Abstract
Aim
To assess the suitability and potential cost savings, from both the hospital and community perspective, of prescribed oral liquid medicine substitution with acceptable solid forms for children over 2 years.
Method
Oral liquid medicines dispensed from a paediatric hospital (UK) in 1 week were assessed by screening for existence of the solid form alternative and evaluating the acceptability of the available solid form, firstly related to the prescribed dose and secondly to acceptable size depending on the child's age. Costs were calculated based on providing treatment for 28 days or prescribed duration for short term treatments.
Results
Over 90% (440/476) of liquid formulations were available as a marketed solid form. Considering dosage acceptability (maximum of 10% deviation from prescribed dosage or 0% for narrow therapeutic range drugs, maximum tablet divisions into quarters) 80% of liquids could be substituted with a solid form. The main limitation for liquid substitution would be solid form size. However, two-thirds of prescribed liquids could have been substituted with a suitable solid form for dosage and size, with estimated savings being of £5K and £8K in 1 week, respectively based on hospital and community costs, corresponding to a projected annual saving of £238K and £410K (single institution).
Conclusion
Whilst not all children over 2 years will be able to swallow tablets, drug cost savings if oral liquid formulations were substituted with suitable solid dosage forms would be considerable. Given the numerous advantages of solid forms compared with liquids, this study may provide a theoretical basis for investing in supporting children to swallow tablets/capsules.
Keywords: drug formulation, oral drug delivery, paediatric, swallowing
What Is Already Known about this Subject
Oral liquid drug formulations, historically considered as the most appropriate medicine for paediatric patients, present numerous disadvantages (low stability, inappropriate excipients).
Solid forms are more convenient, less costly and improve drug compliance in children.
Pill swallowing training significantly improves the ability to swallow orally administered solids from the age of 3 years old.
What this Study Adds
Considering dosage acceptability of solid forms, approximately 80% of prescribed liquid formulations could be substituted with a solid form in children aged over 2 years.
Considering both dosage and size suitability, half of liquid drugs could be substituted, tablet size being the major limitation for solid form use in children.
From both hospital and community perspectives, three quarters of treatment costs may be saved for liquid formulations that could be substituted.
Introduction
Since the adoption of the Paediatric Regulation (EC) no. 1901/2006 and the emergence of expert committees in paediatric medicine, a significant part of research in paediatric pharmacology is now focused on drug formulation, fundamentally determining whether the dose of the active pharmaceutical ingredient can be successfully delivered to children [1–4]. The availability of an appropriate drug form is of major concern for paediatricians, nurses, pharmacists and parents. Paediatric medicines have to be safe, well tolerated, easy to use (palatable and requiring minimal manipulations), transportable, easily produced, cost effective and commercially viable and have a minimal impact on life-style [1,4]. Oral liquid drug formulations, including solutions, syrups, suspensions and emulsions, are considered as the most appropriate oral formulation for children, since they are developed for younger infants unable to swallow tablets or capsules and accommodate palatability changes required by children [1,5,6]. However, liquid formulations present numerous disadvantages, such as low stability, difficulties in taste masking, inappropriate excipients for children (e.g. propylene glycol, benzyl alcohol, sweeteners for diabetics) and low transportability [1,2,7]. In the long term, dose volume and frequency of administration can lead to reduced compliance in children receiving multiple drugs [8]. In addition, liquid drugs for children are often provided as unlicensed medicines, when no licensed suitable alternative is available. Unlicensed medicines are often much more expensive than licensed products. Finally, oral liquid medicine multiple preparation steps, such as suspension reconstitution and homogenization, dose and volume calculation and precision of volume measurement [9], as well as misunderstanding of instruction for liquid medicines use by caregivers [10,11], are risk factors for medicine error in children [12]. Most administration errors in children result from decimal place errors or confusion between mg and ml [11,13].
An alternative to liquid formulations is the conventional solid oral dosage form, such as tablets or capsules. These offer the advantages of greater stability, easy dose selection, improved transportability and ease of storage [2,14]. Solid forms also allow the modification of drug pharmacokinetic parameters, minimizing administration frequency and indirectly improving drug compliance [1,15,16]. Oral solid forms are much less costly than liquid formulations, since they are easier to develop, manufacture, transport, store and dispense. However, despite those significant advantages and the recent change of paradigm in solid dosage form acceptability in children from numerous experts in paediatric pharmacology [1,2,4,8], there is a lack of evidence concerning solid dosage form acceptability in children and in particular their ability to swallow tablets or capsules. Solid dosage forms remain unpopular in paediatric practice. The primary explanation would be the assumed inability for children to swallow whole the solid form safely, avoiding risks of inhalation and choking. Nevertheless, some published studies have shown that solid forms can be swallowed by very young children, including children as young as 6 months of age [4,8,17–19]. Another limitation which may also explain the low use of oral solid forms in paediatrics is the lack of dosing flexibility compared with liquid formulations without crushing tablets [2,20]. Indeed, an important practical issue in paediatrics is to ensure that the chosen formulation can provide an accurate and appropriate dose for the children's needs. To date there are no published studies which have evaluated the concordance between the marketed dosages of oral solid forms and the prescribed doses in paediatrics.
Considering these two assumed limitations for the use of solid forms in children, the objective of this study was to assess the availability and the suitability of solid oral dosage forms for prescribed liquid medicines in children over 2 years of age. Considering that crushing tablets carries risks of preparation error, loss of stability and administration complexities this study has been conducted based on the assumption that tablets have to be swallowed by children, whole, halved or quartered. Results allow estimating the potential cost savings that could be made to the NHS (based on both hospital and community pharmacy prices) if all children over 2 years were converted from liquid to solid formulations where there is an available and acceptable solid form. This cost estimate supports answering the research question asked by the Expert Committee on Selection and Use of Essential Medicines (WHO committee) about dosage forms of medicines for children: ‘What are true component costs of different dosage forms of medicines for children?’ [6].
Methods
Data sources
A cross-sectional single site study was performed on anonymized pharmacy records of dispensed medicines in Birmingham Children's Hospital (BCH) for patients of 2 years old and above during 1 week (7–13th January 2013). Data were extracted from the BCH dispensing software database (Ascribe©, ASC, Bolton, UK). For each dispensed item the following information was recorded: child's age, drug international non-proprietary and commercial names, unit and daily prescribed dosage, dispensed pharmaceutical dosage form, treatment duration, quantity dispensed and cost of total drug quantity delivered from hospital prices.
Approval for this observational study was obtained from the National Research Ethics Service (Manchester, UK).
Suitability of oral solid forms
Substitution suitability with oral solid dosage form for each dispensed liquid medicine was determined:
Firstly by screening for the existence of the prescribed oral liquid drug in a marketed solid form alternative. The existence of an alternative marketed oral solid form was assessed consulting Ascribe© and the electronic Medicines Compendium (eMC) website.
Secondly by evaluating the suitability of available solid form dosage(s) related to the prescribed dose. Solid dosage concordance with prescribed dose was assessed for each dispensed liquid formulation existing as a marketed solid form. The maximum tablet division considered acceptable for appropriately scored tablets was the quarter (coated and time-released tablets were excluded from splitting). We considered that solid forms have to be swallowed (whole, halved or quartered) and not crushed. If, in spite of tablet division, the prescribed dose and available solid form dosage(s) did not fully match, the possibility of rounding the prescribed dose was evaluated by a hospital pharmacist considering the drug therapeutic index, current indication and the recommended dosage in the British National Formulary (BNF) for Children 2012–2013 regarding the child's age. The maximum recommended dose should not be exceeded and the maximum accepted dose deviation from the prescribed dose was 10% with a dosing error being defined as any dose deviation of more than 10% [21,22]. No dose adjustment was envisaged for narrow therapeutic index drugs, such as immunosuppressive medicines (e.g. azathioprine, ciclosporin, tacrolimus), anti-epileptics (e.g. carbamazepine, gabapentin, phenytoin, sodium valproate) or anticoagulants (e.g. warfarin).
Lastly by evaluating the solid size suitability depending on the child's age. The size suitability for solid forms available in an acceptable dosage, exactly or after dose rounding, was determined considering the guideline on pharmaceutical development of medicines for paediatric use of the Committee for Medicinal Products for Human Use (CHMP, European Medicines Agency's committee) of 2011 [23]: 3–5 mm diameter tablets are acceptable above 2 years of age, 5–10 mm diameter tablets are acceptable above 6 years, 10–15 mm diameter tablets are acceptable above 12 years and larger than 15 mm diameter tablets are not acceptable for the entire paediatric population, below 18 years. Despite solid form size suitability guidance having now been removed from the CHMP's draft update (2013) [3], no equivalent recommendation or opinion from any referral paediatric committee has been proposed since. Given the lack of published evidence for solid form size acceptability in children, we considered the CHMP's 2011 guidance to be the most authoritative publication to indicate solid size suitability depending on the child's age. The size of the tablets in mm for available marketed solid forms was obtained from TICTAC© software, a toxicology software referencing the diameter of marketed drugs in UK (London, UK).
Cost estimates
Hospital prices of dispensed liquid medicines during the study period were obtained from Ascribe© software, which holds the hospital prices applicable to the study institution. Cost of treatment with suitable solid forms was calculated by determining the equivalent number of doses required and the price of the unit dosage in Ascribe©. If tablet division was needed, we considered the cost of the section, assuming that all tablet sections were administered. For community prices the same process was implemented using the Drug Tariff published at the time of major hospital contract changes (November 2012, National Health Service Drug Tariff for England and Wales).
Calculated cost differences for each sector corresponded to the ‘treatment cost of liquid formulations available in an acceptable solid form’ minus the ‘treatment cost of corresponding oral solid dosage forms’ for the same dosage and treatment duration. The sum of these cost differences provides an estimate of the potential cost benefits if oral liquid drugs dispensed during the study period and having an acceptable solid form were substituted.
Results
Suitability of oral solid forms
Among the 908 dispensed oral medicine items for children over 2 years during 1 week by the BCH pharmacy (7-13th January 2013), 476 were prescribed as liquids. The mean age of children receiving oral liquid formulation was 7.4 ± 4.2 years. The 10 most frequently prescribed liquid formulations were paracetamol (11%), ibuprofen (11%), codeine (10%), amoxicillin/clavulanic acid (5%), colecalciferol (5%), lactulose (3%), phenoxymethylpenicillin (2%), ondansetron (1%), ranitidine (1%) and omeprazole (1%). Of the dispensed liquid formulations 13% (n = 61) were unlicensed medicines in the UK at the time of data collection.
Suitability of prescribed oral liquid medicine substitution with acceptable solid oral forms is detailed in Figure 1. Almost all (92%, n = 440) dispensed liquid formulations were available as a marketed solid form. Among them, 45% (n = 214) were available in a solid form with a dosage exactly corresponding to the prescribed dose. When additionally considering acceptable dose rounding, a total of 80% (n = 379) of prescribed liquid formulations could be substituted. Among those 379 acceptable solid dosage forms, 51% (n = 195) would need to be divided to ensure dose acceptability, among which 56% (n = 109) in halves and 44% (n = 86) in quarters. The proportion of solid dosage forms that would need division depending on the availability of exact prescribed dosage or adjusted prescribed dosage is detailed in Figure 1. Finally, also considering the size suitability of solid forms available in an acceptable dosage, the substitution rate was reduced to 41% (195/476).
Figure 1.
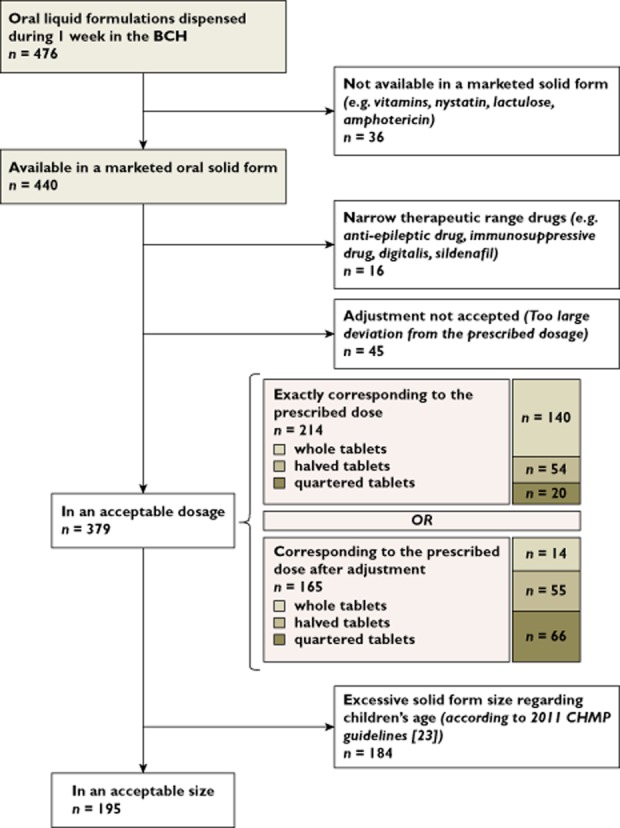
Flow chart of prescribed liquid formulation dispensed during 1 week by the BCH available in a suitable oral solid form for dosage and size
The suitability of oral solid forms evaluated by children's age groups is presented in Table 1. Almost half (46%) of prescribed liquid drugs were intended for pre-school children (2 to 5 years old). The most widely prescribed liquid drugs were comparable among the three age groups. When considering only the dosage acceptability of available solid forms (including dose rounding), the substitution rate of liquid formulations with solid forms would be comparable between the three groups by age (76%, 83% and 81% respectively for pre-school children, school children and adolescents). In pre-school children, 64% (106/165) of acceptable solid dosage forms would require tablet division to achieve the prescribed dose exactly or after rounding the dose, with 61% halved and 39% quartered. The proportion of tablets that would need to be divided was of 46% in school children (65/140) and 27% in adolescents (18/66), with equal proportions of halved and quartered tablets for both age groups. The number of solid dosage forms that would require division depending on dosage suitability and children's age group is presented in Figure 2. When also considering the size suitability, the substitution rate becomes very low in the pre-school children (16.13%), who were considered unable to swallow tablets larger than 5 mm diameter, corresponding to commonly recognized mini-tablet size. The highest substitution rate of liquid formulations with solid forms, considering both dosage and size suitability, was identified for the adolescent group, with 79% of liquid formulations that could be substituted. Interestingly, in the school age group, 52% of prescribed liquid drugs could be converted to a solid form, which is an important finding to accommodate school life style. Finally, two thirds (152/249) of liquid formulations prescribed in children over 6 years could have been substituted with an oral solid form, suitable for both dose and size.
Table 1.
Availability of oral solid forms depending on children's age groups
| Age range* | Liquid formulations | Available in an oral solid form | In an acceptable dosage† | In an acceptable size‡ | |||
|---|---|---|---|---|---|---|---|
| n | n | % | n | % | n | % | |
| All children | 476 | 440 | 92.44 | 379 | 79.62 | 195 | 40.97 |
| Pre-school children (2–5 years) | 217 | 206 | 94.93 | 165 | 76.04 | 35 | 16.13 |
| School children (6–11 years) | 168 | 154 | 91.68 | 140 | 83.33 | 88 | 52.38 |
| Adolescents (over 12 years) | 81 | 70 | 86.42 | 66 | 81.48 | 64 | 79.01 |
| Missing age data | 10 | 10 | – | 8 | – | 8 | – |
Figure 2.
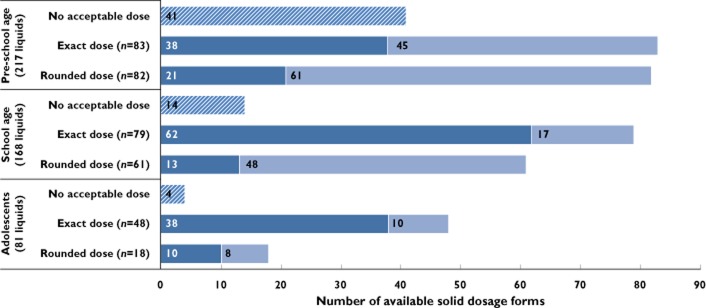
Distribution of available solid dosage forms for prescribed liquid drugs in the different children's age groups, depending on dosage acceptability and need for splitting tablets ( , no acceptable dose;
, no acceptable dose;  , acceptable dose for whole tablet;
, acceptable dose for whole tablet;  , acceptable dose after splitting tablet)
, acceptable dose after splitting tablet)
Cost estimates
The substitution of liquid formulations with solid oral forms available in an acceptable dosage could result in a cost saving of £16K and £18K per week, respectively, for hospital and community sectors, corresponding to 73% and 76% of liquid formulation cost saved (Table 2). However, when additionally considering size suitability of available oral solid forms, these potential cost savings are reduced to £5K and £9K per week, respectively, for hospital and community (Table 3). Treatment costs of solid forms are nevertheless 50% less than treatment costs of liquid medicines.
Table 2.
Costs of dispensed medicines during 1 week in the BCH, considering liquid formulations that could be substituted with a solid form acceptable for dosage (n = 379)
| Age range* | Total cost of oral liquid medicines (£) | Total cost of oral solid form (£) | Potential cost saving (£) | |||
|---|---|---|---|---|---|---|
| Hospital | Community | Hospital | Community | Hospital | Community | |
| All children | 22 192 | 23 454 | 5 893 | 5 685 | 16 299 | 17 769 |
| Pre-school children (2–5 years) | 9 519 | 8 258 | 1 369 | 1 362 | 8 150 | 6 896 |
| School children (6–11 years) | 6 492 | 6 956 | 2 776 | 2 575 | 3 716 | 4 381 |
| Adolescents (over 12 years) | 6 035 | 7 398 | 1 724 | 1 724 | 4 311 | 5 673 |
Defined by the CHMP [2].
Table 3.
Costs of dispensed medicines during 1 week in the BCH, considering liquid formulations that could be substituted with a solid form acceptable both for dosage and size (n = 195)
| Age range* | Total cost of oral liquid medicines (£) | Total cost of oral solid form (£) | Potential cost saving (£) | |||
|---|---|---|---|---|---|---|
| Hospital | Community | Hospital | Community | Hospital | Community | |
| All children | 8 307 | 11 697 | 3 356 | 3 147 | 5 332 | 8 330 |
| Pre-school children (2–5 years) | 205 | 542 | 45 | 36 | 160 | 506 |
| School children (6–11 years) | 1 951 | 3 576 | 1 581 | 1 381 | 370 | 2 195 |
| Adolescents (over 12 years) | 6 018 | 7 348 | 1 216 | 1 719 | 4 802 | 5 629 |
Defined by the CHMP [2].
Discussion
Almost all dispensed oral liquid formulations in children over 2 years during 1 week in a paediatric hospital were available in a marketed solid oral form, and, more surprisingly, they had a dosage suitable for paediatric prescribing. The main limitation for liquid formulation substitution with solid form was the size of solid drugs, especially in children under 6 years of age. Despite this limitation, two-thirds of prescribed liquids could have been substituted with a suitable solid form for both dosage and size, with expected savings of around £5K and £8K in only 1 week, respectively, for hospital and community costs, corresponding to a projected annual saving of £238K and £410K, for only one study site.
These findings support numerous recent study results and expert opinion, which run contrary to the historic argument that liquid formulations are preferable for children. The main disadvantages associated with liquid formulations are related to their stability, potential toxicity, dosing and administration issues. The CHMP highlights the interest in using solid oral forms in young children with long term illness who require continuing medication. It also recommends that, more generally, children be trained to swallow ‘pills’ from a relatively early age of 3–5 years [2]. In parallel, the WHO recommends that children of developing countries be treated with oral solid medicines for drugs that do not require a precise dose titration [6]. In addition, a small number of studies have provided evidence that the use of oral solid forms in children was associated with a greater acceptability and drug compliance than with liquid forms [15,16,18,24,25]. Spomer et al. have shown in an open randomized crossover study that, contrary to their original research hypothesis, mini-tablets (2 mm in diameter) acceptance in 60 children from 6 months to 6 years was at least equal or even better than that of sweet liquid, especially in very young children (0.5–1 years) [18]. Furthermore, complete refusal of oral administration was much higher for liquid than for solid formulation (40% of refusal vs. 10%). In a multicentre randomized study conducted by Van Riet-Nales et al. [24], 405 children from 1 to 4 years were selected to receive four oral placebo dosage forms: small tablet (4 mm), powder, suspension and syrup. The comparison of the dosage form acceptability showed that tablets were significantly better accepted than other forms (P < 0.001). Interestingly, the number of fully swallowed administrations was also significantly higher with tablets (P < 0.05). In Ansah et al.'s study, involving 301 children aged from 0 to 5 years, 91% of children who received pre-packed chloroquine tablets complied with the prescribed treatment, compared with only 42% in children who received chloroquine syrup (P < 0.001) [15]. The economic evaluation in the present study has shown that tablet treatment was five times less costly than current prescribed formulations. Increased patient (child) autonomy, a higher transportability, a single dose packaging and saving time for caregivers seems to be the most likely explanation for better drug compliance with solid oral forms in children [26,27].
One of the limitations of our study was to base the size acceptability substitution on the 2011 edition of CHMP's Pharmaceutical Development of Medicines for Paediatric Use [26]. Although these recommendations were not based on evidence but on expert opinion and are now removed from the updated draft [3], given the lack of published evidence, they provide an insight into suitable solid size in children. In addition, CHMP note that tablet size acceptability may be improved using pill swallowing training and fractioning tablets, allowing very young children to swallow larger size solid forms [2]. Since the potential substitution rate of liquid formulations with solid forms when considering both dosage and size was sharply reduced, this point is of considerable importance. Indeed, the annual projected cost saving if we consider that children would be able to swallow all solid forms with an acceptable dose would be £782K and £853K, respectively, for hospital and community costs, or more than twice that if we also consider the pill size.
The suitability study of liquid drug substitution with solid forms was conducted considering the possibility of splitting tablets to achieve the prescribed dose. This methodological choice reflects a widespread clinical practice in our paediatric hospitals, where tablet splitters are made available when required. Indeed, breaking tablets is the most common oral dosage form manipulation, undertaken by caregivers for home carers or by nurses [28]. The magnitude of splitting tablet practice in paediatric wards has been poorly investigated. Fontan et al. have collected direct observations of nurse's administration habits in 14 French paediatric hospitals. The results revealed that around half (46.7%) of administered tablets were split before administration, most frequently by hand (55.3%) [29]. However, this study concerned young children (12.6 ± 17 months). Richey et al.'s multicentre study conducted in UK paediatric wards has shown that, among 40 observed tablet manipulations, 65% corresponded to splitting tablets, other practices being dispersing (30%) and crushing (2.5%) [30]. The high observed rates of splitting tablet practice in paediatric care compared with adults can easily be explained knowing that oral solid dosage forms are developed to meet the needs of adult patients [31]. From CHMP's reflexion paper on formulation of choice for the paediatric population, scoring tablets should be considered in paediatric care to improve solid form dosage flexibility and make tablets easier to swallow [2]. Although we considered such practice only for appropriate solid forms, coated and time-released tablets being excluded, consequences of solid form splitting on active drug efficacy and safety should, however, be assessed. Splitting tablets relies on the assumption that the active drug is uniformly distributed throughout the product. Nevertheless, several studies have shown that drug amount in tablet segments is often unequal, even using scored tablets. In addition, tablets can be difficult to cut, crumble and finally be inappropriately divided [30]. If tablet splitters seem to improve accuracy of division compared with hand or knife breaking, these devices also have significant segment inequality, in particular when tablets are small and quartered [32–34]. In our study, 23% of acceptable solid forms would need to be quartered. In addition, the need for splitting tablets was higher when the prescribed dose was rounded, adding further deviation from the initially prescribed dose. In practice, paediatricians and pharmacists should consider the need for tablet division, especially for small, unscored and low dosage tablets, prior to formulation choice. As regards children's ability to swallow, it is expected that splitting tablets will reduce palatability. Tablets when divided often have rough edges, may crumble and reveal the active drug taste [33]. However, Richey et al. report the case of a child who preferred split tablets to liquid, due to liquid bad taste and excess liquid volume to be swallowed [30]. Finally, from our results, dividing tablets offers interesting opportunities to adapt solid dosage forms for children and allows raising the rate of acceptable solid forms for dosage from 39% to 80%. This practice, however, has unknown consequences on active drug accuracy, drug stability and bioavailability [20,30,33]. Concern about dose deviations in children should focus on narrow therapeutic range drugs [35]. Among the 195 solid forms that would need to be split to achieve the prescribed dose, only one drug had a narrow therapeutic range (quartered gabapentin) in a 3-year-old child. Lastly, one of the major advantages of oral solid forms, which is that it is ready to use and offers easy dose selection, is reduced using this time-consuming practice. By the same reasoning, the impact of tablet splitting on drug adherence and administration error risks should also be assessed [30]. Splitting tablets, however, remains far preferable to crushing or dispersing tablets, involving greater risks for drug deterioration and administration error [20].
It might be seen as a limitation to conduct the cost study considering that all the split tablet's segments could be administered. Splitting tablets allows significant cost savings when each tablet section is administered, and could interestingly reduce treatment costs [31,33]. Indeed, divided tablets would generally be less costly than the whole solid dosage form. In our own institution using all tablet sections is variable. Tablet splitters have a space to store unused tablet sections and are often provided for use at home, and the pharmacy can provide pre-split tablets if necessary, although this is rare. In the Fontan et al. study, only half of divided tablet's segments were actually kept by nurses and administered [29]. To our knowledge, concerning home administration, no quantified data are available in the literature. When treatment cost is covered by health care insurance, the patient's main reasons for dividing tablets are to achieve the prescribed dose, ease swallowing and reduce dose, without economic considerations [36]. From our experience, we assume that patients with long term treatment are more inclined to keep tablet segments than patients with short term treatments. Finally, considering that every tablet's segment would be administered may have led to an overestimated cost saving associated with solid form use in children.
Some recent solid oral forms (e.g. powders, chewable, melt or oro-dispersible dosage forms) may provide another interesting alternative to liquid formulations in children, providing appropriate dosage and avoiding splitting tablets. However, few have been licensed for children under 4 years old, and must be provided ‘off-label’. Furthermore, in spite of their practical advantages, they are much more costly than conventional solid drugs and, consequently, there are very few available on the market [1]. An evaluation of cost effectiveness regarding compliance improvement and comparing with conventional formulations is needed. More generally, the development of new paediatric medicines is complex, time consuming and costly. Insofar as cost may be a long term obstacle to widespread adoption of these new paediatric drug formulations, the immediate effective solution would be the use of already available alternatives, the most obvious being the solid oral form. Further work is required to identify the limits of oral solid forms use in paediatrics, in order to propose appropriate changes. From our results, the absolute necessity to facilitate solid oral form use in paediatrics is to reduce tablet size, making them more suitable for younger children and to facilitate swallowing learning for children. Developing solid dosage forms adapted to paediatric doses (e.g. mini-tablets) or securing splitting tablets (e.g. highly divisible tablets, scored tablets with drug-free basal compartment) would also help provide more suitable solid forms in children [37]. Awareness of pharmaceutical companies to this critical issue is an essential prerequisite for change. Community and paediatric hospital pharmacists should also consider the size parameter in drug selection. Another particularly attractive solution is the implementation of pill swallowing training in paediatric hospitals, in order to improve children's ability to swallow orally administered solids, especially for children with chronic illnesses requiring long term medication [38–46]. Numerous research teams have worked on this focus and methods for this behavioural change technique are now well defined. In Czyzewski et al.'s study, the largest capsule size was 19.4 mm, showing that pill swallowing training is effective even for large sized solid oral forms [38]. Given the cost savings that could be obtained when substituting liquid formulations with solid form in only 1 week in a single hospital, it is of major interest to evaluate the cost effectiveness of pill swallowing training implementation in a paediatric hospital for children who require long term medication. To conclude, expected practical and clinical advantages of oral solid form use in children have to be carefully considered against challenges related to children's ability to swallow and risks arising from splitting tablets. A larger long term multicentre study would help to investigate further solid forms suitability in children, consider the different therapeutic classes, treatment duration, required solid form manipulations and represent more accurately the diversity of prescribed drugs in paediatrics.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work or activities that could appear to have influenced the submitted work.
References
- 1.Salunke S, Hempenstall J, Kendall R, Roger B, Mroz C, Nunn T, Tuleu C. European Paediatric Formulation Initiative's (EuPFI) 2nd conference commentary – Formulating better medicines for children. Int J Pharm. 2011;419:235–239. doi: 10.1016/j.ijpharm.2011.06.040. [DOI] [PubMed] [Google Scholar]
- 2.European Medicines Agency, Committee for Medicinal Products for Human Use. 2006. Reflection paper: formulations of choice for the paediatric population; EMEA/CHMP/PEG/194810/2005.
- 3.Walsh J, Mills S. Conference report: formulating better medicines for children: 4th European Paediatric Formulation Initiative conference. Ther Deliv. 2013;4:21–25. doi: 10.4155/tde.12.135. [DOI] [PubMed] [Google Scholar]
- 4.Cram A, Breitkreutz J, Desset-Brethes S, Nunn T, Tuleu C European Paediatric Formulation I. Challenges of developing palatable oral paediatric formulations. Int J Pharm. 2009;365:1–3. doi: 10.1016/j.ijpharm.2008.09.015. [DOI] [PubMed] [Google Scholar]
- 5.Nahata MC. Lack of pediatric drug formulations. Pediatrics. 1999;104:607–609. [PubMed] [Google Scholar]
- 6.Schirm E, Tobi H, de Vries TW, Choonara I, De Jong-van den Berg LT. Lack of appropriate formulations of medicines for children in the community. Acta Paediatr. 2003;92:1486–1489. doi: 10.1080/08035250310006728. [DOI] [PubMed] [Google Scholar]
- 7.World Health Organization. Report of the Informal Expert Meeting on Dosage Forms of Medicines for Children. Geneva: WHO Headquarters; 2009. [Google Scholar]
- 8.Yeung VW, Wong IC. When do children convert from liquid antiretroviral to solid formulations? Pharm World Sci. 2005;27:399–402. doi: 10.1007/s11096-005-7911-z. [DOI] [PubMed] [Google Scholar]
- 9.Yin HS, Mendelsohn AL, Wolf MS, Parker RM, Fierman A, van Schaick L, Bazan IS, Kline MD, Dreyer BP. Parents’ medication administration errors: role of dosing instruments and health literacy. Arch Pediatr Adolesc Med. 2010;164:181–186. doi: 10.1001/archpediatrics.2009.269. [DOI] [PubMed] [Google Scholar]
- 10.Bailey SC, Pandit AU, Yin S, Federman A, Davis TC, Parker RM, Wolf MS. Predictors of misunderstanding pediatric liquid medication instructions. Fam Med. 2009;41:715–721. [PubMed] [Google Scholar]
- 11.Schillie SF, Shehab N, Thomas KE, Budnitz DS. Medication overdoses leading to emergency department visits among children. Am J Prev Med. 2009;37:181–187. doi: 10.1016/j.amepre.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 12.European Medicines Agency; Committee for Medicinal Products for Human Use. 2013. Guideline on pharmaceutical development of medicines for paediatric use; EMA/CHMP/QWP/805880/2012 Rev. 2. [DOI] [PubMed]
- 13.French National Agency for Medicines and Health Products Safety (ANSM) Management of Medication Errors Associated with the Use of Delivery Devices for Orally Ingested Liquid Drugs. Available at http://ansm.sante.fr/var/ansm_site/storage/original/application/202d871e1bdcead7004ad0d7b07e2f4d.pdf (last accessed 2 August 2014) [Google Scholar]
- 14.Nunn T, Williams J. Formulation of medicines for children. Br J Clin Pharmacol. 2005;59:674–676. doi: 10.1111/j.1365-2125.2005.02410.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ansah EK, Gyapong JO, Agyepong IA, Evans DB. Improving adherence to malaria treatment for children: the use of pre-packed chloroquine tablets vs. chloroquine syrup. Trop Med Int Health. 2001;6:496–504. doi: 10.1046/j.1365-3156.2001.00740.x. [DOI] [PubMed] [Google Scholar]
- 16.Bagenda A, Barlow-Mosha L, Bagenda D, Sakwa R, Fowler MG, Musoke PM. Adherence to tablet and liquid formulations of antiretroviral medication for paediatric HIV treatment at an urban clinic in Uganda. Ann Trop Paediatr. 2011;31:235–245. doi: 10.1179/1465328111Y.0000000025. [DOI] [PubMed] [Google Scholar]
- 17.Garvie PA, Lensing S, Rai SN. Efficacy of a pill-swallowing training intervention to improve antiretroviral medication adherence in pediatric patients with HIV/AIDS. Pediatrics. 2007;119:e893–899. doi: 10.1542/peds.2006-1488. [DOI] [PubMed] [Google Scholar]
- 18.Spomer N, Klingmann V, Stoltenberg I, Lerch C, Meissner T, Breitkreutz J. Acceptance of uncoated mini-tablets in young children: results from a prospective exploratory cross-over study. Arch Dis Child. 2012;97:283–286. doi: 10.1136/archdischild-2011-300958. [DOI] [PubMed] [Google Scholar]
- 19.Thomson SA, Tuleu C, Wong IC, Keady S, Pitt KG, Sutcliffe AG. Minitablets: new modality to deliver medicines to preschool-aged children. Pediatrics. 2009;123:e235–238. doi: 10.1542/peds.2008-2059. [DOI] [PubMed] [Google Scholar]
- 20.Standing JF, Tuleu C. Paediatric formulations – getting to the heart of the problem. Int J Pharm. 2005;300:56–66. doi: 10.1016/j.ijpharm.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 21.Sam T, Ernest TB, Walsh J, Williams JL European Paediatric Formulation I. A benefit/risk approach towards selecting appropriate pharmaceutical dosage forms – an application for paediatric dosage form selection. Int J Pharm. 2012;435:115–123. doi: 10.1016/j.ijpharm.2012.05.024. [DOI] [PubMed] [Google Scholar]
- 22.Walsh KE, Mazor KM, Stille CJ, Torres I, Wagner JL, Moretti J, Chysna K, Stine CD, Usmani GN, Gurwitz JH. Medication errors in the homes of children with chronic conditions. Arch Dis Child. 2011;96:581–586. doi: 10.1136/adc.2010.204479. [DOI] [PubMed] [Google Scholar]
- 23.European Medicines Agency; Committee for Medicinal Products for Human Use. 2011. Guideline on pharmaceutical development of medicines for paediatric use; EMA/CHMP/QWP/180157/2011.
- 24.Van Riet-Nales DA, de Neef BJ, Schobben AF, Ferreira JA, Egberts TC, Rademaker CM. Acceptability of different oral formulations in infants and preschool children. Arch Dis Child. 2013;98:725–731. doi: 10.1136/archdischild-2012-303303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ameen VZ, Pobiner BF, Giguere GC, Carter EG. Ranitidine (Zantac) syrup versus ranitidine effervescent tablets (Zantac) EFFERdose) in children: a single-center taste preference study. Paediatr Drugs. 2006;8:265–270. doi: 10.2165/00148581-200608040-00005. [DOI] [PubMed] [Google Scholar]
- 26.Chappuy H, Patteau G, Treluyer JM, Cheron G. Medications dosage and adherence. Arch Pediatr. 2009;16:968–969. doi: 10.1016/S0929-693X(09)74221-6. [DOI] [PubMed] [Google Scholar]
- 27.Greenberg RN. Overview of patient compliance with medication dosing: a literature review. Clin Ther. 1984;6:592–599. [PubMed] [Google Scholar]
- 28.Ernest TB, Craig J, Nunn A, Salunke S, Tuleu C, Breitkreutz J, Alex R, Hempenstall J. Preparation of medicines for children – a hierarchy of classification. Int J Pharm. 2012;435:124–130. doi: 10.1016/j.ijpharm.2012.05.070. [DOI] [PubMed] [Google Scholar]
- 29.Fontan JE, Mille F, Brion F, Aubin F, Ballereau F, Benoit G, Brunet ML, Braguier D, Combeau D, Dugast P, Gerout AC, May I, Meunier P, Naveau-Ploux C, Proust V, Samdjee F, Schlatter J, Thebault A, Vie M Groupe Pediatrie de la Societe Francaise de Pharmacie C. [Drug administration to paediatric inpatient] Arch Pediatr. 2004;11:1173–1184. doi: 10.1016/j.arcped.2004.06.024. [DOI] [PubMed] [Google Scholar]
- 30.Richey RH, Shah UU, Peak M, Craig JV, Ford JL, Barker CE, Nunn AJ, Turner MA. Manipulation of drugs to achieve the required dose is intrinsic to paediatric practice but is not supported by guidelines or evidence. BMC Pediatr. 2013;13:81. doi: 10.1186/1471-2431-13-81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Quinzler R, Gasse C, Schneider A, Kaufmann-Kolle P, Szecsenyi J, Haefeli WE. The frequency of inappropriate tablet splitting in primary care. Eur J Clin Pharmacol. 2006;62:1065–1073. doi: 10.1007/s00228-006-0202-3. [DOI] [PubMed] [Google Scholar]
- 32.McDevitt JT, Gurst AH, Chen Y. Accuracy of tablet splitting. Pharmacotherapy. 1998;18:193–197. [PubMed] [Google Scholar]
- 33.Van Santen E, Barends DM, Frijlink HW. Breaking of scored tablets: a review. Eur J Pharm Biopharm. 2002;53:139–145. doi: 10.1016/s0939-6411(01)00228-4. [DOI] [PubMed] [Google Scholar]
- 34.Habib MA, Soofi SB, Bhutta ZA. Effect of zinc in tablet and suspension formulations in the treatment of acute diarrhoea among young children in an emergency setting of earthquake affected region of Pakistan. J Coll Physicians Surg Pak. 2010;20:837–838. [PubMed] [Google Scholar]
- 35.Shah RB, Collier JS, Sayeed VA, Bryant A, Habib MJ, Khan MA. Tablet splitting of a narrow therapeutic index drug: a case with levothyroxine sodium. AAPS PharmSciTech. 2010;11:1359–1367. doi: 10.1208/s12249-010-9515-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rodenhuis N, De Smet PA, Barends DM. The rationale of scored tablets as dosage form. Eur J Pharm Sci. 2004;21:305–308. doi: 10.1016/j.ejps.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 37.Wening K, Breitkreutz J. Oral drug delivery in personalized medicine: unmet needs and novel approaches. Int J Pharm. 2011;404:1–9. doi: 10.1016/j.ijpharm.2010.11.001. [DOI] [PubMed] [Google Scholar]
- 38.Czyzewski DI, Runyan RD, Lopez MA, Calles NR. Teaching and maintaining pill-swallowing in HIV-infected children. AIDS Read. 2000;10:88–95. [Google Scholar]
- 39.Cruz-Arrieta E. Pill-swallowing training: a brief pediatric oncology report. Prim Psychiatry. 2008;15:49–53. [Google Scholar]
- 40.Wright L, Woodcock JM, Scott R. Conditioning children when refusal of oral medication is life threatening. Pediatrics. 1969;44:969–972. [PubMed] [Google Scholar]
- 41.Dahlquist LM, Blount RL. Teaching a six-year-old girl to swallow pills. J Behav Ther Exp Psychiatry. 1984;15:171–173. doi: 10.1016/0005-7916(84)90014-4. [DOI] [PubMed] [Google Scholar]
- 42.Blount RL, Dahlquist LM, Baer RA, Wuori D. A brief, effective method for teaching children to swallow pills. Behav Ther. 1984;15:381–387. doi: 10.1016/0005-7916(84)90014-4. [DOI] [PubMed] [Google Scholar]
- 43.Walco GA. A behavioral treatment for difficulty in swallowing pills. J Behav Ther Exp Psychiatry. 1986;17:127–128. doi: 10.1016/0005-7916(86)90050-9. [DOI] [PubMed] [Google Scholar]
- 44.Pelco LE, Kissel RC, Parrish JM, Miltenberger RG. Behavioral management of oral medication administration difficulties among children: a review of literature with case illustrations. J Dev Behav Pediatr. 1987;8:90–96. [PubMed] [Google Scholar]
- 45.Babbitt RL, Parrish JM, Brierley PE, Kohr MA. Teaching developmentally disabled children with chronic illness to swallow prescribed capsules. J Dev Behav Pediatr. 1991;12:229–235. [PubMed] [Google Scholar]
- 46.Sallows GO. Behavioral treatment of swallowing difficulty. J Behav Ther Exp Psychiatry. 1980;11:45–47. [Google Scholar]


