Abstract
Aims
Platelets play an important role in cardiovascular disease, and β-blockers are often prescribed for cardiovascular disease prevention. β-Blockers may directly affect platelet aggregation, because β-adrenergic receptors are present on platelets. There is uncertainty about the existence and magnitude of an effect of β-blockers on platelet aggregation. The aim of this study was to perform a systematic review and meta-analysis of the effect of β-blockers on platelet aggregation.
Methods
MEDLINE and EMBASE were searched until April 2014. Two reviewers independently performed data extraction and risk of bias assessment. Type of β-blocker, population, treatment duration and platelet aggregation were extracted. Standardized mean differences were calculated for each study and pooled in a random-effects meta-analysis.
Results
We retrieved 31 studies (28 clinical trials and three observational studies). β-Blockers decreased platelet aggregation (standardized mean difference −0.54, 95% confidence interval −0.85 to −0.24, P < 0.0001). This corresponds to a reduction of 13% (95% confidence interval 8–17%). Nonselective lipophilic β-blockers decreased platelet aggregation more than selective nonlipophilic β-blockers.
Conclusions
Clinically used β-blockers significantly reduce platelet aggregation. Nonselective lipophilic β-blockers seem to reduce platelet aggregation more effectively than selective nonlipophilic β-blockers. These findings may help to explain why some β-blockers are more effective than others in preventing cardiovascular disease.
Keywords: β-blockers, meta-analysis, platelet aggregation
What is Already Known about this Subject
Platelets play an important role in the pathogenesis of cardiovascular disease.
β-Blockers are one of the most prescribed drug classes for cardiovascular disease prevention.
β-Blockers may directly affect platelet aggregation, but there is still uncertainty about the existence and magnitude of this effect.
What this Study Adds
This study synthesized all available evidence and estimated the magnitude of the effect of β-blockers on platelet aggregation.
Clinically used β-blockers reduce platelet aggregation. Nonselective, lipophilic β-blockers reduce platelet aggregation most effectively.
These findings may help to explain why some β-blockers are more effective than others in preventing cardiovascular disease.
Introduction
Cardiovascular disease (CVD) is a major cause of mortality and morbidity worldwide [1,2]. Antiplatelet and β-blocking drugs are cornerstones in the secondary prevention of CVD [3–6]. The aim of antiplatelet therapy is to inhibit prothrombotic and anti-inflammatory platelet properties, which contribute to the pathogenesis of CVD [7]. β-Blockers are recommended for secondary prevention of CVD because of their beneficial effects on heart rate, blood pressure and myocardial oxygen demand [5,6,8]. Yet, β-blocking agents may also affect platelet aggregation, because β-adrenergic receptors are present on platelets and catecholamine levels are known to potentiate platelet reactivity [9]. The β-adrenergic receptor on human platelets is mainly the β2 subtype, which is inhibited only by nonselective β-blockers [10]. This suggests that nonselective β-blockers would have a more pronounced effect on platelet aggregation than selective β-blockers [11]. Besides, β-blockers have a membrane-stabilizing effect which, depending on the lipophilicity of the compounds, could also affect platelet aggregation [10,12].
Although β-blockers are one of the most frequently prescribed drug classes for CVD prevention and notwithstanding the mechanistic plausibility, there is still uncertainty about the existence and magnitude of an effect of β-blockers on platelet aggregation [13]. Our aim was to synthesize the currently available evidence on the effect of β-blockers on platelet aggregation and to examine whether this effect is modulated by the selectivity and lipophilicity of the β-blockers.
Methods
Search strategy
MEDLINE and EMBASE were searched until April 2014. The search terms used were ‘platelet aggregation’ and ‘beta blockers’ or ‘adrenergic beta-antagonists’ (an overview of the complete search string is shown in Methods S1). The search was extended by review of bibliographies from articles included in the final selection.
Eligibility criteria
Eligible articles had to report on the effect of β-blockers on platelet aggregation measured by light transmission aggregometry. In vitro studies, defined as studies in which β-blockers were added after blood sampling, were not eligible. Non-English articles, animal studies and platelet aggregation studies performed in conditions affecting platelet aggregation, i.e. physical or psychological stress, acute cardiovascular disease or pregnancy, were not included. Unpublished trials and data presented in short reports, conference abstracts or letters to the editor were also not eligible. Only studies on β-blockers registered by the World Health Organization for clinical use were considered (http://www.whocc.no/atc_ddd_index/). Studies were excluded if platelet aggregation was induced by an infrequently studied agonist, defined as an agonist that was used in one eligible study only.
Assessment of risk of bias
We assessed the risk of bias of the included studies according to the Cochrane Collaboration's tool for assessing risk of bias [14]. For the assessment of cross-over studies, we added the characteristics ‘reported on carry-over effects’ and ‘presence of carry-over effects’ to the domain ‘other bias’. For observational studies, we added the characteristics ‘reporting in- and exclusion criteria’ and ‘adequate control for confounding factors’ to the domain ‘other bias’. In accordance with the tool's instruction, all dimensions were scored as ‘low risk of bias’, ‘high risk of bias’ or ‘unclear risk of bias’ by two independent reviewers (TNB and CEIP). Disagreement occurred in five of 264 (2%) scorings and was resolved by consensus.
To estimate the impact of studies with a high risk of bias, we performed a sensitivity analysis by restricting the analysis to randomized studies, assuming a lower risk of bias for randomized studies. We regarded the domains on blinding as less important in the risk of bias assessment, because platelet aggregation is measured quantitatively. Publication bias was examined using a funnel plot. To estimate the impact of possible publication bias, we performed a cumulative meta-analysis based on study precision (standard error).
Data extraction
Two reviewers (TNB and CEIP) independently extracted data using standardized coding forms. For one-group (single treatment arm, no placebo) and cross-over trials (multiple treatment arms, interventions and placebo in each arm), we extracted data on platelet aggregation before and after intervention. For two-group trials (multiple treatment arms, intervention or placebo in each arm), we extracted data before and after intervention in the intervention and placebo groups. One two-group trial reported only data after intervention for the two trial arms; in this case, we used those data for meta-analysis [15]. Of cross-sectional studies, we compared data on platelet aggregation between exposed and non-exposed groups. If results were presented in figures only, we extracted outcome measures from the appropriate figures where possible [16]. When multiple measurements of platelet aggregation were performed in time, the last measurement was selected for analysis.
Effect size calculation and statistical procedures
Given that the measurement scale of platelet aggregation varied across studies, we calculated standardized mean differences (SMDs) for each study. As four types of study designs were included (cross-sectional, one-group, two-group and cross-over trials), we calculated SMDs using the appropriate formulas for each design (Methods S2) [14,17–19]. In brief, an SMD was calculated by dividing the mean difference by the pretest standard deviation (SD) or SD of the non-exposed group for cross-sectional studies. The SMD values were negative when the intervention (β-blocker) reduced the outcome (platelet aggregation), with effect sizes of −0.20, −0.50 and −0.80 representing small, medium and large reductions, respectively [20]. To facilitate interpretation, we additionally recalculated the overall effect size back to percentage platelet aggregation, a commonly used measurement scale in platelet aggregation studies, by multiplying the overall SMD with the mean SD [and 95% confidence interval (CI)] of the studies which used percentage platelet aggregation as the measurement scale. Most studies used multiple agonists to measure platelet aggregation. First, we analysed the effect of all agonists separately. Second, we calculated the mean of the effect sizes for each study, so that each study contributed with only one effect size to the analysis.
A random-effects meta-analysis was performed by default, because of expected between-study heterogeneity [21]. Subgroup analyses were performed for studies with nonselective vs. selective β-blockers, nonlipophilic vs. lipophilic β-blockers, short- (intake <1 week) vs. long-term exposure (intake >1 week) and healthy vs. diseased study population. Additionally, sensitivity analyses were performed for year of publication, method of platelet aggregation measurement and duration of follow-up. If the duration of exposure was not reported, we assumed long-term exposure [22–24]. Univariate meta-regression analysis was performed for each subgroup. All analyses were performed with STATA 12 (StataCorp LP, College Station, TX, USA).
Results
Literature search and study characteristics
A total of 1065 articles were identified through the literature search, of which 388 were duplicates (n = 156) or contained no English full text (n = 232). After review of the title and abstract, another 614 were excluded, mostly because no β-blocker or platelet aggregation was studied (n = 416) or studies used animals only (n = 121). Subsequently, 70 articles were reviewed in detail, in which no additional publications were identified by review of bibliographies. Finally, a total of 31 studies, reported in 25 articles, were included (Figure 1). We included 13 cross-over trials, eight one-group trials, seven two-group trials and three cross-sectional studies, in which a total of 454 subjects (range 4–43) were studied (details of included studies are shown in Table S1). The majority of the studies (n = 25) included patients with cardiovascular disease or cardiovascular disease risk factors (hypertension, coronary artery disease, diabetes or previous myocardial infarction). Mean age ranged from 33 to 74.5 years. Most studies (n = 26) reported on long-term exposure (>1 week) and nonselective β-blockers such as carvedilol, propranolol, labetalol or timolol (n = 23).
Figure 1.
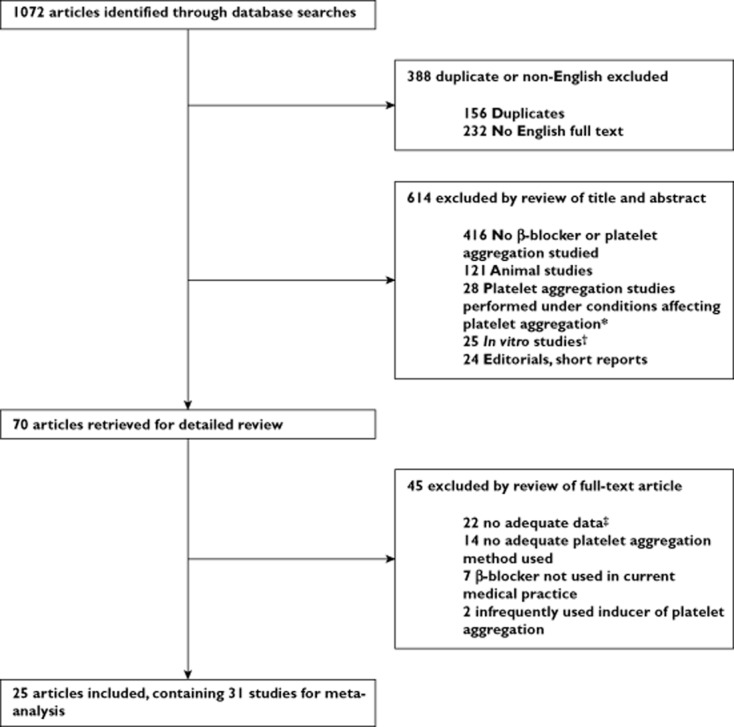
Flow chart of study selection. *Conditions affecting platelet aggregation: physical or psychological stress, acute cardiovascular disease or pregnancy. †In vitro was defined as studies in which β-blockers were added after blood drawing. ‡No outcome measures and/or no measure of variability (SE or SD), t statistic or exact P value reported
Meta-analysis of effect of β-blockers on platelet aggregation
The 31 included studies contained a total of 81 observations on platelet aggregation. The most used agonist to induce platelet aggregation was adenosine diphosphate (ADP; 52%), followed by collagen (20%) and epinephrine (20%), whereas arachidonic acid (n = 5) and thrombin (n = 2) were used in the minority of experiments. The effect of β-blockers on platelet aggregation was maximal with the use of epinephrine as an antagonist (Figure 2).
Figure 2.
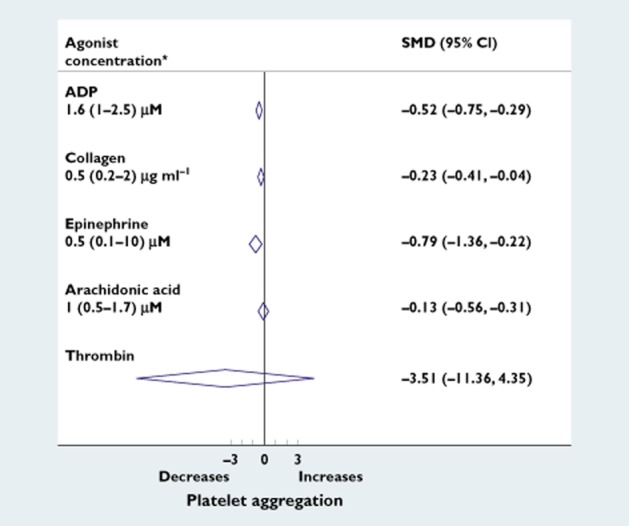
Effect of β-blockers on platelet aggregation by agonists. Forest plot of standardized mean differences (SMD) of the effects of β-blockers on platelet aggregation by different agonists. Abbreviations are as follows, ADP, adenosine diphosphate; CI, confidence interval. *Median (interquartile range) concentrations of the used agonists. Thrombin was used only for measurement of threshold concentration platelet aggregation
When study observations and antagonists were pooled, 13% (n = 4) of the studies showed an increase in platelet aggregation, whereas the majority (84%; n = 26) showed a decrease in platelet aggregation due to β-blockers. Random effects meta-analysis showed a decrease in platelet aggregation with exposure to β-blockers: SMD −0.54 (95% CI −0.85 to −0.24, P < 0.0001; Figure 3). This corresponds to a reduction of 13% (95% CI 8–17%) in platelet aggregation.
Figure 3.
Overall effect over β-blockers on platelet aggregation. Forest plot of standardized mean differences (SMD) of the effects of β-blockers on platelet aggregation. The black diamonds represent the effect estimate (SMD), where a negative SMD represents a decrease of platelet aggregation. The size of the grey squares around the effect estimates corresponds to the weight of the study in the meta-analysis. Horizontal lines represent corresponding 95% confidence intervals (CI). The estimate and CI of the pooled effect is indicated by the diamond
Subgroup and sensitivity analyses
Subgroup analysis showed that nonselective and lipophilic β-blockers [SMD −0.78 (95% CI −1.21 to −0.35) and −0.66 (95% CI −1.04 to −0.27), respectively] decreased platelet aggregation more than selective and nonlipophilic β-blockers [SMD −0.14 (95% CI −0.40 to 0.12) and −0.21 (95% CI −0.51 to 0.08), respectively; Figure 4]. These differences were not statistically significant with meta-regression analysis (nonselective vs. selective, P = 0.18; and nonlipophilic vs. lipophilic, P = 0.47). The effect was not different for studies with healthy or nonhealthy populations and with short- (<1 week) or long-term exposure (>1 week; P = 0.77 and P = 0.94; Figure 4). As a sensitivity analysis, we also analysed the duration of exposure as a continuous variable with meta-regression. Duration of exposure was missing for four studies [15,22–24], and one study [25] reported an exposure duration of 50 weeks. With inclusion of this study with very long exposure, meta-regression suggested that longer duration of follow-up was associated with decreasing platelet aggregation (coefficient −0.086; P = 0.004). However, after exclusion of this study, this was no longer observed (coefficient 0.005, P = 0.869). Thus, the effect of β-blockers on platelet aggregation does not seem to depend on the duration of exposure, which is also supported by the subgroup analysis in which studies with follow-up duration of <1 week and >1 week were compared (Figure 4).
Figure 4.
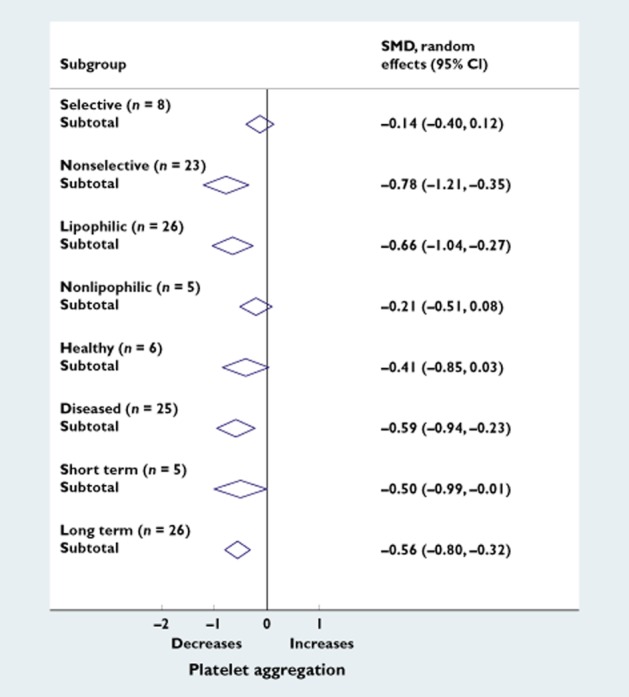
Subgroup analyses. Forest plot of standardized mean differences (SMD) of the effects of subgroups β-blockers on platelet aggregation. The effect estimates and CI of each subgroup are indicated by the diamonds. Subgroups are as follows: selective (metoprolol and atenolol); nonselective (propranolol, labetalol, timolol and carvedilol); lipophilic (propranolol, labetalol, timolol, metoprolol and carvedilol); nonlipophilic (atenolol); healthy subjects; diseased subjects (hypertension, coronary artery disease, diabetes or previous myocardial infarction); short-term treatment (<1 week); and long-term treatment (>1 week)
Platelet aggregation measurement methods as well as clinical use of β-blocking agents may change over time, and the included studies were published over a long period of time (between 1970 and 2005). Therefore, we performed a sensitivity analysis with restriction of studies published in or after 1990. Only seven of 31 (23%) of the studies were published in or after 1990. This analysis still indicated a decrease in platelet aggregation with the use of β-blockers [SMD −0.22 (95% CI −0.47 to 0.03)], although this was not statistically significant, possibly due to the small number of studies.
The included studies used different fixed concentrations of agonists, which affects platelet aggregation and adds to the heterogeneity of the present meta-analysis. The use of a threshold concentration as an outcome measure prevents this heterogeneity, because the inherent sensitivity of platelets to an agonist is tested, instead of the response of platelets to a fixed concentration of agonist. With restriction of the analysis to studies which used threshold concentration as an outcome (n = 11), platelet aggregation was still decreased with the use of β-blockers [SMD −0.63 (95% CI −1.42 to 0.164)] and was similar to the result when the analysis was restricted to studies which used another outcome measure [e.g. percentage platelet aggregation; SMD −0.54 (95% CI −0.80 to −0.27)].
Assessment of risk of bias and publication bias
A summary of the risk of bias assessment for clinical trials and observational studies is shown in Figure S1. Blinding was performed in 21 of 28 (75%) trials, but only 17 of 28 (61%) were double or triple blinded. Of cross-over trials, only six of 13 (46%) reported on carry-over effects. Only 20 of 28 (71%) of all trials were randomized, and only one trial described the randomization procedure. In a sensitivity analysis with restriction to randomized trials only (n = 20), the pooled effect estimate was SMD −0.42 (95% CI −0.80 to −0.04).
The funnel plot showed a relative lack of small studies with negative results (Figure 5). Two studies showed extreme effects, although their contributions to the overall effect were marginal (weights of 1.2 and 0.3%) [25,26]. Exclusion of these two studies from our analysis did not materially affect the pooled effect estimate [SMD after exclusion: −0.41 (95% CI −0.65 to −0.17)]. To estimate the impact of possible publication bias, we conducted a cumulative meta-analysis based on study precision (Figure S2). This indicated that the overall effect estimate was influenced by small studies. However, the effect estimate of the largest studies with the smallest standard errors was robust around SMD −0.25 to −0.30, again indicating a decrease in platelet aggregation with exposure to β-blockers.
Figure 5.
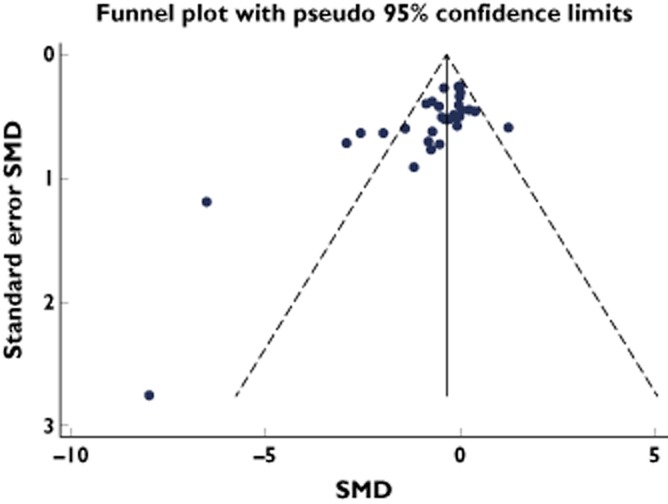
Funnel plot of all included studies. Two studies with extreme effects are the studies of Campbell [26] (1981; SMD −8) and Frishman [25] (1978; SMD −6.5)
A total of 15 studies included in this meta-analysis originated from six authors (Table S1), which raises the possibility that data or patients were used in multiple studies from the same author. To exclude any possible effect of the duplicate use of data, we restricted the analysis to the first study of each author, either historically or within one manuscript. Meta-analysis of the remaining 22 studies indicated a decrease of platelet aggregation with the use of β-blockers [SMD −0.46 (95% CI −0.76 to −0.16)].
Discussion
β-Blockers are one of the most frequently used drug classes in prevention of CVD, and platelets play an important role in the pathogenesis of CVD. Until now it was unclear whether β-blockers affected platelet aggregation. This meta-analysis demonstrates that clinically used doses of β-blockers significantly reduce platelet aggregation. Additionally, it indicates that nonselective lipophilic β-blockers reduce platelet aggregation more effectively than selective and nonlipophilic β-blockers.
Previous studies
Recent meta-analyses found that β-blockers, as a class, effectively reduce cardiac- and all-cause mortality in patients with systolic heart failure and acute myocardial infarction [27,28]. It is known that this is the result of beneficial effects on heart rate, blood pressure and myocardial oxygen demand [5,6,8]. Besides these established beneficial effects, the present meta-analysis suggests that part of the protective effect of β-blockers could be the result of inhibition of platelet aggregation. Interestingly, recent meta-analyses found that the nonselective β-blocker carvedilol was superior to selective β-blockers, which is in line with the results and subgroup analysis of the COMET trial [29,30]. In these studies, it was suggested that the nonselective lipophilic β-blocker carvedilol exerts an additional beneficial effect through improvement of endothelial function, stimulating β-arresting signalling and antioxidant properties [31–33]. Our findings suggest that lipophilic nonselective β-blockers decrease platelet aggregation most effectively, which could explain, in part, the superiority of nonselective lipophilic β-blockers.
Mechanisms of reduction of platelet aggregation by β-blockers
The inhibition of platelet aggregation by β-blockers can be explained by multiple mechanisms. First, β-blockers may exert their antiplatelet effect by a chemical interaction with the platelet cell membrane. The strength of this interaction depends on the lipophilicity of the compound, and results in stabilization of the platelet cell membrane, making it less sensitive to agonists [12]. Second, β2-receptors on platelets may be blocked by nonselective β-blockers. This would affect intraplatelet levels of adenosine 3′,5′-cyclic monophosphate (cAMP), which decreases calcium availability and subsequent activation of platelets [9]. However, compared with the number of α-receptors per platelet, the number of β2-receptors is small [34]. Therefore, it is more logical to assume that the β-blockers exert part of their antiplatelet effect not directly, by binding platelet receptors, but indirectly, by decreasing plasma catecholamine levels. This is also supported by the fact that platelet aggregation was maximally inhibited by β-blockers in experiments where epinephrine was used as an agonist (Figure 2). It is known that the catecholamine levels reached in vivo potentiate platelet aggregation, thereby overcoming even the inhibition of platelet aggregation by aspirin [9]. Interestingly, nonselective β-blockers reduce catecholamine levels more effectively than selective β-blockers, which supports our finding that platelet aggregation is more effectively inhibited by nonselective β-blockers [35,36]. Third, high blood pressure activates platelets through multiple pathways, and a decrease of blood pressure itself could therefore decrease platelet aggregation [13].
Strengths and limitations
A major strength of this meta-analysis is that we excluded in vitro studies from our analysis [37–43]. In these studies, β-blockers were added in concentrations that exceeded those currently used in medical practice, making it difficult to generalize the effects to the clinical situation. Another strength is that we did not exclude any type of study design. Calculation of effects sizes specific for each study design facilitated meta-analysis of the studies.
Given that different agonists induce different platelet activation pathways, this could influence the observed results. Before pooling all agonists, therefore, we first analysed the effect of β-blockers on platelet aggregation separately by all agonists (Figure 2). This analysis showed that platelet aggregation was inhibited with the use of all agonists, and was more pronounced with epinephrine. This could be expected, because platelet sensitivity is modified by catecholamine levels [9]. We think that pooling of all agonists to estimate the overall effect of β-blockers on platelet aggregation is valid because all agonists showed a clear reduction in platelet aggregation. Moreover, ADP was the most frequently used agonist and did not show an extreme effect estimate which could have overestimated our overall pooled effect estimate.
A limitation of this meta-analysis is that the risk of bias of the included studies is quite high. We think this can be explained partly by the fact that most studies were performed in years when reporting according to international standards for clinical trials and observational studies was not yet established. However, we do not think that, for example, nonblinding would have affected our results, because platelet aggregation is not affected by placebo effects and it has been shown that open unblinded studies with objective end-points yield the same results as double-blind placebo-controlled studies [44]. Fifty per cent of the included trials were designed as cross-over experiments, in which carry-over effects could influence the results [45]. However, the treatment periods in all included cross-over studies were long enough to ensure adequate wash-out. This is supported by our finding that the pooled effect did not differ between short-term (<1 week) or long-term administration (>1 week) of β-blockers. Finally, only 65% of the trials were randomized and only one trial described the randomization procedure. However, in a sensitivity analysis we showed that the overall pooled effect size was robust with restriction to randomized studies only. The funnel plot showed evidence for small-study effect, which might be explained by publication bias. Nevertheless, cumulative meta-analysis still indicated a clear and statistically significant effect of β-blockers on platelet aggregation. Finally, it is known that platelet aggregation is influenced by numerous characteristics, which were not all registered in the included studies. However, the majority of included studies were clinical trials, in which randomization ensured that known and unknown variables affecting platelet aggregation were equally distributed over treatment groups and were kept constant during the trial. Therefore, confounding of our results by other platelet-affecting variables is highly unlikely.
Conclusion
In conclusion, this meta-analysis suggests that clinically used doses of β-blockers reduce platelet aggregation. Nonselective, lipophilic β-blockers appear to reduce platelet aggregation most effectively. These findings may help to explain why some β-blockers are more effective than others in preventing CVD.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
This work was supported by a grant from the Netherlands Heart Foundation (grant number 2010B171).
Author Contributions
TNB and CEIP designed research, collected and interpreted data, performed statistical analysis and wrote the manuscript; JDS interpreted data and wrote the manuscript; TS performed statistical analysis and wrote the manuscript; OMD and JGB designed research, interpreted data and wrote the manuscript.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site:
Methods S1
Overview of the complete search strategy per database. Results for search on 1 April 2014
Methods S2
Calculation of effect size estimates (SMDs) and standard errors (SEs). Formulas used to calculate SMDs and SEs for each study design
Figure S1
Risk of bias assessment. Methodological quality graphs review authors' judgements about each methodological quality item presented across included trials and observational studies
Figure S2
Cumulative meta-analysis based on study precision
Table S1
Characteristics of all included studies
References
- 1.Nichols M, Townsend N, Scarborough P, Luengo-Fernandez R, Leal J, Gray A, Rayner M. 2012. European Cardiovascular Disease Statistics 2012. European Heart Network, European Society of Cardiology.
- 2.Roger VL, Go AS, Lloyd-Jones DM, Benjamin EJ, Berry JD, Borden WB, Bravata DM, Dai S, Ford ES, Fox CS, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Makuc DM, Marcus GM, Marelli A, Matchar DB, Moy CS, Mozaffarian D, Mussolino ME, Nichol G, Paynter NP, Soliman EZ, Sorlie PD, Sotoodehnia N, Turan TN, Virani SS, Wong ND, Woo D, Turner MB. Heart disease and stroke statistics − 2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Eikelboom JW, Hirsh J, Spencer FA, Baglin TP, Weitz JI. Antiplatelet drugs: antithrombotic therapy and prevention of thrombosis, 9th ed: American College of Chest Physicians Evidence-Based clinical practice guidelines. Chest. 2012;141:e89S–e119S. doi: 10.1378/chest.11-2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Vandvik PO, Lincoff AM, Gore JM, Gutterman DD, Sonnenberg FA, Alonso-Coello P, Akl EA, Lansberg MG, Guyatt GH, Spencer FA. Primary and secondary prevention of cardiovascular disease. Chest. 2012;141:e637S–668. doi: 10.1378/chest.11-2306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.O'Gara PT, Kushner FG, Ascheim DD, Casey DE, Jr, Chung MK, de Lemos JA, Ettinger SM, Fang JC, Fesmire FM, Franklin BA, Granger CB, Krumholz HM, Linderbaum JA, Morrow DA, Newby LK, Ornato JP, Ou N, Radford MJ, Tamis-Holland JE, Tommaso CL, Tracy CM, Woo YJ, Zhao DX, Anderson JL, Jacobs AK, Halperin JL, Albert NM, Brindis RG, Creager MA, DeMets D, Guyton RA, Hochman JS, Kovacs RJ, Kushner FG, Ohman EM, Stevenson WG, Yancy CW. 2013 ACCF/AHA guideline for the management of ST-elevation myocardial infarction: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines. Circulation. 2013;127:e362–425. doi: 10.1161/CIR.0b013e3182742cf6. [DOI] [PubMed] [Google Scholar]
- 6.Smith SC, Benjamin EJ, Bonow RO, Braun LT, Creager MA, Franklin BA, Gibbons RJ, Grundy SM, Hiratzka LF, Jones DW, Lloyd-Jones DM, Minissian M, Mosca L, Peterson ED, Sacco RL, Spertus J, Stein JH, Taubert KA. AHA/ACCF secondary prevention and risk reduction therapy for patients with coronary and other atherosclerotic vascular disease: 2011 update: a guideline from the American Heart Association and American College of Cardiology Foundation. Circulation. 2011;124:2458–2473. doi: 10.1161/CIR.0b013e318235eb4d. [DOI] [PubMed] [Google Scholar]
- 7.Davi G, Patrono C. Platelet activation and atherothrombosis. N Engl J Med. 2007;357:2482–2494. doi: 10.1056/NEJMra071014. [DOI] [PubMed] [Google Scholar]
- 8.Messerli FH, Grossman E. Beta-blockers in hypertension: is carvedilol different? Am J Cardiol. 2004;93:7B–12B. doi: 10.1016/j.amjcard.2004.01.020. [DOI] [PubMed] [Google Scholar]
- 9.Anfossi G, Trovati M. Role of catecholamines in platelet function: pathophysiological and clinical significance. Eur J Clin Invest. 1996;26:353–370. doi: 10.1046/j.1365-2362.1996.150293.x. [DOI] [PubMed] [Google Scholar]
- 10.Frishman WH. Beta-adrenoceptor antagonists: new drugs and new indications. N Engl J Med. 1981;305:500–506. doi: 10.1056/NEJM198108273050907. [DOI] [PubMed] [Google Scholar]
- 11.Kerry R, Scrutton MC. Platelet beta-adrenoceptors. Br J Pharmacol. 1983;79:681–691. doi: 10.1111/j.1476-5381.1983.tb10005.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imai S. Pharmacologic characterization of beta blockers with special reference to the significance of nonspecific membrane effects. Am J Cardiol. 1991;67:B8–B12. doi: 10.1016/0002-9149(91)90814-2. [DOI] [PubMed] [Google Scholar]
- 13.Blann AD, Nadar S, Lip GY. Pharmacological modulation of platelet function in hypertension. Hypertension. 2003;42:1–7. doi: 10.1161/01.HYP.0000077901.84467.E1. [DOI] [PubMed] [Google Scholar]
- 14.Higgins JPT, Green S. 2011. Cochrane Handbook for Systematic Reviews of Interventions, Version 5.1.0 [updated March 2011].. The Cochrane Collaboration. Available at http://www.cochrane-handbook.org (last accessed 1 March 2014)
- 15.Thaulow E, Kjekshus J, Erikssen J. Effect of timolol on platelet aggregation in coronary heart disease. Acta Med Scand Suppl. 1981;651:101–109. doi: 10.1111/j.0954-6820.1981.tb03640.x. [DOI] [PubMed] [Google Scholar]
- 16.Winther K, Trap-Jensen J. Effects of three beta-blockers with different pharmacodynamic properties on platelet aggregation and platelet and plasma cyclic AMP. Eur J Clin Pharmacol. 1988;35:17–20. doi: 10.1007/BF00555501. [DOI] [PubMed] [Google Scholar]
- 17.Becker BJ. Synthesizing standardized mean-change measures. Br J Math Stat Psychol. 1988;41:257–278. [Google Scholar]
- 18.Elbourne DR, Altman DG, Higgins JP, Curtin F, Worthington HV, Vail A. Meta-analyses involving cross-over trials: methodological issues. Int J Epidemiol. 2002;31:140–149. doi: 10.1093/ije/31.1.140. [DOI] [PubMed] [Google Scholar]
- 19.Morris SB, DeShon RP. Combining effect size estimates in meta-analysis with repeated measures and independent-groups designs. Psychol Methods. 2002;7:105–125. doi: 10.1037/1082-989x.7.1.105. [DOI] [PubMed] [Google Scholar]
- 20.Cohen J. Statistical Power Analysis for the Behavioural Sciences. 2nd edn. Hillsdale, NJ: Lawrence Erlbaum Associates; 1988. [Google Scholar]
- 21.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 22.Mehta J, Mehta P, Pepine CJ. Differences in platelet aggregation in coronary sinus and aortic blood in patients with coronary artery disease: effect of propranolol. Clin Cardiol. 1978;1:96–100. doi: 10.1002/clc.4960010208. [DOI] [PubMed] [Google Scholar]
- 23.Mehta J, Mehta P, Pepine CJ. Platelet aggregation in aortic and coronary venous blood in patients with and without coronary disease. 3. Role of tachycardia stress and propranolol. Circulation. 1978;58:881–886. doi: 10.1161/01.cir.58.5.881. [DOI] [PubMed] [Google Scholar]
- 24.Mehta P, Mehta J, Pepine CJ, Miale TD, Burger C. Platelet aggregation across the myocardial vascular bed in man: I. Normal versus diseased coronary arteries. Thromb Res. 1979;14:423–432. doi: 10.1016/0049-3848(79)90251-2. [DOI] [PubMed] [Google Scholar]
- 25.Frishman WH, Christodoulou J, Weksler B, Smithen C, Killip T, Scheidt S. Abrupt propranolol withdrawal in angina pectoris: effects on platelet aggregation and exercise tolerance. Am Heart J. 1978;95:169–179. doi: 10.1016/0002-8703(78)90460-x. [DOI] [PubMed] [Google Scholar]
- 26.Campbell WB, Johnson AR, Callahan KS, Graham RM. Anti-platelet activity of beta-adrenergic antagonists: inhibition of thromboxane synthesis and platelet aggregation in patients receiving long-term propranolol treatment. Lancet. 1981;2:1382–1384. doi: 10.1016/s0140-6736(81)92800-2. [DOI] [PubMed] [Google Scholar]
- 27.Chatterjee S, Biondi-Zoccai G, Abbate A, D'Ascenzo F, Castagno D, Van TB, Mukherjee D, Lichstein E. Benefits of beta blockers in patients with heart failure and reduced ejection fraction: network meta-analysis. BMJ. 2013;346:f55. doi: 10.1136/bmj.f55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.DiNicolantonio JJ, Lavie CJ, Fares H, Menezes AR, O'Keefe JH. Meta-analysis of carvedilol versus beta 1 selective beta-blockers (atenolol, bisoprolol, metoprolol, and nebivolol) Am J Cardiol. 2013;111:765–769. doi: 10.1016/j.amjcard.2012.11.031. [DOI] [PubMed] [Google Scholar]
- 29.Poole-Wilson PA, Swedberg K, Cleland JG, Di Lenarda A, Hanrath P, Komajda M, Lubsen J, Lutiger B, Metra M, Remme WJ, Torp-Pedersen C, Scherhag A, Skene A. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 30.Remme WJ, Torp-Pedersen C, Cleland JGF, Poole-Wilson PA, Metra M, Komajda M, Swedberg K, Di Lenarda A, Spark P, Scherhag A, Moullet C, Lukas MA. Carvedilol protects better against vascular events than metoprolol in heart failure: results from COMET. J Am Coll Cardiol. 2007;49:963–971. doi: 10.1016/j.jacc.2006.10.059. [DOI] [PubMed] [Google Scholar]
- 31.Intengan HD, Schiffrin EL. Disparate effects of carvedilol versus metoprolol treatment of stroke-prone spontaneously hypertensive rats on endothelial function of resistance arteries. J Cardiovasc Pharmacol. 2000;35:763–768. doi: 10.1097/00005344-200005000-00013. [DOI] [PubMed] [Google Scholar]
- 32.Mochizuki M, Yano M, Oda T, Tateishi H, Kobayashi S, Yamamoto T, Ikeda Y, Ohkusa T, Ikemoto N, Matsuzaki M. Scavenging free radicals by low-dose carvedilol prevents redox-dependent Ca2+ leak via stabilization of ryanodine receptor in heart failure. J Am Coll Cardiol. 2007;49:1722–1732. doi: 10.1016/j.jacc.2007.01.064. [DOI] [PubMed] [Google Scholar]
- 33.Wisler JW, DeWire SM, Whalen EJ, Violin JD, Drake MT, Ahn S, Shenoy SK, Lefkowitz RJ. A unique mechanism of beta-blocker action: carvedilol stimulates beta-arrestin signaling. Proc Natl Acad Sci USA. 2007;104:16657–16662. doi: 10.1073/pnas.0707936104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Kerry R, Scrutton MC, Wallis RB. Mammalian platelet adrenoceptors. Br J Pharmacol. 1984;81:91–102. doi: 10.1111/j.1476-5381.1984.tb10748.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Azevedo ER, Kubo T, Mak S, Al-Hesayen A, Schofield A, Allan R, Kelly S, Newton GE, Floras JS, Parker JD. Nonselective versus selective beta-adrenergic receptor blockade in congestive heart failure: differential effects on sympathetic activity. Circulation. 2001;104:2194–2199. doi: 10.1161/hc4301.098282. [DOI] [PubMed] [Google Scholar]
- 36.Kohno T, Yoshikawa T, Yoshizawa A, Nakamura I, Anzai T, Satoh T, Ogawa S. Carvedilol exerts more potent antiadrenergic effect than metoprolol in heart failure. Cardiovasc Drugs Ther. 2005;19:347–355. doi: 10.1007/s10557-005-4761-9. [DOI] [PubMed] [Google Scholar]
- 37.Berglund U, Wallentin L, von Schenck H. Platelet function and plasma fibrinogen and their relations to gender, smoking habits, obesity and beta-blocker treatment in young survivors of myocardial infarction. Thromb Haemost. 1988;60:21–24. [PubMed] [Google Scholar]
- 38.Jurgensen HJ, Dalsgaard-Nielsen J, Kjoller E, Gormsen J. Effect of long-term beta-blockade with alprenolol on platelet function and fibrinolytic activity in patients with coronary heart disease. Eur J Clin Pharmacol. 1981;20:245–250. doi: 10.1007/BF00618773. [DOI] [PubMed] [Google Scholar]
- 39.Pinterova E, Kacerovsky J, Zadak Z, Maly J, Pidrman V. The effect of bopindolol on lipids and platelet aggregation. Cor Vasa. 1988;30:352–360. [PubMed] [Google Scholar]
- 40.Sacchetti G, Bellani D, Montanari C, Gibelli A. Effects ‘in vitro’ of some cardiovascular drugs and other agents on human platelet aggregation. Thromb Diath Haemorrh. 1973;29:190–195. [PubMed] [Google Scholar]
- 41.Small M, Douglas JT, Aherne GW, Orr M, Lowe GD, Forbes CD, Prentice CR. Effects of the non-selective beta-adrenoceptor blocking agent, carteolol, on platelet function, blood coagulation and viscosity. Thromb Res. 1982;25:351–360. doi: 10.1016/0049-3848(82)90235-3. [DOI] [PubMed] [Google Scholar]
- 42.Sziegoleit W, Block HU, Fahr A, Mest HJ. Serum thromboxane B2 after intravenous administration of talinolol and propranolol in healthy subjects. Pharmazie. 1991;46:58–59. [PubMed] [Google Scholar]
- 43.Virgolini I, Fitscha P, Rauscha F, Sinzinger H. Effects of bopindolol on platelet function in hypertension at rest and during exercise. Prostaglandins Leukot Essent Fatty Acids. 1990;40:125–130. doi: 10.1016/0952-3278(90)90154-d. [DOI] [PubMed] [Google Scholar]
- 44.Smith DH, Neutel JM, Lacourciere Y, Kempthorne-Rawson J. Prospective, randomized, open-label, blinded-endpoint (PROBE) designed trials yield the same results as double-blind, placebo-controlled trials with respect to ABPM measurements. J Hypertens. 2003;21:1291–1298. doi: 10.1097/00004872-200307000-00016. [DOI] [PubMed] [Google Scholar]
- 45.Senn SJ. Cross-over trials, carry-over effects and the art of self-delusion. Stat Med. 1988;7:1099–1101. doi: 10.1002/sim.4780071010. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Methods S1
Overview of the complete search strategy per database. Results for search on 1 April 2014
Methods S2
Calculation of effect size estimates (SMDs) and standard errors (SEs). Formulas used to calculate SMDs and SEs for each study design
Figure S1
Risk of bias assessment. Methodological quality graphs review authors' judgements about each methodological quality item presented across included trials and observational studies
Figure S2
Cumulative meta-analysis based on study precision
Table S1
Characteristics of all included studies



