Abstract
Aims
Olfactory loss impairs the patient's quality of life. In individualized therapies, olfactory drug effects gain clinical importance. Molecular evidence suggests that among drugs with potential olfactory effects is Δ9-tetrahydrocannabinol (THC), which is approved for several indications, including neuropathic pain or analgesia in cancer patients. The present study aimed at assessing the olfactory effects of THC to be expected during analgesic treatment.
Methods
The effects of 20 mg oral THC on olfaction were assessed in a placebo-controlled, randomized cross-over study in healthy volunteers. Using an established olfactory test (Sniffin' Sticks), olfactory thresholds, odour discrimination and odour identification were assessed in 15 subjects at baseline and 2 h after THC administration.
Results
Δ9-Tetrahydrocannabinol impaired the performance of subjects (n = 15) in the olfactory test. Specifically, olfactory thresholds were increased and odour discrimination performance was reduced. This resulted in a significant drop in composite threshold, discrimination, identification (TDI) olfactory score by 5.5 points (from 37.7 ± 4.2 to 32.2 ± 5.6, 95% confidence interval for differences THC vs. placebo, −7.8 to −2.0, P = 0.003), which is known to be a subjectively perceptible impairment of olfactory function.
Conclusions
Considering the resurgence of THC in medical use for several pathological conditions, the present results indicate that THC-based analgesics may be accompanied by subjectively noticeable reductions in olfactory acuity. In particular, for patients relying on their sense of smell, this might be relevant information for personalized therapy strategies.
Keywords: cannabinoids, olfaction, sensory pharmacology, Δ9-tetrahydrocannabinol
What is Already Known about this Subject
Olfactory drug effects have frequently been reported, but controlled evidence is rare.
Several lines of evidence gathered from molecular, animal and human research suggest that Δ9-tetrahydrocannabinol (THC) is likely to alter the human sense of smell.
What this Study Adds
At doses advised for analgesia, THC produced subjectively noticeable effects on olfaction.
In particular, THC pushed olfactory thresholds toward more concentrated odours and reduced the number of correctly discriminated odours.
This led to a reduced overall olfactory score to an extent that is known to be perceptible subjectively, therefore being a subjectively noticeable deterioration of olfactory function.
Introduction
In the current activities in drug research and development directed toward individualized therapies in increasingly preselected patient subpopulations, treatment effects on the quality of life gain importance. In this context, drug effects on the olfactory system, which have been the subject of several reviews [1–5] and are based on various established or likely pharmacodynamics mechanisms [6], are given greater attention. This is because depressed olfactory function impairs the patient's quality of life [7–9]. Although human life seems dominated by the visual sense, smells trigger deep emotional responses [10]. Olfactory loss in otherwise healthy people is a likely cause for suffering, because this is associated with a loss of the enjoyment of foods [11]. Moreover, in certain professionals who rely on the sense of smell, such as enologists, chefs, perfumers and chemists, olfactory loss can lead to professional disability. The assessment of olfactory drug effects is facilitated by the advances in our understanding of the sense of smell [12,13] and the broader accessibility of such effects in humans with validated and well-established tools to test olfactory function [14].
Among drugs with potential olfactory effects are cannabinoids, which display several lines of molecular evidence, including the expression of cannabinoid CB1 receptors in olfactory neurons [15] and the olfactory bulb [16,17], the production of endocannabinoids in olfactory neurons and the decrease in olfactory thresholds as an effect of cannabinoid signalling [15]. In particular, Δ9-tetrahydrocannabinol (THC), the major active ingredient of Cannabis sativa, is approved or considered for approval for several indications, including neuropathic pain or analgesia in cancer patients. Given that anecdotal reports support an action of cannabis on olfaction, olfactory side-effects of THC are likely. Therefore, the present study aimed at assessing the effects of THC on olfaction to be expected during analgesic treatment.
Methods
Subjects, design and medication
The study followed the Declaration of Helsinki and was approved by the Ethics Committee of the Goethe University, Frankfurt am Main, Germany. Informed written consent was obtained from every participant. Their health was assessed by medical history, physical examination, including vital signs, and routine clinical laboratory test results.
The cohort for olfactory testing consisted of seven men and eight women (aged 26.6 ± 2.9 years, mean ± SD; all within ±10% of their ideal body weight). According to a randomized, placebo-controlled, double-blind, two-way cross-over design, they received either an oral dose of 20 mg of THC (two capsules of 10 mg THC dissolved in Adeps solidus manufactured by the Hospital Pharmacy of the University of Heidelberg, Germany) or placebo (two capsules of Adeps solidus only). It has to be mentioned, however, that in a placebo-controlled design with psychoactive drugs, unblinding of the subjects due to side-effects is possible and has to be considered in the interpretation of the results of psychophysical assessments, although the consequences of unblinding in cannabis studies have been judged to be modest [18]. A washout interval of at least 4 weeks was observed. Any medication, alcohol and food were prohibited for 30 days, 24 h and 6 h before the experiments, respectively.
Olfactory function was assessed at baseline, i.e. prior to medication, and 2 h afterwards, when maximal THC effects were expected [19]. In addition, subjects rated ‘tiredness’, ‘drowsiness’, ‘nausea’ and ‘euphoria’ by means of visual analog scales (VAS; length, 100 mm), ranging from ‘very weak’ to ‘very strong’.
Clinical olfactory testing
The validated ‘Sniffin’ Sticks' test (Burghart Messtechnik GmbH, Wedel, Germany) [20,21] was used to assess the three main components of olfactory function (threshold, discrimination and indentification, TDI) birhinally.
Odour thresholds were obtained for the rose-like odour phenylethylalcohol presented in 16 successive 1:2 dilution steps starting from a 4% solution. Using a three-alternative forced-choice task and a staircase protocol starting at low phenylethylalcohol concentrations, one pen with the odorant and two blanks were presented at each dilution step. Two successive correct identifications or one incorrect identification triggered a reversal of the staircase. Odour threshold was estimated using the mean of the last four out of seven staircase reversals (normal values, >6.0 and >6.5 for men and women, respectively [20,22]).
Odour discrimination was determined with 16 triplets of pens, two of each triplet containing the same odorant and the third a different, ‘target’ one (for names of odorants see [21,23]). The discrimination performance was assessed employing a three-alternative forced-choice task (normal score, >10 correct discriminations for both sexes).
Odour identification was determined with 16 odours (for names of odorants see [21,23]) using a four-alternative forced-choice task with presentation of a list of four descriptors for each pen (normal score, >11 correct identifications for males and females) using different stimulus sequences for every measurement. In addition to this scoring, the intensities and pleasantness of the 16 odours were rated on visual analog scales (length, 100 mm) ranging from ‘no odour perception’ to ‘odour perceived at maximal intensity’ or from ‘very unpleasant’ to ‘very pleasant’, respectively.
The evaluation of olfactory performance followed the clinically established procedure consisting of the calculation of a composite ‘TDI score’ (‘threshold, discrimination, identification’) as the sum of the scores from the three subtests [24]. Pathological olfactory function is indicated by TDI values of ≤29.5 and ≤30.5 for men and women, respectively, with the separation of hyposmia (30.5 ≥ TDI > 15.5) from functional anosmia at TDI ≤ 15.5 [20].
Statistics
The study followed a 2 × 2 design, with two baseline measurements and two measurements after administration of the medication. To accommodate this design, the data were submitted to analyses of variance for repeated-measures ANOVA, with ‘drug’ (i.e. THC or placebo; degrees of freedom, d.f. = 1) and ‘measurement’ (i.e. at baseline or subsequent to the administration of medication; d.f. = 1) as within-subject factors. Gender was further included as a between-subject factor (SPSS 21 for Linux; IBM SPSS Statistics, Chicago, IL, USA). This was done separately for each target parameter. In addition, differences from baseline were compared between the THC and placebo conditions by means of Student's paired t tests, and 95% confidence intervals of the differences (95% CIs) were calculated. In addition, changes in olfactory parameters and side-effects were explored for intercorrelations (rank correlation calculating Spearman's ρ [25]) following calculation of the placebo-corrected THC effects as E = ETHC − EBasline,THC − (EPlacebo − EBaseline,Placebo). The α level was set at 0.05, and a correction of the statistics for multiple testing was performed by using the relatively strict Bonferroni procedure [26].
Results
All subjects completed the study without serious side-effects that would have required medical intervention. Normal baseline olfactory function was established in all participants (TDI scores 38.8 ± 3.6 and 37.7 ± 4.2 for the placebo and active drug study day, respectively).
Detailed results of the statistical analysis of the THC effects on main olfactory parameters are shown in Table 1. A significant effect of THC on odour threshold was observed, in comparison to placebo. Specifically, THC administration increased the odour threshold from phenylethylalcohol dilution step 10.1 ± 1.6 to dilution step 7.5 ± 3 (Figure 1). Likewise, a significant effect of THC on odour discrimination was observed, in comparison to placebo. Specifically, THC reduced the number of correct odour discriminations from 13.3 ± 1.9 to 10.1 ± 2.8. The only subtest not affected by THC was odour identification, which changed only from 14.3 ± 1.8 to 14.6 ± 1.4 correctly identified odours. The overall effects on olfactory dimensions resulted in a reduction of the TDI score from 37.7 ± 4.2 to 32.2 ± 5.6. The subject's gender played no role in the results of the olfactory tests (always P > 0.5). Finally, THC did not significantly modulate the ratings of intensity or pleasantness of any of the 16 odours from the identification test (interactions ‘drug’ by ‘measurement’ or effects ‘drug’ always P > 0.05; Figure 2).
Table 1.
Results of the olfactory test (‘Sniffin’ Sticks' test [21]), including the results of the separate olfactory subtests (threshold, discrimination and identification) and the TDI sum score, acquired before administration of the medication (‘Baseline’) and 2 h after drug administration (‘Post’)
| Measure (units) | Drug | Measurement | Mean | SD | Repeated-measures ANOVA effects in a 2 × 2 design | Differences from baseline, THC vs. placebo | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ‘Drug’ | ‘Measurement’ | ‘Drug’ by ‘measurement’ | ‘Gender’* | Mean | 95% CI, lower limit | 95% CI, upper limit | Student's paired t test, P value | |||||
| Olfactory threshold (dilution step) | Placebo | Baseline | 10.8 | 2.4 | d.f. = 1,13, | d.f. = 1,13, | d.f. = 1,13, | d.f. = 1,13, | −2.4 | −4.4 | −0.3 | 0.028 |
| Post | 10.6 | 1.7 | F = 16.218, | F = 25.704, | F = 6.467, | F = 0.117, | ||||||
| THC | Baseline | 10.1 | 1.7 | P = 0.001† | P < 0.001† | P = 0.025 | P = 0.738 | |||||
| Post | 7.5 | 3.0 | ||||||||||
| Odour discrimination (number correct) | Placebo | Baseline | 13.5 | 1.6 | d.f. = 1,13, | d.f. = 1,13, | d.f. = 1,13, | d.f. = 1,13, | −2.7 | −4.6 | −0.9 | 0.006 |
| Post | 12.9 | 1.8 | F = 8.651, | F = 14.378, | F = 9.935, | F = 0.388, | ||||||
| THC | Baseline | 13.3 | 1.9 | P = 0.011† | P = 0.002† | P = 0.008† | P = 0.544 | |||||
| Post | 10.1 | 2.8 | ||||||||||
| Odour identification (number correct) | Placebo | Baseline | 14.5 | 1.6 | d.f. = 1,13, | d.f. = 1,13, | d.f. = 1,13, | d.f. = 1,13, | 0.2 | −0.5 | 0.9 | 0.55 |
| Post | 14.7 | 1.6 | F = 0.58, | F = 1.122, | F = 0.47, | F = 0.151, | ||||||
| THC | Baseline | 14.3 | 1.8 | P = 0.46 | P = 0.309 | P = 0.505 | P = 0.704 | |||||
| Post | 14.6 | 1.4 | ||||||||||
| TDI score | Placebo | Baseline | 38.8 | 3.6 | d.f. = 1,13, | d.f. = 1,13, | d.f. = 1,13, | d.f. = 1,13, | −4.9 | −7.8 | −2.0 | 0.003 |
| Post | 38.2 | 3.7 | F = 16.285, | F = 21.542, | F = 13.065, | F = 0.315, | ||||||
| THC | Baseline | 37.7 | 4.2 | P = 0.001† | P < 0.001† | P = 0.003† | P = 0.584 | |||||
| Post | 32.2 | 5.6 | ||||||||||
Abbreviations are as follows: CI, confidence interval; TDI, threshold, discrimination, identification; THC, Δ9-tetrahydrocannabinol. Results of statistical analysis are shown for the repeated-measures ANOVA in a 2 × 2 design (two measurements per day, two medications) and, in addition, as comparisons between the THC and placebo conditions for the respective differences from baseline.
Given that no effects of ‘gender’ were identified, the interactions including this factor, all having resulted in nonsignificant effects, are omitted from this table.
Significant effect (P < 0.05).
Figure 1.
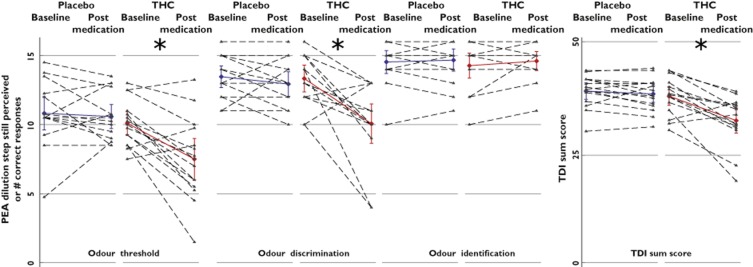
Effects of Δ9-tetrahydrocannabinol (THC; 20 mg oral) on the three major dimensions of olfaction and on the performance score of an established olfactory test (‘Sniffin’ Sticks' test; Burghart Medical Instruments, Wedel, Germany [21]). The olfactory test was applied at baseline and 2 h after oral administration of either 20 mg THC or placebo (‘postmedication’) to 15 healthy volunteers. Individual observations are shown as triangles connected with the respective postmedication observation by a dashed line. Parameter mean values and 95% confidence intervals are overlaid and coloured (blue, placebo condition; red, THC condition). In comparison to placebo, THC significantly increased the detection threshold of phenylethylalcohol (PEA; interaction ‘drug’ by ‘measurement’, P = 0.025), reduced the ability to discriminate between different odours (interaction ‘drug’ by ‘measurement’, P = 0.008) and therefore also decreased the sum TDI score (threshold, discrimination, identification; interaction ‘drug’ by ‘measurement’, P = 0.003), although the identification of 16 different odours was not affected by THC. The asterisks indicate significances (P < 0.05) according to post hoc Student's paired t tests following significant ANOVA effects
Figure 2.
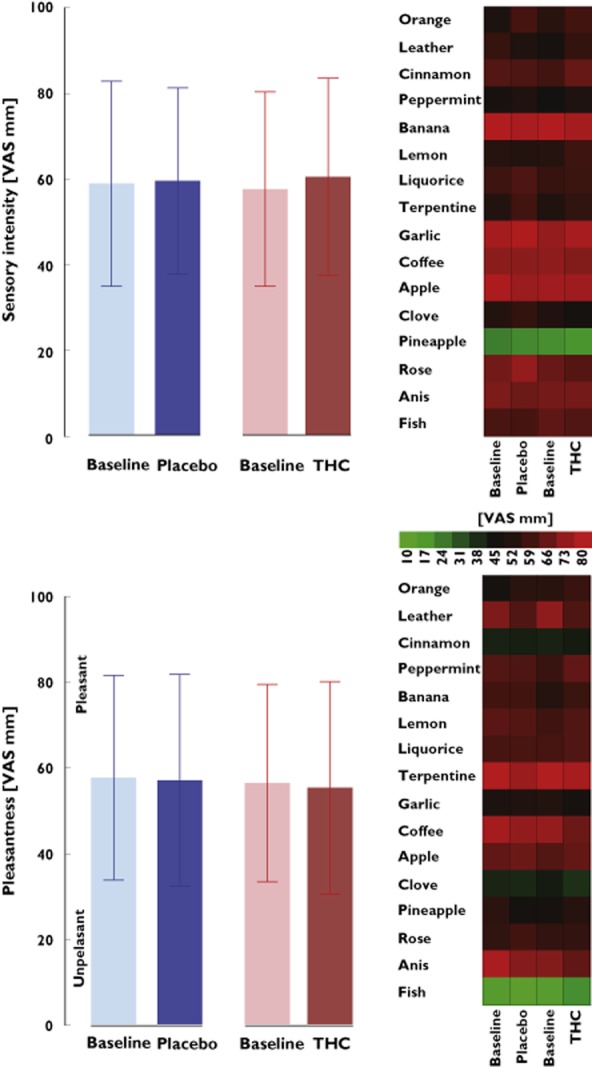
Ratings of the sensory intensity (top) and the pleasantness (bottom) of the 16 odours belonging to the identification subtest of the ‘Sniffin'Stick' test (listed at the left side of the heat plot). Left panels show means and 95% confidence intervals of the ratings obtained at baseline and at 2 h following administration of the medication (THC or placebo), averaged across all 16 odorants and 15 subjects. Right panels are heat plots of the ratings of single odorants of the identification subtest. The colour coding from green, unpleasant or low intensity, via black, middle intensity of hedonically inert, to red, pleasant or high intensity, indicates the visual analog scale (VAS) ratings. None of the intensity ratings were changed by THC, and no pattern shift was observed in this cohort.  , placebo;
, placebo;  , THC
, THC
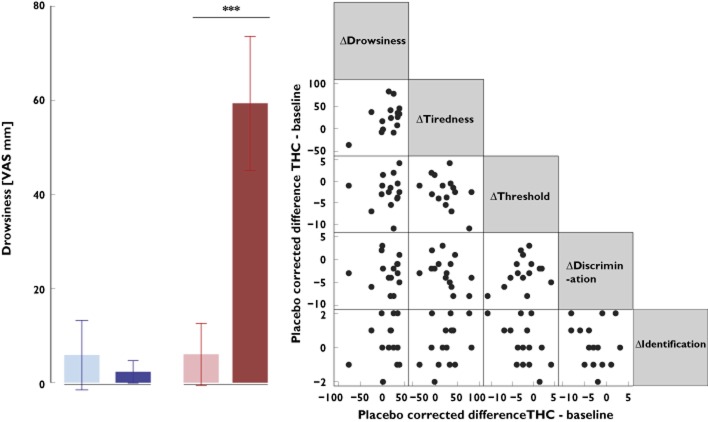
Detailed results of the statistical analysis of selected THC side-effects are shown in Table 2. Δ9-Tetrahydrocannabinol, in comparison to placebo, induced significant increases mainly in drowsiness and tiredness from baseline to 2 h after drug administration, respectively, as rated on a 100 mm VAS, whereas euphoria showed only a trend towards significance. Further side-effects occurring after THC administration were vomiting (n = 2), paranoid reaction (n = 1) and dizziness (n = 1), while none was reported for the placebo condition. Of note, the observed changes in olfactory functions following THC administration were not correlated with changes in the ratings of tiredness and drowsiness (Figure 3), nausea or euphoria (Spearman's rank correlation, P > 0.05).
Table 2.
Results of the ratings of selected THC side-effects acquired by means of visual analog scales (range, 0–100) before administration of the medication (‘Baseline’) and 2 h after drug administration (‘Post’)
| Repeated-measures ANOVA effects in a 2 × 2 design | Differences from baseline, THC vs. placebo | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Measure (mm VAS) | Drug | Measurement | Mean | SD | ‘Drug’ | ‘Measurement’ | ‘Drug’ by ‘measurement’ | ‘Gender’* | Mean | 95% CI, lower limit | 95% CI, upper limit | Student's paired t test, P value |
| Drowsiness | Placebo | Baseline | 5.8 | 14.7 | d.f. = 1,13, | d.f. = 1,13, | d.f. = 1,13, | d.f. = 1,13, | 57.3 | 36.0 | 78.7 | 0 |
| Post | 2.3 | 4.7 | F = 47.239, | F = 19.269, | F = 55.14, | F = 0.05, | ||||||
| THC | Baseline | 6.0 | 13.1 | P < 0.001† | P = 0.001† | P = 0.001† | P = 0.826 | |||||
| Post | 59.5 | 28.2 | ||||||||||
| Tiredness | Placebo | Baseline | 51.5 | 23.3 | d.f. = 1,13, F = 7.787, P = 0.015† | d.f. = 1,13, F = 1.978, P = 0.183 | d.f. = 1,13, F = 9.809, P = 0.008† | d.f. = 1,13, F = 0.636, P = 0.44 | 24.9 | 7.6 | 42.2 | 0.008 |
| Post | 43.5 | 25.1 | ||||||||||
| THC | Baseline | 51.3 | 19.1 | |||||||||
| Post | 68.3 | 17.5 | ||||||||||
| Nausea | Placebo | Baseline | 1.8 | 5.1 | d.f. = 1,13, F = 4.958, P = 0.044† | d.f. = 1,13, F = 2.804, P = 0.118 | d.f. = 1,13, F = 4.326, P = 0.058 | d.f. = 1,13, F = 0.83, P = 0.777 | 13.7 | 0.2 | 27.3 | 0.047 |
| Post | 0.5 | 0.9 | ||||||||||
| THC | Baseline | 1.9 | 4.9 | |||||||||
| Post | 14.3 | 24.1 | ||||||||||
| Euphoria | Placebo | Baseline | 16.7 | 26.4 | d.f. = 1,13, F = 1.259, P = 0.282 | d.f. = 1,13, F = 2.116, P = 0.169 | d.f. = 1,13, F = 3.507, P = 0.084 | d.f. = 1,13, F = 0.668, P = 0.428 | 16.3 | −1.7 | 34.3 | 0.072 |
| Post | 13.0 | 21.4 | ||||||||||
| THC | Baseline | 12.9 | 19.2 | |||||||||
| Post | 25.6 | 23.1 | ||||||||||
Abbreviations are as follows: CI, confidence interval; TDI, threshold, discrimination, identification; THC, Δ9-tetrahydrocannabinol; VAS, visual analog scale. Statistical analysis results are shown for the repeated-measures ANOVA in a 2 × 2 design (two measurements per day, two medications) and, in addition, as comparisons between the THC and placebo conditions for the respective differences from baseline.
Given that no effects of ‘gender’ were identified, the interactions including this factor, all having resulted in nonsignificant effects, are omitted from this table.
Significant effect (P < 0.05).
Figure 3.
Ratings of the drowsiness and association of THC-induced changes in drowsiness and tiredness with THC-induced changes in olfactory test parameters. Left panel shows means and 95% confidence intervals of the ratings of drowsiness, obtained at baseline and at 2 h following administration of the medication (THC or placebo), averaged across the 15 subjects. Right panel is a scatterplot matrix of the THC-induced changes in selected parameters and olfactory test outcomes, calculated as differences of the ratings in the presence of THC from the baseline ratings, corrected for the respective changes in the presence of placebo. The asterisks indicate significances (P = < 0.001) according to post hoc paired t-test following significant ANOVA effects. , placebo;
, placebo;  , THC; *** P = < 0.001
, THC; *** P = < 0.001
Discussion
The present results establish a significant effect of THC on the human sense of smell. At the dosage used in the treatment of pain, the effects are directed toward reduced olfactory acuity associated with a reduction of the ability to distinguish odours. The change by 5.5 points in the composite olfactory TDI score identifies a subjectively noticeable change in olfactory function [27].
Δ9-Tetrahydrocannabinol probably modulated olfaction by altering both the perception and the evaluation of olfactory stimuli. An upstream olfactory functional involvement is suggested by the significant change in odour thresholds that have been reported to be unrelated to higher cognitive factors [28]. However, the same report [28] also established that executive functioning and semantic memory contribute significantly to the performance of odour discrimination and identification. As odour discrimination was also reduced following THC administration, these higher cognitive processes were probably affected by THC. Further support for a cognitive component of the effects can be derived from the absence of changes in odour intensity relations, which indicates that the reduced odour discrimination was not simply due to reduced perception. In contrast, the changes were not correlated with the ratings of tiredness or drowsiness, which emphasizes the specificity of the olfactory effects of THC as opposed to a mere nonspecific influence via altered arousal.
The decrease in the average TDI score by 5.5 points corresponds to the subjectively perceived change in olfactory function found in a longitudinal assessment. As established in 83 patients with impaired olfactory sensitivity of various aetiologies, who performed the ‘Sniffin’ Stick' test battery on two occasions (mean interval 136 days), an increase of at least 5.5 points in TDI was required to be considered by the patients as improved olfactory function [27]. Thus, a decrease of 5.5 TDI as observed in this study represents a subjectively noticeable reduction in olfactory function and contradicts anecdotal information about cannabis-induced positive perceptual changes in olfaction [29].
The present findings of reduced olfactory function as a drug effect of THC reflect the oral dosage of 20 mg, which is recommended for pain treatment [30]. For comparison, cannabis joints have a reported THC content of 13 mg [31]; however, the inhalational route of administration achieves mean peak plasma concentrations of 77 ng ml−1 THC [31], which are 10 times higher than after oral administration [19,32], including the concentrations observed during the present study of 4.8 ± 2.8 ng ml−1 in men and 8.1 ± 4.3 ng ml−1 in women observed 2 h after THC administration [33]. In contrast, as approved initial THC dosages for appetite stimulation and relief of nausea are 2.5 and 5 mg THC [34], respectively, the present findings might not apply to these lower doses. Moreover, it cannot be excluded that the observed effects are limited to the time window assessed in the present study, i.e. close to the time of occurrence of maximal THC plasma concentrations (see also [33]).
However, the observation also disagrees with expectations from research results obtained in animals, which have implicated cannabinoids in increased sensitivity to odorants [15,35]. In Xenopus laevis, 2-arachidonoyl-glycerol produced, depending on the hunger state of the animal, lowered odorant detection thresholds via cannabinoid CB1 receptor activation [15]. Blockade of CB1 receptors at olfactory receptor neurons resulted in diminished responses to odorants [35]. Moreover, cannabinoid signalling plays a role in the regulation of neuronal activity and signalling in mouse olfactory bulb glomeruli, with the consequence that activation of CB1 receptors may increase the overall sensitivity of the glomerulus to sensory inputs [36]. Indeed, CB1 receptor signalling in the olfactory bulb, triggered by both exogenous and endogenous cannabinoids, increased odour detection and promoted food intake in fasted mice [37]. The present observations may hint at a fundamental difference in the effects of cannabis on olfaction between humans and animals, which receives some support from different species expression patterns of CB1 receptors. Specifically, while CB1 receptors are expressed in the olfactory bulb of animals [16,17], they were not in human olfactory bulbs at either the mRNA [38] or the proteomic level [39]. Moreover, cognitive factors may have contributed, such as effects of THC on cerebral information processing [40,41], which might have included executive functioning and semantic memory [42] that are important for odour discrimination and identification [28]. However, the effects were also observed on odour thresholds reported to be unrelated to higher cognitive factors [28].
The present data establish a subjectively noticeable change of the olfactory performance induced by an oral dose of 20 mg THC in humans. Considering the resurgence of THC in medical use for pathological conditions, THC-based analgesics [43] may be accompanied by subjectively noticeable reductions in olfactory acuity that might be relevant for personalized therapy strategies. The sense of smell will be reduced to an extent that is known to be subjectively perceptible, therefore being a noticeable deterioration of olfactory function.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organization that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
References
- 1.Ackerman BH, Kasbekar N. Disturbances of taste and smell induced by drugs. Pharmacotherapy. 1997;17:482–496. [PubMed] [Google Scholar]
- 2.Doty RL, Philip S, Reddy K, Kerr KL. Influences of antihypertensive and antihyperlipidemic drugs on the senses of taste and smell: a review. J Hypertens. 2003;21:1805–1813. doi: 10.1097/00004872-200310000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Henkin RI. Drug effects on smell and taste. In: Pradham SN, Maickel RP, Dutta SN, editors. Pharmacology in Medicine: Principles and Practice. Bethesda: SP Press Int; 1986. pp. 748–753. [Google Scholar]
- 4.Henkin RI. Drug-induced taste and smell disorders. Incidence, mechanisms and management related primarily to treatment of sensory receptor dysfunction. Drug Saf. 1994;11:318–377. doi: 10.2165/00002018-199411050-00004. [DOI] [PubMed] [Google Scholar]
- 5.Schiffman S. Drugs influencing taste and smell perception. In: Getchell TV, Doty RL, Bartoshuk LM, Snow JB, editors. Smell and Taste in Health and Disease. New York: Raven Press; 1991. pp. 845–850. [Google Scholar]
- 6.Lötsch J, Geisslinger G, Hummel T. Sniffing out pharmacology: interactions of drugs with human olfaction. Trends Pharmacol Sci. 2012;33:193–199. doi: 10.1016/j.tips.2012.01.004. [DOI] [PubMed] [Google Scholar]
- 7.Doty RL, Genow A, Hummel T. Scratch density differentiates microsmic from normosmic and anosmic subjects on the University of Pennsylvania Smell Identification Test. Percept Mot Skills. 1998;86:211–216. doi: 10.2466/pms.1998.86.1.211. [DOI] [PubMed] [Google Scholar]
- 8.Frasnelli J, Hummel T. Olfactory dysfunction and daily life. Eur Arch Otorhinolaryngol. 2005;262:231–235. doi: 10.1007/s00405-004-0796-y. [DOI] [PubMed] [Google Scholar]
- 9.Hummel T, Nordin S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngol. 2005;125:116–121. doi: 10.1080/00016480410022787. [DOI] [PubMed] [Google Scholar]
- 10.Rinaldi A. The scent of life. The exquisite complexity of the sense of smell in animals and humans. EMBO Rep. 2007;8:629–633. doi: 10.1038/sj.embor.7401029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hummel T, Nordin S. Olfactory disorders and their consequences for quality of life. Acta Otolaryngol. 2005;125:116–121. doi: 10.1080/00016480410022787. [DOI] [PubMed] [Google Scholar]
- 12.Buck L, Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991;65:175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- 13.Axel R. The molecular logic of smell. Sci Am. 1995;273:154–159. doi: 10.1038/scientificamerican1095-154. [DOI] [PubMed] [Google Scholar]
- 14.Lapid H, Shushan S, Plotkin A, Voet H, Roth Y, Hummel T, Schneidman E, Sobel N. Neural activity at the human olfactory epithelium reflects olfactory perception. Nat Neurosci. 2011;14:1455–1461. doi: 10.1038/nn.2926. [DOI] [PubMed] [Google Scholar]
- 15.Breunig E, Manzini I, Piscitelli F, Gutermann B, Di Marzo V, Schild D, Czesnik D. The endocannabinoid 2-arachidonoyl-glycerol controls odor sensitivity in larvae of Xenopus laevis. J Neurosci. 2010;30:8965–8973. doi: 10.1523/JNEUROSCI.4030-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pettit DA, Harrison MP, Olson JM, Spencer RF, Cabral GA. Immunohistochemical localization of the neural cannabinoid receptor in rat brain. J Neurosci Res. 1998;51:391–402. doi: 10.1002/(SICI)1097-4547(19980201)51:3<391::AID-JNR12>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 17.Cesa R, Mackie K, Beltramo M, Franzoni MF. Cannabinoid receptor CB1-like and glutamic acid decarboxylase-like immunoreactivities in the brain of Xenopus laevis. Cell Tissue Res. 2001;306:391–398. doi: 10.1007/s004410100461. [DOI] [PubMed] [Google Scholar]
- 18.Wright S, Duncombe P, Altman DG. Assessment of blinding to treatment allocation in studies of a cannabis-based medicine (Sativex®) in people with multiple sclerosis: a new approach. Trials. 2012;13:189. doi: 10.1186/1745-6215-13-189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hollister LE, Gillespie HK, Ohlsson A, Lindgren JE, Wahlen A, Agurell S. Do plasma concentrations of delta 9-tetrahydrocannabinol reflect the degree of intoxication? J Clin Pharmacol. 1981;21:171S–177. doi: 10.1002/j.1552-4604.1981.tb02593.x. [DOI] [PubMed] [Google Scholar]
- 20.Hummel T, Kobal G, Gudziol H, Mackay-Sim A. Normative data for the ‘Sniffin’ Sticks' including tests of odor identification, odor discrimination, and olfactory thresholds: an upgrade based on a group of more than 3,000 subjects. Eur Arch Otorhinolaryngol. 2007;264:237–243. doi: 10.1007/s00405-006-0173-0. [DOI] [PubMed] [Google Scholar]
- 21.Hummel T, Sekinger B, Wolf SR, Pauli E, Kobal G. ‘Sniffin’ sticks': olfactory performance assessed by the combined testing of odor identification, odor discrimination and olfactory threshold. Chem Senses. 1997;22:39–52. doi: 10.1093/chemse/22.1.39. [DOI] [PubMed] [Google Scholar]
- 22.Croy I, Lange K, Krone F, Negoias S, Seo HS, Hummel T. Comparison between odor thresholds for phenyl ethyl alcohol and butanol. Chem Senses. 2009;34:523–527. doi: 10.1093/chemse/bjp029. [DOI] [PubMed] [Google Scholar]
- 23.Kobal G, Hummel T, Sekinger B, Barz S, Roscher S, Wolf S. ‘Sniffin’ sticks': screening of olfactory performance. Rhinology. 1996;34:222–226. [PubMed] [Google Scholar]
- 24.Wolfensberger M, Schnieper I, Welge-Lussen A. ‘Sniffin’ Sticks': a new olfactory test battery. Acta Otolaryngol. 2000;120:303–306. doi: 10.1080/000164800750001134. [DOI] [PubMed] [Google Scholar]
- 25.Spearman C. The proof and measurement of association between two things. By C. Spearman, 1904. Am J Psychol. 1987;100:441–471. [PubMed] [Google Scholar]
- 26.Hochberg Y. A sharper bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800–802. [Google Scholar]
- 27.Gudziol V, Lötsch J, Hahner A, Zahnert T, Hummel T. Clinical significance of results from olfactory testing. Laryngoscope. 2006;116:1858–1863. doi: 10.1097/01.mlg.0000234915.51189.cb. [DOI] [PubMed] [Google Scholar]
- 28.Hedner M, Larsson M, Arnold N, Zucco GM, Hummel T. Cognitive factors in odor detection, odor discrimination, and odor identification tasks. J Clin Exp Neuropsychol. 2010;32:1062–1067. doi: 10.1080/13803391003683070. [DOI] [PubMed] [Google Scholar]
- 29.Green B, Kavanagh D, Young R. Being stoned: a review of self-reported cannabis effects. Drug Alcohol Rev. 2003;22:453–460. doi: 10.1080/09595230310001613976. [DOI] [PubMed] [Google Scholar]
- 30.Noyes R, Jr, Brunk SF, Avery DA, Canter AC. The analgesic properties of delta-9-tetrahydrocannabinol and codeine. Clin Pharmacol Ther. 1975;18:84–89. doi: 10.1002/cpt197518184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ohlsson A, Lindgren JE, Wahlen A, Agurell S, Hollister LE, Gillespie HK. Plasma delta-9 tetrahydrocannabinol concentrations and clinical effects after oral and intravenous administration and smoking. Clin Pharmacol Ther. 1980;28:409–416. doi: 10.1038/clpt.1980.181. [DOI] [PubMed] [Google Scholar]
- 32.Walter C, Ferreiros N, Bishay P, Geisslinger G, Tegeder I, Lotsch J. Exogenous Delta9-tetrahydrocannabinol influences circulating endogenous cannabinoids in humans. J Clin Psychopharmacol. 2013;33:699–705. doi: 10.1097/JCP.0b013e3182984015. [DOI] [PubMed] [Google Scholar]
- 33.Walter C, Ferreirós N, Bishay P, Geisslinger G, Tegeder I, Lötsch J. Exogenous delta9-tetrahydrocannabinol influences circulating endogenous cannabinoids in humans. J Clin Psychopharmacol. 2013;33:699–705. doi: 10.1097/JCP.0b013e3182984015. [DOI] [PubMed] [Google Scholar]
- 34.Marinol. 2008. Summary of product characteristics.
- 35.Czesnik D, Schild D, Kuduz J, Manzini I. Cannabinoid action in the olfactory epithelium. Proc Natl Acad Sci U S A. 2007;104:2967–2972. doi: 10.1073/pnas.0609067104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wang Z-J, Sun L, Heinbockel T. Cannabinoid receptor-mediated regulation of neuronal activity and signaling in glomeruli of the main olfactory bulb. J Neurosci. 2012;32:8475–8479. doi: 10.1523/JNEUROSCI.5333-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Soria-Gómez E, Bellocchio L, Reguero L, Lepousez G, Martin C, Bendahmane M, Ruehle S, Remmers F, Desprez T, Matias I, Wiesner T, Cannich A, Nissant A, Wadleigh A, Pape H-C, Chiarlone AP, Quarta C, Verrier D, Vincent P, Massa F, Lutz B, Guzmán M, Gurden H, Ferreira G, Lledo P-M, Grandes P, Marsicano G. The endocannabinoid system controls food intake via olfactory processes. Nat Neurosci. 2014;17:407–415. doi: 10.1038/nn.3647. [DOI] [PubMed] [Google Scholar]
- 38.Lötsch J, Schaeffeler E, Mittelbronn M, Winter S, Gudziol V, Schwarzacher SW, Hummel T, Doehring A, Schwab M, Ultsch A. Functional genomics suggest neurogenesis in the adult human olfactory bulb. Brain Struct Funct. 2013 doi: 10.1007/s00429-013-0618-3. PMID: 23928746, [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 39.Fernandez-Irigoyen J, Corrales FJ, Santamaria E. Proteomic atlas of the human olfactory bulb. J Proteomics. 2012;75:4005–4016. doi: 10.1016/j.jprot.2012.05.011. [DOI] [PubMed] [Google Scholar]
- 40.D'Souza DC, Fridberg DJ, Skosnik PD, Williams A, Roach B, Singh N, Carbuto M, Elander J, Schnakenberg A, Pittman B, Sewell RA, Ranganathan M, Mathalon D. Dose-related modulation of event-related potentials to novel and target stimuli by intravenous Delta(9)-THC in humans. Neuropsychopharmacol. 2012;37:1632–1646. doi: 10.1038/npp.2012.8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Klumpers LE, Cole DM, Khalili-Mahani N, Soeter RP, Te Beek ET, Rombouts SARB, van Gerven JMA. Manipulating brain connectivity with δ9-tetrahydrocannabinol: a pharmacological resting state FMRI study. Neuroimage. 2012;63:1701–1711. doi: 10.1016/j.neuroimage.2012.07.051. [DOI] [PubMed] [Google Scholar]
- 42.Marsicano G, Wotjak CT, Azad SC, Bisogno T, Rammes G, Cascio MG, Hermann H, Tang J, Hofmann C, Zieglgänsberger W, Di Marzo V, Lutz B. The endogenous cannabinoid system controls extinction of aversive memories. Nature. 2002;418:530–534. doi: 10.1038/nature00839. [DOI] [PubMed] [Google Scholar]
- 43.Kraft B. Is there any clinically relevant cannabinoid-induced analgesia? Pharmacology. 2012;89:237–246. doi: 10.1159/000337376. [DOI] [PubMed] [Google Scholar]