Abstract
Aims
The aim was to evaluate the effect of boceprevir and telaprevir on dolutegravir pharmacokinetics (PK); the effect of dolutegravir on boceprevir and telaprevir PK was assessed through comparison with historical data for each hepatitis C virus (HCV) drug's prescribing information alone.
Methods
This was a single-centre, randomized, open-label, two-cohort, two-period, one-way study in healthy adult subjects. Dolutegravir 50 mg once daily was administered for 5 days in Period 1, and dolutegravir 50 mg once daily was coadministered with either boceprevir 800 mg every 8 h (Cohort 1) or telaprevir 750 mg every 8 h (Cohort 2) for 10 days in Period 2.
Results
No deaths or serious adverse events were reported during the study. Four subjects were withdrawn from the study because of adverse events (elevated alanine aminotransferase, cellulitis, increased serum creatinine and dizziness). One subject became pregnant during the study. Coadministration of dolutegravir with boceprevir had no effect on dolutegravir area under the plasma concentration–time curve (AUC) and maximal plasma concentration (Cmax) and caused a small increase in concentration at the end of the dosing interval (Cτ; 8%). Coadministration of dolutegravir with telaprevir resulted in increased dolutegravir plasma exposures compared with those after administration of dolutegravir alone; AUC0–τ, Cmax and Cτ increased by 25, 19 and 37%, respectively. Coadministration of boceprevir or telaprevir with dolutegravir had no clinically significant effect on dolutegravir PK. Plasma boceprevir and telaprevir PK data for either combined treatment were similar to historical data, indicating no effect of dolutegravir on boceprevir or telaprevir exposure.
Conclusions
Dolutegravir can be coadministered with boceprevir or telaprevir in patients coinfected with HIV and HCV with no dose adjustment.
Keywords: dolutegravir, hepatitis C, human immunodeficiency virus, integrase, pharmacokinetics
What is Already Known about this Subject
Hepatitis C virus (HCV) is a highly prevalent comorbidity of human immunodeficiency virus (HIV).
Hepatitis C virus protease inhibitors and many antiretroviral drugs for HIV infection share a common metabolic elimination pathway mediated by cytochrome P450 3A4.
Coadministration of HCV protease inhibitors and antiretroviral drugs is often not recommended or requires dose adjustment because of drug–drug interactions.
What this Study Adds
This research demonstrates that dolutegravir and the recently approved HCV protease inhibitors boceprevir and telaprevir can be coadministered with no concern regarding drug–drug interaction or safety in patients coinfected with HIV and HCV.
Introduction
Dolutegravir (Tivicay®; ViiV Healthcare, Research Triangle Park, NC, USA) is a once-daily inhibitor of the human immunodeficiency virus (HIV) integrase enzyme, with demonstrated efficacy across a broad range of HIV-infected patients and a favourable safety profile [1,2]. While many HIV antiretroviral therapies (ARTs) are metabolized by the cytochrome P450 3A (CYP3A) pathway, dolutegravir is primarily metabolized via glucuronidation by UDP glucuronosyltransferase 1A1 (UGT1A1), with a minor contribution to metabolism by CYP3A [3]. Dolutegravir is likely to be combined with other drugs used to treat HIV and HIV comorbidities, and studies to date demonstrate few unfavourable drug–drug interactions [4]. However, potent enzyme inducers can reduce dolutegravir exposure, necessitating dose adjustments [5]. Therefore, there is a need to continue examining the effects of various classes of drugs on the pharmacokinetics (PK) of dolutegravir.
Approximately 25% of all persons infected with HIV are also infected with hepatitis C virus (HCV) [6]. Coinfection rates are the highest among injection drug users (86–98.5%); however, recent outbreaks of HCV among HIV-infected men who have sex with men suggest that large proportions of persons infected with HIV are at risk of coinfection with HCV [7]. In 2011, two inhibitors of the HCV nonstructural serine protease 3/4A, boceprevir (Victrelis®; Merck Sharpe & Dohme Corp., Whitehouse Station, NJ, USA) and telaprevir (Incivek®; Vertex Pharmaceuticals Inc., Cambridge, MA, USA), were approved to treat HCV and have since emerged as the most effective treatment options [8,9]. Boceprevir and telaprevir are primarily cleared via metabolism with short to moderate half-lives (3–4 h for boceprevir and 9–10 h for telaprevir). Boceprevir is primarily metabolized by aldoketoreductases (AKR1 and AKR3), with a minor contribution from CYP3A4. Telaprevir is primarily metabolized via CYP3A4. Both boceprevir and telaprevir are P-glycoprotein substrates [8]. Many ARTs are subject to cautionary or exclusion statements with boceprevir and telaprevir, raising concerns that coinfection with HCV in patients with HIV could lead to limitation or interruption of ART use and consequent poor clinical outcomes, including development of viral resistance [8]. Given that they are metabolized by distinct pathways, no unfavourable interactions were expected between dolutegravir and either boceprevir or telaprevir. This PK study in healthy subjects was designed to confirm the absence of a clinically significant drug–drug interaction between dolutegravir and either boceprevir or telaprevir.
Methods
Study design and subjects
This was a single-centre, randomized, open-label, two-cohort, two-period, one-way study in healthy adult subjects designed to evaluate the effects of boceprevir and telaprevir on the PK of dolutegravir. Eligibility of male and female healthy subjects between 18 and 65 years of age was determined by evaluation of medical history, physical examinations and laboratory tests. Subjects with bodyweight ≥50 kg for males and ≥45 kg for females and body mass index within the range of 18.5–31.0 kg m−2 were eligible. Subjects with evidence of hepatitis B or HCV infection or both within 3 months of screening, a positive test for HIV, current or chronic liver disease, a positive prestudy drug/alcohol screening result or a history of regular alcohol consumption were excluded. Subjects were also excluded if they participated in a recent clinical trial, and use of prescription and nonprescription drugs, including vitamins and herbal and dietary supplements (including St John's Wort), was prohibited within 14 days of the first dose of study medications and throughout the trial and follow-up visit.
Treatment administration
All subjects provided written informed consent, and the protocol was approved by the institutional review board of the study site (IntegReview, Austin, TX, USA). This study is registered with ClinicalTrials.gov (NCT01563328; protocol number ING115697). All subjects had a screening visit within 30 days of the first dose of study drugs, two treatment periods, and a follow-up visit 7–14 days after the last dose of study drugs. Subjects were randomized into two cohorts before the start of the study. During Period 1, all subjects received dolutegravir 50 mg day−1 for 5 days. During Period 2, Cohort 1 received dolutegravir 50 mg day−1 plus boceprevir 800 mg three times per day for an additional 10 days, and Cohort 2 received dolutegravir 50 mg day−1 plus telaprevir 750 mg three times per day for 10 days. There was no washout between treatment periods. All dolutegravir doses were administered with a moderate-fat meal in the morning, and boceprevir and telaprevir doses were administered with a moderate-fat meal or snack, in accordance with the respective product label. The exact constitution of the meals was left to the discretion of the site, as long as they were designated moderate fat (30% fat, 600 calories).
Bioanalytical methods and pharmacokinetic analysis
Dolutegravir
Plasma concentrations of dolutegravir were determined using a validated liquid chromatography and tandem mass spectrometric (LC-MS/MS) method as described previously [10]. The study-specific bias for the analysis of dolutegravir was −2.2 to 3.9%, with precision values of 1.6–3.6% (within day) and ≤2.1% (between days).
Boceprevir/telaprevir
The measurement of boceprevir or (S)-telaprevir in plasma samples was determined using a validated LC-MS/MS method. Plasma was stabilized to prevent further isomerization by acidification with phosphoric acid. Boceprevir stereoisomers and (S)-telaprevir were extracted from plasma using solid-phase extraction and supported liquid extraction plates, respectively. Extracts were then separated by high-performance LC and analysed with heated nebulizer MS/MS detection in the positive ion mode for boceprevir and TurboIonSpray MS/MS detection in the positive ion mode for (S)-telaprevir. The calibration ranges for the respective boceprevir stereoisomers were 0.0052–5.2 μg ml−1 and 0.0048–4.8 μg ml−1. The calibration range for (S)-telaprevir was 0.1–20 μg ml−1. Quality control samples, prepared separately at low, medium and high regions of the calibration curve, were stored at the same temperature as the study samples and analysed against separately prepared calibration standards. The biases for the boceprevir enantiomers were −3.6 to 0.8%, with a precision of 3.3–9.1%, and −7.7 to 1.6%, with a precision of 1.9–5.1%, and the bias for the analysis of (S)-telaprevir was −2.3 to 8.8%, with a precision of 2.3–8.6%.
Pharmacokinetic analysis
A noncompartmental PK analysis of the concentration–time data was performed with WinNonlin® (version 5.2; Pharsight Corp., Mountain View, CA, USA). Pharmacokinetic parameters for dolutegravir were calculated using the actual recorded times for each treatment and included area under the concentration–time curve from time 0 to the end of the dosing interval (AUC0–τ), observed maximal plasma concentration (Cmax), 24 h postdose concentration at steady state (Cτ), observed minimal plasma concentration (Cmin), apparent oral clearance (CL/F), apparent terminal half-life (t1/2) and time to Cmax (Tmax).
Statistical analysis
Statistical analyses were performed on the log-transformed PK parameters AUC0–τ, Cτ and Cmax. Analysis of variance (anova) was performed using SAS® mixed linear models procedure (SAS, Cary, NC, USA) to assess the effect of boceprevir and telaprevir on the PK of dolutegravir. Telaprevir PK values were compared with historical data [11]. Subject was fitted as a random effect and treatment was fitted as a fixed effect in the model. The ratio of geometric least squares means and associated 95% confidence intervals were estimated for PK parameters of interest. Dolutegravir given alone was considered the reference treatment, and dolutegravir coadministered with either boceprevir or telaprevir were considered the test treatments.
Telaprevir and boceprevir steady-state PK parameters were not subjected to formal statistical analyses but were compared with values in the Incivek and Victrelis labels, respectively [11,12].
Results
Subject disposition and demographics
A total of 32 subjects were enrolled in the study, and 16 were randomized into each cohort. Twenty-eight subjects completed the study, and the remaining four subjects withdrew because of adverse events (AEs). Mean age (±SD) was 42.5 ± 16.6 years, with a mean body mass index of 25.8 ± 3.3 kg m−2, and the majority of subjects were male (59%) and Caucasian (72%). Additional details of subject demographics can be found in Table 1.
Table 1.
Subject disposition and demographics
| Dolutegravir (n = 32) | Dolutegravir + boceprevir (n = 16) | Dolutegravir + telaprevir (n = 16) | Overall (n = 32) | |
|---|---|---|---|---|
| Subject disposition* | ||||
| Planned subjects | 32 | 16 | 16 | 32 |
| Randomized subjects | 32 | 16 | 16 | 32 |
| Safety population | 32 (100) | 16 (100) | 16 (100) | 32 (100) |
| PK concentration population | 32 (100) | 16 (100) | 16 (100) | 32 (100) |
| PK summary population | 32 (100) | 16 (100) | 16 (100) | 32 (100) |
| Subjects completed as planned | 32 (100) | 13 (81) | 15 (94) | 28 (88) |
| Subjects withdrawn | 0 | 3 (19) | 1 (6) | 4 (13) |
| Subjects withdrawn because of AEs | 0 | 3 (19) | 1 (6) | 4 (13) |
| Subject demographics | ||||
| Age (years)† | 42.5 (16.56) | 45.2 (17.71) | 39.9 (15.44) | 42.5 (16.56) |
| Sex* | ||||
| Male | 19 (59) | 10 (63) | 9 (56) | 19 (59) |
| Female | 13 (41) | 6 (38) | 7 (44) | 13 (41) |
| Body mass index (kg m−2)† | 25.8 (3.31) | 25.7 (3.25) | 25.8 (3.47) | 25.8 (3.31) |
| Height (cm)† | 169 (9.99) | 169 (10.3) | 169 (9.98) | 169 (9.99) |
| Weight (kg)† | 73.8 (13.3) | 73.9 (13.4) | 73.7 (13.7) | 73.8 (13.3) |
| Ethnicity* | ||||
| Hispanic or Latino | 11 (34) | 9 (56) | 2 (13) | 11 (34) |
| Not Hispanic or Latino | 21 (66) | 7 (44) | 14 (88) | 21 (66) |
| Race* | ||||
| African American/African heritage | 4 (13) | 1 (6) | 3 (19) | 4 (13) |
| American Indian or Alaskan native | 3 (9) | 2 (13) | 1 (6) | 3 (9) |
| White, Arabic/North African heritage | 1 (3) | 1 (6) | 0 | 1 (3) |
| White, Caucasian/European heritage | 23 (72) | 12 (75) | 11 (69) | 23 (72) |
| Mixed race | 1 (3) | 0 | 1 (6) | 1 (3) |
Abbreviations are as follows: AE, adverse event; PK, pharmacokinetic.
n (%).
Mean (SD).
Pharmacokinetics
Mean concentration–time profiles of dolutegravir alone and in combination with boceprevir or telaprevir are shown in Figure 1. Analyses of PK parameters for dolutegravir are shown in Table 2. Coadministration of dolutegravir with boceprevir had no significant effects on dolutegravir AUC0–τ, Cmax and Cτ, with changes of <10% for each parameter observed when dolutegravir was given alone compared with coadministration. Coadministration of dolutegravir with telaprevir resulted in modest increases in plasma exposure of dolutegravir compared with administration of dolutegravir alone (AUC0–τ, Cmax and Cτ were 25, 19 and 37% higher, respectively). Dolutegravir coadministration did not significantly affect plasma exposure of boceprevir or telaprevir; observed PK parameters were similar to published values (Tables 3 and 4).
Figure 1.
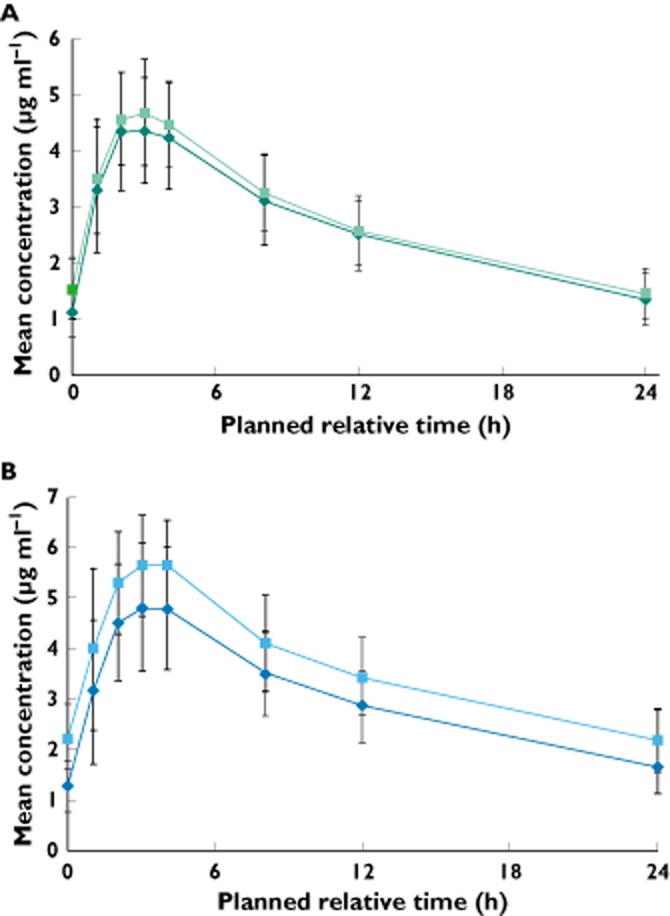
Mean plasma concentration–time profiles of dolutegravir after administration of dolutegravir alone, dolutegravir coadministration with boceprevir (A) and dolutegravir coadministration with telaprevir (B).  , dolutegravir (A);
, dolutegravir (A);  , dolutegravir (B);
, dolutegravir (B);  , dolutegravir + boceprevir (A);
, dolutegravir + boceprevir (A);  , dolutegravir + telaprevir (B)
, dolutegravir + telaprevir (B)
Table 2.
Summary and statistical analysis of dolutegravir pharmacokinetic parameters*
| Pharmacokinetic parameters | Cohort 1 | Cohort 2 | ||||
|---|---|---|---|---|---|---|
| Dolutegravir, Period 1 (n = 16) | Dolutegravir + boceprevir, Period 2 (n = 13) | Dolutegravir + boceprevir vs. dolutegravir GLS mean ratio (95% CI) | Dolutegravir, Period 1 (n = 16) | Dolutegravir + telaprevir, Period 2 (n = 15) | Dolutegravir + telaprevir vs. dolutegravir GLS mean ratio (95% CI) | |
| AUC0–τ (μg h ml−1) | 61.5 (53.5, 70.8) | 65.3 (57.4, 74.3) | 1.07 (0.924, 1.234) | 68.9 (60.3, 78.6) | 84.2 (75.2, 94.2) | 1.25 (1.183, 1.327) |
| Cmax (μg ml−1) | 4.62 (4.13, 5.16) | 4.82 (4.36, 5.33) | 1.05 (0.941, 1.176) | 4.99 (4.43, 5.62) | 5.81 (5.35, 6.31) | 1.19 (1.096, 1.282) |
| Cτ (μg ml−1) | 1.31 (1.08, 1.59) | 1.40 (1.13, 1.73) | 1.08 (0.878, 1.329) | 1.59 (1.33, 1.90) | 2.09 (1.80, 2.44) | 1.37 (1.274, 1.468) |
| Cmin (μg ml−1) | 1.00 (0.79, 1.25) | 1.36 (1.10, 1.68) | 1.36 (1.106, 1.684) | 1.18 (0.96, 1.46) | 2.03 (1.73, 2.38) | 1.76 (1.570, 1.963) |
| t1/2 (h) | 13.2 (12.0, 14.5) | 13.8 (12.3, 15.4) | 1.05 (0.940, 1.166) | 14.5 (13.1, 16.1) | 16.7 (15.0, 18.5)‡ | 1.18 (1.061, 1.305) |
| Tmax (h)† | 2.50 (1.0, 4.0) | 3.00 (1.0, 4.0) | – | 3.00 (1.0, 4.0) | 4.00 (1.1, 4.0) | – |
Abbreviations are as follows: AUC0–τ, area under the concentration–time curve from time 0 to the end of the dosing interval; CI, confidence interval; Cmax, observed maximal plasma concentration; Cmin, observed minimal plasma concentration; Cτ, 24 h postdose concentration at steady state; GLS, geometric least squares; t1/2, apparent terminal half-life; Tmax, time to maximal plasma concentration.
Geometric mean (95% CI).
Presented as median (range).
n = 14.
Table 3.
Summary of boceprevir pharmacokinetic parameters*
| Pharmacokinetic parameters | Cohort 1 dolutegravir + boceprevir (n = 15) | Boceprevir alone‡ |
|---|---|---|
| AUC0–τ (μg h ml−1) | 4.63 (14) | 5.408 |
| Cmax (μg ml−1) | 1.64 (16) | 1.723 |
| Cτ (μg ml−1) | 0.08 (35) | – |
| Cmin (μg ml−1) | 0.07 (23) | 0.08 |
| t1/2 (h) | 1.31 (14) | – |
| Tmax (h)† | 2.0 (1.0–3.0) | – |
Abbreviations are as follows: AUC0–τ, area under the concentration–time curve from time 0 to the end of the dosing interval; Cmax, observed maximal plasma concentration; Cmin, observed minimal plasma concentration; Cτ, 24 h postdose concentration at steady state; t1/2, apparent terminal half-life; Tmax, time to maximal plasma concentration.
Geometric mean (between subject coefficient of variation, %CVb).
Presented as median (range).
Boceprevir package insert, 2013.
Table 4.
Summary of telaprevir pharmacokinetic parameters*
| Pharmacokinetic parameters | Cohort 2 dolutegravir + telaprevir (n = 15) | Telaprevir alone§ |
|---|---|---|
| AUC0–τ (μg h ml−1) | 19.2 (19) | 22.3 |
| Cmax (μg ml−1) | 3.21 (17) | 3.51 |
| Cτ (μg ml−1) | 1.75 (26) | – |
| Cmin (μg ml−1) | 1.72 (25) | 2.03 |
| t1/2 (h)† | 5.14 (27) | – |
| Tmax (h)‡ | 3.00 (2.0, 4.0) | – |
Abbreviations are as for Table 3.
Geometric mean (between subject coefficient of variation, %CVb).
n = 10.
Presented as median (range).
Telaprevir package insert, 2013.
Safety
Study drugs were generally well tolerated, and no grade 4 or serious AEs were reported. The most commonly reported drug-related AEs (headache, anorectal discomfort, dysgeusia and maculopapular rash) were mild in intensity. The majority of AEs occurred during boceprevir (31%) or telaprevir (44%) coadministration compared with dolutegravir alone (6%). A summary of observed AEs can be found in Table 5. Four subjects were discontinued because of the following AEs: cellulitis (Period 2, dolutegravir + boceprevir), increased serum creatinine (Period 2, dolutegravir + boceprevir), dizziness (Period 1, dolutegravir) and increased alanine aminotransferase (Period 2, dolutegravir + boceprevir); one subject for each. Of these, only the grade 3 increase in alanine aminotransferase AE was assessed as related to study drugs (dolutegravir + boceprevir). Increases in alanine aminotransferase began in Period 2 with coadministration of study drugs, peaked on day 5 and resolved within 32 days of discontinuation of study drugs. One subject who received dolutegravir and telaprevir became pregnant during the study. The pregnancy was followed to resolution and resulted in the birth of a healthy infant at 41 weeks of gestation.
Table 5.
Summary of adverse events
| Dolutegravir (n = 32) | Dolutegravir + boceprevir (n = 16) | Dolutegravir + telaprevir (n = 16) | |
|---|---|---|---|
| All adverse events* | |||
| Any adverse event | 4 (13) | 9 (56) | 11 (69) |
| Headache | 2 (6) | 1 (6) | 5 (31) |
| Anorectal discomfort | 0 | 2 (13) | 2 (13) |
| Dysgeusia | 0 | 2 (13) | 1 (6) |
| Maculopapular rash | 1 (3) | 0 | 2 (13) |
| Abdominal pain | 0 | 0 | 2 (13) |
| Anal haemorrhage | 0 | 0 | 2 (13) |
| Haemorrhoids | 0 | 0 | 2 (13) |
| Drug-related adverse events* | |||
| Any adverse event | 2 (6) | 5 (31) | 7 (44) |
| Headache | 1 (3) | 0 | 4 (25) |
| Dysgeusia | 0 | 2 (13) | 1 (6) |
| Maculopapular rash | 1 (3) | 0 | 2 (13) |
| Abdominal pain | 0 | 0 | 2 (13) |
Occurring in two or more subjects in any treatment group. Presented as n (%).
Discussion
Treatment of HIV and HCV coinfection is challenging because of lower response rates and drug interactions [13]. Unlike the majority of currently available HIV ARTs, dolutegravir is predominantly cleared by hepatic metabolism via glucuronidation by UGT1A1 with a minor contribution from CYP3A4 and is therefore a good choice for patients with impaired liver function in whom conjugation mechanisms remain relatively intact, in contrast to other metabolizing functionality [14]. This feature may enable dolutegravir to be combined with many classes of drugs that cannot be coadministered with other HIV ARTs. Dolutegravir, therefore, may be a valuable choice in devising treatment strategies for serious HIV comorbidities, including HCV.
The results presented here show that dolutegravir can be coadministered safely with either of two HCV protease inhibitors that are associated with improved clinical outcomes, boceprevir or telaprevir, with no dose adjustments. Adverse events observed in the study were generally mild and included headache, anorectal discomfort, dysgeusia and maculopapular rash. The majority of these events occurred during combination therapy and have been described with boceprevir and telaprevir [11,12,15]. One subject withdrew in Period 2 while receiving dolutegravir + boceprevir because of a grade 3 elevation in alanine aminotransferase. Elevated alanine aminotransferase has been observed infrequently (<1–2% of subjects) in previous dolutegravir clinical studies [2,15]. The alanine aminotransferase level in the withdrawn subject returned to normal after drug discontinuation. Overall, coadministration of dolutegravir with either boceprevir or telaprevir did not result in any AEs significantly different from those observed during the administration of any of the drugs individually [11,12,15], and no new safety signals emerged.
Coadministration of dolutegravir with boceprevir or telaprevir resulted in modest changes in dolutegravir exposure (25% with telaprevir and 7% with boceprevir) that were not considered clinically significant. The largest change was a 37% increase in dolutegravir Cτ with telaprevir. The exact mechanism for this drug–drug interaction is unknown, although it is likely that telaprevir is inhibiting the minor dolutegravir CYP3A4 metabolic clearance pathway.
Phase III studies in integrase inhibitor-naive subjects did not identify a relationship between dolutegravir exposure and incidence of AEs [16]. This lack of relationship was also observed in studies with higher dolutegravir exposures, including those with twice-daily dosing and when dolutegravir was given with the UGT1A1 inhibitor atazanavir [17,18]. Thus, given the modest increase in exposure observed, no alteration in dose is required when dolutegravir is given with either HCV protease inhibitor. These PK observations are consistent with previous studies that predict dolutegravir exposure will not change significantly when coadministered with drugs metabolized by CYP3A4 [19,20].
Although dolutegravir is not predicted to be a perpetrator of drug interactions, the effect of dolutegravir on the PK parameters of boceprevir and telaprevir was also investigated. Given the limitations of cross-study comparisons, these data suggest no significant effect of dolutegravir on either drug, given that PK parameters differed from historical data by <15% (Tables 3 and 4).
Availability of the HCV protease inhibitors boceprevir and telaprevir has drastically improved clinical outcomes for HCV patients; both are now considered to be the most effective HCV therapies when used in combination with interferon and ribavirin [8,9,21]. Boceprevir and telaprevir are inhibitors of the P-glycoprotein efflux pump and are metabolized by and inhibit the CYP3A4 pathway [8,11,12]. These characteristics result in an extensive list of potential drug–drug interactions that can complicate therapeutic intervention for other disease states in patients with HCV, including HIV [8]. Among these complications are significant fluctuations in plasma exposure to either the HCV drugs or HIV ART, necessitating dose adjustments. On the basis of drug–drug interaction studies to date, combination of boceprevir or telaprevir with HIV ART regimens, including the non-nucleoside reverse transcriptase inhibitor efavirenz (for boceprevir) or ritonavir-boosted protease inhibitors, is generally not recommended [8,21]. One exception to this is ritonavir-boosted atazanavir, because it can be administered with telaprevir.
The data presented here show that dolutegravir can be coadministered with boceprevir or telaprevir without dose adjustment and gives patients who are coinfected with HCV and HIV an important new treatment option for the control of both viruses.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: JB, SC, MJ, SP, PS and BW had support from ViiV Healthcare for the submitted work; JB, SC, MJ, SP and BW are employees of GlaxoSmithKline.
All listed authors meet the criteria for authorship set forth by the International Committee for Medical Journal Editors. The authors wish to acknowledge the following individuals for editorial assistance during the development of this manuscript: Michael Hast and Jennifer Rossi. The authors would also like to thank the principal investigator, Dr Matthew Medlock, and the staff and volunteers at PPD, Austin, TX, USA.
Funding Sources
Funding for this work was provided by ViiV Healthcare.
References
- 1.Eron JJ, Clotet B, Durant J, Katlama C, Kumar P, Lazzarin A, Poizot-Martin I, Richmond G, Soriano V, Ait-Khaled M, Fujiwara T, Huang J, Min S, Vavro C, Yeo J the VIKING Study Group. Safety and efficacy of dolutegravir in treatment-experienced subjects with raltegravir-resistant HIV type 1 infection: 24-week results of the VIKING Study. J Infect Dis. 2013;207:740–748. doi: 10.1093/infdis/jis750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Raffi F, Rachlis A, Stellbrink H-J, Hardy WD, Torti C, Orkin C, Bloch M, Podzamczer D, Pokrovsky V, Pulido F, Almond S, Margolis D, Brennan C, Min S the SPRING-2 Study Group. Once-daily dolutegravir versus raltegravir in antiretroviral-naive adults with HIV-1 infection: 48 week results from the randomised, double-blind, non-inferiority SPRING-2 study. Lancet. 2013;381:735–743. doi: 10.1016/S0140-6736(12)61853-4. [DOI] [PubMed] [Google Scholar]
- 3.Castellino S, Moss L, Wagner D, Borland J, Song I, Chen S, Lou Y, Min SS, Goljer I, Culp A, Piscitelli SC, Savina PM. Metabolism, excretion, and mass balance of the HIV-1 integrase inhibitor dolutegravir in humans. Antimicrob Agents Chemother. 2013;57:3536–3546. doi: 10.1128/AAC.00292-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cottrell ML, Hadzic T, Kashuba AD. Clinical pharmacokinetic, pharmacodynamic and drug-interaction profile of the integrase inhibitor dolutegravir. Clin Pharmacokinet. 2013;52:981–994. doi: 10.1007/s40262-013-0093-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dooley KE, Sayre P, Borland J, Purdy E, Chen S, Song I, Peppercorn A, Everts S, Piscitelli S, Flexner C. Safety, tolerability, and pharmacokinetics of the HIV integrase inhibitor dolutegravir given twice daily with rifampin or once daily with rifabutin: results of a phase 1 study among healthy subjects. J Acquir Immune Defic Syndr. 2013;62:21–27. doi: 10.1097/QAI.0b013e318276cda9. [DOI] [PubMed] [Google Scholar]
- 6.Thomas DL. The challenge of hepatitis C in the HIV-infected person. Annu Rev Med. 2008;59:473–485. doi: 10.1146/annurev.med.59.081906.081110. [DOI] [PubMed] [Google Scholar]
- 7.Thomas DL, Leoutsakas D, Zabransky T, Kumar MS. Hepatitis C in HIV-infected individuals: cure and control, right now. J Int AIDS Soc. 2011;14:22. doi: 10.1186/1758-2652-14-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wilby KJ, Greanya ED, Ford J-A, Yoshida EM, Partovi N. A review of drug interactions with boceprevir and telaprevir: implications for HIV and transplant patients. Ann Hepatol. 2012;11:179–185. [PubMed] [Google Scholar]
- 9.Wilby KJ, Partovi N, Ford J-A, Greanya ED, Yoshida EM. Review of boceprevir and telaprevir for the treatment of chronic hepatitis C. Can J Gastroenterol. 2012;26:205–210. doi: 10.1155/2012/751057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Song IH, Borland J, Chen S, Savina P, Peppercorn AF, Piscitelli S. Effect of prednisone on the pharmacokinetics of the integrase inhibitor dolutegravir. Antimicrob Agents Chemother. 2013;57:4394–4397. doi: 10.1128/AAC.00728-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.2013. Incivek [package insert]. Cambridge, MA: Vertex Pharmaceuticals Inc.
- 12.2014. Victrelis [package insert]. Whitehouse Station, NJ: Merck Sharp & Dohme Corp.
- 13.Barreiro P, Vispo E, Labarga P, Soriano V. Management and treatment of chronic hepatitis C in HIV patients. Semin Liver Dis. 2012;32:138–146. doi: 10.1055/s-0032-1316469. [DOI] [PubMed] [Google Scholar]
- 14.Furlan V, Demirdjian S, Bourdon O, Magdalou J, Taburet A-M. Glucuronidation of drugs by hepatic microsomes derived from healthy and cirrhotic human livers. J Pharmacol Exp Ther. 1999;289:1169–1175. [PubMed] [Google Scholar]
- 15.2013. Tivicay [package insert]. Research Triangle Park, NC: ViiV Healthcare.
- 16.Song I, Chen S, Piscitelli S, Min S. 2013. PK and PK-PD relationship of DTG in INI-naive subjects. Abstract presented at: 53rd Interscience Conference on Antimicrobial Agents and Chemotheraphy; September 10–13,; Denver, CO.
- 17.Castagna A, Maggiolo F, Penco G, Wright D, Mills A, Grossberg R, Molina JM, Chas J, Durant J, Moreno S, Doroana M, Ait-Khaled M, Huang J, Min S, Song I, Vavro C, Nichols G, Yeo JM. Dolutegravir in antiretroviral-experienced patients with raltegravir- and/or elvitegravir-resistant HIV-1: 24-week results of the Phase III VIKING-3 study. J Infect Dis. 2014 doi: 10.1093/infdis/jiu051. Feb 23. [Epub ahead of print] the VIKING-3 Study Group. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cahn P, Pozniak AL, Mingrone H, Shuldyakov A, Brites C, Andrade-Villanueva JF, Richmond G, Buendia CB, Fourie J, Ramgopal M, Hagins D, Felizarta F, Madruga J, Reuter T, Small CB, Lombaard J, Grinsztejn B, Dorey D, Underwood M, Griffith S, Min S the extended SAILING Study Team. Dolutegravir versus raltegravir in antiretroviral-experienced, integrase inhibitor-naive adults with HIV: week 48 results from the randomised, double blind, non-inferiority SAILING study. Lancet. 2013;382:700–708. doi: 10.1016/S0140-6736(13)61221-0. [DOI] [PubMed] [Google Scholar]
- 19.Reese MJ, Savina PM, Generaux GT, Tracey H, Humphreys JE, Kanaoka E, Webster LO, Harmon KA, Clarke JD, Polli JW. In vitro investigations into the roles of drug transporters and metabolizing enzymes in the disposition and drug interactions of dolutegravir, a HIV integrase inhibitor. Drug Metab Dispos. 2013;41:353–361. doi: 10.1124/dmd.112.048918. [DOI] [PubMed] [Google Scholar]
- 20.Song I, Min SS, Borland J, Lou Y, Chen S, Patel P, Ishibashi T, Piscitelli SC. The effect of lopinavir/ritonavir and darunavir/ritonavir on the HIV integrase inhibitor S/GSK1349572 in healthy participants. J Clin Pharmacol. 2011;51:237–242. doi: 10.1177/0091270010371113. [DOI] [PubMed] [Google Scholar]
- 21.Pearlman BL. Protease inhibitors for the treatment of chronic hepatitis C genotype-1 infection: the new standard of care. Lancet Infect Dis. 2012;12:717–728. doi: 10.1016/S1473-3099(12)70060-9. [DOI] [PubMed] [Google Scholar]


