Abstract
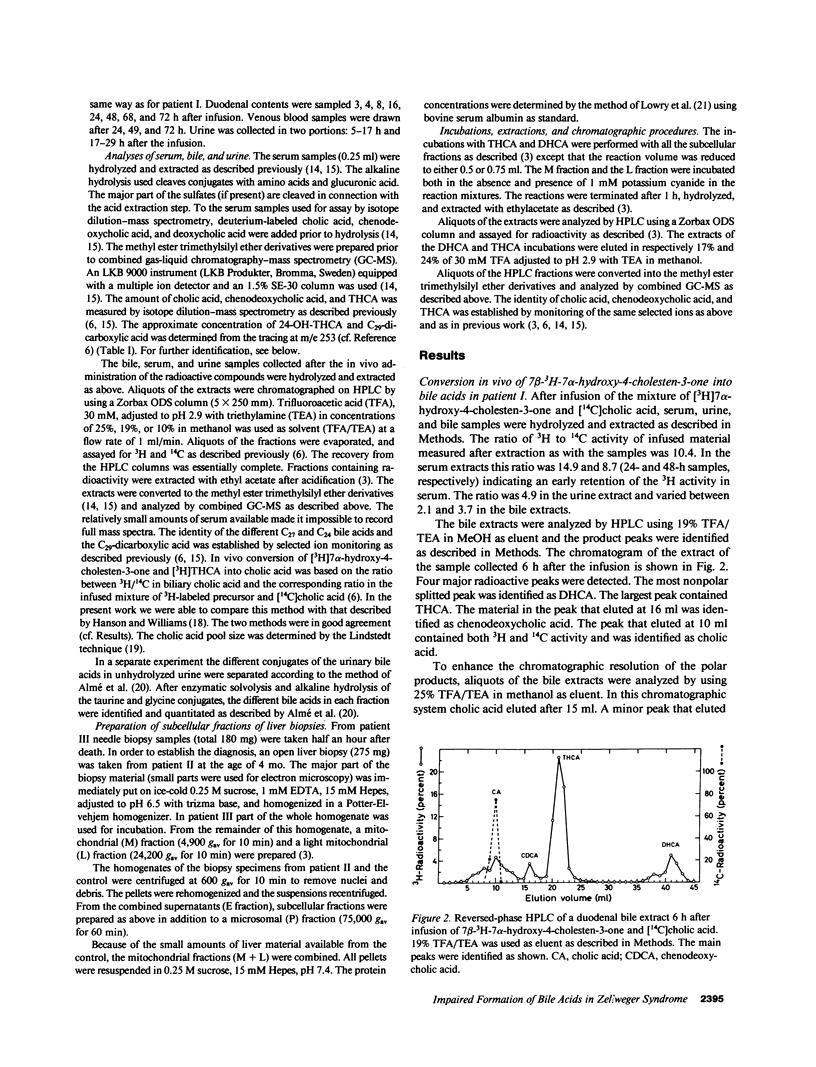
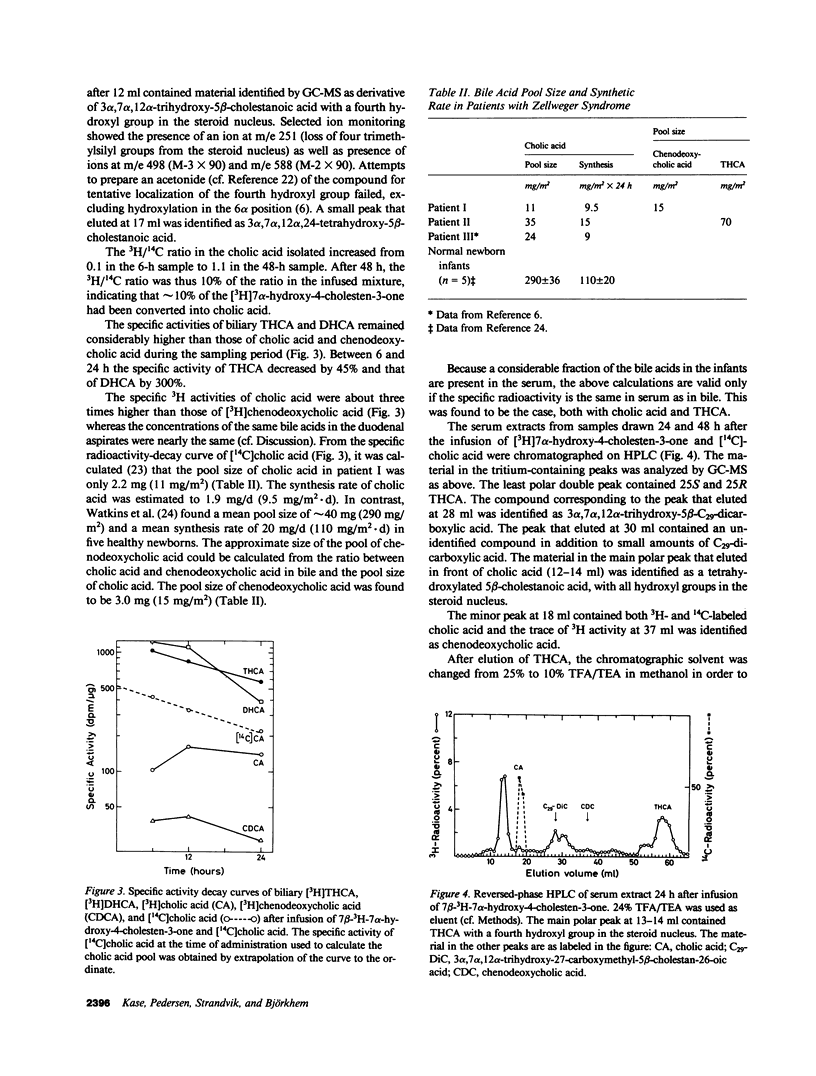
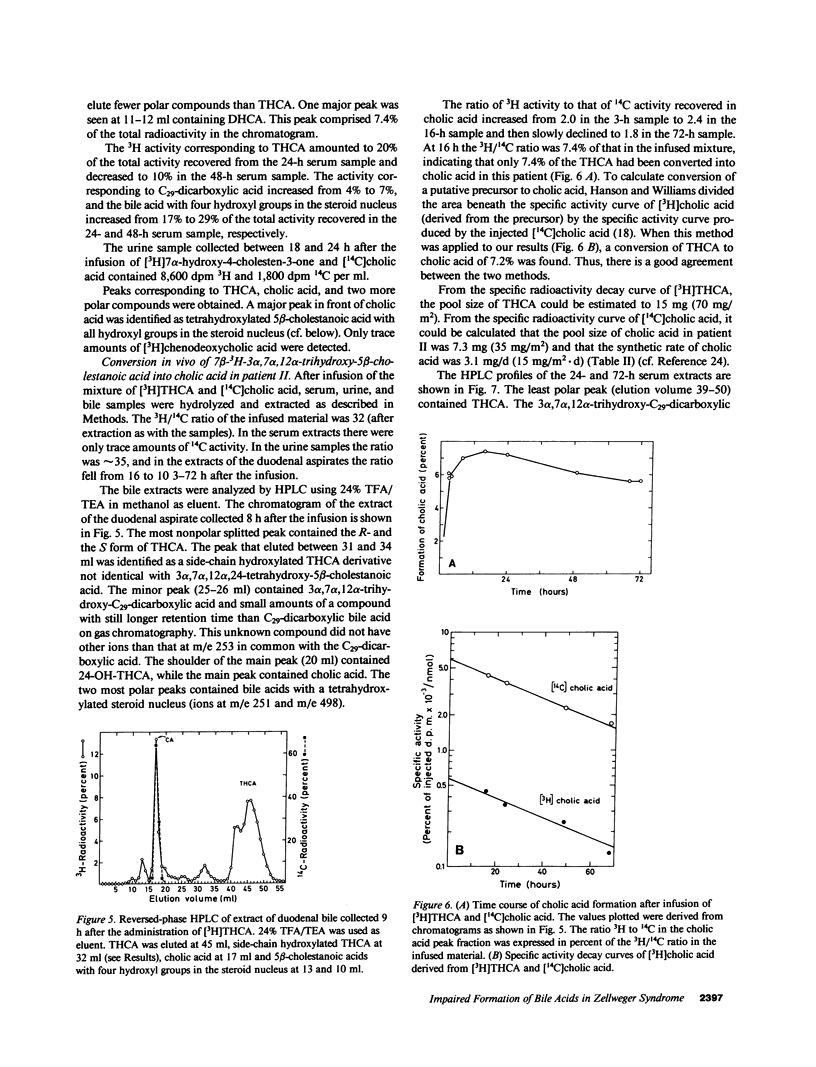
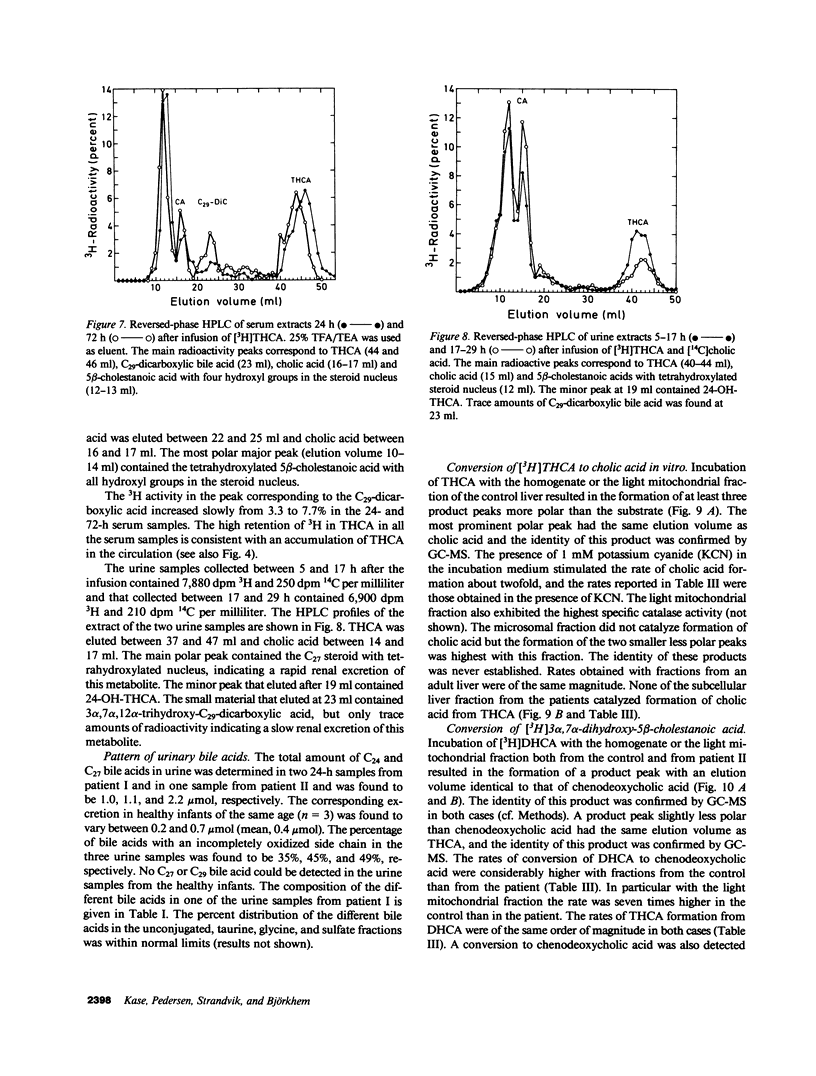
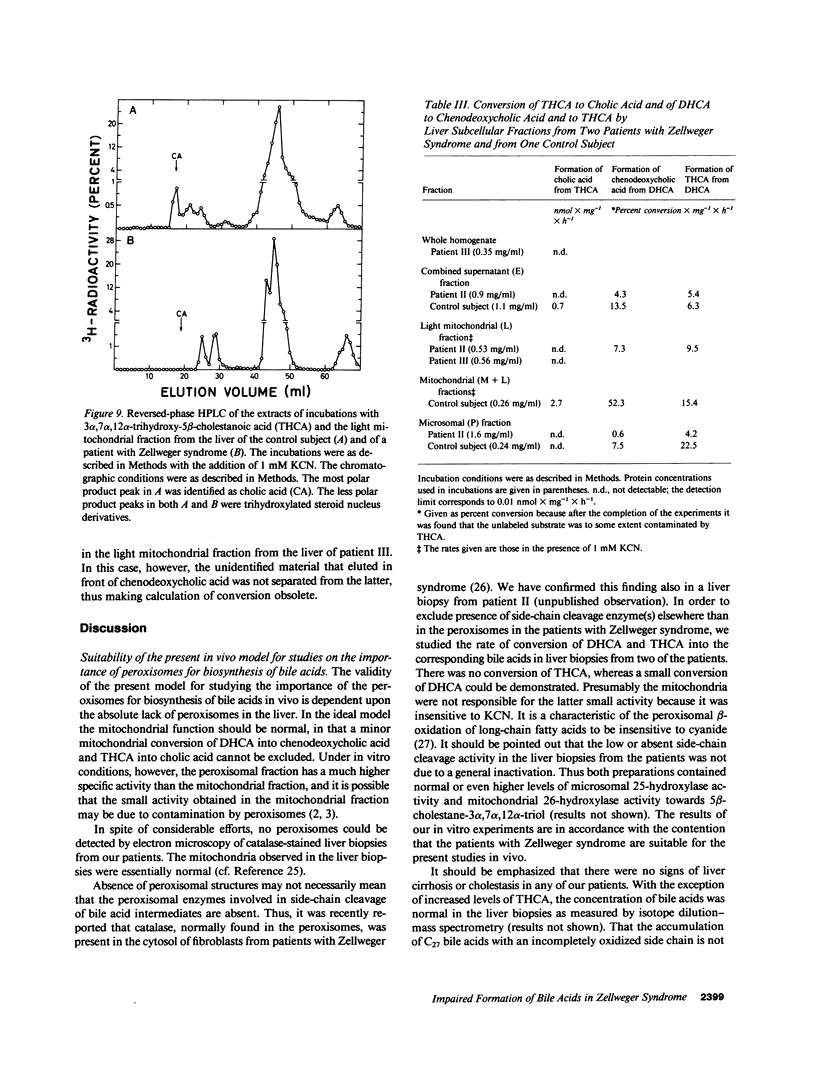
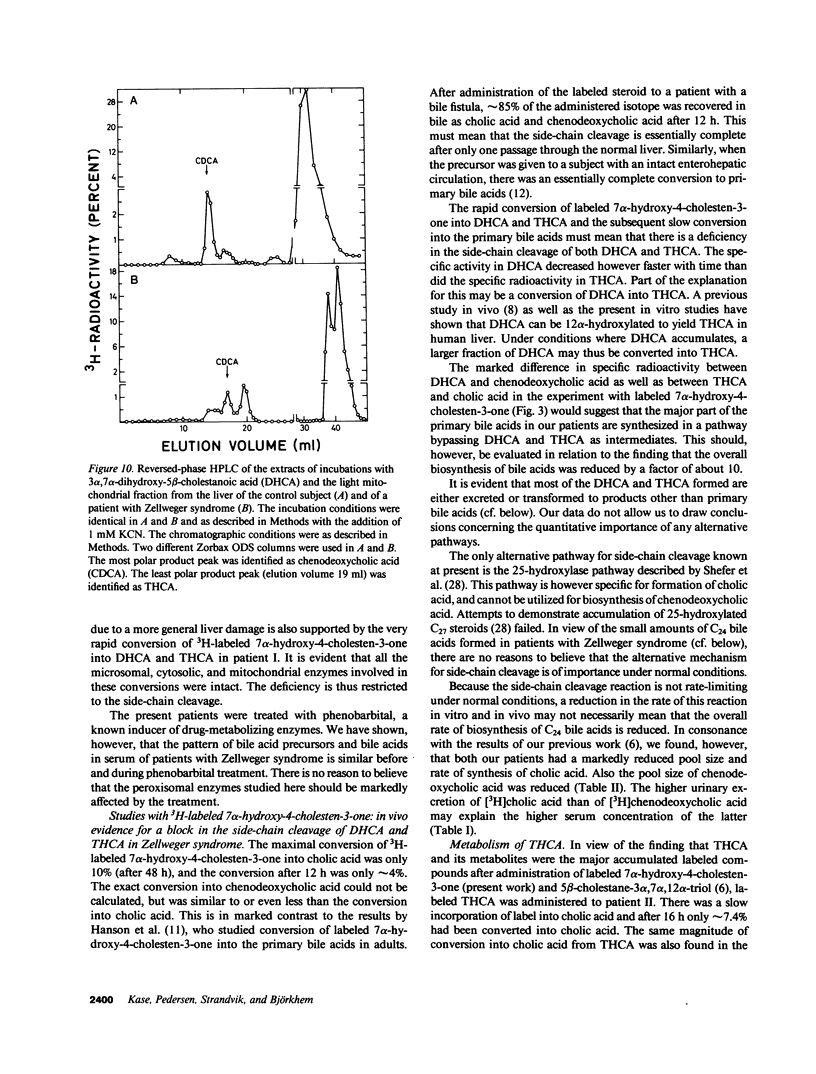
The last step in bile acid formation involves conversion of 3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-cholestanoic acid (THCA) into cholic acid and 3 alpha,7 alpha-dihydroxy-5 beta-cholestanoic acid (DHCA) into chenodeoxycholic acid. The peroxisomal fraction of rat and human liver has the highest capacity to catalyze these reactions. Infants with Zellweger syndrome lack liver peroxisomes, and accumulate 5 beta-cholestanoic acids in bile and serum. We recently showed that such an infant had reduced capacity to convert a cholic acid precursor, 5 beta-cholestane-3 alpha,7 alpha,12 alpha-triol into cholic acid. 7 alpha-Hydroxy-4-cholesten-3-one is a common precursor for both cholic acid and chenodeoxycholic acid. Intravenous administration of [3H]7 alpha-hydroxy-4-cholesten-3-one to an infant with Zellweger syndrome led to a rapid incorporation of 3H into biliary THCA but only 10% of 3H was incorporated into cholic acid after 48 h. The incorporation of 3H into DHCA was only 25% of that into THCA and the incorporation into chenodeoxycholic acid approximately 50% of that in cholic acid. The conversion of intravenously administered [3H]THCA into cholic acid in another infant with Zellweger syndrome was only 7%. There was a slow conversion of THCA into 3 alpha,7 alpha,12 alpha-trihydroxy-5 beta-C29-dicarboxylic acid. The pool size of both cholic- and chenodeoxycholic acid was markedly reduced. Preparations of liver from two patients with Zellweger syndrome had no capacity to catalyze conversion of THCA into cholic acid. There was, however, a small conversion of DHCA into chenodeoxycholic acid and into THCA. It is concluded that liver peroxisomes are important both for the conversion of THCA into cholic acid and DHCA into chenodeoxycholic acid.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Almé B., Bremmelgaard A., Sjövall J., Thomassen P. Analysis of metabolic profiles of bile acids in urine using a lipophilic anion exchanger and computerized gas-liquid chromatorgaphy-mass spectrometry. J Lipid Res. 1977 May;18(3):339–362. [PubMed] [Google Scholar]
- Angelin B., Björkhem I., Einarsson K., Ewerth S. Hepatic uptake of bile acids in man. Fasting and postprandial concentrations of individual bile acids in portal venous and systemic blood serum. J Clin Invest. 1982 Oct;70(4):724–731. doi: 10.1172/JCI110668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkheim I., Danielsson H., Einarsson K., Johansson G. Formation of bile acids in man: conversion of cholesterol into 5-beta-cholestane-3-alpha, 7-alpha, 12-alpha-triol in liver homogenates. J Clin Invest. 1968 Jul;47(7):1573–1582. doi: 10.1172/JCI105849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björkhem I., Blomstrand R., Blomstrand S., Braathen G., Broberger O., Glaumann H., Kase B. F., Pedersen J. I., Strandvik B. Peroxisombrist bakom Zellwegers sjukdom--beskrivning av två svenska fall. Lakartidningen. 1985 May 1;82(18):1674–1677. [PubMed] [Google Scholar]
- Björkhem I., Falk O. Assay of the major bile acids in serum by isotope dilution-mass spectrometry. Scand J Clin Lab Invest. 1983 Apr;43(2):163–170. doi: 10.1080/00365518309168239. [DOI] [PubMed] [Google Scholar]
- Björkhem I., Sisfontes L., Boström B., Kase F., Hagenfeldt L., Blomstrand R. Possibility of prenatal diagnosis of Zellweger syndrome. Lancet. 1984 Jun 2;1(8388):1234–1235. doi: 10.1016/s0140-6736(84)91712-4. [DOI] [PubMed] [Google Scholar]
- Bremmelgaard A., Sjövall J. Hydroxylation of cholic, chenodeoxycholic, and deoxycholic acids in patients with intrahepatic cholestasis. J Lipid Res. 1980 Nov;21(8):1072–1081. [PubMed] [Google Scholar]
- Eyssen H., Parmentier G., Compernolle F., Boon J., Eggermont E. Trihydroxycoprostanic acid in the duodenal fluid of two children with intrahepatic bile duct anomalies. Biochim Biophys Acta. 1972 Jun 26;273(1):212–221. doi: 10.1016/0304-4165(72)90209-7. [DOI] [PubMed] [Google Scholar]
- Goldfischer S., Moore C. L., Johnson A. B., Spiro A. J., Valsamis M. P., Wisniewski H. K., Ritch R. H., Norton W. T., Rapin I., Gartner L. M. Peroxisomal and mitochondrial defects in the cerebro-hepato-renal syndrome. Science. 1973 Oct 5;182(4107):62–64. doi: 10.1126/science.182.4107.62. [DOI] [PubMed] [Google Scholar]
- Gustafsson J. Metabolism of 3 alpha, 7 alpha-dihydroxy-5 beta-cholestanoic acid by rat liver in vivo and in vitro. J Lipid Res. 1979 Feb;20(2):265–270. [PubMed] [Google Scholar]
- Hanson R. F., Klein P. D., Williams G. C. Bile acid formation in man: metabolism of 7 -hydroxy-4-cholesten-3-one in bile fistula patients. J Lipid Res. 1973 Jan;14(1):50–53. [PubMed] [Google Scholar]
- Hanson R. F., Szczepanik P. A., Klein P. D., Johnson E. A., Williams G. C. Formation of bile acids in man. Metabolism of 7alpha-hydroxy-4-cholesten-3-one in normal subjects with an intact enterohepatic circulation. Biochim Biophys Acta. 1976 May 27;431(2):335–346. doi: 10.1016/0005-2760(76)90154-5. [DOI] [PubMed] [Google Scholar]
- Hanson R. F. The formation and metabolism of 3 ,7 -dihydroxy-5 -cholestan-26-oic acid in man. J Clin Invest. 1971 Oct;50(10):2051–2055. doi: 10.1172/JCI106698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson R. F., Williams G. C. Metabolism of 3alpha, 7alpha, 12alpha-trihydroxy-5beta-cholestan-26-oic acid in normal subjects with an intact enterohepatic circulation. J Lipid Res. 1977 Sep;18(5):656–659. [PubMed] [Google Scholar]
- Kase B. F., Björkhem I., Hågå P., Pedersen J. I. Defective peroxisomal cleavage of the C27-steroid side chain in the cerebro-hepato-renal syndrome of Zellweger. J Clin Invest. 1985 Feb;75(2):427–435. doi: 10.1172/JCI111717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kase F., Björkhem I., Pedersen J. I. Formation of cholic acid from 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoic acid by rat liver peroxisomes. J Lipid Res. 1983 Dec;24(12):1560–1567. [PubMed] [Google Scholar]
- LINDSTEDT S. The turnover of cholic acid in man: bile acids and steroids. Acta Physiol Scand. 1957 Sep 17;40(1):1–9. doi: 10.1111/j.1748-1716.1957.tb01473.x. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Lazarow P. B., De Duve C. A fatty acyl-CoA oxidizing system in rat liver peroxisomes; enhancement by clofibrate, a hypolipidemic drug. Proc Natl Acad Sci U S A. 1976 Jun;73(6):2043–2046. doi: 10.1073/pnas.73.6.2043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moser A. E., Singh I., Brown F. R., 3rd, Solish G. I., Kelley R. I., Benke P. J., Moser H. W. The cerebrohepatorenal (Zellweger) syndrome. Increased levels and impaired degradation of very-long-chain fatty acids and their use in prenatal diagnosis. N Engl J Med. 1984 May 3;310(18):1141–1146. doi: 10.1056/NEJM198405033101802. [DOI] [PubMed] [Google Scholar]
- Parmentier G. G., Janssen G. A., Eggermont E. A., Eyssen H. J. C27 bile acids in infants with coprostanic acidemia and occurrence of a 3 alpha,7 alpha,12 alpha-tridhydroxy-5 beta-C29 dicarboxylic bile acid as a major component in their serum. Eur J Biochem. 1979 Dec;102(1):173–183. doi: 10.1111/j.1432-1033.1979.tb06278.x. [DOI] [PubMed] [Google Scholar]
- Pedersen J. I., Gustafsson J. Conversion of 3 alpha, 7 alpha, 12 alpha-trihydroxy-5 beta-cholestanoic acid into cholic acid by rat liver peroxisomes. FEBS Lett. 1980 Dec 1;121(2):345–348. doi: 10.1016/0014-5793(80)80377-2. [DOI] [PubMed] [Google Scholar]
- Reiner J. M. Recent advances in molecular pathology. Isotopic analysis of metabolic systems. I. Exp Mol Pathol. 1974 Feb;20(1):78–108. doi: 10.1016/0014-4800(74)90045-8. [DOI] [PubMed] [Google Scholar]
- Schwartz C. C., Cohen B. I., Vlahcevic Z. R., Gregory D. H., Halloran L. G., Kuramoto T., Mosbach E. H., Swell L. Quantitative aspects of the conversion of 5 beta-cholestane intermediates to bile acids in man. J Biol Chem. 1976 Oct 25;251(20):6308–6314. [PubMed] [Google Scholar]
- Shefer S., Cheng F. W., Dayal B., Hauser S., Tint G. S., Salen G., Mosbach E. H. A 25-hydroxylation pathway of cholic acid biosynthesis in man and rat. J Clin Invest. 1976 Apr;57(4):897–903. doi: 10.1172/JCI108366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watkins J. B., Ingall D., Szczepanik P., Klein P. D., Lester R. Bile-salt metabolism in the newborn. Measurement of pool size and synthesis by stable isotope technic. N Engl J Med. 1973 Mar 1;288(9):431–434. doi: 10.1056/NEJM197303012880902. [DOI] [PubMed] [Google Scholar]



