Abstract
Aims
The aim of this study was to assess opinions of frontline healthcare professionals on the linking of routinely collected national (Scottish) paediatric data for the purpose of identifying earlier signals of adverse drug reactions.
Methods
Stratified purposive sampling led to profession-specific focus groups with pharmacists, nurses and medical doctors from primary and secondary care in different Scottish Health Boards. A topic guide was used to explore the proposed data linkage of routinely collected paediatric data. Discussions were audio recorded and transcribed verbatim. Transcripts were analysed using a framework approach to identify themes. Ethical approval was obtained from the North of Scotland Research Ethics Service.
Results
Six focus groups were conducted in 2011 with 22 participants. Views of the proposed data linkage were generally positive. Several issues were identified, including lack of clarity on data ownership and concerns about diversion of funding. Identified issues were at a practical rather than a strategic level.
Conclusions
This study identified that professional stakeholder groups are likely to find linkage of paediatric patient data acceptable. Barriers identified could be addressed. Focus group participants commented on the importance of informing patients and members of the public about the benefits of linking healthcare data. These findings clarify the steps that should be taken to ensure the acceptability of data linkage for pharmacovigilance.
Keywords: data linkage, pharmacovigilance, qualitative research
What is Already Known about this Subject
Off-label prescribing in children is linked to higher rates of adverse drug reactions.
The linkage of routinely collected healthcare data may provide a new approach for earlier signal generation of adverse drug reactions.
What This Study Adds
Healthcare professionals would generally support data linkage for pharmacovigilance purposes.
Issues of information governance, ethical approval and public information need to be addressed before linking data.
Introduction
The prescribing of unlicensed drugs and the off-label use of drugs, particularly in children, is linked to a higher rate of adverse drug reactions (ADRs) compared with the use of licensed medication [1]. In the UK, between 11 and 26% of children were reported to receive an off-label prescription from their general practitioner (GP) [2–4], and between 48 and 57% [5,6] of children in hospitals receive an off-label or unlicensed treatment at some time [7]. Reported figures differ, but it is estimated that five out of eight severe ADRs in paediatric inpatients are linked to off-label use of drugs [2]. A prospective surveillance study in five paediatric wards in England showed that ADRs were associated with 6% of off-label prescribing compared with only 3.9% of prescriptions for licensed indications [5]. In order to identify ADRs reliably, a cohort of at least 1000 patients is required, but this is challenging in children, especially for those drugs used in rare conditions [8]. Furthermore, off-label or unlicensed drugs, often used in paediatrics, are not subject to the usual rigorous postmarketing surveillance schemes [9].
In the UK, the Yellow Card Scheme (YCS) of the Committee on the Safety of Medicines (UK) is the main pharmacovigilance system of the National Health Service (NHS). This signal generation scheme relies on voluntary reporting of ADRs by healthcare professionals (HCPs) and, since 2005, patients [10]. All reactions associated with medication used in children should be reported, including reactions related to off-label and unlicensed use of medication [11]. Although the YCS is established as the main system for routine pharmacovigilance in the UK, its recognized limitations include lack of a denominator (the total number of people receiving the medicine and the duration of therapy) and the variable quality of the data received [12]. Furthermore, despite widespread off-label prescribing in children as described above, it was reported that of all returned Yellow Cards, only 7–13% related to children [13].
There is therefore a need to identify other complementary approaches to the identification of ADRs, especially those in children. Scotland's routinely collected healthcare data include a unique patient identifier, the Community Health Index number (CHI nr), which could allow data linkage, for example between primary and secondary care. This would include the possibility of following up the patient in real time and provide the required denominators as well as avoid duplicate reporting, i.e. reporting the same reaction twice. Such routine data linkage would permit creation of a continuous virtual cohort to monitor for long-term outcomes, for example after exposure to pharmacotherapy, and enable a more efficient screening for side-effects or ADRs due to an ever-increasing data pool [14]. Combining data sets from primary and secondary care would maximize the potential to identify safety issues around paediatric medication [15] and allow differences to be explained between prescriptions in primary care and outcomes resulting in hospitalization.
Data linkage is, however, a potentially controversial issue. Before introducing a new system, such as a paediatric data linkage system, it is important to assess its acceptability to key stakeholders and to identify potential barriers to effective implementation. This report describes a qualitative study conducted in Scotland to assess the views and opinions of healthcare professionals on the linking of routinely acquired paediatric data in the National Health System in Scotland as a means of identifying adverse drug reactions in children.
Methods
Focus groups were conducted with frontline healthcare professionals to capture their views on a planned data linkage project.
Participants and setting
Nurses, pharmacists and medical doctors with either a current registration to practice in Scotland or recently retired, working in either primary or secondary care, were eligible. Paediatric experience was preferred but not essential.
Sampling and recruitment
Stratified purposive sampling followed by snowballing was used to recruit participants from professional networks. For each profession, a different method of recruitment was used.
Pharmacists: NHS Borders volunteered to recruit for a pharmacist focus group after the study was discussed at the Scottish Directors of Pharmacy meeting. A second pharmacist focus group was conducted with pharmacists from the Scottish Neonatal and Paediatric Pharmacist Group.
Doctors: NHS National Education Scotland distributed invitation packs to their medical registrar trainees within NHS Grampian. Further invitation packs were issued to all paediatricians listed on the NHS Grampian website as working at the Royal Aberdeen Children's Hospital (RACH), followed by an invitation presented at a meeting of paediatric consultants at the same hospital.
Nurses in NHS Greater Glasgow and Clyde were invited via the Nonmedical Prescribing Co-ordinator. A second group was held in NHS Grampian, where the head of paediatric nurses agreed to distribute the invitation to all senior nurses within RACH. Additional nurses were recruited from the MSc class of Advanced Nursing at the University of Aberdeen by distributing invitation packs at the beginning of an evening class.
The aim was to recruit between three and eight participants per group.
Invitation packs consisted of an invitation letter, a study information sheet (available on request) and a consent form. One reminder was sent to nonresponders after 2 weeks. Those interested in participating were asked to return the signed consent form to the researcher using the reply paid envelope provided. Reimbursement for locum costs to aid attendance was offered. A focus group topic guide was developed (available on request), guided by the research question, ‘What are the views of healthcare professionals towards linking NHS data across Scotland for signal generation of adverse drug reactions in children?’ and informed by the findings of an earlier interview study [16]. The topic guide was piloted in April 2010 with five participants (one GP, one community pharmacist, two hospital pharmacists and a health visitor). The discussion was longer than anticipated (90 min), so a revised topic guide focusing on perceived issues with linkage of routinely collected health data was used thereafter. After three focus groups, a question was added to explore which changes to their own behaviour participants considered being necessary to aid the proposed data linkage. To encourage maximal attendance, CPD (Continuous Professional Development) certificates were issued to participants confirming their attendance, the topic and the length of the discussion.
Focus groups
All focus groups were conducted by a trained facilitator (YMH) at a mutually convenient time for the participants in available rooms at the respective Health Boards or hospitals. Refreshments were available throughout the discussion. All groups started with a focusing exercise, asking participants what they associated with the term pharmacovigilance. Answers were written on a flipchart that everyone could see. These answers were then reflected back to the group during the discussion.
Data management and analysis
All focus groups were audio recorded and the recordings transcribed verbatim. The anonymized transcripts were uploaded to NVivo (Qualitative data analysis software, Version 7; QSR International Pty Ltd, Victoria, Australia) to aid data management and analysis. The Framework approach to analysis [17] was used, with main themes being set a priori in addition to identification of emergent themes. Analysis was ongoing as soon as the first focus group was completed to allow iterative refinement of the topic guide. Coding frames were prepared and descriptive accounts created by YMH. Coding frames and descriptive accounts were reviewed by the study team at regular intervals throughout the study period.
Ethical approval
The study was approved by the North of Scotland Research Ethics Service (Reference number 09/S0801/115) and NHS Research and Development.
Results
A total of six focus groups were conducted, with participants (n = 22) recruited from seven different Scottish Health Boards (as shown in Table 1 and Figure 1) between August 2010 and May 2011. This coverage included rural and urban areas, with different proximity to a paediatric hospital/specialist paediatric care. Participants were recruited from both primary and secondary care, as well as from three different professional backgrounds, i.e. medicine, pharmacy and nursing. Numbers of pharmacists were highest (n = 11, 50%), and the majority of participants were female (n = 13, 59%; see Table 2).
Table 1.
Breakdown of participant numbers per group (the pilot focus group was labelled FG01 and is not included)
| Focus group | Number of participants | Total |
|---|---|---|
| Doctors | ||
| General practitioners (FG02) | 2 | |
| Paediatricians (FG07) | 3 | 5 |
| Nurses | ||
| Primary care (FG03) | 2 | |
| Secondary care (FG06) | 4 | 6 |
| Pharmacists | ||
| Secondary care (FG04) | 2 | |
| Paediatric pharmacists (FG05) | 9 | 11 |
| Total number of focus group participants | 22 | |
Figure 1.
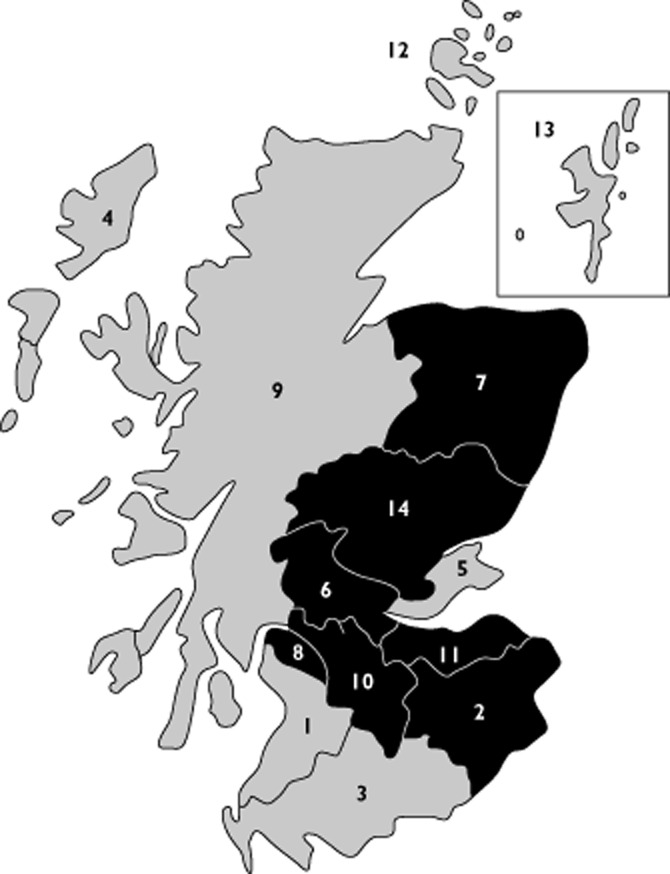
Coverage of NHS Health Boards in focus groups. Participants were recruited from areas shaded in black (2, NHS Borders; 6, NHS Forth Valley; 7, NHS Grampian; 8, NHS Greater Glasgow & Clyde; 10, NHS Lanarkshire; 11, NHS Lothian; and 14, NHS Tayside)
Table 2.
Clinical background and gender breakdown of focus group participants
| Medicine | Nursing | Pharmacy | Total | |
|---|---|---|---|---|
| Male | 4 | 1 | 4 | 9 (41%) |
| Female | 1 | 5 | 7 | 13 (59%) |
| Total | 5 (23%) | 6 (27%) | 11 (50%) | 22 (100%) |
The following four main themes were identified: (i) opinions on available data within the NHS; (ii) understanding of the proposed data linkage; (iii) beliefs about the usage of linked data; and (iv) opinions about the dissemination of feedback from linked data. Direct quotes are distinguished by quotation marks. Sources of the quotes and results are referred to below by the professional background of the focus groups, e.g. paediatricians for FG07 (numbering for each group provided in Table 1).
Opinions on available data within the NHS
A large number of potential data sources were identified for the proposed data linkage, including primary and secondary care data, such as Scottish Morbidity Records, Emergency Care Summaries, prescription data, data from diseases registries and the General Practice Research Database (GPRD):
‘There are hundreds of databases, and the potential for them to be linked has been made possible by the use of CHI …’ (FG07, paediatrician)
Three potential problems with available data were identified, i.e. data quality, data security and availability. A poor quality of available data was presumed:
‘… although [my] experience from what I can gather is that the standard of coding can be quite poor …’ (FG05, paediatric pharmacist)
Understanding of the proposed data linkage
Participants discussed data linkage at length. They perceived data linkage as the collection and combination of data from different sources:
‘I suppose I understand that to have data linkage, you have to have two electronic databases and have the ability to make them comparable.’ (FG02, GP)
Participants demonstrated diverse knowledge of ongoing data linkage in Scotland, citing NHS Information Service Division (ISD) and the Scottish Government as organizations that currently use data linkage, for example in the form of the Scottish Health Informatics Programme (SHIP). There was general support for the proposed data linkage, while acknowledging potential risks, such as anonymization, confidentiality, identifiability and use of data:
‘Well, the question is how are they [ = data sets] linked? [If] it's anonymized then obviously the linkage might throw up interesting data, but there's the potential that it's not anonymized or easily recognized […] say, it's a very rarely prescribed drug then there's a potential that patients and their underlying condition could be easily identified, which would throw up some problems with data protection really.’ (FG07, paediatrician)
‘It's actually the biggest risk to patients because the more you link, the more you risk you're going to find somebody.’ (FG02, GP)
Certain standards that would define access to data, encryption and password protection were expected (see Box 1). The NHS was preferred as facilitator of the linkage, and strictly enforced governance was seen as helpful:
‘If the clinician involved in the study breaches research governance, he loses his job and will never work in medicine again and boy is that the way you get controlled governance.’ (FG02, GP)
Participants also identified potential problems with funding of the proposed data linkage. Ownership issues were discussed widely, with several options, including the Information Service Division of the NHS and the NHS in general, being mentioned by paediatric pharmacists. Primary care nurses rejected general practitioners as data holders:
‘… they [GPs] opted out of the out of hours [service], they actually also opted out of that right to keep that record, as their record.’ (FG03, nurse)
Box 1
Excerpt from the transcript FG06 (paediatric nurses); participants are discussing prerequisites for data linkage
Excerpt of the transcript of Focus Group 06 (paediatric nurses)
A, B, C = paediatric nurses; YMH = researcher
A ‘Who has access to it?’
B ‘Do you mean like password protected and all that kind of stuff?’
YMH ‘That would be expected?’
C ‘Absolutely, yeah!’
YMH ‘It's just a shame I couldn't video your face.’
B ‘Indignant look.’
A ‘C looked shocked.’
YMH ‘Very shocked’.
B ‘As if you even have to ask!’
C ‘Exactly, did we even have to say that yeah, that to me actually goes without saying, that that would be, but. …’
Beliefs about usage of linked data
Participants were very supportive of using linked data for pharmacovigilance:
‘I think it's a good idea. […] The more we are aware of, the more information you have, the better – information is power.’ (FG03, nurses)
Data linkage was thought to be beneficial in providing a more complete picture by enabling population-based research studies and the identification of long-term trends or prescribing patterns:
‘As a professional, I can see the huge advantages of linking the data. From a professional point of view, I think it would be fantastic if it works.’ (FG05, paediatric pharmacist)
But the discussion was perceived as rather hypothetical:
‘But I suppose it, because this [ = discussion about perceived problems] is hypothetical, you could take any of those examples, and you could think there are cases when it would NOT be appropriate, and there could be cases when it would be appropriate and I think it really needs to be in context. …’ (FG03, nurses)
Participants focused more on acceptable than unacceptable uses of linked data. Paediatricians (FG07) were the only group identifying an unacceptable area, i.e. nonmedical research:
‘… basically you have to ensure that it doesn't fall into the false hands, I mean, which may include other services, like social services or tax office or the police or worse, even journalists.’ (FG07, paediatrician)
Acceptable uses included the use of linked data for benchmarking, e.g. for identification of prescribing trends or patterns (FG04), for service evaluation (FG02) and comparison of practices against national standards. Some opposing views were reported, e.g. by the three paediatricians (FG07). Ideas for the use of linked data also covered service evaluations and audits, feedback on referrals, gaining information on side-effects, ADRs and interactions, or to inform medicine use in children. Paediatric pharmacists (FG05) pointed out that any research conducted with the linked data should benefit the NHS.
Remarks on potential use of linked data by pharmaceutical companies were prompted by the facilitator but elicited only moderate reactions amongst paediatric pharmacists (FG05), who reported that companies would be notified automatically of adverse reactions anyway, and nurses (FG03), who were unsure whether or not providing pharmaceutical companies access to the data would be beneficial.
Opinions about the dissemination of feedback from linked data
Feedback on identified reactions and side-effects as well as prescribing trends and the percentage of off-license prescribing to healthcare professionals was requested:
‘Clearer information about adverse drug reactions, particularly if you are going to look at hospital admissions around drug reactions.’ (FG03, nurses)
‘… flag something up that is important …’ (FG05, paediatric pharmacist)
Participants also considered reports at an individual or regional level for information:
‘… very detailed named clinician's report [similar to Practice Team Information feedback] …’ (FG02, GP)
‘[Regionalized results as] it is an important aspect for ensuring consistency across the whole country with best treatment.’ (FG06, paediatric nurse)
Suggested modes for dissemination ranged from reports to peer-reviewed publications. Databases like NHS e-library (now called The Knowledge Network; http://www.knowledge.scot.nhs.uk/home.aspx) were considered, as well as websites similar to NHS Clinical Evidence (http://www.cks.nhs.uk/home). The need for ‘snippets of learning’ (FG02, GP) and ‘short, brief, snappy, […] relevant’ information (FG07, paediatrician) was emphasized, as was the need to gather preferences from potential data users. It was pointed out that HCPs were already inundated with an overload of information to the degree that people ‘just click OK’ (FG05, paediatric pharmacist). It was suggested that information might be better targeted specifically ‘rather than spending money sending the information to me, it needs to be sent to the people who can do something about it’ (FG05, paediatric pharmacist). Results were also expected to be passed on to official authorities, such as the Medicines and Healthcare products Regulating Agency (MHRA), and to respected sources of drug and prescribing information, e.g. the British National Formulary for children or the Scottish Intercollegiate Guidelines Network (SIGN) guidelines.
Discussion
The proposed data linkage was conditionally supported by this diverse sample of clinical stakeholders. Database and information governance were strong facilitators. It was expected that standard governance requirements, such as data security, would be addressed in advance.
Strengths and limitations
Focus groups, as a qualitative method, are limited by the fact that findings are not necessarily generalizable to a more general population. They are very well suited to explore people's opinions and views because they allow observation of the context in which points were raised and the rationale behind participants' thinking [18]. But the interest was to explore the widest range of issues that frontline HCPs might have with the proposed data linkage rather than to create a list of the most common issues.
Recruitment was difficult, despite reimbursement for locum cover, and did not lead to target participant numbers. Nonetheless, the sampling successfully included participants from different healthcare settings, clinical backgrounds and locations (as defined by Health Boards). Usually, focus groups consist of more than three participants because any smaller number may lose the ‘group’ effect and result in a collection of interviews [19]. To avoid this, the facilitator actively encouraged participants in the smaller groups (two to four participants) to comment on each other's observations and views to regain the synergistic effect attributed to focus group discussion where participants work together to reach a more detailed description of issues than when asked in a one-to-one setting. Despite small group sizes, participants engaged well in the group discussions, reflecting on other participants' statements instead of relying on the researcher to sustain the debate.
Participants appeared to struggle with the hypothetical nature of the data linkage. Inviting them to comment on assumed proposed linkage of data and resulting problems might have led to ‘artificial’ answers that were not generalizable and would not be reflected by their views should the proposed system become reality.
A strength of this study was that issues were identified at a very practical rather than a strategic level, thus demonstrating that the discussion reflected the sampling of participants, i.e. frontline HCPs, and hence allowed for wider extrapolation of the data.
Views of healthcare professionals towards linking NHS data across Scotland
Participants reflected on potential issues relating to their work. They identified prerequisites, such as appropriate governance procedures and the importance of anonymization of data. Participants did not disclose any detailed specifications of governance unless prompted, revealing an expectation that current standards of data management and legislation should be imposed and met.
Finances and liability were discussed by nurses and pharmacists, who reflected on the current financial constraints in the NHS. While acknowledging the potential benefits of the linkage, support would be withdrawn if funding the project would mean reduction of the funding available for frontline personnel.
Not all participants perceived data ownership, i.e. responsibility of the data holder, as a barrier, with one group thinking that the linked data should be seen as health service data and consequently would be held in the health service, in this case by NHS ISD. Although data ownership was discussed as a potential issue for GPs by non-GP participants, one focus group rejected a potential claim to ownership of patient data by GPs. It was thought that, whilst acknowledging that it would be necessary to include GPs in any data-sharing discussions, they had forfeited the right to claim ownership of their data when they withdrew from the provision of emergency services as part of the GP contract after 2004. The same group also shared their belief that GPs had become more familiar with data sharing since the implementation of the emergency care summary and PRISMS in Scotland.
The NHS was the preferred option in terms of holding the data. Participants took into account that the NHS, in the form of the Information Services Division, is already responsible for the single data sets that are considered for the linkage. The faith in the NHS displayed by participants might also explain why expected governance standards were not discussed in detail, because participants might be familiar with, and have trust in, the standards applied by NHS organizations.
Issues and concerns were discussed at a pragmatic level across all groups, potentially reflecting their involvement with primary data recording as part of their work routine. This became apparent when discussing potential feedback from the proposed linkage. While generally recognizing the need for feedback to educate practitioners about adverse reactions and side-effects, participants also recognized an existing information overload.
Participants were supportive of the use of linked data for pharmacovigilance if previously discussed risks and concerns had been addressed prior to the planned linkage. The only area that was perceived as unacceptable was nonmedical research, but only one group discussed this. Other groups concentrated more on acceptable areas of research, in particular audits and service evaluations. This idea seemed to be more acceptable to participants working in primary care, whereas one paediatrician felt uncomfortable with the idea of benchmarking. The differences of opinion could be due to the fact that HCPs in primary care are used to routinely collected data informing service evaluations in the form of the Quality and Outcomes Framework or data supplied by Prescribing Information System for Scotland (PRISMS). Benchmarking per se was not broadly discussed, but there was an underlying fear that the linked data could be used to identify and pursue specific prescribers.
In conclusion, issues identified in the focus groups are possible to address. Newly identified concerns related to the funding of the project, fearing a redirection of NHS funds. Identified issues were at a practical rather than a strategic level, reflecting the sample of participants and thus allowing for extrapolation. The use of the data by the pharmaceutical industry was neither opposed nor openly supported by participants. The findings presented here will inform a pan-Scotland Delphi survey and will also be compared with the findings of a parallel study exploring the views of the public on the proposed data linkage (currently ongoing), because any final recommendations for this project should ideally be drawn from both relevant populations, healthcare professionals as data collectors and the public as data providers.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: all authors had support from the Chief Scientist Office Scotland for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
This work was supported by the Chief Scientist Office (Child Medical Records for Safe Medicines (CHIMES) Applied Research Programme, grant number ARPG/07/4). We would like to thank all our participants for their time and contributions as well as the transcribers of the audio material.
References
- 1.Impicciatore P, Choonara I, Clarkson A, Provasi D, Pandolfini C, Bonati M. Incidence of adverse drug reactions in paediatric in/out-patients: a systematic review and meta-analysis of prospective studies. Br J Clin Pharmacol. 2001;52:77–83. doi: 10.1046/j.0306-5251.2001.01407.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Choonara I, Conroy S. Unlicensed and off-label drug use in children: implications for safety. Drug Saf. 2002;25:1–5. doi: 10.2165/00002018-200225010-00001. [DOI] [PubMed] [Google Scholar]
- 3.Ekins-Daukes S, Helms P, Simpson CR, Taylor MW, McLay J. Off-label prescribing to children in primary care: retrospective observational study. Eur J Clin Pharmacol. 2004;60:349–353. doi: 10.1007/s00228-004-0752-1. [DOI] [PubMed] [Google Scholar]
- 4.Stewart D, Rouf A, Snaith A, Elliot K, Helms PJ, McLay J. Attitudes and experiences of community pharmacists towards paediatric off-label prescribing: a prospective survey. Br J Clin Pharmacol. 2007;64:90–95. doi: 10.1111/j.1365-2125.2007.02865.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Turner S, Nunn A, Fielding K, Choonara I. Adverse drug reactions to unlicensed and off-label drugs on paediatric wards: a prospective study. Acta Paediatr. 1999;88:965–968. doi: 10.1080/08035259950168469. [DOI] [PubMed] [Google Scholar]
- 6.Conroy S, Choonara I, Impicciatore P, Mohn A, Arnell H, Rane A, Knoeppel C, Seyberth H, Pandolfini C, Raffaelli MP, Rocchi F, Bonati M, Jong G, de Hoog M, van den Anker J. Survey of unlicensed and off label drug use in paediatric wards in European countries. Br Med J. 2000;210:79–82. doi: 10.1136/bmj.320.7227.79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.McIntyre J, Conroy S, Avery A, Corns H, Choonara I. Unlicensed and off label prescribing of drugs in general practice. Arch Dis Child. 2000;83:498–501. doi: 10.1136/adc.83.6.498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Luo X, Cappelleri JC, Frush K. A systematic review on the application of pharmaco-epidemiology in assessing prescription drug-related adverse events in pediatrics. Curr Med Res Opin. 2007;23:1015–1024. doi: 10.1185/030079907x182211. [DOI] [PubMed] [Google Scholar]
- 9.Neonatal and Paediatric Pharmacists Group. Introduction to Paediatric Pharmaceutical Care. 1st edn. Glasgow: NHS Education for Scotland; 2005. [Google Scholar]
- 10.Blenkinsopp A, Wilkie P, Wang M, Routledge PA. Patient reporting of suspected adverse drug reactions: a review of published literature and international experience. Br J Clin Pharmacol. 2007;63:148–156. doi: 10.1111/j.1365-2125.2006.02746.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Paediatric Formulary Committee 2011. British National Formulary for Children 2011-2012. London: British Medical Association, the Royal Pharmaceutical Society of Great Britain, the Royal College of Paediatrics and Child Health, and the Neonatal and Paediatric Pharmacists Group; 2011. [Google Scholar]
- 12.Clarkson A, Choonara I. Surveillance for fatal suspected adverse drug reactions in the UK. Arch Dis Child. 2002;87:462–466. doi: 10.1136/adc.87.6.462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stewart D, Helms P, McCaig D, Bond C, McLay J. Monitoring adverse drug reactions in children using community pharmacies: a pilot study. Br J Clin Pharmacol. 2005;59:677–683. doi: 10.1111/j.1365-2125.2005.02424.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Glover GR. A comprehensive clinical database for mental health care in England. Soc Psychiatry Psychiatr Epidemiol. 2000;35:523–529. doi: 10.1007/s001270050275. [DOI] [PubMed] [Google Scholar]
- 15.Neubert A, Sturkenboom MC, Murray ML, Verhamme KM, Nicolosi A, Giaquinto C, Ceci A, Wong IC TEDDY Network of Excellence. Databases for paediatric medicine research in Europe-assessment and critical appraisal. Pharmacoepidemiol Drug Saf. 2008;17:1155–1167. doi: 10.1002/pds.1661. [DOI] [PubMed] [Google Scholar]
- 16.Hopf YM, Bond CB, Francis JJ, Haughney J, Helms PJ. Linked health data for pharmacovigilance in children: perceived legal and ethical issues for stakeholders and data guardians. BMJ Open. 2014;4:e003875. doi: 10.1136/bmjopen-2013-003875. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ritchie J, Spencer L. Qualitative data analysis for applied policy research. In: Bryman A, Burgess R, editors. Analyzing Qualitative Data. London: Routledge; 1994. pp. 173–194. [Google Scholar]
- 18.Kitzinger J. Qualitative Research: introducing focus groups. Br Med J. 1995;311:299–302. doi: 10.1136/bmj.311.7000.299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finch H, Lewis J. Focus groups. In: Ritchie J, Lewis J, editors. Qualitative Research Practice-A Guide for Social Science Students and Researchers. 1st edn. London: SAGE Publications; 2003. pp. 170–198. [Google Scholar]


