Abstract
Aim
The objective of this systematic review was to characterize the pharmacokinetics and pharmacodynamics of denosumab (XGEVA®), a fully human IgG2 monoclonal antibody which binds to receptor activator of nuclear factor kappa-B ligand (RANKL), for the treatment of skeletal-related events (SREs) in patients with advanced cancer and bone metastases.
Methods
A total of 708 patients (116 healthy patients and 592 patients with solid tumours or multiple myeloma and bone metastases) included in seven clinical studies were evaluated for denosumab pharmacokinetics. Denosumab was administered as a single subcutaneous (s.c.) dose or multiple s.c. doses, ranging from 0.1 to 3.0 mg kg−1 or 30 mg to 180 mg fixed dosing, every 1 or 3 months for up to 45 months.
Results
Consistent with the results in healthy adults, single s.c. doses of denosumab demonstrated dose-dependent, non-linear pharmacokinetics in advanced cancer patients with bone metastases across a wide dose range (0.1–3.0 mg kg−1). Reductions in levels of the bone turnover marker, uNTx/Cr, were observed within 1 day. The duration of reductions generally increased with dose and dosing frequency. In patients with solid tumours and bone metastases, pharmacokinetics and pharmacodynamic comparisons across tumour types and concomitant cancer therapies (chemotherapies and/or hormone therapies) suggest that neither tumour type nor type of concomitant therapy markedly affects denosumab pharmacokinetics or pharmacodynamics.
Conclusions
Denosumab displayed non-linear pharmacokinetics at doses below 60 mg but at higher doses, denosumab exposure increased approximately dose-proportionally in advanced cancer patients with bone metastases. Following a 120 mg, every 4 weeks dosing schedule, similar denosumab pharmacokinetics and pharmacodynamics were observed across tumour types and were independent of concomitant cancer therapies.
Keywords: advanced cancer patients, bone metastases, denosumab, pharmacodynamics, pharmacokinetics
Introduction
For patients with advanced cancers, the skeleton represents one of the most common sites of tumour metastasis 1. Bone metastases occur in over 1.5 million patients and presents a major challenge in disease management 2. Patients diagnosed with bone metastases often have poor prognosis and significant morbidity including bone-related pain, hypercalcaemia of malignancy and skeletal-related events (SREs) such as pathological fractures and spinal cord compression 3. Surgery or radiation to the bone is often required for effective treatment of bone-related pain or pathological fractures 4.
The pathophysiology of bone metastases disrupts the balanced and tightly coordinated interplay between osteoclast-mediated bone resorption and osteoblast-mediated bone formation. In advanced cancer patients with bone metastases, malignancy often leads to a dramatic increase in osteoclast activity and bone resorption, resulting in a significant loss of structural integrity and ultimately bone stability/strength 5. Receptor activator of nuclear factor kappa-B ligand (RANKL) plays a central role in the mediation of bone resorption and remodeling 6. Upon binding to RANK receptors on the surface of osteoclast precursors and mature osteoclasts, RANKL induces both differentiation and activation of osteoclasts 7. This in turn leads to increased bone resorption and metabolism, reflected by increases in the levels of the bone turnover marker, creatinine-corrected urinary N-telopeptide of type I collagen (uNTx/Cr) 8. In early experimental models involving bone metastasis, antagonists of RANKL completely prevented tumour-associated osteolysis 9, suggesting that inhibition of RANKL reduces bone resorption and has the potential to do so more effectively than other classes of antiresorptives 10.
Preclinical studies have shown that levels of bone resorption markers, particularly uNTx, can correlate with the presence and extent of bone metastases 11,12 and skeletal complications 8,13 in patients with advanced cancer or multiple myeloma. Based on data from several large, randomized phase 3 trials of zoledronic acid, results from Brown et al. suggested that patients with a baseline uNTx/Cr value of >100 nmol/mmol were more likely to experience a SRE or death during the first 3 months of intravenous (i.v.) bisphosphonate therapy. In addition, patients with on-study uNTx/Cr concentrations of >50 nmol/mmol were found to have a two-fold or greater risk of skeletal complications, disease progression, and death. Therefore, among patients with advanced cancer and bone metastases who also have elevated uNTx/Cr concentrations, the normalization of uNTx as a result of osteoclast inhibition may have clinical benefit in the reduction of SREs. As such, uNTx was utilized as a pharmacodynamic marker for suppression of bone resorption in patients with bone metastases from solid tumours.
Denosumab (XGEVA®) is a fully human monoclonal IgG2 antibody that binds with high affinity ([Kd] = 3 × 10−12 mol l−1) and specificity to RANKL 14. By inhibiting the binding of RANKL to RANK, denosumab can prevent the formation, activation and survival of osteoclasts and significantly decrease bone resorption. As a consequence, bone resorption and cancer-induced bone destruction is reduced. Recently, the results of several large phase 3 clinical studies demonstrated that denosumab treatment consistently and significantly reduced uNTx/Cr concentrations in patients with solid tumours or multiple myeloma and bone metastases, reflecting denosumab’s significant antiresorptive properties 15–17. Furthermore, the duration of maximal uNTx/Cr suppression increased with denosumab dosing, which ranged from 30 to 180 mg subcutaneous (s.c.) every 4 weeks or 60 to 180 mg s.c. every 12 weeks. Expected reversibility of uNTx/Cr suppression was observed after stopping treatment 18,19. Currently, denosumab dosing at 120 mg s.c. administered every 4 weeks is approved in the United States and other countries for the prevention of SREs in patients with bone metastases from solid tumours.
Denosumab pharmacokinetics (PK) and suppression of uNTx/Cr time course have been investigated in clinical studies of advanced cancer patients with bone metastases. Recent population PK and population PK-pharmacodynamic (PD) analyses, which included data from patients with advanced cancer, were used to determine population PK and PD parameter estimates and also investigated the effects of patient demographic covariates on the parameter estimates 20. In the population PK analysis performed by Gibiansky et al., non-linear PK of denosumab were characterized. Bioavailability following s.c. administration was determined to be 61% and the mean half-life, corresponding to linear elimination, was approximated as 25–30 days 20. Repeated monthly administration of denosumab resulted in an approximate two-fold accumulation at steady-state and time-dependent PK were not observed under the dose regimens examined. Furthermore, the effects of body weight, age, race and tumour type on denosumab PK and PD were evaluated by Gibiansky et al. 20. Body weight was identified as an important covariate on denosumab PK, but with limited clinical relevance, given the large inter-subject variability in PK and lack of difference in predicted uNTx/Cr values 21. The present review was conducted to evaluate and summarize denosumab PK and PD data across phase 1, 2, and 3 clinical studies in advanced cancer patients with bone metastases, including data in healthy patients, and to assess the potential clinical importance of reported, significant covariates (i.e. body weight and tumour type) on denosumab exposures and observed PD effects.
Methods
Clinical data
A randomized, double-blind, active-controlled, single dose phase 1 study (study 1, Table 1) was conducted in patients with breast cancer and bone metastases or multiple myeloma 22. Each patient (breast cancer, n = 26; multiple myeloma, n = 28) received both a single s.c. injection of denosumab or placebo (3:1 ratio) and an intravenous (i.v.) bisphosphonate infusion. Denosumab doses were evaluated at 0.1, 0.3, 1.0 and 3.0 mg kg−1 and intense PK and PD (uNTx/Cr) samplings were performed up to study day 85. The safety, PK, and uNTx/Cr data for this study have been previously reported 22.
Table 1.
Summary of clinical studies
| Study number | n* | Study patient population† | Dose regimen (s.c.) | Weight‡ (kg) | References |
|---|---|---|---|---|---|
| Phase 1 | |||||
| 1 | 43 | Breast cancer and multiple myeloma | 0.1, 0.3, 1, 3 mg kg−1 (single dose) | 58 (10) [35–75] | 22 |
| Phase 2 | |||||
| 2 | 194 | Breast cancer | 30, 120, or 180 mg every 4 weeks (x6) | 69 (14) [43–127] | 19 |
| 60 or 180 mg every 12 weeks (x2) | |||||
| 3 | 60 | Breast cancer, prostate cancer, other solid tumours or multiple myeloma | 180 mg every 4 weeks (x6) | 64 (12) [36–87] | 23 |
| 180 mg every 12 weeks (x2) | |||||
| Bioequivalence study | |||||
| 4 | 116 | Healthy | 120 mg every 4 weeks (single dose) | 33 (11) [18–61] | Data not published |
| Phase 3 | |||||
| 5 | 92 | Breast cancer | 120 mg every 4 weeks | 64 (15) [40–121] | 15 |
| 6 | 82 | Solid tumour or multiple myeloma (excluding breast and prostate cancer) | 120 mg every 4 weeks | 76 (16) [40–113] | 17 |
| 7 | 71 | Prostate cancer | 120 mg every 4 weeks | 87 (14) [53–135] | 16 |
Number of patients with pharmacokinetic and/or pharmacodynamic sampling.
Cancer patients with bone metastases.
Presented as mean (SD) and [range]. s.c., subcutaneous.
Study 2 was conducted as a phase 2, randomized, partially-blinded, active-controlled, multiple dose, parallel group study in patients with advanced breast cancer naïve to previous i.v. bisphosphonate treatment 19. Patients were randomized to one of six treatment groups (n = 42–43 patients per cohort), receiving denosumab doses of 30, 120 or 180 mg every 4 weeks (six doses), denosumab 60 or 180 mg every 12 weeks (two doses) or i.v. bisphosphonate every 4 weeks per package insert (open label). Randomization was stratified by the anti-neoplastic therapy, either chemotherapy (with or without hormonal therapy) or hormonal therapy alone. Patients participated in the study for 57 weeks, including a 25 week treatment period followed by three post-treatment follow-up visits at weeks 33, 45, and 57. Limited PK and PD (uNTx/Cr) samplings were performed after the first dose and up to study week 57.
Study 3 was conducted as a phase 2, randomized, open-label, active-controlled, multiple dose, parallel group study in patients with advanced cancer who had uNTx/Cr concentrations of >50 nmol bone collagen equivalents (BCE)/mmol during pre-study i.v. bisphosphonate treatment 23. Patients were randomized in a 1:1:1 ratio (n = 35–38 patients per cohort) to receive either denosumab 180 mg s.c. every 12 weeks (two doses) or 180 mg s.c. every 4 weeks (six doses) or to continue on i.v. bisphosphonate every 4 weeks for 25 weeks (study treatment period). Randomization was stratified by cancer type (breast, prostate, solid tumours [except lung] or multiple myeloma) and screening uNTx/Cr (50–100 nmol BCE/mmol or >100 nmol BCE/mmol) using an equal allocation ratio. Patients participated in the study for 57 weeks, including a 25 week treatment period. Post-treatment follow-up visits were scheduled at weeks 33, 45 and 57 and limited PK and PD (uNTx/Cr) samplings were performed after the first dose and up to study week 57.
Study 4 evaluated the single-dose PK of 120 mg s.c. denosumab in healthy women (n = 60) and men (n = 56), age = 18–61 years. Intense serum PK samplings were performed and evaluated up to study day 127 (19 weeks post-dose, results not published).
Studies 5, 6, and 7 were conducted as phase 3, randomized, double-blind, active-controlled, multiple dose studies in advanced cancer patients with bone metastases, including histologically or cytologically confirmed breast cancer (study 5) 15, solid tumours or multiple myeloma (excluding breast and prostate cancer) (study 6) 17 and prostate cancer (study 7) 16. Patients in these studies were randomized to receive either denosumab 120 mg s.c. or i.v. bisphosphonate followed by a 2 year survival period. In study 5, randomization of patients was stratified by whether patients were receiving on-study breast cancer chemotherapy (with or without hormone therapy) or hormone therapy alone at study entry. On-study breast cancer hormonal and chemotherapy used by ≥ 20% of patients in study 5 consisted of capecitabine, letrozole, anastrozole and paclitaxel. A subset of patients participated in a PK substudy and had sparse PK and PD (uNTx/Cr) sampling conducted through the end of the blinded treatment phase. The PK analyses sets comprised 92, 73 and 82 patients for studies 5, 6 and 7, respectively.
Assay methodology
Serum denosumab concentrations were determined using a validated, conventional sandwich enzyme-linked immunosorbent assay (ELISA) method at Amgen Inc. (Thousand Oaks, CA) or PPD (Richmond, VA). The principle of the assay was based on a bridging format, where denosumab would bind to an immobilized osteoprotegerin ligand (OPGL = RANKL) and horseradish peroxidase (HRP)-labelled OPGL. Procedural details of the assay have previously been developed and described by Chen et al. 24. The lower and upper limit of quantification (LLOQ and ULOQ) of the assay were 0.8 and 35 ng ml−1, respectively.
Urinary NTx concentrations were determined using a validated, ELISA method at Amgen Inc. (Thousand Oaks, CA, USA) or PPD (Richmond, VA, USA). Procedural details of the assay have previously been developed and described by Hanson et al. 25. For studies 1 to 3, a Logistics-Auto Estimate model with a weighting factor of 1 using Watson LIMS version 7.0.0.01 data reduction package was used for data regression analyses. For studies 4 to 6, a 4-Paramater Logistics with a weighting factor of 1 using PPD’s Assist LIMS version 3.08 to 5.00 data reduction package was used for data regression. The limit of quantification (LOQ) of the uNTx assay was 30 nmol l−1 BCE for study 1 and 62.5 nmol BCE for all other studies. The ULOQ was 2857 nmol l−1 BCE. Creatinine was measured photometrically on Roche Modular Analyzers, with a linear assay range of 0.00884 to 2.21 mmol l−1.
Pharmacokinetics and uNTx/Cr data analyses
Individual serum denosumab concentration–time data were analyzed by non-compartmental PK analysis methods using WinNonlin Professional v3.3 (Pharsight, Mountain View, CA, USA) for study 1 and WinNonlin v4.1e (Pharsight, Mountain View, CA, USA) for studies 2 to 4. Summary statistics for serum denosumab concentrations were calculated using SAS version 9.1 (SAS Institute Inc., Cary, NC, USA) and Excel 2003 (Microsoft Corporation, Redmond, WA, USA) for studies 4 to 6. Actual sampling times were used in the analysis and denosumab serum concentrations below the LOQ were converted to zero for the calculation of summary statistics. For purposes of analysis, uNTx values determined to be below the LOQ were assigned the value 62.5 nmol l−1. Urine NTx concentrations were corrected for urine creatinine (Cr) concentrations using the following equation: uNTx/Cr (nmol BCE/mmol) = uNTx/(Cr × 0.0884).
The maximum observed serum denosumab concentration (Cmax) and corresponding time of Cmax (tmax) after dosing was identified by inspection of the data. The area under the concentration–time curve from time zero to end of dosing interval (AUC(0,τ)) was calculated by the linear-log trapezoidal method, which applies the linear trapezoidal rule up to the first occurrence of Cmax and then the log trapezoidal rule for the remainder of the curve. The apparent clearance (CL/F) was determined by dividing the dose by AUC(0,∞). A linear regression of the log-transformed data points vs. time was used to estimate the terminal phase rate constant (λz). Cmax was not included for λz estimates. The terminal phase half-life, t1/2,z, was calculated as 0.693/λz. PK parameters for a particular patient were excluded from the analysis (treated as missing values) if the parameters could not be calculated (e.g. the number of samples within a given dosing interval was insufficient or λz could not be estimated).
Results
Single and multiple dose pharmacokinetics
Similar to results in healthy volunteers 14, single s.c. doses of denosumab demonstrated non-linear PK in advanced cancer patients with bone metastases (study 1, Figure 1) across a wide dose range (0.1–1.0 mg kg−1) but increased approximately dose-proportionally for 1.0 and 3.0 mg kg−1 (Figure 1). The slower elimination of denosumab at higher serum concentrations resulted in an approximately five-fold increase in half-life with increasing dose (0.1–3.0 mg kg−1) for both multiple myeloma and breast cancer patients. In addition, for patients with breast cancer, AUC exposure increased approximately 25-fold for a 10-fold increase in dose (0.1–1.0 mg kg−1), but approximately 4.6-fold for a three-fold increase in dose (1.0–3.0 mg kg−1). In patients with multiple myeloma, AUC exposure increased approximately 22-fold and 1.8-fold over the same dose ranges. High inter-subject variability in exposure was observed across the dose groups (percent coefficient of variation [%CV] for AUC was 31.9% to 53.9%). On average, patients with breast cancer had lower serum denosumab concentrations (∼50%) than patients with multiple myeloma at doses between 0.1 and 1.0 mg kg−1. However, at the highest dose administered (3.0 mg kg−1), serum denosumab exposures were similar between patients with breast cancer and those with multiple myeloma.
Figure 1.
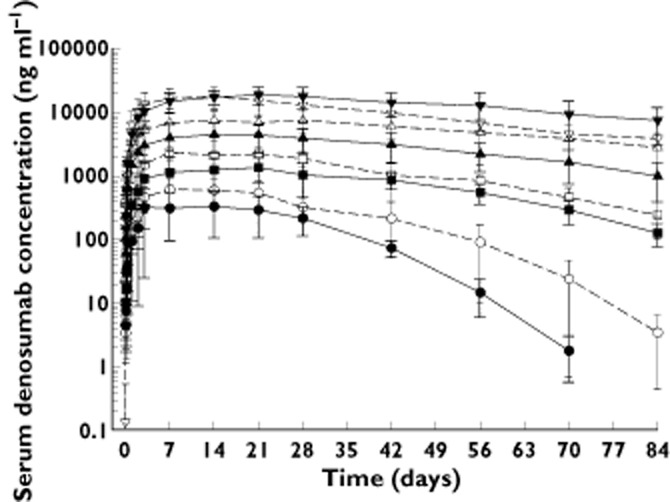
Mean (SD) denosumab serum concentrations following single subcutaneous administration to patients with multiple myeloma (MM) or breast cancer (BC).  , 0.1 mg kg−1 MM (n = 3);
, 0.1 mg kg−1 MM (n = 3);  , 0.3 mg kg−1 MM (n = 4);
, 0.3 mg kg−1 MM (n = 4);  , 1.0 mg kg−1 MM (n = 3);
, 1.0 mg kg−1 MM (n = 3);  , 3.0 mg kg−1 MM (n = 7–9);
, 3.0 mg kg−1 MM (n = 7–9);  , 0.1 mg kg−1 BC (n = 5–7);
, 0.1 mg kg−1 BC (n = 5–7);  , 0.3 mg kg−1 BC (n = 5–7);
, 0.3 mg kg−1 BC (n = 5–7);  , 1.0 mg kg−1 BC (n = 6–7);
, 1.0 mg kg−1 BC (n = 6–7);  , 3.0 mg kg−1 BC (n = 3)
, 3.0 mg kg−1 BC (n = 3)
Following multiple s.c. doses of denosumab in patients with breast cancer and bone metastases (study 2), mean Cmax and AUC values increased 5.8- to 6.5-fold for a six-fold increase in fixed dose (30–180 mg, Table 2), consistent with approximately dose-proportional increases in exposure with weight-based doses above 1.0 mg kg−1. Mean dose-normalized AUC and Cmax (nCmax) values were observed to be similar (<18% and <16% different, respectively) for doses between 30 and 180 mg, with notable overlap in interindividual variability. Thus, denosumab exposure, based on data for both Cmax and AUC, increased approximately dose-proportionally over the range of fixed doses that were assessed in patients with breast cancer and bone metastasis. In addition, an approximate two-fold accumulation was observed across all every 4 weeks dose groups after the third and fifth doses, reaching steady-state levels by 6 months. This was expected based on denosumab’s single dose PK profile and indicates that denosumab PK do not significantly change with time. At the end of the treatment period serum denosumab levels declined, with a mean half-life of approximately 29 days for the 120 mg every 4 weeks cohort (range = 25–35 days across dose cohorts).
Table 2.
Mean (± SD) denosumab PK parameters after multiple dose subcutaneous administration of 30, 120, and 180 mg Q4W
| Dose 1 | Dose 3 | Dose 5 | Dose 6 | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Dose (mg) | C1 week (ng ml−1) | AUC(0,τ) (ng ml−1 day) | C2 weeks (ng ml−1) | AUC(0,τ) (ng ml−1day) | AR1 | C2 weeks (ng ml−1) | AUC(0,τ) (ng ml−1 day) | AR2 | C2 weeks (ng ml−1) | t1/2,z (day) | |
| 30 | n | 33 | 33 | 39 | 39 | – | 39 | 39 | – | 34 | 19 |
| Mean | 3190 | 67900 | 7300 | 152000 | 2.24 | 7830 | 168000 | 2.47 | 6090 | 26.1 | |
| SD | 1390 | 28600 | 3940 | 81300 | 2.84 | 4320 | 80100 | 2.80 | 3360 | 10.1 | |
| %CV* | 43.4 | 42.1 | 54.0 | 53.4 | 1.27 | 55.2 | 47.8 | 1.14 | 55.1 | 38.8 | |
| 120 | n | 34 | 34 | 36 | 36 | – | 35 | 35 | – | 35 | 29 |
| Mean | 13500 | 287000 | 22800 | 539000 | 1.88 | 27100 | 723000 | 2.52 | 20500 | 28.8 | |
| SD | 6140 | 130000 | 9440 | 215000 | 1.66 | 14500 | 684000 | 5.26 | 13500 | 9.5 | |
| %CV* | 45.5 | 45.3 | 41.4 | 40.0 | 0.883 | 54.7 | 94.5 | 2.09 | 65.8 | 32.9 | |
| 180 | n | 36 | 36 | 39 | 39 | – | 38 | 38 | – | 33 | 28 |
| Mean | 22000 | 478000 | 46300 | 1030000 | 2.15 | 45300 | 1090000 | 2.27 | 38200 | 34.8 | |
| SD | 7300 | 154000 | 20300 | 381000 | 2.48 | 15300 | 356000 | 2.31 | 13000 | 12.4 | |
| %CV* | 33.2 | 32.1 | 43.9 | 37.1 | 1.16 | 33.8 | 32.7 | 1.02 | 34.0 | 35.6 | |
Percent coefficient of variation = (SD/Mean) × 100. SD, standard deviation; PK, pharmacokinetics; C1 week, serum concentration 1 week post-dose; C2 weeks, serum concentration 2 weeks post-dose; AUC(0,τ), area under the concentration–time curve from time zero to day 84; t1/2,z, terminal phase half-life; accumulation ratio (AR1) = (AUC(0,τ), dose 3)/(AUC(0,τ), dose 1); accumulation ratio (AR2) = (AUC(0,τ), dose 5)/(AUC(0,τ), dose 1).
After multiple 120 mg s.c. denosumab administrations during studies 5 to 7, steady-state serum trough exposures were observed to be similar (<15% difference) between months 6 and 12 (weeks 25–49) across advanced cancer patients with bone metastases (breast, prostate, other solid tumours or multiple myeloma), consistent with a lack of change in PK with time. In addition, after single dose administration of 120 mg denosumab, no notable differences in exposures based on AUC and Cmax were observed (<23% and <16% different, respectively) between healthy men and women (data not shown). This is comparable with earlier population PK/PD analyses which indicated that gender was not a significant covariate for denosumab PK or uNTx/Cr suppression in cancer patients with bone metastases 20.
Pharmacodynamic properties
Reductions in the bone resorption marker, uNTx/Cr, were observed across the weight-based or fixed doses evaluated during the phase 1 and 2 studies (studies 1 to 3), with significant decreases noted as early as 1 day after administration of denosumab in advanced cancer patients with bone metastases. While the rate and maximum extent of uNTx/Cr suppression at doses of 0.3 mg kg−1 and above were similar, the duration of maximal uNTx/Cr suppression generally increased with dose 22.
In the phase 2 trial of breast cancer patients with bone metastases who had not previously received i.v. bisphosphonate treatment (study 2), rapid and sustained suppression of uNTx/Cr concentrations were observed across the range of denosumab doses tested (30, 120, or 180 mg every 4 weeks, 60 or 180 mg every 12 weeks) 19. A similar level of suppression was noted across the dose cohorts. Data for the 120 mg denosumab every 4 weeks dose cohort (approved clinical dose for the advanced cancer indication), illustrates the relationship between the PK and PD of denosumab (Figure 2). Subcutaneous doses of denosumab 120 mg every 4 weeks resulted in a rapid and significant reduction (82%) of uNTx/Cr concentrations within 1 week. These reductions were maintained, with median reductions of 74% to 82% from weeks 2 to 25 of continued 120 mg every 4 weeks dosing (Figure 2).
Figure 2.
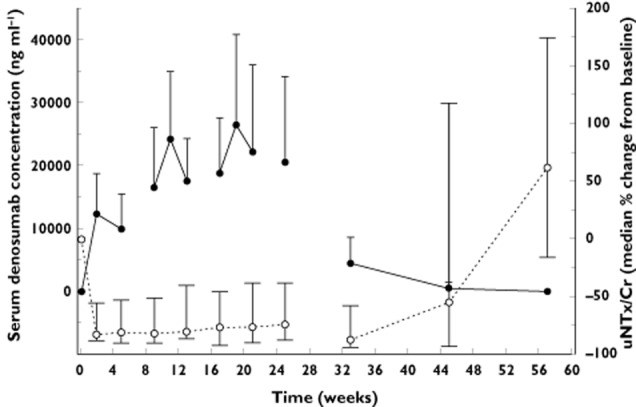
Mean (SD) denosumab serum concentrations and median percent change from baseline (Q1, Q3) in uNTX/Cr following subcutaneous administration of 120 mg denosumab every 4 weeks for 6 months in patients with breast cancer and bone metastasis (study 2). uNTx/Cr = creatine-corrected urinary N-telopeptide of type I collagen.  , denosumab (n = 38);
, denosumab (n = 38);  , uNTx/Cr (n = 39)
, uNTx/Cr (n = 39)
Similarly, in patients with bone metastases from solid tumours or multiple myeloma who were receiving i.v. bisphosphonate treatment yet had elevated uNTX/Cr concentrations (>50 nmol BCE/mmol), multiple s.c. dosing of denosumab (every 4 weeks or every 12 weeks) resulted in an approximate 80% reduction in uNTx/Cr concentrations from baseline after 3 and 6 months of treatment, respectively (study 3). Overall, 97% of patients in the denosumab groups had at least one uNTx/Cr value <50 nmol BCE/mmol up to week 25 of the study. Across studies 5 to 7, an 80% median reduction in uNTx/Cr concentrations (from baseline) was observed after 3 months of treatment in 2075 denosumab-treated cancer patients.
Pharmacokinetics and uNTx/Cr concentrations across tumour types
Comparison of denosumab exposures between advanced cancer patients with bone metastases (breast, prostate and others solid tumours; studies 5 to 7) and healthy adults (men and women; study 4) indicated that median trough serum denosumab concentrations at month 1 after a 120 mg dose differed by <34%, with extensive overlap in the 10th to 90th percentile ranges (Figure 3). In addition, median trough serum denosumab concentrations at month 3 (data not shown) and 6 (Figure 4) differed by <22%, for patients with breast cancer (studies 2 and 5), prostate cancer (study 7) and other solid tumours (study 6), with notable overlap observed in the 10th to 90th percentile ranges (Figure 4). Although serum trough exposures varied across tumour types (< 34% different) at months 1 and 6, they are not likely to translate into clinically meaningful differences given the large intersubject variability and extensive overlap across tumour types. Additionally, phase 3 clinical study results published to date in breast cancer, prostate cancer and other solid tumour types demonstrated comparable efficacy (i.e. time to first SRE; HR range: 0.82–0.84 [95% CI, 0.72, 0.98]) across these indications, which further supports the conclusion that the observed PK differences are not likely to translate into differences in clinical efficacy or outcome 15–17.
Figure 3.
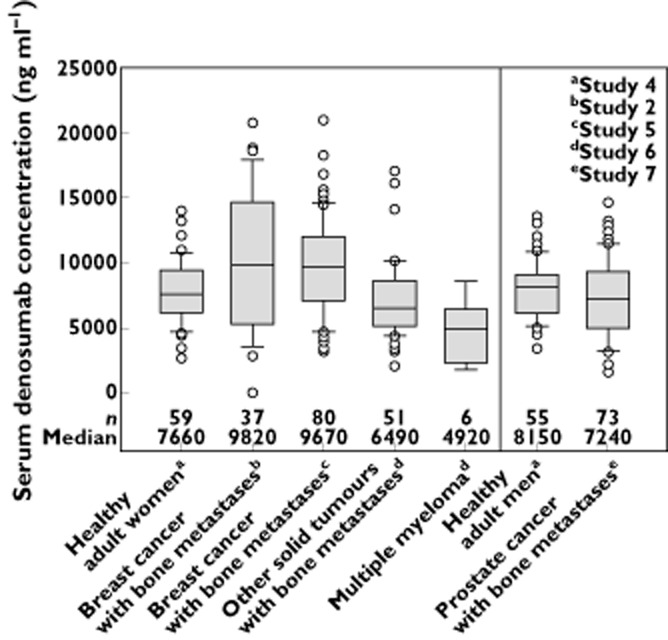
Box plots for serum denosumab concentration 1 month after subcutaneous administration of 120 mg to healthy adults and patients with cancer and bone metastases (breast, prostate, other solid tumours or multiple myeloma). Box = interquartile range; line = median; whiskers = 10th and 90th percentiles; dots = outliers
Figure 4.
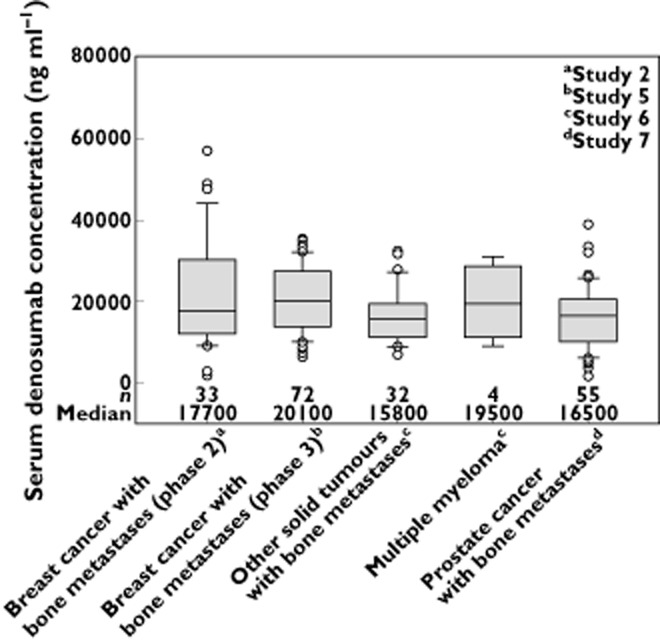
Box plots for trough serum denosumab concentrations at month 6 during subcutaneous administration of 120 mg every 4 weeks to patients with cancer and bone metastases (breast, prostate, other solid tumours or multiple myeloma). Box = interquartile range; line = median; whiskers = 10th and 90th percentiles; dots = outliers
Study 6 included patients with multiple myeloma, non-small cell lung cancer and a range of other solid tumours (breast and prostate cancer were excluded). Due to the large number of different solid tumour types coupled with the small number of patients per tumour type, comparisons of exposure between all individual tumour types in this study were not performed. Only data for patients with multiple myeloma were assessed separately. Collectively, these results indicate that, relative to healthy adults, tumour type (breast cancer, prostate cancer or other solid tumours) does not markedly affect the PK of denosumab.
The median serum denosumab trough concentrations in patients with multiple myeloma were moderately lower (by 42% to 50%) compared with either healthy patients or advanced cancer patients with other solid tumours (including breast and prostate cancer) at months 1 and 3, but were comparable at month 6 (Figures 3 and 4). However, the results should be interpreted with caution given the small number of patients with multiple myeloma (n = 4–7 patients per time point). Furthermore, these results were in contrast to study 1 (single dose), where exposures based on Cmax and AUC were similar or higher in patients with multiple myeloma vs. those with advanced breast cancer. Despite observed discrepancies in PK, the PD response (based on uNTx/Cr suppression) was similar in patients with multiple myeloma when compared with advanced cancer patients (Table 3).
Table 3.
Summary statistics for change (%) in uNTx/Cr concentrations at week 13 during administration of subcutaneous120 mg denosumab every 4 weeks
| Advanced breast cancer* | Other solid tumours (excluding breast/prostate)† | Multiple myeloma‡ | Hormone-refractory prostate cancer‡ | |
|---|---|---|---|---|
| n | 824 | 473 | 63 | 738 |
| Reduction in uNTx/Cr concentrations (median, %) | −80 | −77 | −73 | −84 |
| Q1, Q3 (%) | −89, −56 | −88, −58 | −83, −49 | −92, −66 |
| Min, Max (%) | −100, 4730 | −99, 798 | −96, 66 | −100, 1720 |
Studies 2 (N = 39; n = 38) and 5 (N = 859; n = 786).
Study 6: Other solid tumours (N = 525; n = 473) and multiple myeloma (N = 71; n = 63).
Study 7 (N = 950; n = 738). n, number of patients at time point; N, total number of patients; uNTx/Cr, creatinine-corrected urinary N-telopeptide of type I collagen.
As presented in Table 3, the median reductions in uNTx/Cr at week 13 were similar between advanced cancer patients with different tumour types, including breast (studies 2 and 5; combined due to similar exposures), hormone refractory prostate cancer (study 6) and other solid tumours or multiple myeloma (study 7). In addition, inter-subject variability (Q1, Q3) in the extent of uNTx/Cr reduction was similar across different patient populations.
Effects of concomitant drug exposure on denosumab pharmacokinetics and pharmacodynamics
Serum denosumab trough concentrations were found to be similar (17 700 vs. 20 100 ng ml−1; month 6) for breast cancer patients in studies 2 and 5, respectively. Patients were stratified by chemotherapy (with or without hormone therapy) or hormone therapy alone at study entry. Therefore, patient data from these two studies were combined to perform an analysis on the effects of concomitant administration of chemotherapy or hormone therapy on denosumab PK. Assessment of the effect of concomitant treatment with chemotherapy and/or hormone therapy vs. no concomitant treatment was not performed, as PK data were not collected for the few patients in study 5 who received neither therapy.
As shown in Figure 5, serum denosumab trough concentrations at months 1 and 3 were similar between patients receiving chemotherapy (with or without hormone therapy) and those receiving hormone therapy alone (<19% difference), with extensive overlap in inter-quartile ranges. In addition, decreases in uNTx/Cr concentrations at week 13 were similar between these groups (<8% difference; Table 4). These results suggest that for patients with advanced breast cancer, the type of concomitant cancer therapy received did not affect denosumab PK or PD.
Figure 5.
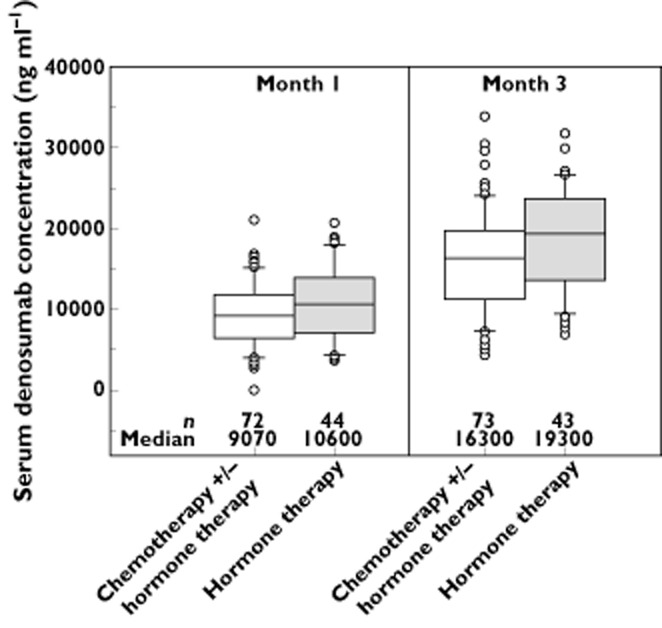
Box plots of trough serum denosumab concentrations at 1 and 3 months during subcutaneous administration of 120 mg denosumab every 4 weeks to patients with breast cancer and bone metastases receiving chemotherapy (with or without hormone therapy) vs. hormone therapy alone (studies 2 and 5). Box = interquartile range; line = median; whiskers = 10th and 90th percentiles; dots = outliers
Table 4.
Summary statistics for change (%) in uNTx/Cr concentrations at week 13 during subcutaneous administration of 120 mg denosumab every 4 weeks in patients with breast cancer and bone metastases
| Chemotherapy with or without hormone therapy (N = 82) | Hormone therapy (N = 50) | |
|---|---|---|
| n | 71 | 41 |
| Median (%) | −79.6 | −85.3 |
| Q1, Q3 (%) | −87, −66 | −89, −55 |
| Min, Max (%) | −98, 134 | −95, 133 |
n, number of patients at time point; N, total number of patients; uNTx/Cr, creatinine-corrected urinary N-telopeptide of type I collagen.
Discussion
Denosumab displayed non-linear PK at doses below 60 mg, but approximately dose-proportional increases in exposure at higher doses. After multiple s.c., 120 mg, every 4 weeks doses, a mean 2.52-fold accumulation in serum denosumab concentrations was observed and steady-state was achieved by 6 months. At steady-state, the mean (SD) serum trough concentration was 20.5 (13.5) μg ml−1, and the mean elimination terminal phase half-life (t1/2,z) was 29 days. Based on results from the population PK analysis published by Gibiansky et al. 20, the reported bioavailability of 61% for denosumab after 120 mg s.c. dosing was found to be similar to that reported for other IgG antibodies such as omalizumab (62%) and ustekinumab (57%). Additionally, denosumab clearance and volume of distribution were found to be proportional to body weight, as generally observed for other IgG antibodies 26.
The typical central volume of distribution (Vc) range for IgG antibodies has been reported to be 2–3 l, with the volume of distribution at steady-state (Vss) ranging from 2.5 to 7 l, depending on the affinity for and capacity binding to cellular antigens 27. Based on the population PK analysis, the Vc for denosumab was estimated to be 2.62 l/66 kg and Vss was estimated to be 3.96 l/66 kg, consistent with the values summarized by Roskos et al. 27. In addition, the population PK-derived median linear clearance (CLL) estimate of monoclonal Abs was 0.31 l day−1 (range 0.066–0.535) 28, while for denosumab the CLL was estimated to be 0.078 l day−1 20. Although denosumab clearance is within the range observed for other mAbs, the relatively slower clearance may be attributed to the high binding affinity of denosumab for its target, RANKL, as well as FcRn (neonatal Fc receptor for IgG) 29. Additional hypothetical explanations include more potent binding of denosumab to RANKL in its membrane-bound form in tissue vs. the soluble form (similar to other TNF ligands, and possibly related to its ability to cluster RANK), resulting in a limited availability of target in the primary distribution space. By and large, these data suggest that denosumab extravascular distribution (e.g. reticuloendothelial cells) and the subsequent elimination that occurs from these sites via catabolism, target-mediated elimination or other routes 28,30, appears to be similar to that reported for other therapeutic antibodies.
The non-linear PK attributes of denosumab observed in clinical studies at lower doses or serum concentrations may be explained by a saturable mechanism of elimination based on specific binding and complex formation of denosumab to its target ligand, RANKL. This mechanism of target-mediated elimination has been proposed for several antibodies 31. Denosumab exhibits non-saturable, linear PK at doses greater than 1.0 mg kg−1 which, as discussed above, may be explained by proteolytic catabolism via the reticuloendothelial system and endothelial cells or binding to FcRn. These proposed mechanisms of elimination are further confirmed by the observation of similar mean apparent CL/F values (0.07–0.10 ml h−1 kg−1) in clinical studies of denosumab at higher doses (1.0–3.0 mg kg−1) to that reported for other endogenous immunoglobulins (CL/F = 0.09–0.12 ml h−1 kg−1) 32.
Given that body weight has been reported to be an important demographic predictor of monoclonal antibody PK, denosumab PK parameters were evaluated by body weight. Exposure, based on AUC and Cmax, tended to be lower for heavier patients than lower weight patients following administration of denosumab 120 mg s.c. to healthy adult patients. However, due to large inter-subject variability in exposure, there was extensive overlap in exposure across the range of body weight explored. Moreover, this observed trend had no impact on the reduction of uNTx/Cr concentrations 20. Therefore, the relationship between body weight and denosumab exposure has limited clinical relevance, as the exposure attained with 120 mg every 4 weeks dosing is sufficient to suppress maximally uNTx/Cr concentrations across the wide body weight range evaluated. To investigate further the differences of denosumab weight-based dosing vs. fixed dosing, Doshi et al. 21 performed population PK/PD model-based simulations of uNTx/Cr concentration–time profiles comparing 2 mg kg−1 weight-based dosing vs. 120 mg every 4 weeks fixed-dosing. Results indicate that at week 25, predicted uNTx/Cr concentrations were similar for both the 2 mg kg−1 and 120 mg dosing regimens, with a median (Q1, Q3) concentration of 4.91 (2.26, 10.16) and 5.21 (2.31, 12.01) nmol BCE/mmol, respectively. Therefore, comparison of weight-based vs. fixed dosing of denosumab did not result in any relevant differences in denosumab PD effects as determined by uNTx/Cr suppression.
As elevated levels of bone turnover markers (i.e. uNTx/Cr) correlate with disease progression and poorer patient prognosis 8,11–13, the efficacy of denosumab on uNTx/Cr suppression was assessed during studies 2 and 3 of patients with tumours involving bone. Rapid and maximal suppression of uNTx/Cr concentrations was observed after the first dose across the range of denosumab doses tested (30, 120 or 180 mg every 4 weeks; 60 or 180 mg every 12 weeks). Although uNTx/Cr suppression was sustained throughout the entire dosing interval in the every 4 weeks cohorts, evidence of escape from suppression was observed in the every 12 weeks cohorts at week 13. In studies 5 to 7, a similar degree of uNTx/Cr reduction was also observed across the three phase 3 studies by week 13 in advanced cancer patients with bone metastases receiving 120 mg denosumab every 4 weeks, indicating sustained and maximal uNTx/Cr suppression across tumour types. On average, although the maximum level of uNTx/Cr suppression appeared similar for all dose groups in studies 2 and 3, the duration of maximum suppression was prolonged in the every 4 weeks (vs. every 12 weeks) group and in the higher dose groups (i.e. 120 and 180 mg), likely attributed to greater circulating systemic concentrations of denosumab. Given the high inter-subject variability observed in both denosumab PK and PD, the 120 mg every 4 weeks dosing regimen was selected for phase 3 clinical trials (studies 5 to 7) in order to maintain serum denosumab concentrations that provided maximal uNTx/Cr suppression in the highest number of patients throughout the entire dosing interval. A population PK/PD analysis that included the phase 3 PK and PD study data was also subsequently conducted 20, which further supported selection of the dose regimen. Results from this analysis indicated that the 120 mg every 4 weeks dose regimen resulted in a greater proportion of patients with normalized uNTx/Cr concentrations (<50 nmol BCE/mmol) (relative to 30 mg every 4 weeks) and with substantial (>90%) suppression of uNTx/Cr (relative to every 12 weeks dosing). Furthermore, every 4 weeks doses above 120 mg provided limited additional benefit with regard to the proportion of patients with >90% uNTx/Cr suppression.
The efficacy and safety for denosumab have been demonstrated in studies 5 to 7 15–17 for the 120 mg s.c. every 4 weeks denosumab dosing regimen, in addition to significant and clinically relevant reductions in the risk of SREs compared with zoledronic acid for advanced cancer patients with bone metastases from solid tumours. Importantly, this dosing regimen also led to significantly greater (P < 0.0001) suppression of uNTx/Cr concentrations when compared with zoledronic acid treatment (80% median reduction with denosumab vs. 68% median reduction with zoledronic acid at week 13) 33.
Conclusions
In this review, denosumab PK and PD data are described and summarized across phase 1, 2 and 3 clinical studies in advanced cancer patients with bone metastases, including data for healthy patients. The limited clinical importance of weight, as an identified covariate from the population PK/PD analyses, was evaluated in relation to the observed data. Similar to other monoclonal antibodies, lower denosumab dosing resulted in non-linear PK, but increased dose-proportionally in exposure for doses at and above 60 mg in advanced cancer patients with bone metastases. Clinically meaningful differences in denosumab PK or PD (uNTx/Cr suppression) were not observed across tumour types or with on-study chemotherapy and/or hormone therapies. In conclusion, given the observed efficacy and safety of denosumab in addition to the robust denosumab PK and PD data, these findings demonstrate that targeting maximal suppression of uNTx/Cr was an appropriate strategy for dose selection.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no additional support from any organization for the submitted work. WS, GJ, SH, MAS and KJ are employees of Amgen and received Amgen stocks and/or stock options in the previous 3 years. There are no other relationships or activities that could appear to have influenced the submitted work.
The authors would like to thank the patients and investigators who made important contributions across all the studies. We also thank Albert Y. Rhee (Amgen Inc.) for medical writing assistance and Nilima Vyas (CACTUS Communications) with manuscript formatting.
References
- Coleman RE. Clinical features of metastatic bone disease and risk of skeletal morbidity. Clin Cancer Res. 2006;12:6243s–6249. doi: 10.1158/1078-0432.CCR-06-0931. [DOI] [PubMed] [Google Scholar]
- Coleman RE, Plunkett TA, Rubens RD. Clinical features and prognosis of bone metastases. In: Coleman RE, editor. Textbook of Bone Metastases. Chichester: John Wiley and Sons, Ltd; 2006. pp. 65–75. [Google Scholar]
- Theriault RL. Biology of bone metastases. Cancer Control. 2012;19:92–101. doi: 10.1177/107327481201900203. [DOI] [PubMed] [Google Scholar]
- Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer treatment reviews. 2001;27:165–176. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- Kanis JA, McCloskey EV, Taube T, O’Rourke N. Rationale for the use of bisphosphonates in bone metastases. Bone. 1991;12(Suppl. 1):S13–18. doi: 10.1016/8756-3282(91)90061-m. [DOI] [PubMed] [Google Scholar]
- Tanaka S, Nakamura K, Takahasi N, Suda T. Role of RANKL in physiological and pathological bone resorption and therapeutics targeting the RANKL-RANK signaling system. Immunol Rev. 2005;208:30–49. doi: 10.1111/j.0105-2896.2005.00327.x. [DOI] [PubMed] [Google Scholar]
- Yasuda H, Shima N, Nakagawa N, Yamaguchi K, Kinosaki M, Mochizuki S, Tomoyasu A, Yano K, Goto M, Murakami A, Tsuda E, Morinaga T, Higashio K, Udagawa N, Takahashi N, Suda T. Osteoclast differentiation factor is a ligand for osteoprotegerin/osteoclastogenesis-inhibitory factor and is identical to TRANCE/RANKL. Proc Natl Acad Sci U S A. 1998;95:3597–3602. doi: 10.1073/pnas.95.7.3597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JE, Thomson CS, Ellis SP, Gutcher SA, Purohit OP, Coleman RE. Bone resorption predicts for skeletal complications in metastatic bone disease. Br J Cancer. 2003;89:2031–2037. doi: 10.1038/sj.bjc.6601437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roodman GD, Dougall WC. RANK ligand as a therapeutic target for bone metastases and multiple myeloma. Cancer Treat Rev. 2008;34:92–101. doi: 10.1016/j.ctrv.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Morony S, Warmington K, Adamu S, Asuncion F, Geng Z, Grisanti M, Tan HL, Capparelli C, Starnes C, Weimann B, Dunstan CR, Kostenuik PJ. The inhibition of RANKL causes greater suppression of bone resorption and hypercalcemia compared with bisphosphonates in two models of humoral hypercalcemia of malignancy. Endocrinology. 2005;146:3235–3243. doi: 10.1210/en.2004-1583. [DOI] [PubMed] [Google Scholar]
- Costa L, Demers LM, Gouveia-Oliveira A, Schaller J, Costa EB, de Moura MC, Lipton A. Prospective evaluation of the peptide-bound collagen type I cross-links N-telopeptide and C-telopeptide in predicting bone metastases status. J Clin Oncol. 2002;20:850–856. doi: 10.1200/JCO.2002.20.3.850. [DOI] [PubMed] [Google Scholar]
- Lipton A, Costa L, Ali SM, Demers LM. Bone markers in the management of metastatic bone disease. Cancer Treat Rev. 2001;27:181–185. doi: 10.1053/ctrv.2000.0212. [DOI] [PubMed] [Google Scholar]
- Menssen HD, Sakalova A, Fontana A, Herrmann Z, Boewer C, Facon T, Lichinitser MR, Singer CR, Euller-Ziegler L, Wetterwald M, Fiere D, Hrubisko M, Thiel E, Delmas PD. Effects of long-term intravenous ibandronate therapy on skeletal-related events, survival, and bone resorption markers in patients with advanced multiple myeloma. J Clin Oncol. 2002;20:2353–2359. doi: 10.1200/JCO.2002.02.032. [DOI] [PubMed] [Google Scholar]
- Bekker PJ, Holloway DL, Rasmussen AS, Murphy R, Martin SW, Leese PT, Holmes GB, Dunstan CR, DePaoli AM. A single-dose placebo-controlled study of AMG 162, a fully human monoclonal antibody to RANKL, in postmenopausal women. J Bone Miner Res. 2004;19:1059–1066. doi: 10.1359/JBMR.040305. [DOI] [PubMed] [Google Scholar]
- Stopeck AT, Lipton A, Body JJ, Steger GG, Tonkin K, de Boer RH, Lichinitser M, Fujiwara Y, Yardley DA, Viniegra M, Fan M, Jiang Q, Dansey R, Jun S, Braun A. Denosumab compared with zoledronic acid for the treatment of bone metastases in patients with advanced breast cancer: a randomized, double-blind study. J Clin Oncol. 2010;28:5132–5139. doi: 10.1200/JCO.2010.29.7101. [DOI] [PubMed] [Google Scholar]
- Fizazi K, Carducci M, Smith M, Damiao R, Brown J, Karsh L, Milecki P, Shore N, Rader M, Wang H, Jiang Q, Tadros S, Dansey R, Goessl C. Denosumab versus zoledronic acid for treatment of bone metastases in men with castration-resistant prostate cancer: a randomised, double-blind study. Lancet. 2011;377:813–822. doi: 10.1016/S0140-6736(10)62344-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry DH, Costa L, Goldwasser F, Hirsh V, Hungria V, Prausova J, Scagliotti GV, Sleeboom H, Spencer A, Vadhan-Raj S, von Moos R, Willenbacher W, Woll PJ, Wang J, Jiang Q, Jun S, Dansey R, Yeh H. Randomized, double-blind study of denosumab versus zoledronic acid in the treatment of bone metastases in patients with advanced cancer (excluding breast and prostate cancer) or multiple myeloma. J Clin Oncol. 2011;29:1125–1132. doi: 10.1200/JCO.2010.31.3304. [DOI] [PubMed] [Google Scholar]
- Body JJ, Facon T, Coleman RE, Lipton A, Geurs F, Fan M, Holloway D, Peterson MC, Bekker PJ. A study of the biological receptor activator of nuclear factor-kappaB ligand inhibitor, denosumab, in patients with multiple myeloma or bone metastases from breast cancer. Clin Cancer Res. 2006;12:1221–1228. doi: 10.1158/1078-0432.CCR-05-1933. [DOI] [PubMed] [Google Scholar]
- Lipton A, Steger GG, Figueroa J, Alvarado C, Solal-Celigny P, Body JJ, de Boer R, Berardi R, Gascon P, Tonkin KS, Coleman R, Paterson AH, Peterson MC, Fan M, Kinsey A, Jun S. Randomized active-controlled phase II study of denosumab efficacy and safety in patients with breast cancer-related bone metastases. J Clin Oncol. 2007;25:4431–4437. doi: 10.1200/JCO.2007.11.8604. [DOI] [PubMed] [Google Scholar]
- Gibiansky L, Sutjandra L, Doshi S, Zheng J, Sohn W, Peterson MC, Jang GR, Chow AT, Perez-Ruixo JJ. Population pharmacokinetic analysis of denosumab in patients with bone metastases from solid tumours. Clin Pharmacokinet. 2012;51:247–260. doi: 10.2165/11598090-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Doshi S, Sutjandra L, Zheng J, Sohn W, Peterson M, Jang G, Chow AT, Perez-Ruixo JJ. Denosumab dose selection for patients with bone metastases from solid tumors. Clin Cancer Res. 2012;18:2648–2657. doi: 10.1158/1078-0432.CCR-11-2944. [DOI] [PubMed] [Google Scholar]
- Body JJ, Greipp P, Coleman RE, Facon T, Geurs F, Fermand JP, Harousseau JL, Lipton A, Mariette X, Williams CD, Nakanishi A, Holloway D, Martin SW, Dunstan CR, Bekker PJ. A phase I study of AMGN-0007, a recombinant osteoprotegerin construct, in patients with multiple myeloma or breast carcinoma related bone metastases. Cancer. 2003;97:887–892. doi: 10.1002/cncr.11138. [DOI] [PubMed] [Google Scholar]
- Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, Gao G, Wu L, Sohn W, Jun S. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–1571. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- Chen D, Sarikaya NA, Gunn H, Martin SW, Young JD. ELISA methodology for detection of modified osteoprotegerin in clinical studies. Clin Chem. 2001;47:747–749. [PubMed] [Google Scholar]
- Hanson DA, Weis MA, Bollen AM, Maslan SL, Singer FR, Eyre DR. A specific immunoassay for monitoring human bone resorption: quantitation of type I collagen cross-linked N-telopeptides in urine. J Bone Miner Res. 1992;7:1251–1258. doi: 10.1002/jbmr.5650071119. [DOI] [PubMed] [Google Scholar]
- Anderson BJ, Holford NH. Mechanism-based concepts of size and maturity in pharmacokinetics. Annu Rev Pharmacol Toxicol. 2008;48:303–332. doi: 10.1146/annurev.pharmtox.48.113006.094708. [DOI] [PubMed] [Google Scholar]
- Tabrizi MA, Roskos LK. Preclinical and clinical safety of monoclonal antibodies. Drug Discov Today. 2007;12:540–547. doi: 10.1016/j.drudis.2007.05.010. [DOI] [PubMed] [Google Scholar]
- Dirks NL, Meibohm B. Population pharmacokinetics of therapeutic monoclonal antibodies. Clin Pharmacokinet. 2010;49:633–659. doi: 10.2165/11535960-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Waldmann TA. Disorders of immunoglobulin metabolism. N Engl J Med. 1969;281:1170–1177. doi: 10.1056/NEJM196911202812107. [DOI] [PubMed] [Google Scholar]
- Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–558. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- Wileman T, Harding C, Stahl P. Receptor-mediated endocytosis. Biochem J. 1985;232:1–14. doi: 10.1042/bj2320001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thurmann PA, Sonnenburg C, Valentova K, Gregora E, Freischlager F, Lissner R. Pharmacokinetics of viral antibodies after administration of intravenous immunoglobulin in patients with chronic lymphocytic leukaemia or multiple myeloma. Eur J Clin Pharmacol. 2001;57:235–241. doi: 10.1007/s002280100305. [DOI] [PubMed] [Google Scholar]
- XGEVA® (Denosumab) Package Insert. Thousand Oaks, CA: Amgen, Inc; 2012. [Google Scholar]


