseline period for each treatment in each experiment. Summary statistical results for body temperature and heart rate during the post-injection period for each experiment are presented in Tables 2 and 3 respectively.
Table 1.
Average body temperature and heart rate during the baseline period
| Experiment | Body temperature (°C) | Heart rate (beats min−1) |
|---|---|---|
| 1. OT dose–response | ||
| (i) VEH | 38.11 ± 0.05 | 438.52 ± 42.89 |
| (ii) 0.01 mg·kg−1 | 38.18 ± 0.04 | 453.82 ± 24.08 |
| (iii) 0.1 mg·kg−1 | 38.10 ± 0.05 | 435.48 ± 26.88 |
| (iv) 1 mg·kg−1 | 38.11 ± 0.10 | 419.73 ± 19.18 |
| 2A. C25 (5 and 10 mg·kg−1) and OT (1 mg·kg−1) | ||
| (i) VEH + VEH | 37.78 ± 0.10 | 395.26 ± 18.30 |
| (ii) C25 (5) + OT | 37.71 ± 0.02 | 414.00 ± 7.12 |
| (iii) C25 (10) + OT | 37.64 ± 0.09 | 403.84 ± 3.25 |
| (iv) VEH + OT | 37.75 ± 0.10 | 399.29 ± 14.64 |
| 2B. C25 (5 and 10 mg·kg−1) and VEH | ||
| (i) VEH + VEH | 37.77 ± 0.10 | 418.65 ± 8.50 |
| (ii) C25 (5) + VEH | 37.79 ± 0.07 | 428.77 ± 8.69 |
| (iii) C25 (10) + VEH | 37.78 ± 0.05 | 413.70 ± 10.02 |
| 3A. SR (1 and 10 mg·kg−1) and OT (1 mg·kg−1) | ||
| (i) VEH + VEH | 38.22 ± 0.06 | 396.62 ± 10.53 |
| (ii) SR (1) + OT | 38.19 ± 0.11 | 432.36 ± 10.33 |
| (iii) SR (10) + OT | 38.26 ± 0.12 | 445.15 ± 4.20 |
| (iv) VEH + OT | 38.34 ± 0.16 | 431.16 ± 6.84 |
| 3B. SR (1 and 10 mg·kg−1) and VEH | ||
| (i) VEH + VEH | 37.91 ± 0.12 | 393.91 ± 6.24 |
| (ii) SR (1) + VEH | 37.97 ± 0.04 | 395.66 ± 8.28 |
| (iii) SR (10) + VEH | 38.08 ± 0.08 | 427.40 ± 9.69 |
| 4. AVP dose–response | ||
| (i) VEH | 38.19 ± 0.02 | 444.52 ± 12.01 |
| (ii) 0.001 mg·kg−1 | 38.05 ± 0.10 | 429.50 ± 7.58 |
| (iii) 0.01 mg·kg−1 | 38.19 ± 0.10 | 441.09 ± 9.73 |
| (iv) 0.1 mg·kg−1 | 38.17 ± 0.09 | 428.73 ± 9.67 |
| 5. SR (1 and 10 mg·kg−1) and AVP (0.1 mg·kg−1) | ||
| (i) VEH + VEH | 38.04 ± 0.09 | 397.61 ± 9.68 |
| (ii) SR (1) + AVP | 38.07 ± 0.10 | 411.80 ± 5.44 |
| (iii) SR (10) + AVP | 38.24 ± 0.04 | 409.37 ± 15.57 |
| (iv) VEH + AVP | 38.23 ± 0.10 | 402.63 ± 7.22 |
| 6. WAY dose–response | ||
| (i) VEH | 37.98 ± 0.12 | 414.54 ± 10.73 |
| (ii) 10 mg·kg−1 | 38.03 ± 0.12 | 425.14 ± 13.99 |
| (iii) 100 mg·kg−1 | 38.04 ± 0.15 | 424.45 ± 11.63 |
| 7. C25 (10 mg·kg−1) and WAY (100 mg·kg−1) | ||
| (i) VEH + VEH | 37.88 ± 0.21 | 389.29 ± 14.19 |
| (ii) C25 + WAY | 37.90 ± 0.09 | 432.70 ± 15.72* |
| (iii) VEH + WAY | 38.05 ± 0.17 | 417.44 ± 14.40 |
| 8. WAY (10 and 100 mg·kg−1) and OT (1 mg·kg−1) | ||
| (i) VEH + VEH | 37.82 ± 0.12 | 396.01 ± 9.39 |
| (ii) WAY (10) + OT | 37.98 ± 0.10 | 418.04 ± 11.14 |
| (iii) WAY (100) + OT | 37.90 ± 0.09 | 403.36 ± 11.39 |
| (iv) VEH + OT | 37.71 ± 0.07 | 398.29 ± 9.86 |
| 9. WAY (10 and 100 mg·kg−1) and AVP (0.1 mg·kg−1) | ||
| (i) VEH + VEH | 37.86 ± 0.12 | 402.79 ± 15.02 |
| (ii) WAY (10) + AVP | 37.92 ± 0.13 | 395.87 ± 18.54 |
| (iii) WAY (100) + AVP | 38.04 ± 0.09 | 422.58 ± 10.87 |
| (iv) VEH + AVP | 37.93 ± 0.05 | 381.51 ± 4.58 |
Values represent the mean ± SEM.
Asterisk represents a significant difference in heart rate:
P < 0.025.
C25, Compound 25; SR, SR49059; VEH, vehicle; WAY, WAY 267,464.
Table 2.
Treatment effects on body temperature during the 180 min post-injection period
| Experiment | Treatment | Treatment × Time |
|---|---|---|
| 1. OT dose–response | ||
| (i) Linear trend | F(1,2) = 21.15* | – |
| (ii) Quadratic trend | F(1,2) = 23.64* | – |
| 2A. C25 (5 and 10 mg·kg−1) and OT (1 mg·kg−1) | ||
| (i) VEH versus OT | F(1,3) = 33.93** | F(1.49,4.46) = 28.52** |
| (ii) OT versus C25 (5) + OT | F(1,3) = 1.00 | F(1.84,5.52) = 0.40 |
| (iii) OT versus C25 (10) + OT | F(1,3) = 0.01 | F(1.82,5.47) = 1.29 |
| 2B. C25 (5 and 10 mg·kg−1) and VEH | ||
| (i) VEH versus C25 (5) + VEH | F(1,3) = 3.16 | F(2.25,6.75) = 0.62 |
| (ii) VEH versus C25 (10) + VEH | F(1,3) = 1.11 | F(2.35,7.05) = 1.67 |
| 3A. SR (1 and 10 mg·kg−1) and OT (1 mg·kg−1) | ||
| (i) VEH versus OT | F(1,3) = 32.71* | F(1.56,4.67) = 17.22** |
| (ii) OT versus SR (1) + OT | F(1,3) = 22.56 | F(1.68,5.03) = 14.81** |
| (iii) OT versus SR (10) + OT | F(1,3) = 26.76* | F(1.91,5.72) = 30.34*** |
| 3B. SR (1 and 10 mg·kg−1) and VEH | ||
| (i) VEH versus SR (1) + VEH | F(1,3) = 0.91 | F(1.90,5.69) = 0.41 |
| (ii) VEH versus SR (10) + VEH | F(1,3) = 9.19 | F(2.29,6.86) = 2.51 |
| 4. AVP dose–response | ||
| (i) Linear trend | F(1,3) = 56.08** | – |
| (ii) Quadratic trend | F(1,3) = 15.07* | – |
| 5. SR (1 and 10 mg·kg−1) and AVP (0.1 mg·kg−1) | ||
| (i) VEH versus AVP | F(1,3) = 132.56*** | F(2.27,6.81) = 16.44** |
| (ii) AVP versus SR (1) + AVP | F(1,3) = 33.18** | F(1.92,5.77) = 32.63*** |
| (iii) AVP versus SR (10) + AVP | F(1,3) = 102.27** | F(2.14,6.41) = 22.11*** |
| 6. WAY dose–response | ||
| (i) Linear trend | F(1,3) = 22.15* | – |
| (ii) Quadratic trend | F(1,3) = 10.56* | – |
| 7. C25 (10 mg·kg−1) and WAY (100 mg·kg−1) | ||
| (i) VEH versus WAY | F(1,2) = 59.67* | F(1.27,2.54) = 14.21 |
| (ii) WAY versus C25 + WAY | F(1,2) = 0.34 | F(1.30,2.59) = 0.23 |
| 8. WAY (10 and 100 mg·kg−1) and OT (1 mg·kg−1) | ||
| (i) VEH versus OT | F(1,3) = 104.03** | F(2.22,6.67) = 47.58*** |
| (ii) OT versus WAY (10) + OT | F(1,3) = 44.30** | F(2.17,6.49) = 19.26** |
| (iii) OT versus WAY (100) + OT | F(1,3) = 27.22* | F(2.56,7.69) = 50.66*** |
| 9. WAY (10 and 100 mg·kg−1) and AVP (0.1 mg·kg−1) | ||
| (i) VEH versus AVP | F(1,3) = 32.19* | F(1.72,5.17) = 7.00 |
| (ii) AVP versus WAY (10) + AVP | F(1,3) = 4.77 | F(1.33,4.00) = 1.56 |
| (iii) AVP versus WAY (100) + AVP | F(1,3) = 0.99 | F(1.48,4.44) = 1.87 |
Asterisks represent significant differences in body temperature:
P < 0.05,
P < 0.01 and
P < 0.001.
C25, Compound 25; SR, SR49059; VEH, vehicle; WAY, WAY 267,464.
Table 3.
Treatment effects on heart rate during the 180 min post-injection period
| Experiment | Treatment | Treatment × Time |
|---|---|---|
| 1. OT dose–response | ||
| (i) Linear trend | F(1,2) = 17.26 | – |
| (ii) Quadratic trend | F(1,2) = 18.45* | – |
| 2A. C25 (5 and 10 mg·kg−1) and OT (1 mg·kg−1) | ||
| (i) VEH versus OT | F(1,3) = 57.03** | F(2.38,7.15) = 7.84* |
| (ii) OT versus C25 (5) + OT | F(1,3) = 4.24 | F(2.67,8.00) = 1.16 |
| (iii) OT versus C25 (10) + OT | F(1,3) = 19.40 | F(2.39,7.16) = 1.17 |
| 2B. C25 (5 and 10 mg·kg−1) and VEH | ||
| (i) VEH versus C25 (5) + VEH | F(1,3) = 20.66* | F(2.90,8.69) = 1.41 |
| (ii) VEH versus C25 (10) + VEH | F(1,3) = 47.06** | F(2.73,8.18) = 1.51 |
| 3A. SR (1 and 10 mg·kg−1) and OT (1 mg·kg−1) | ||
| (i) VEH versus OT | F(1,3) = 126.43** | F(2.27,6.80) = 7.56* |
| (ii) OT versus SR (1) + OT | F(1,3) = 21.78 | F(2.52,7.55) = 10.22** |
| (iii) OT versus SR (10) + OT | F(1,3) = 59.30** | F(2.62,7.86) = 13.03** |
| 3B. SR (1 and 10 mg·kg−1) and VEH | ||
| (i) VEH versus SR (1) + VEH | F(1,3) = 0.32 | F(2.15,6.45) = 0.86 |
| (ii) VEH versus SR (10) + VEH | F(1,3) = 0.09 | F(2.36,7.08) = 1.67 |
| 4. AVP dose–response | ||
| (i) Linear trend | F(1,3) = 84.17** | – |
| (ii) Quadratic trend | F(1,3) = 13.67* | – |
| 5. SR (1 and 10 mg·kg−1) and AVP (0.1 mg·kg−1) | ||
| (i) VEH versus AVP | F(1,3) = 571.38*** | F(2.21,6.63) = 2.98 |
| (ii) AVP versus SR (1) + AVP | F(1,3) = 41.28** | F(2.49,7.46) = 2.22 |
| (iii) AVP versus SR (10) + AVP | F(1,3) = 92.61** | F(2.25,6.76) = 4.72 |
| 6. WAY dose–response | ||
| (i) Linear trend | F(1,3) = 5.23 | – |
| (ii) Quadratic trend | F(1,3) = 12.63* | – |
| 7. C25 (10 mg·kg−1) and WAY (100 mg·kg−1) | ||
| (i) VEH versus WAY | F(1,2) = 19.16 | F(1.96,3.92) = 3.98 |
| (ii) WAY versus C25 + WAY | F(1,2) = 0.09 | F(1.66,3.33) = 0.57 |
| 8. WAY (10 and 100 mg·kg−1) and OT (1 mg·kg−1) | ||
| (i) VEH versus OT | F(1,3) = 217.31*** | F(2.62,7.85) = 6.64* |
| (ii) OT versus WAY (10) + OT | F(1,3) = 58.17** | F(2.54,7.61) = 3.43 |
| (iii) OT versus WAY (100) + OT | F(1,3) = 31.52* | F(2.11,6.34) = 8.49* |
| 9. WAY (10 and 100 mg·kg−1) and AVP (0.1 mg·kg−1) | ||
| (i) VEH versus AVP | F(1,3) = 108.58** | F(2.57,7.70) = 4.63 |
| (ii) AVP versus WAY (10) + AVP | F(1,3) = 2.28 | F(2.54,7.62) = 3.01 |
| (iii) AVP versus WAY (100) + AVP | F(1,3) = 18.28 | F(1.85,5.54) = 2.92 |
Asterisks represent significant differences in heart rate:
P < 0.05,
P < 0.01 and
P < 0.001.
C25, Compound 25; SR, SR49059; VEH, vehicle; WAY, WAY 267,464.
Dose-dependent effects of OT on body temperature and heart rate
The effects of OT on body temperature and heart rate are shown in Figure 1A and B respectively. Polynomial contrast analysis showed a significant linear and quadratic trend (both P < 0.05) in body temperature across ascending doses of OT, with the highest dose (1 mg·kg−1) causing a strong hypothermic effect over the post-injection period. For heart rate, there was a strong tendency towards a linear trend (P = 0.053), and a significant quadratic trend (P < 0.05), over ascending doses of OT, which again reflected the highest dose strongly reducing heart rate in the post-treatment period.
Figure 1.
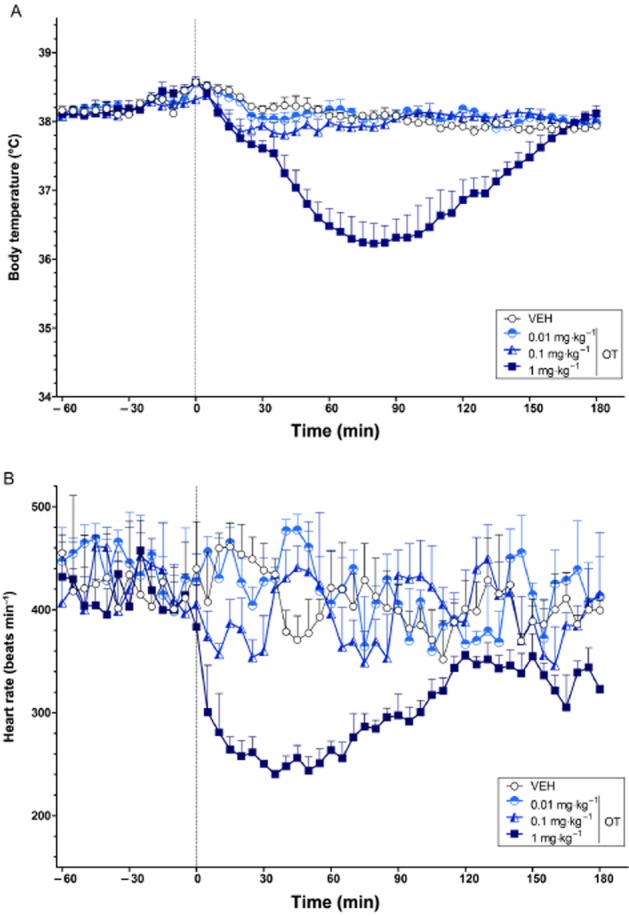
The dose-dependent effects of OT on body temperature (°C) (A) and heart rate (beats min−1) (B) over time (min). The vertical grey hashed line on the X-axis at 0 indicates the time of vehicle or OT administration. Data are the means + SEM. VEH, vehicle.
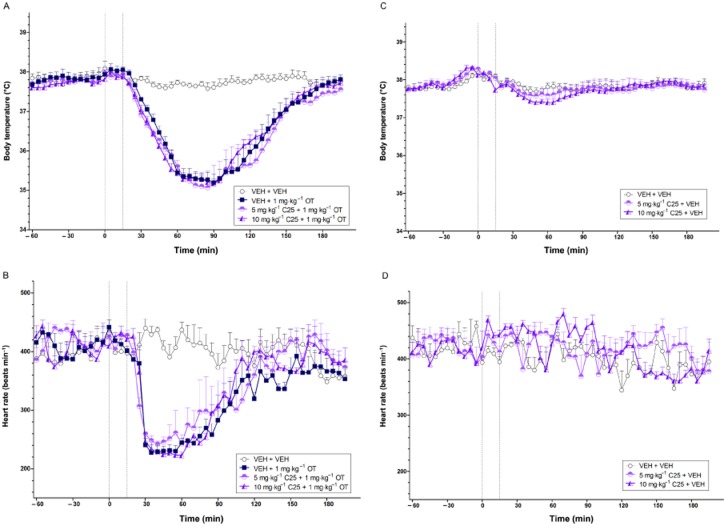
Effects of C25 on OT-induced hypothermia and bradycardia
The effects of C25 on the hypothermia and bradycardia induced by OT (1 mg·kg−1) are shown in Figure 2A and B. Consistent with Experiment 1, OT produced a strong hypothermic and bradycardic response relative to vehicle (both P < 0.01) that recovered to baseline levels after 2–3 h (treatment × time interaction, body temperature: P < 0.01; heart rate: P < 0.017). Pretreatment with either dose of C25 (i.e. 5 and 10 mg·kg−1) did not significantly affect these actions of OT (1 mg·kg−1) (both doses P > 0.017; Bonferroni-corrected).
Figure 2.
The body temperature (°C) (A, C) and heart rate (beats min−1) (B, D) effects of the non-peptide OT receptor antagonist C25 in combination with OT (A, B) and vehicle (C, D) over time (min). The vertical grey hashed line on the X-axis at 0 indicates the time of vehicle or C25 administration, while the second line at X = 15 indicates the time of vehicle or OT injection. Data are the means + SEM. VEH, vehicle.
When given alone, C25 at 5 and 10 mg·kg−1 did not significantly affect body temperature relative to vehicle (both doses P > 0.025; Bonferroni-corrected) (Figure 2C). However, both doses caused a subtle increase in heart rate over the 180 min post-injection period relative to vehicle (5 mg·kg−1: P < 0.025; 10 mg·kg−1: P < 0.01) (Figure 2D).
Effects of SR49059 on OT-induced hypothermia and bradycardia
The effects of SR49059 on OT-induced hypothermia and bradycardia are illustrated in Figure 3A and B respectively. A high dose of OT (1 mg·kg−1) again induced potent hypothermia (P < 0.017) and bradycardia (P < 0.01) relative to vehicle. SR49059 at 1 and 10 mg·kg−1 prevented the hypothermia (treatment effect, 1 mg·kg−1: P = 0.018; 10 mg·kg−1: P < 0.017; treatment × time interaction, 1 mg·kg−1: P < 0.01; 10 mg·kg−1: P < 0.001) and bradycardia (treatment effect, 10 mg·kg−1: P < 0.01; treatment × time interaction, both P < 0.01) caused by OT.
Figure 3.
The body temperature (°C) (A, C) and heart rate (beats min−1) (B, D) effects of the non-peptide V1A receptor antagonist SR49059 in combination with OT (A, B) and vehicle (C, D) over time (min). The vertical grey hashed line on the X-axis at 0 indicates the time of vehicle or SR49059 administration, while the second line at X = 15 indicates the time of vehicle or OT injection. Data are the means + SEM. VEH, vehicle; SR, SR49059.
SR49059 given alone produced no significant effects on body temperature or heart rate, all P > 0.025 (Bonferroni-corrected; Figure 3C and D respectively). There was however, a trend towards a hypothermic effect with the highest dose (10 mg·kg−1), P = 0.056.
Dose-dependent effects of AVP on body temperature and heart rate
The effects of AVP on body temperature and heart rate are illustrated in Figure 4A and B respectively. Polynomial contrast analysis identified a significant linear (P < 0.01) and quadratic (P < 0.05) trend in body temperature and heart rate across ascending doses of AVP. All doses induced a drop in body temperature and heart rate, with the highest dose (0.1 mg·kg−1) producing sustained hypothermia and bradycardia across the entire post-injection period.
Figure 4.
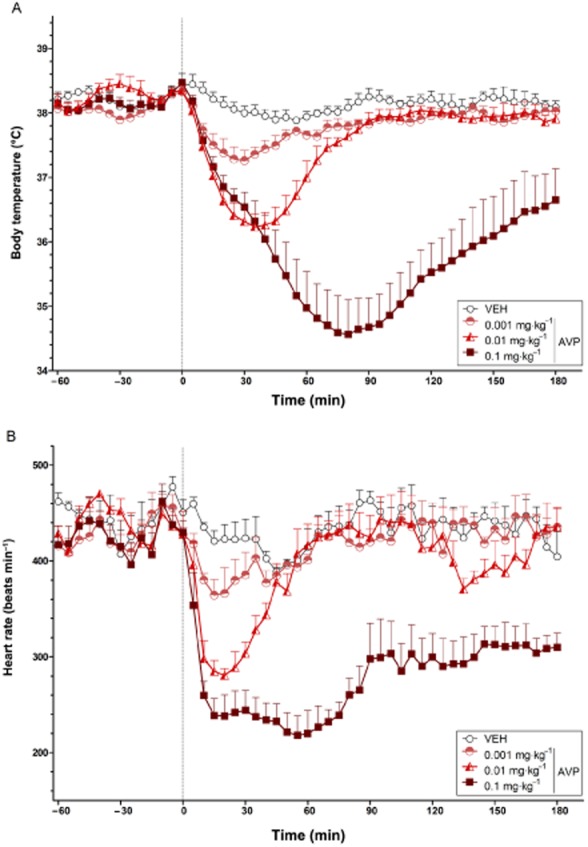
The dose-dependent effects of AVP on body temperature (°C) (A) and heart rate (beats min−1) (B) over time (min). The vertical grey hashed line on the X-axis at 0 indicates the time of vehicle or AVP administration. Data are the means + SEM. VEH, vehicle.
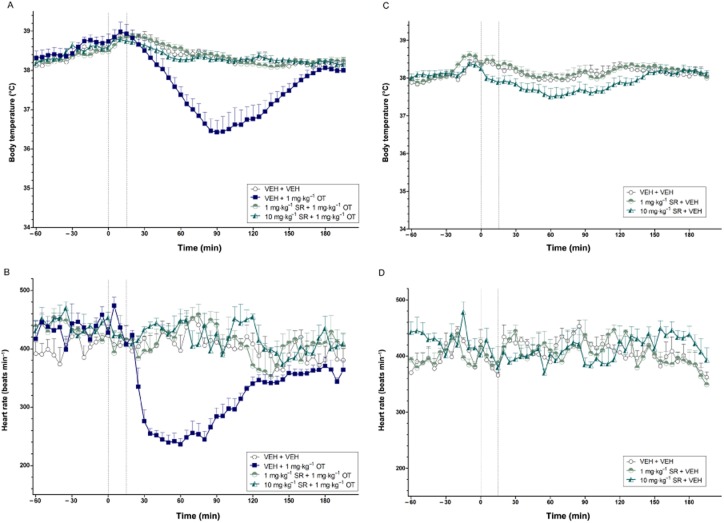
Effects of SR49059 on AVP-induced hypothermia and bradycardia
Consistent with Experiment 4, AVP at a high dose (0.1 mg·kg−1) induced potent and prolonged hypothermia and bradycardia (both P < 0.001). Pretreatment with either dose of SR49059 (i.e. 1 and 10 mg·kg−1) reduced the hypothermic (treatment effect, both P < 0.01; treatment × time interaction, both P < 0.001) and bradycardic (treatment effect, both P < 0.01) effects of AVP (Figure 5A and B respectively). The higher dose of SR49059 (10 mg·kg−1) was required to completely prevent the body temperature and heart rate effects of AVP (0.1 mg·kg−1).
Figure 5.
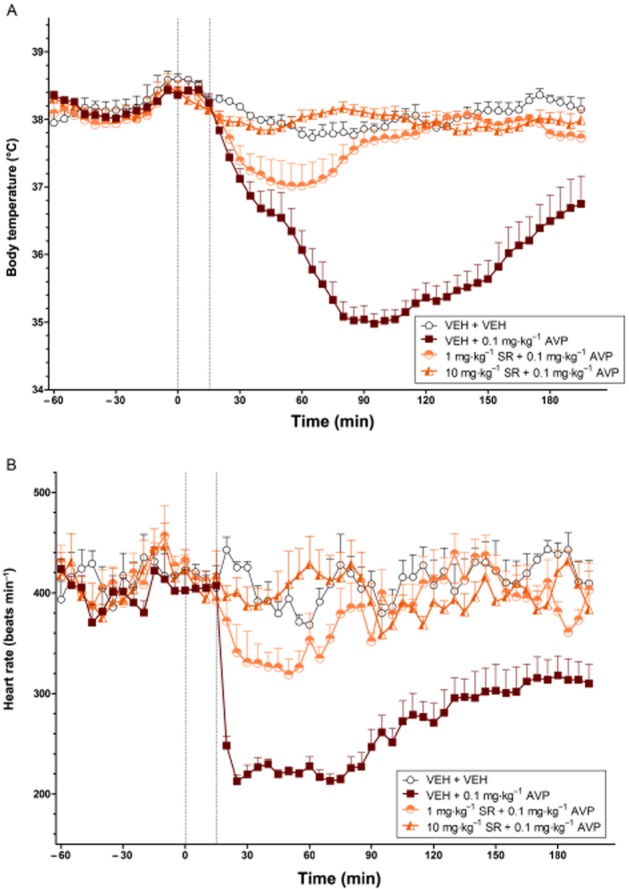
The effects of SR49059 on the body temperature (°C) (A) and heart rate (beats min−1) (B) changes induced by AVP over time (min). The vertical grey hashed line on the X-axis at 0 indicates the time of vehicle or SR49059 administration, while the second line at X = 15 indicates the time of vehicle or AVP injection. Data are the means + SEM. VEH, vehicle; SR, SR49059.
Dose-dependent effects of WAY 267,464 on body temperature and heart rate
Polynomial contrast analysis identified a significant linear and quadratic trend (both P < 0.05) in body temperature over the two doses of WAY 267,464, with the higher dose (100 mg·kg−1) producing hypothermia that lasted across much of the test period (Figure 6A). There was also a significant quadratic trend (P < 0.05) in heart rate with WAY 267,464 reflecting a transient reduction in heart rate with the higher dose (Figure 6B).
Figure 6.
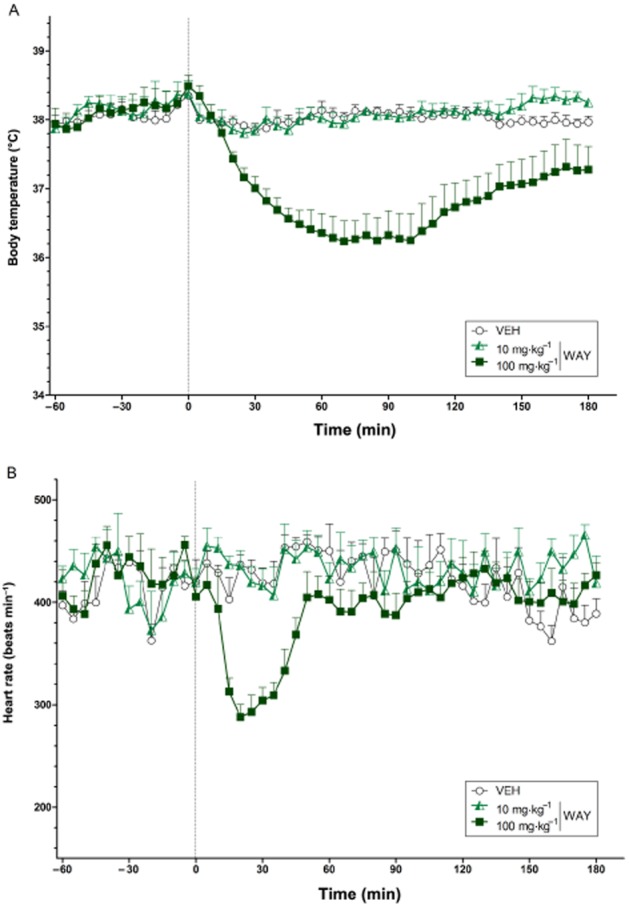
The dose-dependent effects of WAY 267,464 on body temperature (°C) (A) and heart rate (beats min−1) (B) over time (min). The vertical grey hashed line on the X-axis at 0 indicates the time of vehicle or WAY 267,464 administration. Data are the means + SEM. VEH, vehicle; WAY, WAY 267,464.
Effects of C25 on WAY 267,464-induced hypothermia and bradycardia
There were no significant differences in pretreatment body temperature (all P > 0.025; Bonferroni-corrected). However, heart rate during the baseline before the C25 + WAY 267,464 treatment was slightly elevated relative to the baseline for the vehicle + WAY 267,464 condition [F(1,2) = 54.91, P < 0.025]. A high dose of WAY 267,464 (100 mg·kg−1) again caused prolonged hypothermia relative to vehicle (P < 0.025) (Figure 7A), while the transient bradycardic effect approached significance (P = 0.048) (Figure 7B). Pretreatment with C25 at 10 mg·kg−1 failed to attenuate the hypothermic and bradycardic effects of WAY 267,464 (all P > 0.025; Bonferroni-corrected).
Figure 7.
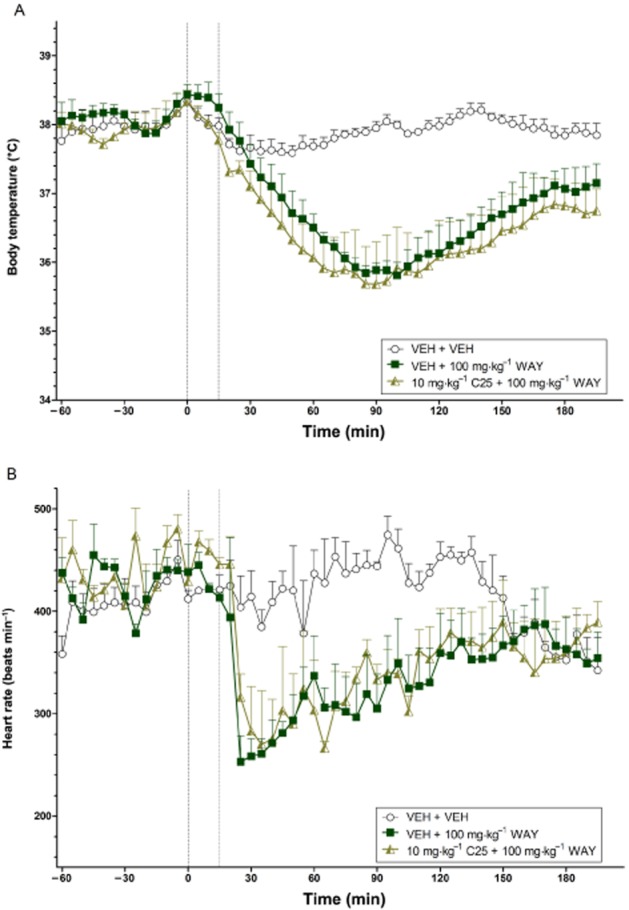
The effects of C25 on the body temperature (°C) (A) and heart rate (beats min−1) (B) changes induced by WAY 267,464 over time (min). The vertical grey hashed line on the X-axis at 0 indicates the time of vehicle or C25 administration, while the second line at X = 15 indicates the time of vehicle or WAY 267,464 injection. Data are the means + SEM. VEH, vehicle; WAY, WAY 267,464.
Effects of WAY 267,464 on OT-induced hypothermia and bradycardia
OT (1 mg·kg−1) again produced a robust hypothermic (P < 0.01) and bradycardic (P < 0.001) effect compared with vehicle that followed a characteristic U-shaped response over time. Pretreatment with 10 or 100 mg·kg−1 WAY 267,464 significantly attenuated the body temperature (treatment effect, 10 mg·kg−1: P < 0.01; 100 mg·kg−1: P < 0.017; treatment × time interaction, 10 mg·kg−1: P < 0.01; 100 mg·kg−1: P < 0.001) and heart rate (treatment effect, 10 mg·kg−1: P < 0.01; 100 mg·kg−1: P < 0.017; treatment × time interaction, 100 mg·kg−1: P < 0.017) effects induced by OT (Figure 8A and B respectively).
Figure 8.
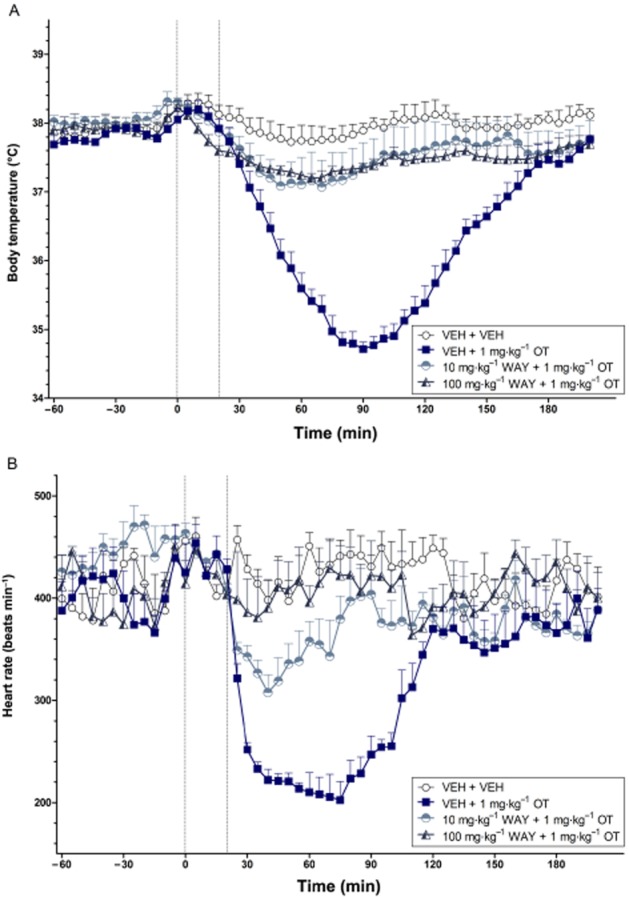
The effects of WAY 267,464 on the body temperature (°C) (A) and heart rate (beats min−1) (B) changes induced by OT over time (min). The vertical grey hashed line on the X-axis at 0 indicates the time of vehicle or WAY 267,464 administration, while the second line at X = 20 indicates the time of vehicle or OT injection. Data are the means + SEM. VEH, vehicle; WAY, WAY 267,464.
Effects of WAY 267,464 on AVP-induced hypothermia and bradycardia
A high dose of AVP (0.1 mg·kg−1) again caused a robust decrease in body temperature (P < 0.017) and heart rate (P < 0.01) relative to vehicle that remained across the test period (Figure 9A and B, respectively). Pretreatment with 10 or 100 mg·kg−1 WAY 267,464 did not significantly reduce the strong hypothermic and bradycardic effects of AVP (all P > 0.017; Bonferroni-corrected), although an apparent antagonist effect on heart rate was close to significance (P = 0.023).
Figure 9.
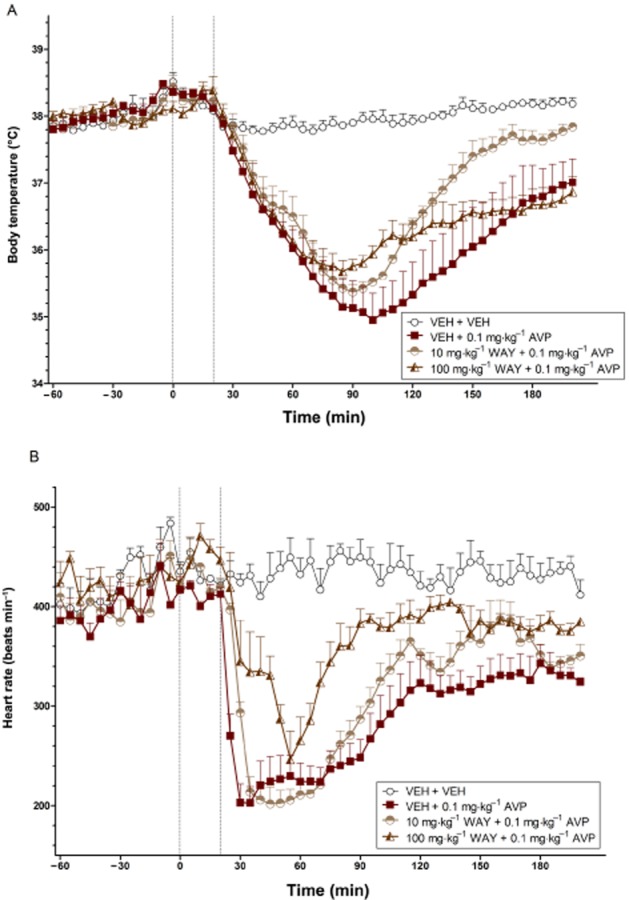
The effects of WAY 267,464 on the body temperature (°C) (A) and heart rate (beats min−1) (B) changes induced by AVP over time (min). The vertical grey hashed line on the X-axis at 0 indicates the time of vehicle or WAY 267,464 administration, while the second line at X = 20 indicates the time of vehicle or AVP injection. Data are the means + SEM. VEH, vehicle; WAY, WAY 267,464.
Discussion and conclusions
The present study used biotelemetry in freely moving rats to characterize the effects of peripherally administered OT, AVP and WAY 267,464 on body temperature and heart rate, and to assess the relative involvement of the OT and V1A receptors in their effects. Results show that OT and AVP cause a dose-dependent reduction of body temperature and heart rate, with the highest doses (i.e. 1 and 0.1 mg·kg−1, respectively) producing effects for up to 3 h after injection. WAY 267,464 (100 mg·kg−1) also induces hypothermia, but with a more transient bradycardic effect than OT or AVP. Remarkably, pretreatment with a V1A receptor antagonist, but not an OT receptor antagonist, prevented the body temperature and cardiovascular effects of OT (and AVP), suggesting these effects are V1A receptor mediated. Moreover, pretreatment with WAY 267,464 partially prevented the effects of OT, and to a certain extent AVP, providing some functional evidence of V1A receptor antagonist actions of this compound.
Our primary findings of hypothermia and bradycardia with OT and AVP are in general agreement with prior studies of cardiovascular and thermoregulatory function that have involved peripheral injection of these neuropeptides (Okuno et al., 1965; Chernoff and Grabowski, 1971; Jodogne et al., 1991; Lundeberg et al., 1994; Xiao-Jun and Wiesenfeld-Hallin, 1994; Ring et al., 2006). However, most of these previous studies utilized traditional procedures that do not allow for continuous monitoring of physiological parameters, or involve stressful interventions (e.g. rectal probes) that can themselves affect body temperature and heart rate (Bouwknecht et al., 2000). In addition, previous studies were often conducted within the context of thermoregulatory and cardiovascular responses to stress, injury or illness-related challenges. For example, Grippo et al. (2009) showed that chronic subcutaneous injection of OT reduces the tachycardia induced by chronic social isolation in prairie voles. Peripherally injected OT also reduced the hyperthermia induced by successive rectal temperature measurements in mice (Ring et al., 2006). Peripheral OT also produces cardioprotective effects in myocardial ischaemia (Houshmand et al., 2009), while systemically delivered AVP has a well-documented antipyretic action (Richmond, 2003). It is particularly striking then, that here we have documented potent thermoregulatory and cardiovascular effects of OT and AVP under basal conditions, thus demonstrating the capacity of OT, AVP and WAY 267,464 to influence tonic thermoregulatory and cardiac functions of rats at doses that are typically used in behavioural studies.
It is important to consider whether the effects of OT, AVP and WAY 267,464 on body temperature and heart rate might, to a certain extent, reflect locomotor hypoactivity observed after exogenous administration of these drugs. OT has well-established sedative effects in rodents following central (Uvnäs-Moberg et al., 1992) or peripheral administration (Carson et al., 2010; Hicks et al., 2012), while AVP (Andrews et al., 1983), and AVP-like analogues (Krejci et al., 1979), also reduce locomotor activity in rats when given peripherally. Similarly, WAY 267,464 induces locomotor hypoactivity at the dose of 100 mg·kg−1 used in the present study (Hicks et al., 2012). However, it seems unlikely that sedation accounts for the hypothermia and bradycardia induced by OT, AVP and WAY 267,464. Notably, hypothermic and bradycardic effects were obtained in the current study with lower doses of OT and AVP that are unlikely to sedate (Andrews et al., 1983; Hicks et al., 2012). Moreover, observations suggest that sleep (i.e. complete immobility), without drug treatment, only reduces body temperature by 1.25 °C and heart rate by 75 beats min−1 (Sei et al., 1997), which is less than the effects reported here. The hypothermia, bradycardia and locomotor hypoactivity seen with OT and AVP, has been suggested to reflect a common shift from sympathetic to parasympathetic autonomic control with an inhibition of the hypothalamic–pituitary–adrenal axis (Uvnäs-Moberg, 1998), to facilitate a passive and non-defensive physiological and behavioural state that permits effective social engagement (Porges, 2003).
An interesting question from the current study relates to the relative involvement of central and peripheral mechanisms in the observed effects. There is increasing evidence that circulating OT directly penetrates the blood–brain barrier in small, but physiologically significant amounts (Neumann et al., 2013), and may also indirectly increase brain OT levels via stimulation of vagal afferents (McEwen, 2004). It appears possible then that a significant portion of the thermoregulatory and cardiovascular effects of OT, and AVP too, may be centrally mediated. Indeed, central infusion of high doses of OT and AVP induce body temperature and heart rate effects that mimic those produced after peripheral injection (Rogers and Hermann, 1985; Diamant and De Wied, 1993; Drago et al., 1997). Moreover, peripheral administration of OT, AVP and WAY 267,464 at the doses used in the current study stimulates neuronal activity in key autonomic centres in the brain including the hypothalamus and the nucleus of the solitary tract (NTS) (Wu et al., 1995; Hicks et al., 2012). When infused directly into the NTS, OT can potentiate the bradycardic response to a pressor challenge (Higa et al., 2002). Furthermore, the hypothermic effect of i.v. AVP was significantly reduced by i.c.v. infusion of an L-glutamate receptor antagonist, suggesting a central site of action of peripheral AVP (Paro et al., 2003).
However, there is also evidence that OT and AVP act directly on peripheral tissues to regulate body temperature and heart rate. Okuno et al. (1965) showed that bilateral electrolesions of the anterior hypothalamus did not prevent the fall in body temperature after i.v. infusion of AVP, which the authors suggested was due to a reduction in brown adipose tissue (BAT)-mediated thermogenesis. Indeed, i.v. administration of AVP significantly reduces BAT temperature (Paro et al., 2003), while OT receptor knockout mice show altered interscapular BAT morphology accompanied by impaired cold-induced thermogenesis (Takayanagi et al., 2008). There is also evidence suggesting that OT and AVP can act directly on cardiac receptors to modulate heart rate, as both neuropeptides reduced heart rate and the force of atrial contractions in isolated atria from perfused rat hearts (Favaretto et al., 1997; Kaygisiz et al., 2001). Future studies might disentangle the sites at which OT, AVP and WAY 267,464 act to influence thermoregulation and heart rate using peripheral administration of these compounds in combination with direct intracranial administration of specific receptor antagonists.
The V1A receptor antagonist SR49059 caused a striking cancellation of OT- and AVP-induced hypothermia and bradycardia at doses that had no significant intrinsic effects. In contrast, the body temperature and heart rate effects of OT were not prevented by pretreatment with the selective OT receptor antagonist C25. These results suggest a primary role for the V1A receptor, rather than the OT receptor, in the hypothermic and bradycardic effects of peripherally administered OT (and AVP). The V1A receptor is widely distributed in the brain and on peripheral organs including the heart, kidney and adrenal glands, and has a well-documented role in thermoregulation and cardiac function (Frank and Landgraf, 2008). Importantly, the present findings contribute to an emerging body of research showing that major functional effects of OT are mediated by AVP receptors (Gupta et al., 2009; Schorscher-Petcu et al., 2010; Loyens et al., 2011; Sala et al., 2011; Ramos et al., 2013).
SR49059 is a potent V1A receptor antagonist that shows >50-fold selectivity over the OT, V1B and V2 receptors in a number of species (Serradeil-Le Gal et al., 1993). The effects of OT were completely abolished with a 1 mg·kg−1 dose of SR49059, although a higher dose (10 mg·kg−1) was necessary to obtain a full antagonism of AVP's effects. This may reflect a difference in the potency of the maximal doses of OT and AVP employed in the present study. Indeed, a prior study showed that a 100-fold higher dose of OT was required to induce cardiovascular effects comparable with AVP (Petty, 1987). At a dose of 10 mg·kg−1, SR49059 may also act on OT and V1B receptors, and therefore the possibility that AVP may act on these receptors, in addition to the V1A receptor, to alter body temperature and heart rate cannot be discounted. In a reciprocal manner to OT, AVP exhibits significant cross-talk with the OT receptor (Gimpl and Fahrenholz, 2001), and some reports have implicated the V1B receptor in the blood pressure changes (Milutinovic et al., 2006), and the OT receptor in the tachycardic effects (Roozendaal et al., 1993), induced by central infusion of AVP.
Although blood pressure data were not collected in the present study, it is likely that the potent bradycardia induced by OT and AVP may be a consequence of increased arterial pressure leading to baroreflex-mediated reductions in heart rate. Peripheral administration of high doses of OT and AVP acutely increase blood pressure (Ludwig et al., 2013), and enhance baroreceptor control of heart rate (Michelini, 2007). The hypertensive actions of OT and AVP are likely mediated by the V1A receptor as they can be prevented by pretreatment with the V1A receptor antagonist (d(CH2)51,Tyr(Me)2,Arg8)-AVP (Costa-e-Sousa et al., 2005), or SR49059 (Serradeil-Le Gal et al., 1993) respectively. It appears plausible then, that SR49059 prevented OT- and AVP-induced bradycardia in the present study by inhibiting the associated increase in arterial pressure induced by these neuropeptides through a V1A receptor-dependent mechanism.
A particularly novel finding in the present study was the antagonist action of WAY 267,464 on OT-induced hypothermia and bradycardia. This provides further evidence of a V1A receptor-mediated mechanism underlying the hypothermic and bradycardic effects of OT, and consolidate our recent observations that WAY 267,464 functions as a V1A receptor antagonist in vitro (Hicks et al., 2012). Interestingly, WAY 267,464 only tended to reduce the effects of AVP, which might again conceivably be attributable to a difference in potency between OT and AVP at the maximal doses used in the present study.
In contrast to the effects of SR49059, the OT receptor antagonist C25 failed to prevent the hypothermic and bradycardic effects of the maximal dose of OT, and WAY 267,464. Given that C25 is relatively uncharacterized in vivo it is possible that antagonist effects might have been achieved with a higher dose. However, C25 given alone caused a subtle alteration of heart rate, indicating functional effectiveness, as well as a role for endogenous OT in cardiovascular homeostasis. Such a role is supported by observations that direct infusion of an OT receptor antagonist into the NTS disrupts the baroreflex response on heart rate to exercise (Braga et al., 2000) or a pressor challenge (Higa et al., 2002), and that i.c.v. administration of an OT receptor antagonist significantly potentiates the tachycardia induced by air jet stress (Wsol et al., 2008).
When interpreting the current findings, it is important to consider the apparent contradiction whereby WAY 267,464 produces an intrinsic hypothermic and bradycardic effect, while largely preventing these effects with peripheral OT and AVP. Although the mechanisms underlying this are not entirely clear, it likely relates to the subtly different pharmacological properties of WAY 267,464 compared with OT and AVP. So while OT and AVP exhibit agonist properties at both the OT and V1A receptors, WAY 267,464 functions as a selective OT receptor agonist but also as a V1A receptor antagonist. In addition, Grundschober et al. (2012) recently suggested that WAY 267,464 may also act as a potent V2 receptor agonist in vitro. The V2 receptor is also involved in thermoregulation and cardiovascular control: i.c.v. administration of a V2 receptor antagonist blocks the antipyretic effect of AVP (Kovacs et al., 1992), while i.v infusion of the V2 receptor agonist 1-deamino-4-valin-8-D-arginine-AVP increases baroreflex-mediated bradycardia in normotensive rats (Budzikowski et al., 1992). Therefore, WAY 267,464, at higher doses, may reduce body temperature and heart rate via a V2 receptor-mediated agonist action while attenuating the effects of OT and AVP at the V1A receptor. The enormous complexity of the peptide and non-peptide ligand-receptor interactions reported in the current study, highlights the need to develop more selective pharmacological research tools that demonstrate high binding and functional selectivity across several species both in vitro and in vivo (Manning et al., 2012).
In conclusion, we show here that peripherally administered OT, AVP and WAY 267,464 have powerful autonomic effects that include hypothermia and bradycardia. These actions should be taken into account in studies involving the administration of high peripheral doses of these drugs to rodents, as they may impair performance on complex behavioural and cognitive tasks. Our study also shows that OT-induced effects on thermoregulation and heart rate after peripheral administration are most likely mediated by the V1A receptor, rather than the OT receptor, contributing to an expanding body of research showing interplay between OT and AVP systems. Finally, our findings confirm our previous in vitro work suggesting important functional actions of WAY 267,464 at the V1A receptor.
Acknowledgments
Research was funded by a National Health and Medical Research Council (NHMRC) grant to ISM and MK. CH is the recipient of an Australian Postgraduate Award. ISM has been supported by an Australian Professorial Fellowship from the Australian Research Council and is currently an NHMRC Principal Research Fellow.
Glossary
- AVP
vasopressin
- BAT
brown adipose tissue
- C25
Compound 25
- NTS
nucleus of the solitary tract
- OT
oxytocin
Conflicts of interest
The authors declare no conflicts of interest.
References
- Alexander SPH, Benson HE, Faccenda E, Pawson AJ, Sharman JL, Spedding M, Peters JA, Harmar AJ CGTP Collaborators. The Concise Guide to PHARMACOLOGY 2013/14: G-Protein Coupled Receptors. Br J Pharmacol. 2013;170:1459–1581. doi: 10.1111/bph.12445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andrews J, Newton B, Sahgal A. The effects of vasopressin on positively rewarded responding and on locomotor activity in rats. Neuropeptides. 1983;4:17–29. doi: 10.1016/0143-4179(83)90004-5. [DOI] [PubMed] [Google Scholar]
- Benarroch EE. Oxytocin and vasopressin: social neuropeptides with complex neuromodulatory functions. Neurology. 2013;80:1521–1528. doi: 10.1212/WNL.0b013e31828cfb15. [DOI] [PubMed] [Google Scholar]
- Borrow AP, Cameron NM. The role of oxytocin in mating and pregnancy. Horm Behav. 2011;61:266–276. doi: 10.1016/j.yhbeh.2011.11.001. [DOI] [PubMed] [Google Scholar]
- Bouwknecht JA, Hijzen TH, van der Gugten J, Maes RA, Olivier B. Stress-induced hyperthermia in mice: effects of flesinoxan on heart rate and body temperature. Eur J Pharmacol. 2000;400:59–66. doi: 10.1016/s0014-2999(00)00387-3. [DOI] [PubMed] [Google Scholar]
- Braga DC, Mori E, Higa KT, Morris M, Michelini LC. Central oxytocin modulates exercise-induced tachycardia. Am J Physiol Regul Integr Comp Physiol. 2000;278:R1474–R1482. doi: 10.1152/ajpregu.2000.278.6.R1474. [DOI] [PubMed] [Google Scholar]
- Brown A, Brown TB, Calabrese A, Ellis D, Puhalo N, Ralph M, et al. Triazole oxytocin antagonists: identification of an aryloxyazetidine replacement for a biaryl substituent. Bioorg Med Chem Lett. 2010;20:516–520. doi: 10.1016/j.bmcl.2009.11.097. [DOI] [PubMed] [Google Scholar]
- Budzikowski A, Lon S, Paczwa P, Szczepanska-Sadowska E. Differential effects of V2 vasopressin agonist and antagonists on blood pressure regulation in normotensive (WKY) and spontaneously hypertensive (SHR) rats. J Auton Nerv Syst. 1992;39:87–95. doi: 10.1016/0165-1838(92)90048-l. [DOI] [PubMed] [Google Scholar]
- Carson DS, Hunt GE, Guastella AJ, Barber L, Cornish JL, Arnold JC, et al. Systemically administered oxytocin decreases methamphetamine activation of the subthalamic nucleus and accumbens core and stimulates oxytocinergic neurons in the hypothalamus. Addict Biol. 2010;15:448–463. doi: 10.1111/j.1369-1600.2010.00247.x. [DOI] [PubMed] [Google Scholar]
- Carson DS, Guastella AJ, Taylor ER, McGregor IS. A brief history of oxytocin and its role in modulating psychostimulant effects. J Psychopharmacol. 2013;27:231–247. doi: 10.1177/0269881112473788. [DOI] [PubMed] [Google Scholar]
- Chernoff N, Grabowski C. Responses of the rat foetus to maternal injections of adrenaline and vasopressin. Br J Pharmacol. 1971;43:270–278. [PMC free article] [PubMed] [Google Scholar]
- Costa-e-Sousa R, Pereira-Junior P, Oliveira P, Olivares E, Werneck-de-Castro J, Mello D, et al. Cardiac effects of oxytocin: is there a role for this peptide in cardiovascular homeostasis? Regul Pept. 2005;132:107–112. doi: 10.1016/j.regpep.2005.09.011. [DOI] [PubMed] [Google Scholar]
- Diamant M, De Wied D. Differential effects of centrally injected AVP on heart rate, core temperature, and behavior in rats. Am J Physiol Regul Integr Comp Physiol. 1993;264:R51–R61. doi: 10.1152/ajpregu.1993.264.1.R51. [DOI] [PubMed] [Google Scholar]
- Domes G, Heinrichs M, Kumbier E, Grossmann A, Hauenstein K, Herpertz SC. Effects of intranasal oxytocin on the neural basis of face processing in autism spectrum disorder. Biol Psychiatry. 2013;74:164–171. doi: 10.1016/j.biopsych.2013.02.007. [DOI] [PubMed] [Google Scholar]
- Drago F, Stanciu M, Salehi S, Scapagnini U. The block of central vasopressin V1 but not V2 receptors suppresses grooming behavior and hypothermia induced by intracerebroventricular vasopressin in male rats. Peptides. 1997;18:1389–1392. doi: 10.1016/s0196-9781(97)00203-9. [DOI] [PubMed] [Google Scholar]
- Favaretto A, Ballejo G, Albuquerque-Araujo W, Gutkowska J, Antunes-Rodrigues J, McCann S. Oxytocin releases atrial natriuretic peptide from rat atria in vitro that exerts negative inotropic and chronotropic action. Peptides. 1997;18:1377–1381. doi: 10.1016/s0196-9781(97)00209-x. [DOI] [PubMed] [Google Scholar]
- Feifel D, MacDonald K, Cobb P, Minassian A. Adjunctive intranasal oxytocin improves verbal memory in people with schizophrenia. Schizophr Res. 2012;139:207–210. doi: 10.1016/j.schres.2012.05.018. [DOI] [PubMed] [Google Scholar]
- Frank E, Landgraf R. The vasopressin system – from antidiuresis to psychopathology. Eur J Pharmacol. 2008;583:226–242. doi: 10.1016/j.ejphar.2007.11.063. [DOI] [PubMed] [Google Scholar]
- Gimpl G, Fahrenholz F. The oxytocin receptor system: structure, function, and regulation. Physiol Rev. 2001;81:629–683. doi: 10.1152/physrev.2001.81.2.629. [DOI] [PubMed] [Google Scholar]
- Grippo AJ, Trahanas DM, Zimmerman RR, 2nd, Porges SW, Carter CS. Oxytocin protects against negative behavioral and autonomic consequences of long-term social isolation. Psychoneuroendocrinology. 2009;34:1542–1553. doi: 10.1016/j.psyneuen.2009.05.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundschober C, Risterucci C, Mueggler T, Biemans B, Bissantz C, Belli S, et al. 2012. The oxytocin agonist WAY267464 is also a potent vasopressin V1a antagonist and V2 agonist. In: International Meeting for Autism Research (IMFAR). Sheraton Hall (Sheraton Centre Toronto)
- Gupta J, Russell R, Wayman C, Hurley D, Jackson V. Oxytocin-induced contractions within rat and rabbit ejaculatory tissues are mediated by vasopressin V1A receptors and not oxytocin receptors. Br J Pharmacol. 2009;155:118–126. doi: 10.1038/bjp.2008.226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutkowska J, Jankowski M. Oxytocin revisited: its role in cardiovascular regulation. J Neuroendocrinol. 2012;24:599–608. doi: 10.1111/j.1365-2826.2011.02235.x. [DOI] [PubMed] [Google Scholar]
- Hicks C, Jorgensen W, Brown C, Fardell J, Koehbach J, Gruber CW, et al. The nonpeptide oxytocin receptor agonist WAY 267,464: receptor-binding profile, prosocial effects and distribution of c-Fos expression in adolescent rats. J Neuroendocrinol. 2012;24:1012–1029. doi: 10.1111/j.1365-2826.2012.02311.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higa KT, Mori E, Viana FF, Morris M, Michelini LC. Baroreflex control of heart rate by oxytocin in the solitary–vagal complex. Am J Physiol Regul Integr Comp Physiol. 2002;282:R537–R545. doi: 10.1152/ajpregu.00806.2000. [DOI] [PubMed] [Google Scholar]
- Houshmand F, Faghihi M, Zahediasl S. Biphasic protective effect of oxytocin on cardiac ischemia/reperfusion injury in anaesthetized rats. Peptides. 2009;30:2301–2308. doi: 10.1016/j.peptides.2009.09.010. [DOI] [PubMed] [Google Scholar]
- Hudson P, Pitt G, Batt R, Roe M. 2005. Piperazines as oxytocin agonists. In: PCT (ed). Patent fetcher, edn. International Application: Ferring BV.
- Jodogne C, Tirelli E, Klingbiel P, Legros JJ. Oxytocin attenuates tolerance not only to the hypothermic but also to the myorelaxant and akinesic effects of ethanol in mice. Pharmacol Biochem Behav. 1991;40:261–265. doi: 10.1016/0091-3057(91)90549-h. [DOI] [PubMed] [Google Scholar]
- Kaygisiz Z, Kabadere T, Dernek S, Erden S. The effects of vasopressin in isolated rat hearts. Indian J Physiol Pharmacol. 2001;45:54–62. [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG. Animal research: reporting in vivo experiments: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1577–1579. doi: 10.1111/j.1476-5381.2010.00872.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klenerova V, Krejci I, Sida P, Hlinak Z, Hynie S. Modulary effects of oxytocin and carbetocin on stress-induced changes in rat behavior in the open-field. J Physiol Pharmacol. 2009;60:57–62. [PubMed] [Google Scholar]
- Kovacs GL, Baars AM, De Wied D. Antipyretic effect of central arginine8-vasopressin treatment: V1 receptors specifically involved? Life Sci. 1992;50:1625–1630. doi: 10.1016/0024-3205(92)90448-x. [DOI] [PubMed] [Google Scholar]
- Krejci I, Kupkova B, Metys J, Barth T, Jost K. Vasopressin analogs: sedative properties and passive avoidance behavior in rats. Eur J Pharmacol. 1979;56:347–353. doi: 10.1016/0014-2999(79)90265-6. [DOI] [PubMed] [Google Scholar]
- Loyens E, Vermoesen K, Schallier A, Michotte Y, Smolders I. Proconvulsive effects of oxytocin in the generalized pentylenetetrazol mouse model are mediated by vasopressin 1a receptors. Brain Res. 2011;1436:43–50. doi: 10.1016/j.brainres.2011.11.059. [DOI] [PubMed] [Google Scholar]
- Ludwig M, Tobin VA, Callahan MF, Papadaki E, Becker A, Engelmann M, et al. Intranasal application of vasopressin fails to elicit changes in brain immediate early gene expression, neural activity and behavioural performance of rats. J Neuroendocrinol. 2013;25:655–667. doi: 10.1111/jne.12046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundeberg T, Uvnäs-Moberg K, Agren G, Bruzelius G. Anti-nociceptive effects of oxytocin in rats and mice. Neurosci Lett. 1994;170:153–157. doi: 10.1016/0304-3940(94)90262-3. [DOI] [PubMed] [Google Scholar]
- Manning M, Misicka A, Olma A, Bankowski K, Stoev S, Chini B, et al. Oxytocin and vasopressin agonists and antagonists as research tools and potential therapeutics. J Neuroendocrinol. 2012;24:609–628. doi: 10.1111/j.1365-2826.2012.02303.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BB. Brain–fluid barriers: relevance for theoretical controversies regarding vasopressin and oxytocin memory research. Adv Pharmacol. 2004;50:531–592. doi: 10.1016/S1054-3589(04)50014-5. [DOI] [PubMed] [Google Scholar]
- McGrath J, Drummond G, McLachlan E, Kilkenny C, Wainwright C. Guidelines for reporting experiments involving animals: the ARRIVE guidelines. Br J Pharmacol. 2010;160:1573–1576. doi: 10.1111/j.1476-5381.2010.00873.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor IS, Bowen MT. Breaking the loop: oxytocin as a potential treatment for drug addiction. Horm Behav. 2012;61:331–339. doi: 10.1016/j.yhbeh.2011.12.001. [DOI] [PubMed] [Google Scholar]
- Mens WBJ, Witter A, Van Wimersma Greidanus TB. Penetration of neurohypophyseal hormones from plasma into cerebrospinal fluid (CSF): half-times of disappearance of these neuropeptides from CSF. Brain Res. 1983;262:143–149. doi: 10.1016/0006-8993(83)90478-x. [DOI] [PubMed] [Google Scholar]
- Michelini LC. Differential effects of vasopressinergic and oxytocinergic pre-autonomic neurons on circulatory control: reflex mechanisms and changes during exercise. Clin Exp Pharmacol Physiol. 2007;34:369–376. doi: 10.1111/j.1440-1681.2007.04589.x. [DOI] [PubMed] [Google Scholar]
- Milutinovic S, Murphy D, Japundzic-Zigon N. The role of central vasopressin receptors in the modulation of autonomic cardiovascular controls: a spectral analysis study. Am J Physiol Regul Integr Comp Physiol. 2006;291:R1579–R1591. doi: 10.1152/ajpregu.00764.2005. [DOI] [PubMed] [Google Scholar]
- Neumann ID, Maloumby R, Beiderbeck DI, Lukas M, Landgraf R. Increased brain and plasma oxytocin after nasal and peripheral administration in rats and mice. Psychoneuroendocrinology. 2013;38:1985–1993. doi: 10.1016/j.psyneuen.2013.03.003. [DOI] [PubMed] [Google Scholar]
- Okuno A, Yamamoto M, Itoh S. Lowering of the body temperature induced by vasopressin. Jpn J Physiol. 1965;15:378–387. [Google Scholar]
- Olszewski PK, Klockars A, Schiöth HB, Levine AS. Oxytocin as feeding inhibitor: maintaining homeostasis in consummatory behavior. Pharmacol Biochem Behav. 2010;97:47–54. doi: 10.1016/j.pbb.2010.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paro FM, Almeida MC, Carnio EC, Branco LG. Role of L-glutamate in systemic AVP-induced hypothermia. J Appl Physiol. 2003;94:271–277. doi: 10.1152/japplphysiol.00291.2002. [DOI] [PubMed] [Google Scholar]
- Petty MA. The cardiovascular effects of the neurohypophysial hormone oxytocin. J Auton Pharmacol. 1987;7:97–104. doi: 10.1111/j.1474-8673.1987.tb00137.x. [DOI] [PubMed] [Google Scholar]
- Porges SW. The polyvagal theory: phylogenetic contributions to social behavior. Physiol Behav. 2003;79:503–513. doi: 10.1016/s0031-9384(03)00156-2. [DOI] [PubMed] [Google Scholar]
- Ramos L, Hicks C, Kevin R, Caminer A, Narlawar R, Kassiou M, et al. Acute prosocial effects of oxytocin and vasopressin when given alone or in combination with 3,4-methylenedioxymethamphetamine in rats: involvement of the V1A receptor. Neuropsychopharmacology. 2013;38:2249–2259. doi: 10.1038/npp.2013.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richmond CA. The role of arginine vasopressin in thermoregulation during fever. J Neurosci Nurs. 2003;35:281–286. doi: 10.1097/01376517-200310000-00007. [DOI] [PubMed] [Google Scholar]
- Ring RH. A complicated picture of oxytocin action in the central nervous system revealed. Biol Psychiatry. 2011;69:818–819. doi: 10.1016/j.biopsych.2011.03.020. [DOI] [PubMed] [Google Scholar]
- Ring RH, Malberg JE, Potestio L, Ping J, Boikess S, Luo B, et al. Anxiolytic-like activity of oxytocin in male mice: behavioral and autonomic evidence, therapeutic implications. Psychopharmacology (Berl) 2006;185:218–225. doi: 10.1007/s00213-005-0293-z. [DOI] [PubMed] [Google Scholar]
- Ring RH, Schechter LE, Leonard SK, Dwyer JM, Platt BJ, Graf R, et al. Receptor and behavioral pharmacology of WAY-267464, a non-peptide oxytocin receptor agonist. Neuropharmacology. 2010;58:69–77. doi: 10.1016/j.neuropharm.2009.07.016. [DOI] [PubMed] [Google Scholar]
- Rogers RC, Hermann GE. Dorsal medullary oxytocin, vasopressin, oxytocin antagonist, and TRH effects on gastric acid secretion and heart rate. Peptides. 1985;6:1143–1148. doi: 10.1016/0196-9781(85)90441-3. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Schoorlemmer G, Koolhaas J, Bohus B. Cardiac, neuroendocrine, and behavioral effects of central amygdaloid vasopressinergic and oxytocinergic mechanisms under stress-free conditions in rats. Brain Res Bull. 1993;32:573–579. doi: 10.1016/0361-9230(93)90157-7. [DOI] [PubMed] [Google Scholar]
- Sala M, Braida D, Lentini D, Busnelli M, Bulgheroni E, Capurro V, et al. Pharmacologic rescue of impaired cognitive flexibility, social deficits, increased aggression, and seizure susceptibility in oxytocin receptor null mice: a neurobehavioral model of autism. Biol Psychiatry. 2011;69:875–882. doi: 10.1016/j.biopsych.2010.12.022. [DOI] [PubMed] [Google Scholar]
- Schorscher-Petcu A, Sotocinal S, Ciura S, Dupre A, Ritchie J, Sorge RE, et al. Oxytocin-induced analgesia and scratching are mediated by the vasopressin-1A receptor in the mouse. J Neurosci. 2010;30:8274–8284. doi: 10.1523/JNEUROSCI.1594-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sei H, Furuno N, Morita Y. Diurnal changes of blood pressure, heart rate and body temperature during sleep in the rat. J Sleep Res. 1997;6:113–119. doi: 10.1046/j.1365-2869.1997.00038.x. [DOI] [PubMed] [Google Scholar]
- Serradeil-Le Gal C, Wagnon J, Garcia C, Lacour C, Guiraudou P, Christophe B, et al. Biochemical and pharmacological properties of SR 49059, a new, potent, nonpeptide antagonist of rat and human vasopressin V1a receptors. J Clin Invest. 1993;92:224–231. doi: 10.1172/JCI116554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi Y, Kasahara Y, Onaka T, Takahashi N, Kawada T, Nishimori K. Oxytocin receptor-deficient mice developed late-onset obesity. Neuroreport. 2008;19:951–955. doi: 10.1097/WNR.0b013e3283021ca9. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K. Oxytocin may mediate the benefits of positive social interaction and emotions. Psychoneuroendocrinology. 1998;23:819–835. doi: 10.1016/s0306-4530(98)00056-0. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Alster P, Hillegaart V, Ahlenius S. Oxytocin reduces exploratory motor behaviour and shifts the activity towards the centre of the arena in male rats. Acta Physiol Scand. 1992;145:429–430. doi: 10.1111/j.1748-1716.1992.tb09385.x. [DOI] [PubMed] [Google Scholar]
- Uvnäs-Moberg K, Ahlenius S, Hillegaart V, Alster P. High doses of oxytocin cause sedation and low doses cause an anxiolytic-like effect in male rats. Pharmacol Biochem Behav. 1994;49:101–106. doi: 10.1016/0091-3057(94)90462-6. [DOI] [PubMed] [Google Scholar]
- Wsol A, Cudnoch-Jedrzejewska A, Szczepanska-Sadowska E, Kowalewski S, Puchalska L. Oxytocin in the cardiovascular responses to stress. J Physiol Pharmacol. 2008;59(Suppl. 8):123–127. [PubMed] [Google Scholar]
- Wu PH, Lança AJ, Liu JF, Man CF, Kalant H. Peripheral injection of arginine8-vasopressin increases Fos in specific brain areas. Eur J Pharmacol. 1995;281:263–269. doi: 10.1016/0014-2999(95)00258-m. [DOI] [PubMed] [Google Scholar]
- Xiao-Jun X, Wiesenfeld-Hallin Z. Is systemically administered oxytocin an analgesic in rats? Pain. 1994;57:193–196. doi: 10.1016/0304-3959(94)90223-2. [DOI] [PubMed] [Google Scholar]