Abstract
Aims
The evidence of hepatotoxicity of antithyroid drugs (ATDs) is limited to case reports or spontaneous reporting. This study aimed to quantify the incidence and comparative risks of hepatotoxicity for methimazole (MMI)/carbimazole (CBM) vs. propylthiouracil (PTU) in a population-based manner.
Methods
We conducted a cohort study of hyperthyroidism patients initially receiving MMI/CBM or PTU between 1 January 2004 and 31 December 2008 using the Taiwan National Health Insurance Research Database. The examined hepatotoxicity consisted of cholestasis, non-infectious hepatitis, acute liver failure and liver transplant, with the incidences and relative risks being quantified by Poisson exact methods and Cox proportional hazard models, respectively.
Results
The study cohort comprised 71 379 ATD initiators, with a median follow-up of 196 days. MMI/CBM vs. PTU users had a higher hepatitis incidence rate (3.17/1000 vs. 1.19/1000 person-years) but a lower incidence of acute liver failure (0.32/1000 vs. 0.68/1000 person-years). The relative risk analysis indicated that any use of MMI/CBM was associated with a 2.89-fold (95% CI 1.81, 4.60) increased hepatitis risk compared with PTU, with the risk increasing to 5.08-fold for high dose MMI/CBM (95% CI 3.15, 8.18). However, any MMI/CBM use vs. PTU was not related to an increased risk of cholestasis (adjusted hazard ratio [HR] 1.14, 95% CI 0.40, 3.72) or acute liver failure (adjusted HR 0.54, 95% CI 0.24, 1.22).
Conclusions
MMI/CBM and PTU exert dissimilar incidence rates of hepatotoxicity. Compared to PTU, MMI/CBM are associated in a dose-dependent manner with an increased risk for hepatitis while the risks are similar for acute liver failure and cholestasis.
Keywords: antithyroid drugs, hepatotoxicity, hyperthyroidism, population-based cohort study
What is Already Known about this subject —
The hepatotoxicity concerns of antithyroid drugs (ATDs), consisting of propylthiouracil (PTU), methimazole (MMI) and its prodrug carbimazole (CBM), have been raised primarily based on case reports and spontaneous reporting.
Precise estimates of incidence rates and comparative risks of hepatotoxicity by individual ATDs are accordingly lacking.
What this Study Adds —
This study quantifies the incidence rates of hepatotoxicity associated with ATDs and observes that hepatitis is the most frequently occurring hepatotoxicity in both PTU and MMI/CBM users.
Compared with PTU, MMI/CBM are associated in a dose-dependent manner with an increased risk for hepatitis while the risks are similar for acute liver failure and cholestasis.
Introduction
Antithyroid drugs (ATDs) are the mainstay treatment for hyperthyroidism and include propylthiouracil (PTU), methimazole (MMI) and its prodrug carbimazole (CBM) 1. However, concerns have been raised about hepatotoxicity by ATDs 2,3 due to numerous reported PTU-induced 4–6 and MMI/CBM-induced hepatotoxic cases 7,8. Additionally, PTU was reported to cause 23 liver transplants from 1990 to 2007 in the US and was ranked as the third most common cause of drug-induced liver failure requiring transplants 3,9. Thirty-four cases of PTU-induced severe or fatal liver injury were also identified by analyzing the US Adverse Event Reporting System (AERS) database, which outnumbered the MMI-related hepatotoxic cases by approximately seven-fold 10. Consequently, the US Food and Drug Administration (FDA) issued a boxed warning of PTU-induced severe hepatotoxicity in April 2010 10.
To date, previous studies evaluating ATD-induced hepatotoxicity were primarily limited to case reports 4–8,11–13, retrospective chart reviews 14,15 and small sample clinical studies 16,17, which only observed few hepatic adverse events. Accordingly, population-based estimates of incidences of hepatotoxicity by ATDs are lacking. An inspection of previous reports 4–6,11,12 seemed to indicate differential hepatotoxicity profiles for the individual ATDs: severe hepatocellular necrosis related to PTU vs. relatively mild cholestatic liver injury by MMI. However, several case reports claimed that MMI/CBM could cause severe liver failure or hepatitis 7,8,13. Though analyses of the AERS data revealed a higher reporting rate of severe hepatotoxicity with PTU than MMI in children and adolescents 18, few hepatotoxic events precluded a robust assessment of relative hepatotoxic safety of ATDs in adults. Accordingly, it is still uncertain as to whether PTU and MMI/CBM carry different hepatotoxicity risks in the present clinical setting. We, therefore, conducted an observational cohort study to assess the incidence for individual ATDs and to evaluate the relative risks of hepatotoxicity associated with PTU and MMI/CBM in all hyperthyroidism patients.
Methods
Data source
This was a retrospective population-based cohort study analyzing the data retrieved from the National Health Insurance Research Database (NHIRD) between 1 January 2004 and 31 December 2008 in Taiwan. The NHIRD contains comprehensive information on demographic characteristics, records of prescription drugs and medical services from inpatient, outpatient and emergency care under a universal health insurance programme enrolling up to 99% of Taiwan inhabitants by 2009 19. Additionally, the analyzed database has been widely used for studying drug safety, including drug-induced hepatotoxicity 20,21. All patients’ identifiers in NHIRD are double encrypted to ensure patient confidentiality. Therefore, this study was exempt from review by the Institutional Review Board when it was performed. The study complies with the current laws of Taiwan where it was performed. The access to and analyses of the database were approved by the National Health Research Institutes (NHRI; registered number: 99154).
Study cohort
All patients diagnosed with hyperthyroidism (International Classification of Diseases, Ninth Revision, Clinical Modifications [ICD-9-CM] codes 242.xx, 775.3) and initiating an ATD between 1 January 2005 and 30 June 2008 were eligible to be part of the study cohort. The date of the initial ATD treatment, without any ATD prescription record in the previous year, marked the cohort entry date. Patients were further excluded if they had less than 1 year continuous NHI enrolment, any record of thyroidectomy, iodine-131 therapy or thyroid cancer or any previous diagnosis of examined hepatotoxicity or underlying liver diseases (see details in Table S1) during the year preceding cohort entry.
We followed the study cohort, categorized into MMI/CBM or PTU users, from the entry date until an ATD discontinuation, combination with or switch to a different ATD, occurrence of hospitalized hepatotoxic outcomes or 31 December 2008, whichever came first. Discontinuation of ATD treatment was defined as > 90 days elapsed between the calculated end date and the subsequent start date of ATD prescriptions. We extended an additional follow-up period of 90 days after the end of the last ATD prescription for any discontinued ATD therapy to observe hepatotoxicity that may occur after discontinued therapy.
Study variables
The hepatotoxic outcomes were identified from inpatient services as any diagnosis of cholestasis (ICD-9-CM code 576.8) 22, non-infectious hepatitis (ICD-9-CM code 573.3) 20,23, acute liver failure (ICD-9-CM code 570) 20,23 and liver transplant (ICD-9-CM procedure code 50.5) 24. In order to avoid misclassification of hepatotoxicity and to increase accuracy of the employed codes, only hospitalized hepatotoxic events were analyzed in this study.
We considered several potential confounders including gender, age (< 65, ≥ 65 years) at study entry and presence of pregnancy during the 280 days prior to cohort entry. History of hyperthyroidism and comorbidities potentially related to liver injury, including thyrotoxic storm, diabetes mellitus, heart failure, chronic kidney disease and hyperlipidaemia were also measured in the year preceding cohort entry. Any diagnosis of underlying liver diseases during follow-up was additionally identified. Furthermore, we examined potentially hepatotoxic medications consisting of antimicrobial agents, non-opioid analgesics, neurological drugs, Chinese herbal medication and miscellaneous hepatotoxic drugs during the 6 months preceding cohort entry. All confounders are detailed in Table S1.
Statistical analysis
The baseline characteristics between PTU and MMI/CBM users were compared using standardized mean differences, calculated as a mean difference or proportion difference of a variable across groups divided by the pooled estimate of the standard deviations 25. This measure is employed because use of a significant test to compare characteristics between groups with very large numbers of subjects will often lead to the detection of differences that are statistically significant (P < 0.05) but not clinically important 26. Standardized mean differences of greater than 0.1 generally represent a clinically meaningful difference in a variable across groups 25. Crude incidence rates of the hepatotoxic events per 1000 person-years were calculated for the two groups and stratified by age (<18, 18–64, ≥65 years), gender, duration of continuous use (0–15, 16–30, 31–60, 61–90, 91–180, >180 days) and average daily dose of ATDs (PTU: ≤100, 100–150, >150 mg day−1; MMI/CBM: ≤10, 10–15, >15 mg day−1) by the Poisson exact method. The average daily dose was calculated based on all ATD prescriptions during the 6 months preceding the end of follow-up.
We performed mutually exclusive Cox proportional hazard analyses to estimate the crude and adjusted hazard ratios (HRs) for each hepatotoxicity outcome associated with MMI/CBM relative to PTU. All covariates listed in Table 1 were adjusted for during multivariate analyses. We validated Cox proportional hazards models by confirming that the Schoenfeld residuals were independent of time. A dose–response assessment was further performed by evaluating the comparative hepatotoxicity of low dose MMI/CBM (≤13 mg), low dose PTU (≤138 mg) and high dose MMI/CBM (>13 mg) vs. any use of PTU and high dose PTU (PTU>138 mg), respectively. The cut-off values used for dose categorizations were determined based on the median daily doses of ATD treatments. Moreover, the individual comparative hepatotoxicity of MMI and CBM vs. PTU, respectively, was also assessed.
Table 1.
Baseline and clinical characteristics of PTU and MMI/CBM users
| Characteristic | PTU (n = 24 941) | MMI/CBM (n = 46 438) | Standardized difference |
|---|---|---|---|
| Age group (years) | |||
| <65 | 22 930 (91.9%) | 42 637 (91.8%) | 0.004 |
| ≥65 | 2011 (8.1%) | 3801 (8.2%) | |
| Female gender | 20 378 (81.7%) | 35 617 (76.7%) | 0.12 |
| Pregnancy | 1105 (4.4%) | 624 (1.3%) | 0.20 |
| Medical comorbidities | |||
| History of hyperthyroidism | 2143 (8.6%) | 3867 (8.3%) | 0.01 |
| Thyrotoxic storm | 1426 (5.7%) | 2899 (6.2%) | 0.02 |
| Heart failure | 803 (3.2%) | 1346 (2.9%) | 0.02 |
| Chronic kidney diseases | 170 (0.7%) | 250 (0.5%) | 0.02 |
| Diabetes | 2014 (8.1%) | 4018 (8.7%) | 0.02 |
| Hyperlipidaemia | 1677 (6.7%) | 3425 (7.4%) | 0.03 |
| Underlying liver diseases* | |||
| In hepatitis outcome | 1364 (5.5%) | 2305 (5.0%) | 0.02 |
| In acute liver failure outcome | 1366 (5.5%) | 2316 (5.0%) | 0.02 |
| In cholestasis outcome | 1368 (5.5%) | 2318 (5.0%) | 0.02 |
| In liver transplant outcome | 1370 (5.5%) | 2319 (5.0%) | 0.02 |
| Hepatotoxic comedications | |||
| Antimicrobial agents | 1684 (6.8%) | 2838 (6.1%) | 0.03 |
| Non-opioid analgesics | 9252 (37.1%) | 17 107 (36.8%) | 0.005 |
| Neurological drugs | 1378 (5.5%) | 2461 (5.3%) | 0.01 |
| Other hepatotoxic drugs | 1920 (7.7%) | 3578 (7.7%) | <0.001 |
| Chinese herbs | 1832 (7.4%) | 3343 (7.2%) | 0.006 |
CBM, carbimazole; MMI, methimazole; PTU, propylthiouracil.
Any diagnosis of underlying liver diseases was recorded during follow-up for the outcomes of hepatitis, acute liver failures, cholestasis and liver transplant, respectively.
Sensitivity analysis
Several sensitivity analyses were conducted: (i) excluding hepatotoxic hospitalizations with any diagnosis of viral hepatitis and/or alcoholic hepatitis in the same admission, (ii) redefining a 30 day gap for continuous use of ATDs, (iii) excluding patients who were diagnosed with underlying liver diseases during follow-up, (iv) restricting patients to those without thyrotoxic storms at baseline, (v) excluding subjects with previous hyperthyroidism, (vi) conducting stratified analyses by age and gender and (vii) employing different model selections in separate Cox models, which adjusted for variables with a standardized mean difference over 0.1 (model 1), as well as controlled for covariates with univariate P values < 0.05 (model 2). Data cleaning was performed using SAS version 9.2 (SAS Institute, Cary, NC, USA) and statistical analyses were conducted using STATA version 10.1 (STATA, College Station, TX, USA).
Results
The study cohort consisted of 71 379 ATD initiators with hyperthyroidism, of whom 24 941 started on PTU and 46 438 began MMI/CBM treatment (Figure 1). The mean age of the study cohort was 41.3 years and 78.5% were female. We followed patients on PTU for a median of 173 (interquartile range 112–342) days and those treated with MMI/CBM for a median of 208 (119–390) days. Table 1 indicates that the examined characteristics were quite balanced between the two groups, except for the higher proportions of females and pregnancy in PTU users.
Figure 1.
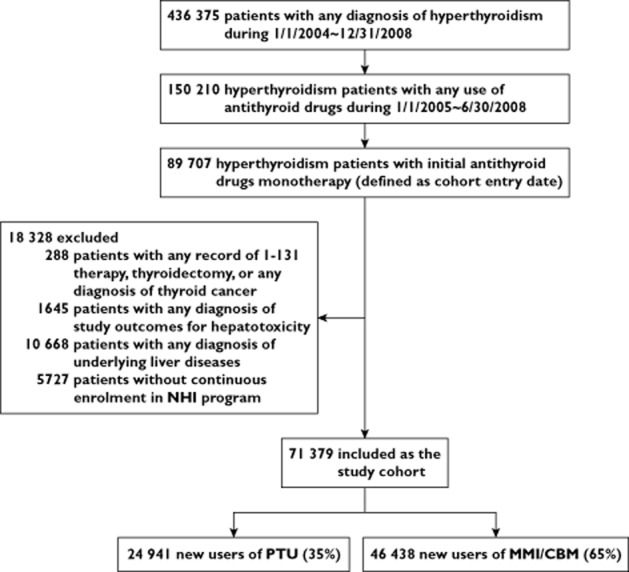
Study flow diagram. NHI, National Health Insurance; PTU, propylthiouracil; MMI, methimazole; CBM, carbimazole
Table 2 indicates that the ranking order of the hepatotoxicity incidence rates from highest to lowest was the same across PTU and MMI/CBM users. The incidence of hepatitis was the highest in both groups (PTU: 1.19/1000 person-years; MMI/CBM: 3.17/1000 person-years), followed by acute liver failure and cholestasis. Only one liver transplant case was found in the PTU users.
Table 2.
Crude incidence of hepatotoxic events associated with individual antithyroid drugs
| Events | Events/PYs | Rate/1000 PYs (95% CI) |
|---|---|---|
| PTU (n = 24 941) | ||
| Hepatitis | 21/17 683 | 1.19 (0.74, 1.82) |
| Acute liver failure | 12/17 683 | 0.68 (0.35, 1.19) |
| Cholestasis | 4/17 685 | 0.23 (0.06, 0.58) |
| Liver transplant | 1/17 688 | 0.06 (0.01, 0.32) |
| MMI/CBM (n = 46 438) | ||
| Hepatitis | 118/37 273 | 3.17 (2.62, 3.79) |
| Acute liver failure | 12/37 308 | 0.32 (0.17, 0.56) |
| Cholestasis | 9/37 307 | 0.24 (0.11, 0.46) |
| Liver transplant | 0/37 310 | NA |
CBM, carbimazole; CI, confidence interval; MMI, methimazole; NA, not applicable; PTU, propylthiouracil; PYs, person-years.
For the incidence rates stratified by age (Figure 2A), patients aged over 65 years generally had higher hepatotoxicity incidence rates than those with younger ages, with the exception of hepatitis incidence among MMI/CBM users aged <65 years old. Figure 2B indicates that the hepatotoxicity seemed to be more gender sensitive in PTU users than that in MMI/CBM users. Particularly, a 4.8-fold increased incidence rate of hepatitis was observed in females compared with males among PTU users. Additionally, the hepatotoxicity incidence rates peaked within 30 days of continuous treatment of both ATDs and tended to decline afterwards (Figure 2C). The incidence of hepatitis appeared to be the most ATD dose-sensitive, especially during MMI/CBM treatment (Figure 2D).
Figure 2.
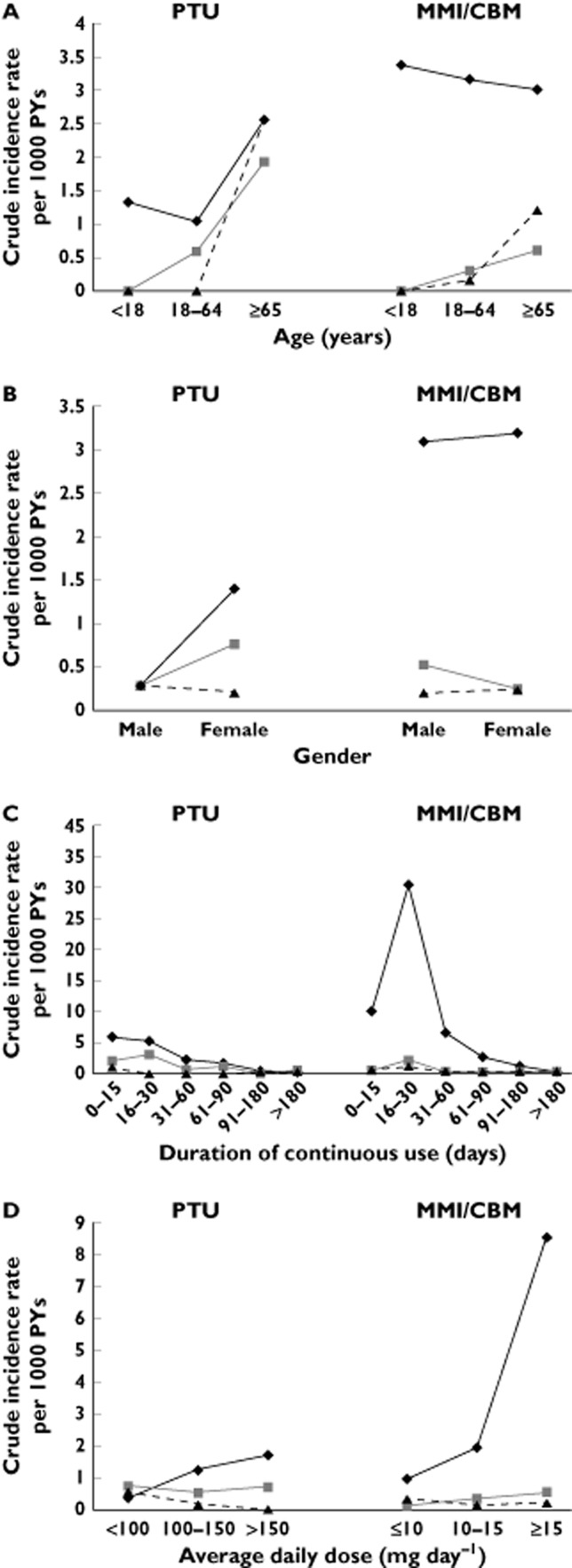
Crude incidence rates per 1000 PYs by age (A) gender (B), duration of continuous use (C) and average daily dose (D) among PTU and MMI/CBM users. PTU, propylthiouracil; MMI, methimazole; CBM, carbimazole; PYs, person-years.  , hepatitis;
, hepatitis;  , acute live failure;
, acute live failure;  , cholestasis
, cholestasis
Table 3 indicates that any MMI/CBM use was associated with a 2.89-fold increased risk of hepatitis compared with any PTU use (95% confidence interval [CI], 1.81, 4.60) after adjustment of all potential confounders. Conversely, any use of MMI/CBM vs. PTU was not related to an increased risk of acute liver failure (adjusted HR 0.54, 95% CI 0.24, 1.22) or risk of cholestasis (adjusted HR 1.14, 95% CI 0.40, 3.72). The full multivariate models analyzing risk of hepatotoxicity associated with MMI/CBM vs. PTU are shown in Table S2.
Table 3.
Relative risk of hepatotoxicity associated with MMI/CBM compared with PTU
| Number of users | Number of events | Crude HR (95% CI) | Adjusted HR (95% CI)* | |
|---|---|---|---|---|
| Hepatitis | ||||
| PTU | 24 941 | 21 | 1.00 | 1.00 |
| MMI/CBM | 46 438 | 118 | 2.91 (1.83, 4.63) | 2.89 (1.81, 4.60)† |
| Acute liver failure | ||||
| PTU | 24 941 | 12 | 1.00 | 1.00 |
| MMI/CBM | 46 438 | 12 | 0.50 (0.22, 1.10) | 0.54 (0.24, 1.22) |
| Cholestasis | ||||
| PTU | 24 941 | 4 | 1.00 | 1.00 |
| MMI/CBM | 46 438 | 9 | 1.10 (0.34, 3.59) | 1.14 (0.40, 3.72) |
CBM, carbimazole; CI, confidence interval; HR, hazard ratio; MMI, methimazole; PTU, propylthiouracil.
Adjusted for all variables listed in Table 1, with the exceptions of history of hyperthyroidism, hyperlipidaemia and use of neurological drugs for the outcome of acute liver failure as well as presence of pregnancy and chronic kidney diseases for the cholestasis outcome due to small sample size.
P < 0.05.
As shown in Figure 3A, high dose MMI/CBM was associated with a 5.08-fold (95% CI 3.15, 8.18) increased risk of hepatitis compared with any use of PTU, whereas the risk was absent for use of low dose MMI/CBM (adjusted HR 1.15, 95% CI 0.64, 2.04). Notably, even compared with high dose PTU, high dose MMI/CBM was consistently associated with an increased hepatitis risk (adjusted HR 3.55, 95% CI 2.05, 6.13) (Figure 3B).
Figure 3.
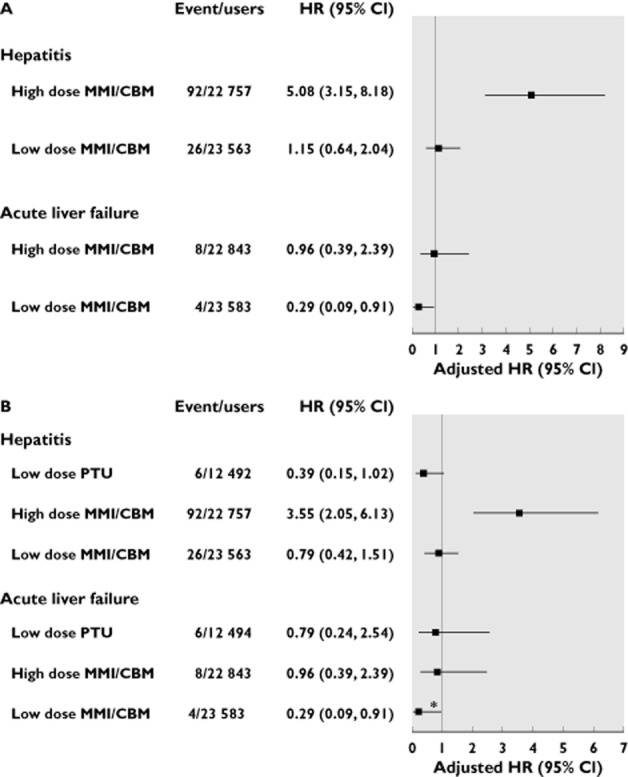
Risk of hepatotoxicity associated with use of high or low dose MMI/CBM compared with any use of PTU (A) and high dose PTU (B). High dose MMI/CBM: MMI equivalent dose >13 mg day−1; low dose MMI/CBM: MMI equivalent dose ≤13 mg day−1. The cholestasis outcome was not analyzed due to the small sample size. *P < 0.05. PTU, propylthiouracil; MMI, methimazole; CBM, carbimazole; HR, hazard ratio; CI, confidence interval
Table 4 presents the relative risks of hepatotoxicity of individual MMI or CBM vs. PTU. Compared with PTU, MMI still incurred an increased risk of hepatitis (adjusted HR 3.54, 95% CI 2.21, 5.65). Nevertheless, CBM was not associated with the hepatitis risk (adjusted HR 1.04, 95% CI 0.50, 2.16).
Table 4.
Relative risk of hepatotoxicity associated with individual MMI or CBM compared with PTU
| Events/PTU users | Events/MMI users† | Events/CBM users† | MMI vs. PTU | CBM vs. PTU | |
|---|---|---|---|---|---|
| Adjusted HR (95% CI)* | Adjusted HR (95% CI)* | ||||
| Hepatitis | 21/24 941 | 107/34 361 | 11/12 077 | 3.54 (2.21, 5.65)‡ | 1.04 (0.50, 2.16) |
| Acute liver failure | 12/24 941 | 9/34 361 | 3/12 077 | 0.55 (0.23, 1.32) | 0.53 (0.15, 1.88) |
| Cholestasis | 4/24 941 | 6/34 361 | 3/12 077 | 1.04 (0.29, 3.72) | 1.41 (0.31, 6.33) |
CBM, carbimazole; CI, confidence interval; HR, hazard ratio; MMI, methimazole; PTU, propylthiouracil.
Adjusted for all variables listed in Table 1, with the exceptions of history of hyperthyroidism, hyperlipidaemia and use of neurological drugs for the outcome of acute liver failure as well as presence of pregnancy and chronic kidney diseases for the cholestasis outcome due to small sample size.
The censorship of switch between use of MMI and CBM was considered during analyses of hepatotoxicity risk from individual MMI or CBM.
P < 0.05.
The main findings remained robust during the majority of the sensitivity analyses (Figure 4). Notably, the risk of acute liver failure was significantly reduced (adjusted HR 0.34, 95% CI 0.12, 0.98, P = 0.045) in MMI/CBM vs. PTU users by excluding patients with underlying liver diseases during follow-up.
Figure 4.
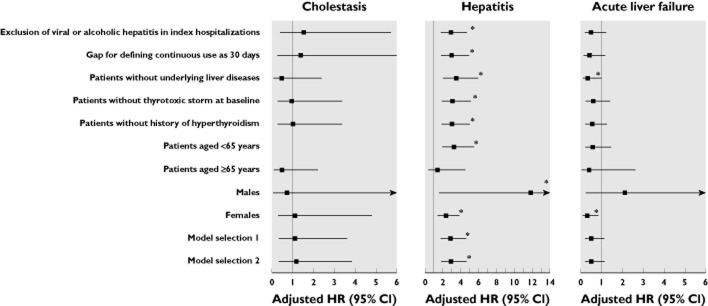
Risk of hepatotoxicity associated with MMI/CBM as compared with PTU in the sensitivity analyses. *P < 0.05. HR, hazard ratio; CI, confidence interval
Discussion
Analyzing the administrative health records of 23 million Taiwan inhabitants, we revealed the similarities and dissimilarities of hepatotoxicity profiles of ATDs. The incidence rates of the examined hepatotoxicity peaked in the first 30 days of treatment and ranked consistently for both PTU and MMI/CBM, with hepatitis being the most frequently occurring outcome. Nevertheless, MMI/CBM and PTU exert dissimilar hepatotoxicity incidence profiles. MMI/CBM users had a higher hepatitis incidence but a lower incidence rate of acute liver failure than PTU users. The comparative risks of ATDs varied by types of hepatotoxicity. MMI/CBM vs. PTU was associated with a dose-dependent increased hepatitis risk but was not related to the risk of cholestasis or acute liver failure. Our findings contribute significantly to the current literature limited to scattered case reports and spontaneous reporting.
Our estimated incidence rate of PTU-related hepatitis (equal to 0.08%) is smaller than what has been estimated in Asian populations, ranging from 1.0 to 1.2% 14,16. The inconsistent findings might result from restriction of hepatotoxicity to hospitalized hepatotoxic events in our study or inclusion of any liver injury case with transaminase elevation in previous studies 14,16. MMI/CBM was surprisingly observed to induce more hepatitis events than cholestasis, the reported major type of MMI-associated hepatotoxicity 11,12. Prolonged cholestasis may progress to further development of hepatocellular insufficiency 27, but the extent to which delayed treatment of cholestasis led to our observed hepatitis by MMI/CBM is unclear. Additionally, our finding on the incidence rate of ATD-related hepatotoxicity peaking in the first 30 days of treatment extends the previous findings 4,11.
Our observed hepatotoxicity profiles were dissimilar to those reported by Rivkees & Szarfman 18. They reported MMI incurred a higher rate (0.09% vs. 0.04%) of composite mild liver injury (including increased hepatic enzymes, jaundice and cholestasis) but a lower rate (0.05% vs. 0.17%) of composite severe liver injury (including hepatitis, acute liver failure and liver transplant) than PTU with analyses of the AERS database 18. These discrepant findings might result from identification of composite hepatotoxic outcomes or over-reporting of healthcare professionals’ perception of PTU-induced hepatitis and MMI-induced cholestasis in the AERS database.
Additionally, two randomized controlled trials conducted in Japan reported a much higher hepatotoxicity rate with PTU than MMI use. Otsuka et al. 28 reported a higher proportion of hepatotoxic events in patients receiving PTU 300 mg day−1 than in those treated with MMI 30 mg day−1 or 15 mg day−1 (PTU: 25.8% vs. MMI: 8.7 and 9.0%) among 449 patients with Grave’s disease. Inclusion of asymptomatic hepatotoxicity and existence of imbalanced baseline characteristics, such as gender, might contribute to their discrepant findings from ours. Notably, a 15% higher proportion of females in the PTU group than in MMI group (15 mg day−1) probably contributed to the conflicting results by Otsuka et al. 28, given that there is an approximately five-fold increased hepatitis rate in females than in males among PTU users (Figure 2B). These abovementioned reasons could also contribute to the discrepant data reported by Nakamura et al. due to the overlap between the two trials 28,29.
ATD-induced hepatotoxicity might be affected by different dose levels. We observed a dose sensitive incidence rate of hepatitis associated with ATDs, especially for MMI/CBM. Additionally, only high dose MMI/CBM was observed to increase the risk of hepatitis even compared with high dose PTU. These data indicate a dose-dependent hepatitis by MMI/CBM. Furthermore, our findings of MMI and its prodrug CBM differing in their relative hepatitis risk might result from dose effects. MMI and CBM are commonly used at the same doses in the clinical setting. However, a 65% serum MMI concentrations only is reached via delivering the same dose of CBM 30, probably causing the observed null association between CBM use and hepatitis risk. On the other hand, Werner et al. 31 reported that high dose PTU (728 ± 216 mg day−1) but not low dose PTU could incur a higher risk of hepatotoxicity than MMI prescribed either at low doses (23 ± 11 mg day−1) or high doses (60 ± 20 mg day−1). Notably, their observed daily doses of ATDs were much higher than our observed doses (median daily dose of PTU = 138 mg day−1; MMI = 13 mg day−1), which fall in the range of the suggested maintenance daily doses of ATDs 1. Accordingly, the dose findings of Werner et al. 31 might not be generalizable to the present clinical setting.
After excluding patients who were diagnosed with underlying liver diseases during follow-up, PTU was found to increase statistically the acute liver failure risk as compared with MMI/CBM in our study, which is consistent with reports of severe hapatotoxicity related to PTU 3–6,32. Collectively, there are more than 30 case reports of PTU-related severe liver failure 4–6,32 and accumulating death reports from PTU use 32. Our data also provide additional evidence supporting severe hepatotoxicity resulting from PTU use.
Our results are probably generalizable to other ethnic groups based on the following reasons. First, the characteristics of our study cohort, such as the female: male ratio and age distribution are similar to patients with hyperthyroidism observed in other countries 33. Second, case reports of ATD-related hepatotoxicity were observed in a diversity of populations 6,7,13 rather than limited to a Taiwanese population 34,35. Third, the examined hepatotoxicity was restricted to hospitalized events, which represent severe hepatotoxicity that is less prone to coding misclassification. Fourth, MMI was observed as the most frequently prescribed ATD, consistent with the ATD prescribing pattern in Western countries 36.
Our study has several unique attributes. This is the first population-based cohort study to quantify the incidence of hepatotoxicity and delineate hepatotoxicity profiles by ATDs. Additionally, the confounding of hepatic comorbidities was minimized by exclusion of patients with underlying liver diseases. Furthermore, we analyzed the data before the warning of PTU-related hepatotoxicity by the FDA, avoiding the effect of the changed ATD prescribing on our findings. Moreover, our study was not threatened by immortal time bias 37 because we followed up all MMI/CBM or PTU users right after the first prescription, without a wait period during which ATD use was defined, and the hepatotoxicity consequently could not occur. Finally, the observed dose−response relationship further enhances the causal inference of hepatotoxic risks in relation to ATD use.
Several limitations merit emphasis. First, although the employed ICD-9-CM codes have been widely used to evaluate drug-induced liver injury 20,24, error in hepatotoxicity ascertainment in the NHIRD is possible due to lack of rigorous performance evaluation of the codes in the Taiwanese population. To reduce coding error, we restricted the hepatotoxicity outcomes to the hospitalized events because each diagnosis coding made in inpatient settings is based on results of laboratory data and clinical findings, and is under strict regulation from the National Health Insurance Administration in Taiwan. We also explored a more complex operational definition of hepatotoxicity, in which hepatotoxic cases were defined as those who discontinued an ATD therapy or switched to a different ATD within 7 days after hospital admission for hepatotoxicity. Approximately 90% of the originally identified hepatotoxic cases met this definition and restriction of hepatotoxic events to these cases led to similar findings (details in Table S3). These data seem to support the validity of our identified hepatotoxic cases associated with ATDs. Second, our findings might be biased from other possible causes of hepatotoxicity such as alcoholic hepatitis and hepatitis B virus (HBV) infection 38,39. We did exclude patients with alcoholic hepatitis or viral hepatitis measured both at the baseline and during the follow-up period. We further examined the extent to which alcoholic hepatitis or HBV infection contributed to the observed hepatotoxicity. Only one and three patients were observed to have such diseases for cholestasis and acute liver failure outcome, respectively. Exclusion of these patients from the analyses led to the consistent findings. On the other hand, people with HBV infection may not know about their HBV status, and these patients would not be identified from the database. However, the undiagnosed HBV is not likely to be disproportionately distributed between PTU and MMI/CBM users, potentially causing an underestimate of the relative risk of hepatotoxicity from ATD use. Third, confounding by indication bias might pose another threat because patients treated with MMI/CBM could have milder hyperthyroidism 32 and experience better outcomes than those treated with PTU. In this study, we adopted a new user design and analyzed over 90% of patients first diagnosed with hyperthyroidism as well as obtaining robust results while excluding patients with previous thyrotoxic storms. Additionally, the well-balanced characteristics between MMI/CBM and PTU users in Table 1 further precluded the possibility of such bias. Fourth, we excluded patients with underlying liver diseases during the follow-up period in the sensitivity analysis, which might be biased due to violation of the intention-to-treat principle. Nevertheless, a similar proportion of patients were excluded from both ATD treatments (5.5% in PTU and 5.0% in MMI/CBM users), and therefore the potential bias was minimized. Fifth, misclassification of MMI/CBM vs. PTU users could occur. However, it is believed to be non-differential and might bias our results towards to the null.
In conclusion, PTU and MMI/CBM exert different comparative hepatotoxicity risks. Our findings suggest that healthcare professionals should not only be vigilant for any symptom related to acute liver failure during PTU treatment, but also be cautious in MMI/CBM-induced hepatitis, especially for those receiving a higher dose of MMI/CBM. Notably, given that PTU is not currently recommended as the first choice of ATD treatment and an increasing population receiving MMI is expected, our observed increased risk of hepatitis by MMI/CBM warrants caution.
Authorship
All authors had access to the data and played a role in writing this manuscript.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare no support from any other organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years and no other relationships or activities that could appear to have influenced the submitted work.
This study was supported by grant NSC100-2320-B016-002 from the National Science Council (NSC) in Taiwan. We appreciate the National Health Insurance Administration (NHIA) and NHRI for providing the database. The interpretation and conclusions contained herein do not represent those of the NSC, NHIA or NHRI.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher’s web-site:
Diagnosis codes of diseases or procedures in the exclusion criteria, medical comorbidities and individual drugs of comedications in potential confounders
Full results from multivariate Cox proportional hazard analyses of hepatotoxicity associated with MMI/CBM compared with PTU
Adjusted hazard ratios for risk of hepatotoxicity associated with MMI/CBM vs. PTU using an operational definition of hepatotoxicity as hepatotoxicity cases with discontinued ATD therapy or switch to another ATD within 7 days after hospital admission for hepatotoxic events
References
- Bahn RS, Burch HB, Cooper DS, Garber JR, Greenlee MC, Klein I, Laurberg P, McDougall IR, Montori VM, Rivkees SA, Ross DS, Sosa JA, Stan MN. Hyperthyroidism and other causes of thyrotoxicosis: management guidelines of the American Thyroid Association and American Association of Clinical Endocrinologists. Thyroid. 2011;21:593–646. doi: 10.1089/thy.2010.0417. [DOI] [PubMed] [Google Scholar]
- Bahn RS, Burch HS, Cooper DS, Garber JR, Greenlee CM, Klein IL, Laurberg P, McDougall IR, Rivkees SA, Ross D, Sosa JA, Stan MN. The role of propylthiouracil in the management of Graves’ disease in adults: report of a meeting jointly sponsored by the American Thyroid Association and the Food and Drug Administration. Thyroid. 2009;19:673–674. doi: 10.1089/thy.2009.0169. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Mattison DR. Propylthiouracil (PTU) hepatoxicity in children and recommendations for discontinuation of use. Int J Pediatr Endocrinol. 2009;2009:132041. doi: 10.1155/2009/132041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrion AF, Czul F, Arosemena LR, Selvaggi G, Garcia MT, Tekin A, Tzakis AG, Martin P, Ghanta RK. Propylthiouracil-induced acute liver failure: role of liver transplantation. Int J Endocrinol. 2010;2010:910636. doi: 10.1155/2010/910636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Testa G, Trevino J, Bogetti D, Layden T, Wiley T, Sankary H, Benedetti E. Liver transplantation for propylthiouracil-induced acute hepatic failure. Dig Dis Sci. 2003;48:190–191. doi: 10.1023/a:1021767322245. [DOI] [PubMed] [Google Scholar]
- Ruiz JK, Rossi GV, Vallejos HA, Brenet RW, Lopez IB, Escribano AA. Fulminant hepatic failure associated with propylthiouracil. Ann Pharmacother. 2003;37:224–228. doi: 10.1177/106002800303700213. [DOI] [PubMed] [Google Scholar]
- Kang H, Choi JD, Jung IG, Kim DW, Kim TB, Shin HK, Kim BT, Park CK, Yoo JY. A case of methimazole-induced acute hepatic failure in a patient with chronic hepatitis B carrier. Korean J Intern Med. 1990;5:69–73. doi: 10.3904/kjim.1990.5.1.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vilchez FJ, Torres I, Garcia-Valero A, Lopez-Tinoco C, de Los Santos A, Aguilar-Diosdado M. Concomitant agranulocytosis and hepatotoxicity after treatment with carbimazole. Ann Pharmacother. 2006;40:2059–2063. doi: 10.1345/aph.1G720. [DOI] [PubMed] [Google Scholar]
- Russo MW, Galanko JA, Shrestha R, Fried MW, Watkins P. Liver transplantation for acute liver failure from drug induced liver injury in the United States. Liver Transpl. 2004;10:1018–1023. doi: 10.1002/lt.20204. [DOI] [PubMed] [Google Scholar]
- US Food and Drug Administration MedWatch. 2010. FDA Drug Safety Communication: New Boxed Warning on severe liver injury with propylthiouracil Available at http://www.fda.gov/Drugs/DrugSafety/PostmarketDrugSafetyInformationforPatientsandProviders/ucm209023.htm (last accessed 18 September 2013)
- Mikhail NE. Methimazole-induced cholestatic jaundice. South Med J. 2004;97:178–182. doi: 10.1097/01.SMJ.0000054690.98272.B1. [DOI] [PubMed] [Google Scholar]
- Woeber KA. Methimazole-induced hepatotoxicity. Endocr Pract. 2002;8:222–224. doi: 10.4158/EP.8.3.222. [DOI] [PubMed] [Google Scholar]
- Baker B, Shapiro B, Fig LM, Woodbury D, Sisson JC, Beierwaltes WH. Unusual complications of antithyroid drug therapy: four case reports and review of literature. Thyroidology. 1989;1:17–26. [PubMed] [Google Scholar]
- Kim HJ, Kim BH, Han YS, Yang I, Kim KJ, Dong SH, Chang YW, Lee JI, Chang R. The incidence and clinical characteristics of symptomatic propylthiouracil-induced hepatic injury in patients with hyperthyroidism: a single-center retrospective study. Am J Gastroenterol. 2001;96:165–169. doi: 10.1111/j.1572-0241.2001.03469.x. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Stephenson K, Dinauer C. Adverse events associated with methimazole therapy of graves’ disease in children. Int J Pediatr Endocrinol. 2010;2010:176970. doi: 10.1155/2010/176970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang MJ, Li KL, Wei JS, Wu SS, Fan KD, Liaw YF. Sequential liver and bone biochemical changes in hyperthyroidism: prospective controlled follow-up study. Am J Gastroenterol. 1994;89:1071–1076. [PubMed] [Google Scholar]
- Liaw YF, Huang MJ, Fan KD, Li KL, Wu SS, Chen TJ. Hepatic injury during propylthiouracil therapy in patients with hyperthyroidism. A cohort study. Ann Intern Med. 1993;118:424–428. doi: 10.7326/0003-4819-118-6-199303150-00005. [DOI] [PubMed] [Google Scholar]
- Rivkees SA, Szarfman A. Dissimilar hepatotoxicity profiles of propylthiouracil and methimazole in children. J Clin Endocrinol Metab. 2010;95:3260–3267. doi: 10.1210/jc.2009-2546. [DOI] [PubMed] [Google Scholar]
- Chen YC, Yeh HY, Wu JC, Haschler I, Chen TJ, Wetter T. Taiwan’s National Health Insurance Research Database: administrative health care database as study object in bibliometrics. Scientometrics. 2011;86:365–380. [Google Scholar]
- Lee CH, Wang JD, Chen PC. Increased risk of hospitalization for acute hepatitis in patients with previous exposure to NSAIDs. Pharmacoepidemiol Drug Saf. 2010;19:708–714. doi: 10.1002/pds.1966. [DOI] [PubMed] [Google Scholar]
- Chang CH, Lin JW, Wu LC, Lai MS, Chuang LM, Chan KA. Association of thiazolidinediones with liver cancer and colorectal cancer in type 2 diabetes mellitus. Hepatology. 2012;55:1462–1472. doi: 10.1002/hep.25509. [DOI] [PubMed] [Google Scholar]
- Shaib YH, El-Serag HB, Davila JA, Morgan R, McGlynn KA. Risk factors of intrahepatic cholangiocarcinoma in the United States: a case-control study. Gastroenterology. 2005;128:620–626. doi: 10.1053/j.gastro.2004.12.048. [DOI] [PubMed] [Google Scholar]
- Enger C, Gately R, Ming EE, Niemcryk SJ, Williams L, McAfee AT. Pharmacoepidemiology safety study of fibrate and statin concomitant therapy. Am J Cardiol. 2010;106:1594–1601. doi: 10.1016/j.amjcard.2010.07.041. [DOI] [PubMed] [Google Scholar]
- Chan KA, Truman A, Gurwitz JH, Hurley JS, Martinson B, Platt R, Everhart JE, Moseley RH, Terrault N, Ackerson L, Selby JV. A cohort study of the incidence of serious acute liver injury in diabetic patients treated with hypoglycemic agents. Arch Intern Med. 2003;163:728–734. doi: 10.1001/archinte.163.6.728. [DOI] [PubMed] [Google Scholar]
- Austin PC. Using the standardized difference to compare the prevalence of a binary variable between two groups in observational research. Commun Stat Simul Comput. 2009;38:1228–1234. [Google Scholar]
- Mamdani M, Sykora K, Li P, Normand SL, Streiner DL, Austin PC, Rochon PA, Anderson GM. Reader’s guide to critical appraisal of cohort studies: 2. Assessing potential for confounding. BMJ. 2005;330:960–962. doi: 10.1136/bmj.330.7497.960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papatheodoridis G. Current progress in our understanding of cholestasis and chronic cholestatic disorders. Ann Gastroenterol. 2001;14:281–287. [Google Scholar]
- Otsuka F, Noh JY, Chino T, Shimizu T, Mukasa K, Ito K, Taniyama M. Hepatotoxicity and cutaneous reactions after antithyroid drug administration. Clin Endocrinol. 2012;77:310–315. doi: 10.1111/j.1365-2265.2012.04365.x. [DOI] [PubMed] [Google Scholar]
- Nakamura H, Noh JY, Itoh K, Fukata S, Miyauchi A, Hamada N. Comparison of methimazole and propylthiouracil in patients with hyperthyroidism caused by Graves’ disease. J Clin Endocrinol Metab. 2007;92:2157–2162. doi: 10.1210/jc.2006-2135. [DOI] [PubMed] [Google Scholar]
- Kampmann JP, Hansen JM. Clinical pharmacokinetics of antithyroid drugs. Clin Pharmacokinet. 1981;6:401–428. doi: 10.2165/00003088-198106060-00001. [DOI] [PubMed] [Google Scholar]
- Werner MC, Romaldini JH, Bromberg N, Werner RS, Farah CS. Adverse effects related to thionamide drugs and their dose regimen. Am J Med Sci. 1989;297:216–219. doi: 10.1097/00000441-198904000-00003. [DOI] [PubMed] [Google Scholar]
- Cooper DS, Rivkees SA. Putting propylthiouracil in perspective. J Clin Endocrinol Metab. 2009;94:1881–1882. doi: 10.1210/jc.2009-0850. [DOI] [PubMed] [Google Scholar]
- Abraham-Nordling M, Bystrom K, Torring O, Lantz M, Berg G, Calissendorff J, Nystrom HF, Jansson S, Jorneskog G, Karlsson FA, Nystrom E, Ohrling H, Orn T, Hallengren B, Wallin G. Incidence of hyperthyroidism in Sweden. Eur J Endocrinol. 2011;165:899–905. doi: 10.1530/EJE-11-0548. [DOI] [PubMed] [Google Scholar]
- Chen WC, Zhub ZX, Wang CH, Chien MN. Cholestasis and acute cholecystitis in hyperthyroidism treated with methimazole. Int J Gerontol. 2009;3:248–250. [Google Scholar]
- Wang MC, Ko WS, Chen CY, Luan TS, Ho CH. Propylthiouracil-induced toxic hepatitis – report of one case. J Intern Med Taiwan. 2008;19:266–269. [Google Scholar]
- Emiliano AB, Governale L, Parks M, Cooper DS. Shifts in propylthiouracil and methimazole prescribing practices: antithyroid drug use in the United States from 1991 to 2008. J Clin Endocrinol Metab. 2010;95:2227–2233. doi: 10.1210/jc.2009-2752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suissa S. Immortal time bias in pharmaco-epidemiology. Am J Epidemiol. 2008;167:492–499. doi: 10.1093/aje/kwm324. [DOI] [PubMed] [Google Scholar]
- den Brinker M, Wit FW, Wertheim-van Dillen PM, Jurriaans S, Weel J, van Leeuwen R, Pakker NG, Reiss P, Danner SA, Weverling GJ, Lange JM. Hepatitis B and C virus co-infection and the risk for hepatotoxicity of highly active antiretroviral therapy in HIV-1 infection. AIDS. 2000;14:2895–2902. doi: 10.1097/00002030-200012220-00011. [DOI] [PubMed] [Google Scholar]
- Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. 2009;360:2758–2769. doi: 10.1056/NEJMra0805786. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Diagnosis codes of diseases or procedures in the exclusion criteria, medical comorbidities and individual drugs of comedications in potential confounders
Full results from multivariate Cox proportional hazard analyses of hepatotoxicity associated with MMI/CBM compared with PTU
Adjusted hazard ratios for risk of hepatotoxicity associated with MMI/CBM vs. PTU using an operational definition of hepatotoxicity as hepatotoxicity cases with discontinued ATD therapy or switch to another ATD within 7 days after hospital admission for hepatotoxic events


