Abstract
Introduction
Primary lung adenocarcinoma is extremely rare in the pediatric age group. There have been anecdotal reports of lesions that are histologically indistinguishable from adult-type pulmonary adenocarcinoma in young patients after treatment for nonpulmonary cancers. Herein, we present clinical, histopathologic, and molecular data on eight such cases.
Methods
Histopathologic evaluation of the tumors was performed according to the World Health Organization classification. Molecular studies for EGFR and KRAS mutations were performed on six patients with sufficient material.
Results
All eight patients were never smokers, four males and four females. Median age at nonpulmonary cancer diagnosis was 14 years (range, 3–23 years). Pulmonary adenocarcinomas were diagnosed at a median age of 15 years (range, 10–24 years); tumors were 0.1 to 2.0 cm in size and in some cases coexisted with metastases from the original cancer. Retrospective review showed that in at least three patients, the nodules were radiographically present before chemotherapy. Of six patients whose tumors were tested for common EGFR and KRAS mutations, two were positive for the former and one for the latter. At a median follow-up of 11 months (range, 2–29 months), six patients remained well without lung nodules and two had additional small, peripheral lung nodules that have not been biopsied.
Conclusions
Pulmonary lesions found in young patients with pediatric cancers can be histologically indistinguishable from lung adenocarcinoma seen in adults, may display typical adenocarcinoma-associated mutations of EGFR and KRAS, and may precede the administration of cytotoxic chemotherapy.
Keywords: Bronchioloalveolar carcinoma, Lung cancer, Adenocarcinoma, Secondary malignancies, Osteosarcoma, EGFR, KRAS
Primary adenocarcinoma of the lung is exceedingly rare in pediatric patients. In 1982, a review of the 230 pediatric cases from the English-language literature identified only 47 classifiable as “bronchogenic carcinoma.”1 Although a small number of sporadic pulmonary adenocarcinomas have been reported since then,2–5 the preponderance have been found in conjunction with congenital cystic adenomatoid malformations (CCAM) of the lung.6–16 Beyond this, given the scarcity of cases, it has not been possible to determine other risk factors for pulmonary adenocarcinoma in children or adolescents.
In 1988, Travis et al.17 reported two adolescent cancer patients who had lesions that histologically resembled adult-type bronchioloalveolar carcinoma (BAC) in the setting of treatment for other, nonpulmonary malignancies. These lung lesions were termed “pulmonary nodules resembling bronchioloalveolar carcinoma,” despite their identical morphology to adult-type BAC, because of the question whether biologically these represented true lung adenocarcinomas. Since that initial report, similar lesions have been described in six other pediatric cancer patients (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/JTO/A37).18–23 All the patients had been treated for other pediatric malignancies of nonpulmonary origin. Until now, it had seemed reasonable to consider these lesions as secondary malignancies; such a concept is grounded in the observation that survivors of childhood cancer have a greater than 14-fold increase in the relative risk of developing second malignant neoplasms24 and by the recent observation of renal translocation-related carcinomas in children after the administration of cytotoxic chemotherapy for another cancer.25 Herein, we describe the largest series of such cases assembled to date, eight patients between the age of 10 and 24 years with pulmonary lesions indistinguishable from adult lung adenocarcinomas, including invasive tumors as well as BAC (adenocarcinoma in situ) that were detected either synchronously with, or subsequent to, treatment for primary pediatric tumors of nonpulmonary origin.
PATIENTS AND METHODS
Cases were identified in the course of clinical care or consultation activities by the authors during the 7-year period, 2003–2009. In compliance with institutional protocols and with guidelines of the Health Insurance Portability and Accountability Act, a waiver was obtained from the Institutional Review Board of Memorial Sloan-Kettering Cancer Center (MSKCC) to conduct this retrospective review. Clinical histories, radiologic studies, operative notes, pathologic analyses, and medical records were evaluated.
Because each of these lesions was discovered incidentally in the course of lung surgery for presumed metastatic disease, operations were conducted in keeping with published practices for pediatric patients with lung metastases.26 In particular, lobectomy and hilar lymph node dissection were not performed, as pulmonary lymphatic spread is not typical for the histologies expected to be seen in pediatrics.
Thoracotomies were performed in six of the seven cases described here, and the histopathologic findings in the wedge resection specimens were evaluated by pathologists with special expertise in thoracic oncologic pathology (M.F.Z, A.L.M., and W.D.T.). In the one patient seen at MSKCC whose lung resection had been performed elsewhere (#2), pathology slides from an outside institution were available for review.
For analysis of EGFR and KRAS mutations, pulmonary nodules from five cases (patients 3 to 7) were macrodissected from formalin-fixed, paraffin-embedded sections, and genomic DNA was extracted. Mutational analysis of EGFR was performed as published.27 Briefly, EGFR exon 19 deletions were detected by length analysis of polymerase chain reaction (PCR) products. EGFR exon 21 L858R mutations were analyzed using PCR followed by Sau96I endonuclease digestion of PCR products. The mutant EGFR L858R PCR product shows two peaks (87 bp and 173 bp), whereas the digested negative control (wild type) shows only the 173 bp peak.
In addition, EGFR exon 21 L858R mutations and KRAS codons 12 and 13 mutations were also analyzed by mass spectrometry-based genotyping on a Sequenom platform (San Diego, CA), as described in detail elsewhere.28
RESULTS
Clinical characteristics of the patients are described in Table 1. All were never smokers. Median age at original cancer diagnosis was 14 years (range, 3–23 years). Median age at the diagnosis of pulmonary adenocarcinoma was 15 years (range, 10–24 years). Six of the eight patients had a primary diagnosis of a bone sarcoma (osteosarcoma, n = 5; chondrosarcoma, n = 1); the others had Wilms’ tumor (n = 1) or neuroblastoma (n = 1). Individual case summaries are given below. Histopathology and EGFR and KRAS mutational status are summarized in Table 2. A total of six BACs were found in five patients and five invasive adenocarcinomas in four patients. One patient had two BACs, one patient had two invasive adenocarcinomas, and one had both a BAC and an invasive adenocarcinoma. The three tumors that showed EGFR or KRAS mutations were adenocarcinomas with an invasive growth pattern.
TABLE 1.
Clinical Characteristics, Treatments, and Temporal Relationship Between Pulmonary Adenocarcinoma, Chemotherapy, and Radiotherapy of Patients
| Patient No. | Primary Oncologic Diagnosis | Age at Primary Diagnosis | Sex | Age at Thoracotomy | Pulmonary Adenocarcinoma Preceeded or Followed Cancer Therapy? | Prior Chemotherapeutic Agents | Pulmonary Irradiation |
|---|---|---|---|---|---|---|---|
| 1 | Wilms’ tumor | 7 | M | 13 | Unknown | Actinomycin-D | 1200 cGy |
| Vincristine | |||||||
| Doxorubicin | |||||||
| Carboplatin | |||||||
| Etoposide | |||||||
| Cyclophosphamide | |||||||
| 2 | Neuroblastoma | 3 | F | 10a | Followed | Vincristine | None |
| Cyclophosphamide | |||||||
| Cisplatin | |||||||
| Carboplatin | |||||||
| Doxorubicin | |||||||
| Etoposide | |||||||
| Ifosphamide | |||||||
| Topotecan | |||||||
| 3 | Osteosarcoma | 19 | M | 20 | Unknown | Cisplatin | None |
| Doxorubicin | |||||||
| High-dose methotrexate | |||||||
| 4 | Osteosarcoma | 18 | M | 19 | Preceded | Cisplatin | None |
| Doxorubicin | |||||||
| High-dose methotrexate | |||||||
| Pamidronate | |||||||
| 5 | Osteosarcoma | 23 | F | 24 | Preceded | Cisplatin | None |
| Doxorubicin | |||||||
| High-dose methotrexate | |||||||
| 6 | Osteosarcoma | 11 | F | 12 | Preceded | Cisplatin | None |
| Doxorubicin | |||||||
| High-dose methotrexate | |||||||
| L-MTP-PE | |||||||
| 7 | Chondrosarcoma | 14 | F | 15 | Unknown | Vincristine | None |
| Doxorubicin | |||||||
| Cyclophosphamide | |||||||
| Ifosfamide | |||||||
| Etoposide | |||||||
| 8 | Osteosarcoma | 14 | M | 14 | Preceded | COG AOST0121 | None |
Underwent thoracoscopic biopsy at outside institution.
L-MTP-PE, liposomal muramyltripeptide phosphatidylethanolamine; COG, Children’s Oncology Group.
TABLE 2.
Histology and Molecular Pathology of Lung Lesions
| Patient No. | Histopathology | EGFR | KRAS |
|---|---|---|---|
| 1 | Metastatic Wilms’ tumor | n/a | n/a |
| Bronchioloalveolar carcinomaa (2 foci of 0.1 cm each) | |||
| 2 | Bronchioloalveolar carcinoma (<0.5 cm) | n/a | n/a |
| 3 | Solitary focus of bronchioloalveolar carcinoma (0.25 cm) | Negative | Negative |
| Multiple foci of benign metaplastic ossification in lung | |||
| 4 | Bronchioloalveolar carcinoma (0.26 cm) | Negative | Negative |
| 5 | Well-differentiated, acinar-type adenocarcinoma (0.6 cm) | L858R mutation | Negative |
| 6 | Moderately differentiated adenocarcinoma, mixed subtype, with acinar and nonmucinous bronchioloalveolar patterns (0.5 cm) | Negative | Negative |
| Adenocarcinoma, acinar subtype (0.3 cm) | |||
| Metastatic osteosarcoma (0.15 cm) | |||
| 7 | Adenocarcinoma, mixed subtype with papillary and bronchioloalveolar patterns (0.2 cm) | Negative | G12V mutation in adenocarcinoma |
| Bronchioloalveolar carcinoma (0.2 cm) | |||
| Metastatic mesenchymal chondrosarcoma (0.6 cm) | |||
| 8 | Moderately differentiated adenocarcinoma, mixed subtype, predominantly acinar and papillary (2.0 cm) | Exon 19 deletion in adenocarcinoma | Not done |
| Metastatic osteosarcoma (0.3 cm) |
Bronchioloalveolar carcinoma is currently called adenocarcinoma in situ in the new classification.32
n/a, specimen insufficient or unavailable for molecular study.
Case 1
A 7-year-old boy was diagnosed with stage IV Wilms’ tumor and was treated with right radical nephrectomy, regimen DD-4A (vincristine, dactinomycin, and doxorubicin), and 1200 cGy whole lung irradiation. Five years later, pulmonary relapse was diagnosed, and a fine needle aspiration confirmed malignant cells. He received chemotherapy according to Stratum C of the National Wilms’ Tumor Study-5 consisting of cyclophosphamide, etoposide, and carboplatin. Thoracoscopic lung resection confirmed metastatic Wilms’ tumor. During cycle 2 of maintenance, he was switched to a regimen of oral cyclophosphamide and oral etoposide. Computed tomographic (CT) imaging (6 years after initial diagnosis) showed possible progression in the lung, and he was referred to us for thoracotomy (Figure 1A). At surgery, all suspicious nodules were removed, including a 0.8-cm focus of metastatic Wilms’ tumor and two separate foci of BAC, each measuring 1 mm (Figures 1B, C). No further surgery was carried out. At 11 months after thoracotomy, the patient developed radiologic evidence of a solitary, enlarging, 6-mm lung nodule, the histology of which is not known.
FIGURE 1.
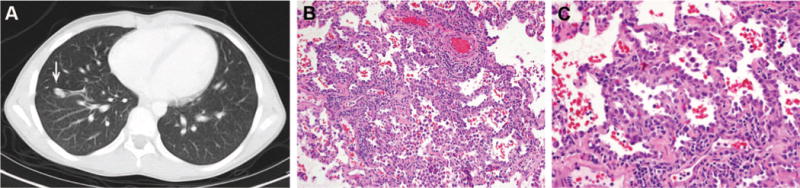
Case 1. A, Computed tomographic (CT) image of new lung nodule (arrow) that prompted thoracotomy. B and C, Bronchioloalveolar carcinoma of 1 mm showing crowded cuboidal to columnar cells growing along alveolar walls in a lepidic fashion, with cellular stratification, overlapping of nuclei, and coarse nuclear chromatin (hematoxylin and eosin; original magnifications, 20× and 40×).
Case 2
A 3-year-old girl received multiagent chemotherapy for an intermediate-grade, stage 3 neuroblastoma according to Pediatric Oncology Group #9244 using combination vincristine, cisplatin, etoposide, and cyclophosphamide alternating with vincristine, carboplatin, etoposide, and cyclophosphamide. This was changed owing to insufficient response to vincristine, doxorubicin, and cyclophosphamide, followed by ifosfamide and etoposide; and finally she received topotecan (Pediatric Oncology Group #9361), but still no responses in urine catecholamines were noted. After referral to our center, gross total resection of the left adrenal mass was performed, showing ganglioneuroblastoma. She received no subsequent chemotherapy. Four years later, minute pulmonary nodules were found. Thoracoscopic biopsy performed at an outside institution was forwarded to our center for review and was classified as BAC. No additional treatment was rendered, and a follow-up CT scan 5 months later showed no progression in the remaining pulmonary nodules.
Case 3
A 19-year-old man was diagnosed with osteosarcoma of the right proximal humerus. Calcified, subcentimeter lung nodules were seen at diagnosis. He received combination therapy with cisplatin, doxorubicin, and high-dose methotrexate. Seven months into treatment, he underwent thoracotomy with eight wedge resections. No osteosarcoma was found, and the majority of the specimens proved to be minute foci of benign lamellar bone, consistent with metaplastic ossification. One wedge resection contained a 0.25-cm nodule of BAC. No additional chest surgery was performed. At 29 months of follow-up since lung surgery, the patient remained well; a small number of tiny, highly calcified nodules were seen on chest CT that have been stable in size and appearance over 2 years and were thought to represent additional foci of benign ossification.
Case 4
An 18-year-old man presented with a mass of the right iliac bone and multiple subcentimeter pulmonary nodules. Biopsy of the pelvic mass showed high-grade osteosarcoma with chondroblastic elements. He received combination cisplatin, doxorubicin, high-dose methotrexate, and pamidronate according to an in-house osteosarcoma protocol. At thoracotomy 5 months after diagnosis, six suspicious nodules were resected. None showed osteosarcoma; one was a focus of BAC measuring 0.26 cm. Without subsequent surgical intervention, the patient has developed no new or progressive disease 20 months after thoracotomy.
Case 5
A 23-year-old woman was diagnosed with high-grade osteosarcoma, osteoblastic type, of the proximal humerus. Chest CT at diagnosis showed a pulmonary nodule (Figure 2A). She received combination cisplatin, doxorubicin, and high-dose methotrexate. Thoracotomy was performed 8 months into treatment, with wedge resection of the right middle lobe nodule showing a 0.6 cm, well-differentiated, acinar-type adenocarcinoma of the lung (Figures 2B–D). Molecular analysis revealed the tumor to be positive for EGFR exon 21 L858R mutation by both the PCR/restriction enzyme assay and Sequenom genotyping (Figure 2E). No further surgical therapy was performed, and the patient has no evidence of disease with 11 months of follow-up since thoracotomy.
FIGURE 2.
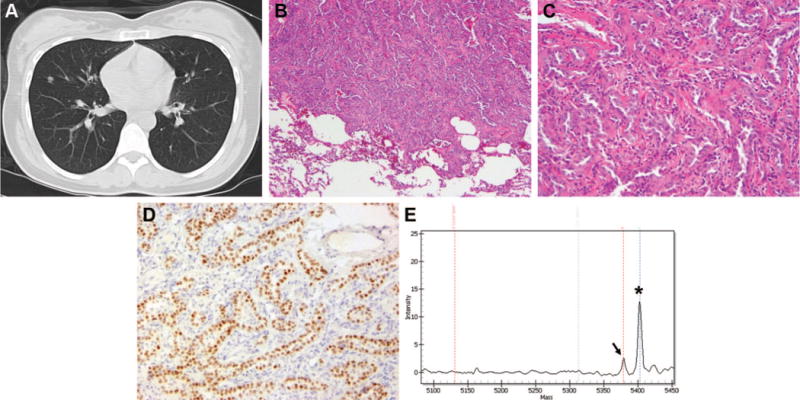
Case 5. A, Suspicious density seen on CT scan in the right middle lobe of the lung at the time of diagnosis of osteosarcoma of the humerus. B and C, Invasive adenocarcinoma of the right middle lobe of the lung. Note the presence of invasive glands with fibrotic stroma and sharp demarcation between the tumor cells and the normal pulmonary parenchyma (hematoxylin and eosin; original magnifications, 10× and 40×). D, Immunohistochemical stain for TTF-1. This photomicrograph shows that the invasive glands are positive for TTF-1, thus confirming a pulmonary origin for this adenocarcinoma. E, Mass spectrometry-based genotyping for EGFR exon 21 L858R mutation on Sequenom platform confirming the presence of L858R mutation. The germline peak is indicated by an asterisk and the mutant peak by an arrow. CT, computed tomography; TTF, thyroid transcription factor-1.
Case 6
An 11-year-old girl presented with a lytic lesion of the distal femur, biopsy of which showed high-grade osteosarcoma, osteoblastic type. Lung lesions were present on CT scanning at diagnosis. She was treated with combination therapy using cisplatin, doxorubicin, and high-dose methotrexate. She also received liposomal muramyltripeptide phosphatidylethanolamine. At thoracotomy, the radiographically identified nodule in the left upper lobe was resected and found to be a 5-mm focus of moderately differentiated adenocarcinoma, mixed subtype, with acinar and nonmucinous bronchioloalveolar patterns. Two nodules were found in the left lower lobe that had not been detected radiographically: a 3-mm acinar subtype adenocarcinoma, and separately, a 1.5-mm focus of metastatic osteosarcoma. The upper lobe adenocarcinoma was tested for EGFR and KRAS mutations and found to be negative for both. No additional surgery was carried out for the pulmonary adenocarcinomas, but staged, contralateral thoracotomy was performed (in keeping with our practice to rule out osteosarcoma metastatic to the other lung). The contralateral lung was free of both osteosarcoma and adenocarcinoma. She has no evidence of disease 8 months after the initial thoracotomy.
Case 7
A 14-year-old girl presented with a 17-cm mass of the tibia and a simultaneously diagnosed solitary pulmonary nodule. Biopsy of the tibial lesion showed mesenchymal chondrosarcoma. Systemic therapy was administered using combination vincristine, doxorubicin, cyclophosphamide, ifosfamide, and etoposide. Wedge resection of the previously identified lung nodule was performed, revealing metastatic chondrosarcoma. At thoracotomy, the discovery of additional nodules, not identified on preoperative imaging, prompted the performance of additional wedge resections. Findings included one focus of adenocarcinoma in the right lower lobe, of mixed subtype with papillary and bronchioloalveolar patterns, and a separate 2-mm BAC-like nodule (Figure 3A, B). The focus of mixed type adenocarcinoma of the right lower lobe was positive for the KRAS codon 12 G→V mutation by Sequenom genotyping (Figure 3C). No additional surgery was carried out. With 11 months of follow-up, she remained free of disease.
FIGURE 3.
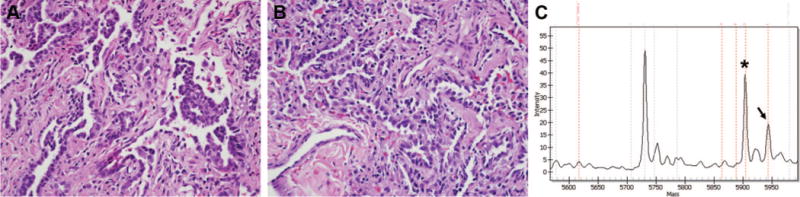
Case 7. A, Right lower lobe adenocarcinoma, mixed subtype with papillary and bronchioloalveolar patterns (hematoxylin and eosin; original magnification, 40). B, Bronchioloalveolar carcinoma-like nodule in the right middle lobe not seen on preoperative CT scan (hematoxylin and eosin; original magnification, 40). C, Sequenom genotyping assay showing the presence of G 3 T mutation at position 35 in KRAS codon 12 (G12V mutation) in the mixed-type adenocarcinoma of the right lower lobe. The germline peak is indicated by an asterisk and the mutant peak by an arrow. CT, computed tomography.
Case 8
A 14-year-old boy was diagnosed with high-grade osteosarcoma of the right femur. A PET scan at diagnosis showed, in addition to the primary lesion in the right lower extremity, a 2.7-cm left lower lobe hypermetabolic mass in the lung that was considered likely to be a metastasis. He underwent limb salvage surgery and treatment according to COG protocol AOST0121 was initiated. At 3 months, a chest CT revealed the left lower lobe somewhat reduced in size (1.6 cm) as well as some small nodules in the lingual and right lung base. Thoracotomy was performed 5 months into treatment, with wedge resection of two lingular nodules and left lower lobectomy. The lingular lesions were a calcified nodule and a nodule with osteoid, consistent with treated osteosarcoma. The left lower lesion was a 2.0 cm, predominantly acinar-type adenocarcinoma of the lung (Figure 4) immunoreactive for thyroid transcription factor-1 and CK7 and negative for CDX2 and CK20. Molecular analysis revealed the lung adenocarcinoma to be positive for EGFR exon 19 deletion. The patient is at only 2 months postthoracotomy.
FIGURE 4.
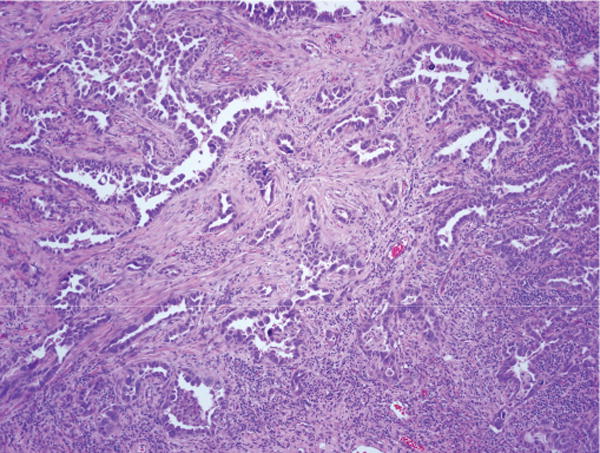
Case 8. Left lower lobe adenocarcinoma, mixed subtype with predominant acinar pattern (hematoxylin and eosin; original magnification, 40×). This tumor contained an EGFR mutation (exon 19 deletion) (see text). EGFR, epidermal growth factor receptor.
EGFR and KRAS Mutations
Table 2 summarizes the findings with respect to EGFR and KRAS mutations. Of the six patients whose tumors were tested, three were found negative for the common mutations of both EGFR and KRAS. The adenocarcinomas in patients 5 and 8 were positive for mutation of EGFR. In patient 7, the adenocarcinoma showed a G12V mutation in KRAS.
Temporal Relationship to Chemotherapy
Temporal relationship between the administration of chemotherapy and the finding of adenocarcinoma in the lung is shown in Table 1. In at least four of the eight patients, pulmonary nodules that later proved to be adenocarcinoma were detected by imaging before the administration of potentially mutagenic chemotherapy.
Clinical Follow-Up
Additional surgery related to the pulmonary adenocarcinomas, such as lobectomy or hilar lymph node dissection, was not carried out in any of the patients. Additional chemotherapy, beyond the substantial multiagent regimens already prescribed for the nonpulmonary primary cancers, was not administered. Median duration of follow-up is 11 months (range, 5–29 months) (Table 3). During this follow-up, two patients had persistent or recurrent radiographic evidence of small lung nodules (histology unknown). Five patients remained free of disease with respect to both primary tumors.
TABLE 3.
Clinical Outcomes
| Patient Thoracotomy No. | Follow-Up Since (mo) | Additional Treatment for Disease Pulmonary Adenocarcinoma | Status of Primary Tumor | Disease Status of Pulmonary Adenocarcinoma |
|---|---|---|---|---|
| 1 | 11 | None | AWD | AWDa |
| 2 | 5 | None | NED | AWD |
| 3 | 29 | None | NED | NED |
| 4 | 20 | None | NED | NED |
| 5 | 11 | None | NED | NED |
| 6 | 8 | None | NED | NED |
| 7 | 11 | None | NED | NED |
| 8 | 2 | None | NED | NED |
Patient 1 has a solitary enlarging lung nodule at last follow-up, the histology of which is not yet determined.
AWD, alive with disease, NED, no evidence of disease.
DISCUSSION
Our analysis of this series indicates that the tumors previously described as BAC-like nodules found in pediatric cancer patients are (1) adenocarcinomas with a spectrum of BAC (adenocarcinoma in situ) and invasive histologic patterns and (2) may be present before administration of chemotherapy for the underlying malignancy and therefore are not always or necessarily secondary malignancies, as had been previously believed. Specifically, patients 4, 5, 6, and 8 had radiographic evidence of pulmonary nodules at the time of their original cancer diagnosis before any treatment. In two patients, these nodules proved to be adenocarcinomas, and in two cases, there was adenocarcinoma coexisting with lung metastases from osteosarcoma. The observation of eight young patients with incidental pulmonary adenocarcinomas raises the possibility that the contemporaneous presence of a primary pediatric malignancy and pulmonary adenocarcinoma may reflect more than coincidence.
Our study clearly documents that invasive patterns of adenocarcinoma occur in the setting of pediatric cancer patients in addition to BAC/adenocarcinoma in situ. Three of the seven patients had areas of invasion in addition to the BAC/adenocarcinoma in situ. How to properly classify the lung tumors with BAC/adenocarcinoma in situ histology in young cancer patients has been problematic since their original description in 1988, because of their noninvasive growth pattern, their presentation as incidental findings, and the young age of the patients. For these reasons in 1988, we (W.D.T.) were reluctant to call these tumors BAC and qualified the terminology for these cases as “BAC-like nodule.” However, in 1995, it was recognized that patients with small solitary adenocarcinomas with a pure BAC histology in the absence of any invasion have a 100% 5-year survival.29 This led to a strict redefinition of the use of the term BAC by 1999 World Health Organization classification as a noninvasive tumor with pure lepidic growth, and this concept was maintained in the 2004 classification.30,31 Furthermore, the excellent survival for small solitary BACs has been validated in multiple subsequent studies, so these tumors are currently being proposed to be called “adenocarcinoma in situ” in the next classification of lung adenocarcinoma.32 Notably, however, we found invasive patterns of lung adenocarcinoma in four of the eight patients in this study.
Pediatric cancer patients undergo lung imaging and thoracotomy at a rate vastly exceeding that of healthy children, so it may be argued that incidentally found lung lesions such as these may have a different natural history from usual adult lung cancer. However, incidental pulmonary adenocarcinomas have so far not been reported among children undergoing workup and surgery for benign etiologies such as trauma, bleb disease, or empyema. There is, however, a well-established association between CCAM and pulmonary adenocarcinoma. However, CCAM-associated adenocarcinomas are typically mucinous lesions and likely stem from a different underlying molecular pathway. Mucinous BACs are associated with higher frequencies of KRAS mutations compared with nonmucinous tumors,33 and mucinous carcinomas seen in association with CCAMs have been shown to exhibit loss of heterozygosity at the p16INK4 locus.34 Such lesions should be contrasted with the nonmucinous lesions exhibited by the patients in this series, as well as by most of the pediatric cancer patients previously reported to have developed pulmonary adenocarcinoma (Supplemental Table 1, Supplemental Digital Content 1, http://links.lww.com/JTO/A37). The coexistence of primary pediatric malignancies and early pulmonary adenocarcinomas as seen here may reflect the presence of an underlying germline predisposition or a common carcinogenic exposure. Additional cases may help to define this, but we should note that none of the patients had family histories suggestive of known familial cancer syndromes. In this regard, a locus associated with increased risk of lung cancer in never smokers has recently been reported.35
To our knowledge, mutations in EGFR or KRAS have not been previously documented in pediatric pulmonary adenocarcinoma. The mutational analysis presented here provides a starting point for elucidating the genetic alterations driving lung neoplasia in this clinical setting. Two of the eight with predominantly acinar histology displayed the EGFR mutations, and another mixed subtype adenocarcinoma showed the KRAS G12V mutation. All three of these tumors had an invasive growth pattern. This mutation rate is not statistically different from the incidence of such mutations among adult North American lung cancer patients, where 20% of lung adenocarcinomas harbor mutations of EGFR36 and about 25 to 30% contain KRAS mutations.37,38 The EGFR mutations have a negative association with smoking, whereas KRAS G12V lacks a clear negative or positive association with smoking.39 The presence of these mutations, in addition to the histology, provides further evidence that these tumors are clonal neoplasms, rather than reactive atypical pneumocyte proliferations. Finally, Park et al40 recently reported two adolescents with lung adenocarcinoma without a prior or concurrent pediatric cancer who both died of their disease.
After surgical resection, the appropriate follow-up treatment for an incidentally discovered, subcentimeter pulmonary adenocarcinoma is not defined in pediatrics. The principles in surgery for lung cancer are founded on data from adults with tumors large enough to be detected on chest radiographs, rather than the minute tumors found by CT scanning or by palpation at the time of thoracotomy.41,42 The surgical management of incidentally discovered pulmonary adenocarcinomas less than 2 cm in size has been significantly revisited in recent years by Rusch and others, with an emphasis on sublobar resection.43–49 Despite the persistence of pulmonary nodules of unknown histology in two of our patients, we did not observe definite malignant behavior such as recurrence or metastases in any of our cases.
Seven of the eight patients presented here were comprehensively discussed at a multidisciplinary tumor board. In each case, the consensus was reached that preservation of pulmonary parenchyma and of lung function were overriding priorities, especially in light of the potential need for additional pulmonary metastatectomies in the future, given these patients’ underlying high-grade malignancies. Consequently, we have chosen close surveillance and have specifically avoided repeat thoracotomies for lobectomies or hilar lymph node dissections. No additional chemotherapy was thought to be warranted. Most of the patients were still in the process of receiving aggressive combination chemotherapy regimens for their primary tumors when the diagnosis of pulmonary adenocarcinoma was made. Continued follow-up and molecular studies will be needed to more definitively comment on the success of this approach.
Supplementary Material
Acknowledgments
Supported by the Anbinder Fund (MSKCC Sequenom facility) and National Cancer Institute (P01-CA129243 to M.L.).
The authors thank Dr. Paul Meyers for encouraging the compilation of this series for publication; Dr. Marian Fleming (Banner Desert Medical Center, Mesa, AZ) for submitting one of the cases; and Dr. Greg Riely for helpful comments. They also thank Dr. Laetitia Borsu for assistance with Sequenom assays.
Footnotes
Disclosure: The authors declare no conflicts of interest.
References
- 1.Hartman GE, Shochat SJ. Primary pulmonary neoplasms of childhood: a review. Ann Thorac Surg. 1983;36:108–119. doi: 10.1016/s0003-4975(10)60664-9. [DOI] [PubMed] [Google Scholar]
- 2.Epstein DM, Aronchick JM. Lung cancer in childhood. Med Pediatr Oncol. 1989;17:510–513. doi: 10.1002/mpo.2950170532. [DOI] [PubMed] [Google Scholar]
- 3.Dosanjh A. Bronchioalveolar carcinoma in a 15-year-old girl. Clin Pediatr (Phila) 1992;31:253–254. doi: 10.1177/000992289203100414. [DOI] [PubMed] [Google Scholar]
- 4.Kantar M, Çetingül N, Veral A, et al. Rare tumors of the lung in children. Pediatr Hematol Oncol. 2002;19:421–428. doi: 10.1080/08880010290097189. [DOI] [PubMed] [Google Scholar]
- 5.Lal DR, Clark I, Shalkow J, et al. Primary epithelial lung malignancies in the pediatric population. Pediatr Blood Cancer. 2005;45:683–686. doi: 10.1002/pbc.20279. [DOI] [PubMed] [Google Scholar]
- 6.Kaslovsky RA, Purdy S, Dangman BC, et al. Bronchioloalveolar carcinoma in a child with congenital cystic adenomatoid malformation. Chest. 1997;112:548–551. doi: 10.1378/chest.112.2.548. [DOI] [PubMed] [Google Scholar]
- 7.Benjamin DR, Cahill JL. Bronchioloalveolar carcinoma of the lung and congenital cystic adenomatoid malformation. Am J Clin Pathol. 1991;95:889–892. doi: 10.1093/ajcp/95.6.889. [DOI] [PubMed] [Google Scholar]
- 8.Ribet ME, Copin M-C, Soots JG, et al. Bronchioloalveolar carcinoma and congenital cystic adenomatoid malformation. Ann Thorac Surg. 1995;60:1126–1128. doi: 10.1016/0003-4975(95)00494-6. [DOI] [PubMed] [Google Scholar]
- 9.Granata C, Gambini C, Balducci T, et al. Bronchioloalveolar carcinoma arising in congenital cystic adenomatoid malformation in a child: a case report and review on malignancies originating in congenital cystic adenomatoid malformation. Pediatr Pulmonol. 1998;25:62–66. doi: 10.1002/(sici)1099-0496(199801)25:1<62::aid-ppul8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 10.Ohye RG, Cohen DM, Caldwell S, et al. Pediatric bronchioloalveolar carcinoma: a favorable pediatric malignancy? J Pediatr Surg. 1998;33:730–732. doi: 10.1016/s0022-3468(98)90200-7. [DOI] [PubMed] [Google Scholar]
- 11.Papagiannopoulos K, Hughes S, Nicholson AG, et al. Cystic lung lesions in the pediatric and adult population: surgical experience at the Brompton Hospital. Ann Thorac Surg. 2002;73:1594–1598. doi: 10.1016/s0003-4975(02)03469-0. [DOI] [PubMed] [Google Scholar]
- 12.MacSweeney F, Papagiannopoulis K, Goldstraw P, et al. An assessment of the expanded classification of congenital cystic adenomatoid malformations and their relationship to malignant transformation. Am J Surg Pathol. 2003;27:1139–1146. doi: 10.1097/00000478-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 13.Sudou M, Sugi K, Murakami T. Bronchioloalveolar carcinoma arising from a congenital cystic adenomatoid malformation in an adolescent: the first case report from the Orient. J Thorac Cardiovas Surg. 2003;126:902–903. doi: 10.1016/s0022-5223(03)00397-0. [DOI] [PubMed] [Google Scholar]
- 14.Lantuejoul S, Ferretti GR, Goldstraw P, et al. Metastases from bronchioloalveolar carcinomas associated with long-standing type 1 congenital cystic adenomatoid malformations. A report of two cases. Histopathology. 2006;48:204–206. doi: 10.1111/j.1365-2559.2005.02206.x. [DOI] [PubMed] [Google Scholar]
- 15.Ramos SG, Barbosa GH, Tavora FR, et al. Bronchioloalveolar carcinoma arising in a congenital pulmonary airway malformation in a child: case report with an update of this association. J Pediatr Surg. 2007;42:E1–E4. doi: 10.1016/j.jpedsurg.2007.02.003. [DOI] [PubMed] [Google Scholar]
- 16.West D, Nicholson AG, Colquhoun I, et al. Bronchioloalveolar carcinoma in congenital cystic adenomatoid malformation of lung. Ann Thorac Surg. 2007;83:687–689. doi: 10.1016/j.athoracsur.2006.06.029. [DOI] [PubMed] [Google Scholar]
- 17.Travis WD, Linnoila RI, Horowitz M, et al. Pulmonary nodules resembling bronchioloalveolar carcinoma in adolescent cancer patients. Mod Pathol. 1988;1:372–377. [PubMed] [Google Scholar]
- 18.Kowalski P, Rodziewicz B, Pejcz J. Bilateral bronchioloalveolar carcinoma of the lungs in a 7 year old girl treated for Hodgkin’s disease. Tumori. 1989;75:449–451. doi: 10.1177/030089168907500509. [DOI] [PubMed] [Google Scholar]
- 19.Nonomura A, Mizukami Y, Shimizu J, et al. Simultaneous occurrence of lung adenocarcinoma and fibular osteosarcoma in a 13-year-old girl. Thorac Cardiovasc Surg. 1994;42:61–63. doi: 10.1055/s-2007-1016458. [DOI] [PubMed] [Google Scholar]
- 20.Kuttesch JF, Jr, Wexler LH, Marcus RB, et al. Second malignancies after Ewing’s sarcoma: radiation dose-dependency of secondary sarcomas. J Clin Oncol. 1996;14:2818–2825. doi: 10.1200/JCO.1996.14.10.2818. [DOI] [PubMed] [Google Scholar]
- 21.Spaner SJ, Raymond G, Puttagunta L, et al. Bronchioloalveolar cell carcinoma in a child with hepatoblastoma: case report. Can Assoc Radiol J. 1999;50:343–345. [PubMed] [Google Scholar]
- 22.Neusuess A, Claviez A, Schroeter T, et al. Synchronous detection of a pulmonary papillary adenoma and lung metastases in a patient with osteosarcoma in relapse. Med Pediatr Oncol. 2002;38:125–127. doi: 10.1002/mpo.1287. [DOI] [PubMed] [Google Scholar]
- 23.Longhi A, Bertoni F, Bacchini P, et al. Simultaneous osteosarcoma lung metastasis and second primary lung cancer. J Pediatr Hematol Oncol. 2004;26:457–461. doi: 10.1097/00043426-200407000-00013. [DOI] [PubMed] [Google Scholar]
- 24.Oeffinger KC, Mertens AC, Sklar CA, et al. Chronic health conditions in adult survivors of childhood cancer. N Engl J Med. 2006;355:1572–1582. doi: 10.1056/NEJMsa060185. [DOI] [PubMed] [Google Scholar]
- 25.Argani P, Laé M, Ballard ET, et al. Translocation carcinomas of the kidney after chemotherapy in childhood. J Clin Oncol. 2006;24:1529–1534. doi: 10.1200/JCO.2005.04.4693. [DOI] [PubMed] [Google Scholar]
- 26.Kayton ML. Pulmonary metastasectomy in pediatric patients. Thorac Surg Clin. 2006;16:167–183. doi: 10.1016/j.thorsurg.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 27.Pan Q, Pao W, Ladanyi M. Rapid polymerase chain reaction-based detection of epidermal growth factor receptor gene mutations in lung adenocarcinomas. J Mol Diagn. 2005;7:396–403. doi: 10.1016/S1525-1578(10)60569-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Chitale D, Gong Y, Taylor BS, et al. An integrated genomic analysis of lung cancer reveals loss of DUSP4 in EGFR-mutant tumors. Oncogene. 2009;28:2773–2783. doi: 10.1038/onc.2009.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Noguchi M, Morikawa A, Kawasaki M, et al. Small adenocarcinoma of the lung. Histologic characteristics and prognosis. Cancer. 1995;75:2844–2852. doi: 10.1002/1097-0142(19950615)75:12<2844::aid-cncr2820751209>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 30.Travis WD, Colby TV, Corrin B, et al. Histological Typing of Lung and Pleural Tumors. Berlin: Springer; 1999. [Google Scholar]
- 31.Travis WD, Brambilla E, Müller-Hermelink HK, et al. Pathology and Genetics: Tumours of the Lung, Pleura, Thymus and Heart. Lyon: IARC; 2004. [Google Scholar]
- 32.Travis WD, Brambilla E, Noguchi M, et al. The new IASLC/ATS/ERS international multidisciplinary lung adenocarcinoma classification. J Thoracic Oncol. In press. [Google Scholar]
- 33.Wislez M, Beer DG, Wistuba I, et al. Molecular biology, genomics, and proteomics in bronchioloalveolar carcinoma. J Thorac Oncol. 2006;1(9 Suppl):S8–S12. [PubMed] [Google Scholar]
- 34.Lantuéjoul S, Nicholson AG, Sartori G, et al. Mucinous cells in type I pulmonary congenital cystic adenomatoid malformation as mucinous bronchioloalveolar carcinoma precursors. Am J Surg Pathol. 2007;31:961–969. doi: 10.1097/01.pas.0000249444.90594.27. [DOI] [PubMed] [Google Scholar]
- 35.Li Y, Sheu CC, Ye Y, et al. Genetic variants and risk of lung cancer in never smokers: a genome-wide association study. Lancet Oncol. 2010;11:321–330. doi: 10.1016/S1470-2045(10)70042-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shigematsu H, Lin L, Takahashi T, et al. Clinical and biological features associated with epidermal growth factor receptor gene mutations in lung cancers. J Natl Cancer Inst. 2005;97:339–346. doi: 10.1093/jnci/dji055. [DOI] [PubMed] [Google Scholar]
- 37.Rodenhuis S, Slebos RJC, Boot AJM, et al. Incidence and possible clinical significance of K-ras oncogene activation in adenocarcinoma of the human lung. Cancer Res. 1988;48:5738–5741. [PubMed] [Google Scholar]
- 38.Ahrendt SA, Decker PA, Alawi EA, et al. Cigarette smoking is strongly associated with mutation of the K-ras gene in patients with primary adenocarcinoma of the lung. Cancer. 2001;92:1525–1530. doi: 10.1002/1097-0142(20010915)92:6<1525::aid-cncr1478>3.0.co;2-h. [DOI] [PubMed] [Google Scholar]
- 39.Riely GJ, Kris MG, Rosenbaum D, et al. Frequency and distinctive spectrum of KRAS mutations in never smokers with lung adenocarcinoma. Clin Cancer Res. 2008;14:5731–5734. doi: 10.1158/1078-0432.CCR-08-0646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Park J-A, Park H-J, Lee J-S, et al. Adenocarcinoma of the lung in never smoked children. Lung Cancer. 2008;61:266–269. doi: 10.1016/j.lungcan.2008.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Ginsberg RJ, Rubeinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Ann Thorac Surg. 1995;60:615–622. doi: 10.1016/0003-4975(95)00537-u. [DOI] [PubMed] [Google Scholar]
- 42.Okubo K, Mark EJ, Flieder D, et al. Bronchoalveolar carcinoma: clinical, radiologic, and pathologic factors and survival. J Thorac Cardiovasc Surg. 1999;118:702–709. doi: 10.1016/S0022-5223(99)70016-4. [DOI] [PubMed] [Google Scholar]
- 43.Okada M, Yoshikawa K, Hatta T, et al. Is segmentectomy with lymph node assessment an alternative to lobectomy for non-small cell lunch cancer of 2 cm or smaller? Ann Thorac Surg. 2001;71:956–961. doi: 10.1016/s0003-4975(00)02223-2. [DOI] [PubMed] [Google Scholar]
- 44.Watanabe S-I, Watanabe T, Arai K, et al. Results of wedge resection for focal bronchioloalveolar carcinoma showing pure ground-glass attenuation on computed tomography. Ann Thorac Surg. 2002;73:1071–1075. doi: 10.1016/s0003-4975(01)03623-2. [DOI] [PubMed] [Google Scholar]
- 45.Yoshikawa K, Tsubota N, Kodama K, et al. Prospective study of extended segmentectomy for small lung tumors: the final report. Ann Thorac Surg. 2002;73:1055–1059. doi: 10.1016/s0003-4975(01)03466-x. [DOI] [PubMed] [Google Scholar]
- 46.Koike T, Yamato Y, Yoshiya K, et al. Intentional limited pulmonary resection for peripheral T1N0M0 small-sized lung cancer. J Thorac Cardiovasc Surg. 2003;125:924–928. doi: 10.1067/mtc.2003.156. [DOI] [PubMed] [Google Scholar]
- 47.Nakamura H, Saji H, Ogata H, et al. Lung cancer patients showing pure ground-glass opacity on computerized tomography are good candidates for wedge resection. Lung Cancer. 2004;44:61–68. doi: 10.1016/j.lungcan.2003.09.025. [DOI] [PubMed] [Google Scholar]
- 48.Watanabe T, Okada A, Imakiire T, et al. Intentional limited resection for small peripheral lung cancer based on intraoperative pathologic exploration. Jpn J Thorac Cardiovasc Surg. 2004;53:29–35. doi: 10.1007/s11748-005-1005-7. [DOI] [PubMed] [Google Scholar]
- 49.Rusch VW, Tsuchiya R, Tsuboi M, et al. Surgery for bronchioloalveolar carcinoma and “very early” adenocarcinoma: an evolving standard of care? J Thorac Oncol. 2006;1(9 Suppl):S27–S31. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


