Abstract
Aims
In patients with atrial fibrillation prescribed dabigatran, the aim was to examine the correlation between plasma dabigatran concentrations and the three screening coagulation assays [international normalized ratio (INR), activated partial thromboplastin time (aPTT) and thrombin time (TT)] as well as the dilute thrombin time (dTT) and to examine the contribution of plasma fibrinogen concentrations to the variability in TT results.
Methods
Plasma from patients with atrial fibrillation on dabigatran were analysed for clotting times and concentrations of fibrinogen and dabigatran. Correlation plots (and associated r2 values) were generated using these data. The variability in TT results explained by fibrinogen concentrations was quantified using linear regression.
Results
Fifty-two patients (38–94 years old) contributed 120 samples, with plasma dabigatran concentrations ranging from 9 to 408 μg l−1. The r2 values of INR, aPTT, TT and dTT against plasma dabigatran concentrations were 0.49, 0.54, 0.70 and 0.95, respectively. Plasma fibrinogen concentrations explained some of the residual variability in TT values after taking plasma dabigatran concentrations into account (r2 = 0.12, P = 0.02).
Conclusions
Of the screening coagulation assays, the TT correlated best with plasma dabigatran concentrations. Variability in fibrinogen concentrations accounts for some of the variability in the TT.
Keywords: atrial fibrillation, blood coagulation, dabigatran, fibrinogen
What Is Already Known about this Subject —
The impact of dabigatran on coagulation assays has been examined mainly using data from healthy volunteers.
The dilute thrombin time (dTT) is highly correlated with plasma dabigatran concentrations (r2 > 0.90).
The dTT involves the dilution of test plasma with normal pooled plasma, which is thought to minimize the variability in fibrinogen concentrations.
What this Study Adds —
We corroborate the previously reported r2 values of the screening coagulation assays for plasma dabigatran concentrations in real-world patients on dabigatran.
We show that plasma fibrinogen concentrations contribute to the variability in TT, which helps to explain the high r2 of the dTT for plasma dabigatran concentrations.
Introduction
Dabigatran is a direct reversible thrombin inhibitor that has become established as an alternative to warfarin for anticoagulation in the setting of atrial fibrillation (AF) 1–6. Some authors recommend that routine laboratory monitoring of coagulation is not required for patients treated with dabigatran 7–10; however, there is increasing appreciation that laboratory monitoring of coagulation is desirable, at least in specific settings, such as guiding management in the setting of an acute thromboembolic or haemorrhagic event 7–9,11,12. Most of the published reports examining the impact of plasma dabigatran concentrations on coagulation assay results have either used data from plasma spiked with dabigatran (in vitro) or have been from healthy volunteers administered dabigatran 7,13–18. There is also an emerging body of literature consisting of reports using ex vivo data from patients treated with dabigatran, outside of drug-development studies 19–22.
Of the assays that have been examined, the dilute thrombin time (dTT) is often highlighted as the best coagulation assay for assessing individuals treated with dabigatran, because it has a high correlation with plasma dabigatran concentrations (r2 > 0.90) 7,16,17,19,20,22. A commercialized example of the dTT is the Hemoclot® Thrombin Inhibitor assay (HTI; Hyphen BioMed, Neuville-sur-Oise, France). The thrombin time (TT) assay involves the addition of exogenous thrombin to test plasma and measurement of the clotting time. The HTI has an additional step involving an eightfold dilution of the test plasma in saline followed by a further twofold dilution in normal pooled plasma 17. This step is thought to minimize the variance in the resulting clotting time stemming from interindividual variation in plasma fibrinogen concentrations 16.
We are aware of only one ex vivo real-world paper that examined all the readily available screening coagulation assays [international normalized ratio (INR), activated partial thromboplastin time (aPTT) and TT] in relationship to patients treated with dabigatran 19, and none has examined plasma fibrinogen concentrations. We aimed to add to the existing published real-world experience with data we collected as part of an observational study. Furthermore, we aimed to test the hypothesis that some of the residual variability in the measured TT between patients can be explained by variability in plasma fibrinogen concentrations.
Methods
Study design
This was an observational study conducted in Christchurch, New Zealand from July 2012 to May 2013. The overarching goal was to assess real-world dabigatran pharmacokinetics and pharmacodynamics in relationship to renal function. Aspects of the data relevant to the aforementioned aims are presented here (other data and analyses from this study will be published elsewhere). Ethical approval for this study was obtained from the Upper South B Regional Ethics Committee, New Zealand (URB/12/02/009 and URB/12/02/009 AM01). Written consent was obtained from each individual who participated in the study.
Participants
Patients with AF who were ≥18 years old were included if they were on dabigatran etexilate at the same dose rate for ≥7 days and had not missed any doses in the 7 days prior to the study day (by their own report). Recorded details included demographics, dabigatran etexilate dose rates and thromboembolic and haemorrhagic risks according to published scoring systems 23,24. Estimated glomerular filtration rates were calculated using the Chronic Kidney Disease Epidemiology Collaboration (CKD-EPI) equation 25. Body surface area, calculated using Mosteller’s equation 26, was used to convert the CKD-EPI result from units of millilitres per minute per 1.73 square metres to millilitres per minute.
Sample collection and laboratory analysis
Each recruited patient either provided two (2 and 10–16 h postdose) or five venous blood samples (1, 2, 4, 8 and 10–16 h postdose) in a single day. At each time point, both plasma dabigatran concentrations (BD Vacutainer® K2 EDTA tubes) and clotting times (BD Vacutainer® citrate tubes) were measured. For each patient, a venous blood sample at one time point was collected to measure plasma creatinine concentrations (BD Vacutainer® lithium heparin tubes).
Dabigatran concentrations in human plasma were analysed by a validated liquid chromatography–tandem mass spectrometry method based on a previously published method 27. Briefly, 50 μl of plasma was added to 450 μl of the internal standard, [13C6]-dabigatran (10 μg l−1 in methanol and 0.1 mmol/L aqueous HCl (9:1, v/v)). The mixture was vortexed and then centrifuged at 15 000g for 5 min to precipitate the proteins. A 50 μl aliquot of clear supernatant was mixed with 500 μl of water, and 10 μl was injected into the liquid chromatography–tandem mass spectrometry system (Agilent 1290 Infinity Series High Performance Liquid Chromatograph connected to an Agilent 6460 Series Triple Quadrupole Mass Spectrometer; Agilent Technologies, Santa Clara, CA, USA). A Poroshell 120 EC C18 2.7 μm, 50 mm × 3.0 mm column (Agilent Technologies) was used for separation under gradient elution with acetonitrile increasing from 1 to 90% within 2 min in 0.2% formic acid and 10 mmol l−1 ammonium formate. The total analysis time was 5 min. Mass spectrometric detection was in the positive mode with dabigatran and [13C6]-dabigatran monitored at m/z 471.5→289.1 and m/z 477.5→295.1, respectively. For the range of 5–1000 μg l−1, the interday precision [coefficient of variation (CV)] values were ≤11.8% (see Table 1) and bias was ≤8.3%. These performance characteristics were calculated from the results of quality control samples that were analysed during patient sample runs. All patient samples were analysed in triplicate.
Table 1.
Details of dabigatran and coagulation-related assays
| Assay | Clot-activator reagent | Interday CV | Reference range* |
|---|---|---|---|
| Dabigatran | Not applicable | 11.8% at 5.0 μg l−1 | Not applicable |
| 4.3% at 50 μg l−1 | |||
| 2.9% at 200 μg l−1 | |||
| 2.9% at 1000 μg l−1 | |||
| INR | HemosIL Recombiplastin 2G | 2.4% at 1.0 | 0.8–1.2 |
| 4.4% at 2.5 | |||
| aPTT | Triniclot APTT HS | 2.5% at 29 s | 23–35 s |
| 4.5% at 70 s | |||
| TT | HemosIL Thrombin Time | 5.3% at 30 s | 18–28 s |
| HTI | HTI α-thrombin | 10.8% at 100 μg l−1 | Not available |
| 4.7% at 280 μg l−1 | |||
| Fibrinogen | HemosIL Fibrinogen C | 5.5% at 2.2 g l−1 | 1.5–4.0 g l−1 |
| 6.0% at 1.0 g l−1 |
Abbreviations are as follows: aPTT, activated partial thromboplastin time; CV, coefficient of variation; HTI, Hemoclot® Thrombin Inhibitor; INR, international normalized ratio; TT, thrombin time.
Canterbury Health Laboratories values for normal adults.
Samples were analysed using the conventional screening assays, including the INR, aPTT and TT. Details of the reagents and precision of these assays are provided in Table 1. For the TT, our laboratory has a maximal reported time of 300 s. For the purpose of presentation in the correlation plots, these have been set to 300 s, but have not been included in the linear regression analyses involving TT. Additionally, samples were analysed using the HTI assay. Plasma fibrinogen concentrations were measured using the Clauss method 28. All of these coagulation-related assays were performed on an ACL TOP 700 instrument (Instrumentation Laboratory, Bedford, MA, USA).
Serum creatinine was measured using an Abbott® Aeroset analyser (Abbott Park, IL, USA) by the modified Jaffe reaction. This was isotope dilution mass spectrometry (IDMS) aligned for the period of this study and had an interday CV of <4.0%.
All samples were analysed at Canterbury Health Laboratories (Christchurch, New Zealand). Apart from the dabigatran assay and the HTI, all other assays were the same as those employed in routine clinical work.
Statistical analysis
Statistical analysis was performed using GraphPad Prism (version 6.03; GraphPad Software, La Jolla, CA, USA; http://www.graphpad.com) and SPSS (IBM SPSS Statistics for Windows, version 20.0.0.2; IBM Corporation, Armonk, NY, USA). A value of P < 0.05 was considered statistically significant.
Linear regression analyses were used to determine the lines of best fit, and the coefficient of determination (r2) was used to describe the relationships between the coagulation assays and plasma dabigatran concentrations. The exception was the aPTT, for which a square root regression model was employed, as per Stangier et al. 13. We repeated the analyses involving aPTT and TT using ratios rather than the reported values. The ratios were calculated by dividing the reported values by the midrange value of the reference ranges for aPTT (23–35 s) and TT (18–28 s), which were 29 and 23 s, respectively. Linear regression analysis was also used to determine the line of best fit and the r2 to describe the relationship between TT and plasma fibrinogen concentrations.
To discern the contribution of plasma fibrinogen concentrations to the variability in measured TT, the plasma fibrinogen concentrations were transformed into standardized values (z-scores) 29. Furthermore, the standardized residuals from the linear regression of TT against plasma dabigatran concentrations were generated 29. The standardized fibrinogen values and, separately, the standardized residuals, were tested for normality using the D’Agostino–Pearson omnibus test (with P > 0.05 indicating that the data passed the normality test). If normality was demonstrable, the standardized fibrinogen values were then linearly regressed against the standardized residuals. Finally, the r2 of a linear regression model including both plasma dabigatran and fibrinogen concentrations against TT was generated.
Results
There were 52 individuals (age range of 38–94 years) recruited, whose characteristics are described in Table 2. Six individuals provided five samples, 44 provided two samples, and two provided one sample each (troughs only), for a total of 120 samples.
Table 2.
Patient characteristics (n = 52)
| Characteristic | Mean (SD)* |
|---|---|
| Age (years) | 65 (12) |
| Male [n (%)] | 41 (79) |
| Weight (kg) | 98 (23) |
| Height (m) | 1.75 (0.08) |
| BMI (kg m−2) | 31.8 (7.1) |
| BSA (m2) | 2.17 (0.28) |
| Estimated GFR [ml min−1 (1.73 m2)−1] | 72 (13) |
| Estimated GFR (ml min−1) | 91 (23) |
| CHA2DS2-VASc | 2.5 (1.7) |
| HAS-BLED | 1.0 (1.0) |
| Dabigatran etexilate dose rate | |
| 75 mg twice daily [n (%)] | 3 (6) |
| 110 mg twice daily [n (%)] | 24 (46) |
| 150 mg twice daily [n (%)] | 25 (48) |
The correlation plots and r2 values between the four coagulation assays and plasma dabigatran concentrations are shown in Figure 1 (plasma dabigatran concentrations 9–408 μg l−1). For the analyses involving the aPTT and TT assays, the use of ratios instead of reported values did not alter the results (data not shown). Forty-five TT values were <300 s (plasma dabigatran concentrations 9–74 μg l−1), while the remaining 75 values were >300 s (plasma dabigatran concentrations 61–408 μg l−1).
Figure 1.
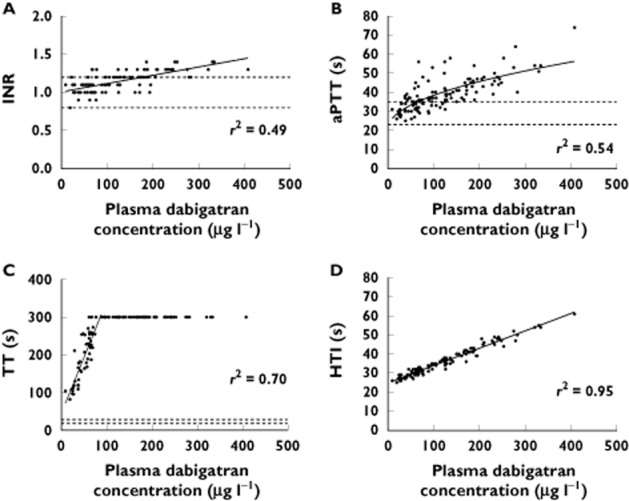
Correlation plots for 120 samples of international normalized ratio (INR; A), activated partial thromboplastin time (aPTT; B), thrombin time (TT; C) and Hemoclot® Thrombin Inhibitor (HTI; D) against plasma dabigatran concentrations. For TT, all values of TT >300 s have been set to 300 s. Continuous lines represent the lines of best fit. Dashed lines for (A), (B) and (C) are the references ranges at Canterbury Health Laboratories. The r2 values are for the line of best fit, which for (C) does not include those data with TT > 300 s in the linear regression
The median (range) of the 120 plasma fibrinogen concentrations was 2.3 (1.1–3.9) g l−1. The standardized fibrinogen values and standardized residuals (from the linear regression of plasma dabigatran concentrations against TT) both passed the normality test, with P = 0.09 and 0.10, respectively. All 45 samples with associated TT < 300 s were associated with plasma fibrinogen concentrations within the laboratory reference range, with a median (range) of 2.5 (1.7–3.9) g l−1. Figure 2 shows the plots using these 45 plasma fibrinogen concentrations. We were unable to demonstrate a significant relationship between plasma fibrinogen concentrations and TT (Figure 2A). However, we found that plasma fibrinogen concentrations explained a small but significant part of the variability in the residuals from the linear regression of plasma dabigatran concentrations against TT, with r2 = 0.12 (P = 0.019; Figure 2B). The linear regression model for the TT including plasma dabigatran and fibrinogen concentrations is shown in Table 3 (r2 = 0.74).
Figure 2.
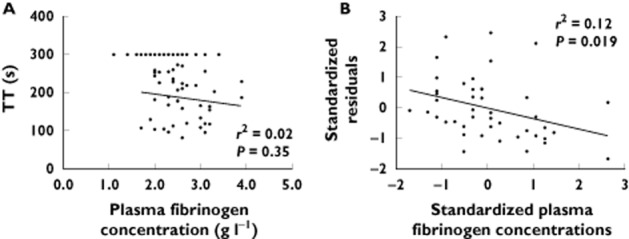
(A) Correlation plot of plasma fibrinogen concentrations against thrombin time (TT; for 120 samples, TT > 300 s have been set to 300 s). (B) Correlation plot of standardized residuals (from the linear regression of plasma dabigatran concentrations against TT) against standardized plasma fibrinogen concentrations (z-scores, for 45 samples with TT < 300 s). Lines of best fit are determined using the 45 samples with TT <300 s. The r2 values are for the line of best fit, and P values are for the hypothesis that the slope of the linear regression is not zero
Table 3.
Linear regression model for thrombin time
| Predictor | B | SE (B) | P value | r2 change |
|---|---|---|---|---|
| Constant | 100.0 | 26.7 | 0.001 | |
| Plasma dabigatran concentration | 3.0 | 0.3 | <0.001 | 0.70 |
| Plasma fibrinogen concentration | −22.0 | 9.1 | 0.02 | 0.04 |
Abbreviations are as follows: B, unstandardized coefficients; SE, standard error.
Discussion
This observational study represents, to our knowledge, the largest single data set (in terms of samples analysed) of patients with AF prescribed dabigatran etexilate outside of phase I–III studies. Furthermore, we are the first to analyse the additional contribution, over and above plasma dabigatran concentrations, of plasma fibrinogen concentrations to explaining TT results in patients on dabigatran etexilate.
Our r2 values, obtained by regressing the results from coagulation assays against plasma dabigatran concentrations measured using liquid chromatography–tandem mass spectrometry, were 0.49, 0.54, 0.70 and 0.95 for the INR, aPTT, TT and HTI, respectively. These compare with reported r2 values from other ex vivo studies of 0.48–0.86 13,19,22, 0.52–0.85 13,20,22, 0.75–0.97 13,19 and 0.92–0.99 7,19,20,22 respectively. Together, these show that all of the screening coagulation assays have a significant relationship with plasma dabigatran concentrations.
For the clinician managing a patient treated with dabigatran etexilate, it is important that the coagulation assay being employed is at least capable of detecting the presence and effect of dabigatran over the range of dabigatran concentrations that are likely to be observed in clinical practice. Our correlation plots in Figure 1 are useful in this regard. These demonstrate that plasma dabigatran concentrations as high as 200 μg l−1 are associated with INR and aPTT values within the local reference ranges (reflecting apparently normal coagulation). Less than 10% of individuals given dabigatran etexilate in the RE-LY trial of dabigatran vs. warfarin for AF had trough concentrations in excess of 200 μg l−1 30. Hence, the INR and aPTT have been regarded as being relatively insensitive to plasma dabigatran concentrations, particularly in comparison to the TT and HTI assays 31. In contrast, the TT is clearly very sensitive, with all TT values in our study being well above the local reference range, even at plasma dabigatran concentrations as low as 9 μg l−1.
At the present time, in terms of the conventional screening assays, Hawes et al. have suggested that a combination of all three is used if laboratory coagulation monitoring is deemed necessary in real-world patients, where the HTI and dabigatran assays are less accessible 19. In this setting, it has been suggested that TT would be useful to cover the lower, and the aPTT and INR to cover the higher, plasma dabigatran concentrations. This is contingent upon the widely disseminated notion that the TT is too sensitive to dabigatran and is unable to gauge the higher concentrations accurately. The TT assay we used consistently resulted in times greater than the maximal reported time at plasma dabigatran concentrations >74 μg l−1 in our study, while Hawes et al. reported that that this value was 138 μg l−1 with the TT assay they used [19]. In contrast, data from Stangier et al. demonstrate that the TT assay they used (Biomatic B10 coagulometer; Desaga, Wiesloch, Germany) could measure TT values for plasma dabigatran concentrations of 0 to ∼400 μg l−1 7,13. This range encompasses >90% of the plasma dabigatran concentrations reported in each of the real-world dabigatran studies, including the present study 19–22. This should be explored further.
Douxfils et al. have previously reported that there is no significant correlation between plasma dabigatran and fibrinogen concentrations, which is in keeping with what might be expected biologically 16. Given that one of the major differences between the HTI and TT assays is that the interindividual variability in plasma fibrinogen concentration is minimized in the HTI (r2 = 0.95 against plasma dabigatran concentrations) compared with the TT (r2 = 0.70), we hypothesized that including fibrinogen concentrations in the regression analysis would account for some of the explained variability of plasma dabigatran concentrations in relationship to the TT. While we demonstrated a statistically significant contribution to the explained variability of TT with plasma fibrinogen concentrations, the resulting r2 = 0.74 for the combination of plasma fibrinogen and dabigatran concentrations for TT was only slightly greater than that observed with plasma dabigatran concentrations alone (r2 = 0.70).
The apparent lack of proportional variance in the relationship between the HTI and plasma dabigatran concentrations is an interesting phenomenon (Figure 1D). This was also demonstrated by van Ryn et al. with a much larger number of samples encompassing plasma dabigatran concentrations up to 300 μg l−1, albeit in healthy volunteers 7. As discussed earlier, the dilution step in the HTI effectively renders the assay into a measure of all thrombin inhibitors in the test plasma, including dabigatran itself 17. In contrast, the proportional variance that is expected in most assays 32 was demonstrated by van Ryn et al. in their report concerning the relationships between the screening coagulation assays (INR, aPTT and TT) and plasma dabigatran concentrations 7. Furthermore, the dabigatran assay published by Delavenne et al., which we also used in this study, also displayed proportional variance 27.
Obesity was a significant feature of our participants (Table 2). This is relevant to consider in the context of this study. Total body weight is a key component in estimating creatinine clearance using the Cockcroft–Gault equation 33, which was found to be a strong determinant of dabigatran concentrations in the RE-LY trial 34. Greater body weight is associated with higher estimated creatinine clearance, and hence lower dabigatran concentrations 30. Furthermore, obesity is associated with increased plasminogen activator inhibitor 1 and plasma fibrinogen concentrations 35,36. Hence, obesity would be expected to be associated with increased thromboembolic risk in the setting of dabigatran therapy for AF 37. As a post hoc analysis, we plotted body mass index against plasma fibrinogen concentrations for the samples used in the analyses against TT, and were unable to demonstrate a significant relationship [Pearson’s r (95% confidence interval) = 0.14 (−0.16, 0.42), P = 0.36].
Our study has a number of limitations. Firstly, the relationships we examined for the coagulation-related assays are dependent upon the coagulometer and clot-activator reagent used 16,19. Nonetheless, as discussed, our results in terms of r2 values are similar to what has been reported. Secondly, the INR was reported to one decimal place, as is routine at Canterbury Health Laboratories, and prothrombin time in seconds was not recorded, which would have provided more precision. This may have contributed to a value for r2 less than that reported in the literature, as mentioned above. Thirdly, the unequal contribution to the 120 samples by the 52 patients may have biased the results. As a post hoc analysis, we re-examined the data, using only the 2 and 10–16 h samples (102 samples). The r2 values for the coagulation assays were very similar to those reported above for the 120 samples, including INR (r2 = 0.44), aPTT (0.55), TT (0.70, using the same 45 samples with TT < 300 s) and HTI (0.95). Fourthly, we measured only the plasma dabigatran concentration, without accounting for the dabigatran glucuronides, which are active metabolites of dabigatran 38,39. The glucuronides may be important, because the percentage contribution of the glucuronides to total active drug exposure ranges from 10 to 35% 13,40. The HTI is thought to account for all thrombin inhibition, irrespective of whether it is from dabigatran or its glucuronides 17; therefore, the r2 of 0.95 for HTI against plasma dabigatran concentrations in our study suggests that the percentage contribution from the glucuronides is relatively constant across the 120 samples analysed. Consequently, we do not believe that explicitly including the glucuronides in our plasma dabigatran concentrations would have significantly altered the relationships, in terms of r2 values. Finally, some fibrinogen assays using the Clauss method, including the one we used, have been reported to suffer from interference from dabigatran, with higher dabigatran concentrations associated with factitiously reduced plasma fibrinogen concentrations 15,41,42. The Clauss method employs the addition of a high thrombin concentration to the test plasma to incite clot formation; the time taken for this is compared with a calibration curve, from which the test fibrinogen concentration is determined. Hence, the presence of thrombin inhibitors is expected to prolong the time to clot formation, and thus falsely depress the measured fibrinogen concentration. Plasma dabigatran concentrations of 100 μg l−1 have been found to be associated with a 12% decrease in plasma fibrinogen concentrations when measured by the fibrinogen assay that we used 15,41,42. This enables comparison with 74 μg l−1 (or around 100 μg l−1 if an additional 30% of the dabigatran glucuronides is included as an extreme estimate), which was the highest dabigatran concentration in the 45 samples we used for the fibrinogen vs. TT analyses. This interference would be expected to obscure the contribution of plasma fibrinogen concentrations to the variability of the TT and may explain the smaller than expected contribution we found of plasma fibrinogen concentrations for TT.
In conclusion, we have corroborated the published data on the relationship between coagulation assays and plasma dabigatran concentrations with our data set of real-world patients with AF. Furthermore, we have found a small but statistically significant contribution of plasma fibrinogen concentrations to TT in these patients. The TT is a widely available coagulation assay, and at least one version of this is capable of measuring TT values in plasma dabigatran concentrations encompassing >90% of the published concentrations observed in clinical practice. It will thus be useful for further work to examine the TT assays in patients on dabigatran to elucidate the causes of variance in the TT, including plasma fibrinogen concentrations. Furthermore, modifications of the TT assay that reduce its excessive sensitivity to dabigatran, while maintaining its sensitivity to intraindividual variance in fibrinogen concentrations, should be tested.
Competing Interests
All authors have completed the Unified Competing Interest form at http://www.icmje.org/coi_disclosure.pdf (available on request from the corresponding author) and declare: no support from any organization for the submitted work; no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
We would like to thank Mark Lewis and Grant Moore (Canterbury Health Laboratories) for assistance with the dabigatran assay; and Stephanie Rose, Amjad Hamid, Amr BinSadiq and Lorraine Skelton (Christchurch Hospital) for assistance with patient recruitment. PKLC is a recipient of the Health Research Council of New Zealand Clinical Research Training Fellowship (2012–2014).
References
- Connolly SJ, Ezekowitz MD, Yusuf S, Eikelboom J, Oldgren J, Parekh A, Pogue J, Reilly PA, Themeles E, Varrone J, Wang S, Alings M, Xavier D, Zhu J, Diaz R, Lewis BS, Darius H, Diener HC, Joyner CD, Wallentin L. Dabigatran versus warfarin in patients with atrial fibrillation. N Engl J Med. 2009;361:1139–1151. doi: 10.1056/NEJMoa0905561. [DOI] [PubMed] [Google Scholar]
- Connolly SJ, Wallentin L, Ezekowitz MD, Eikelboom J, Oldgren J, Reilly PA, Brueckmann M, Pogue J, Alings M, Amerena JV, Avezum A, Baumgartner I, Budaj AJ, Chen JH, Dans AL, Darius H, Di Pasquale G, Ferreira J, Flaker GC, Flather MD, Franzosi MG, Golitsyn SP, Halon DA, Heidbuchel H, Hohnloser SH, Huber K, Jansky P, Kamensky G, Keltai M, Kim SS, Lau CP, Le Heuzey JY, Lewis BS, Liu L, Nanas J, Omar R, Pais P, Pedersen KE, Piegas LS, Raev D, Smith PJ, Talajic M, Tan RS, Tanomsup S, Toivonen L, Vinereanu D, Xavier D, Zhu J, Wang SQ, Duffy CO, Themeles E, Yusuf S. The long-term multicenter observational study of dabigatran treatment in patients with atrial fibrillation (RELY-ABLE) study. Circulation. 2013;128:237–243. doi: 10.1161/CIRCULATIONAHA.112.001139. [DOI] [PubMed] [Google Scholar]
- Metcalfe S, Moodie P. National prescribing data for dabigatran. N Z Med J. 2012;125:97–105. [PubMed] [Google Scholar]
- Southworth MR, Reichman ME, Unger EF. Dabigatran and postmarketing reports of bleeding. N Engl J Med. 2013;368:1272–1274. doi: 10.1056/NEJMp1302834. [DOI] [PubMed] [Google Scholar]
- Sorensen R, Gislason G, Torp-Pedersen C, Olesen JB, Fosbøl EL, Hvidtfeldt MW, Karasoy D, Lamberts M, Charlot M, Køber L, Weeke P, Lip GY, Hansen ML. Dabigatran use in Danish atrial fibrillation patients in 2011: a nationwide study. BMJ Open. 2013;3:e002758. doi: 10.1136/bmjopen-2013-002758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Holbrook AM, Simpson CS, Dowlatshahi D, Johnson AP. Prescribing patters of novel oral anticoagulants following regulatory approval for atrial fibrillation in Ontario, Canada: a population-based descriptive analysis. Can Med Assoc J Open. 2013;1:E115–119. doi: 10.9778/cmajo.20130032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ryn J, Stangier J, Haertter S, Liesenfeld KH, Wienen W, Feuring M, Clemens A. Dabigatran etexilate – a novel, reversible, oral direct thrombin inhibitor: interpretation of coagulation assays and reversal of anticoagulant activity. Thromb Haemost. 2010;103:1116–1127. doi: 10.1160/TH09-11-0758. [DOI] [PubMed] [Google Scholar]
- Gulseth MP, Wittkowsky AK, Fanikos J, Spinler SA, Dager WE, Nutescu EA. Dabigatran etexilate in clinical practice: confronting challenges to improve safety and effectiveness. Pharmacotherapy. 2011;31:1232–1249. doi: 10.1592/phco.31.12.1232. [DOI] [PubMed] [Google Scholar]
- Huisman MV, Lip GY, Diener HC, Brueckmann M, van Ryn J, Clemens A. Dabigatran etexilate for stroke prevention in patients with atrial fibrillation: resolving uncertainties in routine practice. Thromb Haemost. 2012;107:838–847. doi: 10.1160/TH11-10-0718. [DOI] [PubMed] [Google Scholar]
- Diener HC. Pro: ‘The novel oral anticoagulants should be used as 1st choice for secondary prevention in patients with atrial fibrillation.’. Thromb Haemost. 2013;110:493–495. doi: 10.1160/TH13-04-0277. [DOI] [PubMed] [Google Scholar]
- Duffull SB, Wright DF, Al-Sallami HS, Zufferey PJ, Faed JM. Dabigatran: rational dose individualisation and monitoring guidance is needed. N Z Med J. 2012;125:148–154. [PubMed] [Google Scholar]
- Gong IY, Kim RB. Importance of pharmacokinetic profile and variability as determinants of dose and response to dabigatran, rivaroxaban, and apixaban. Can J Cardiol. 2013;29:S24–33. doi: 10.1016/j.cjca.2013.04.002. [DOI] [PubMed] [Google Scholar]
- Stangier J, Rathgen K, Stahle H, Gansser D, Roth W. The pharmacokinetics, pharmacodynamics and tolerability of dabigatran etexilate, a new oral direct thrombin inhibitor, in healthy male subjects. Br J Clin Pharmacol. 2007;64:292–303. doi: 10.1111/j.1365-2125.2007.02899.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wienen W, Stassen JM, Priepke H, Ries UJ, Hauel N. In-vitro profile and ex-vivo anticoagulant activity of the direct thrombin inhibitor dabigatran and its orally active prodrug, dabigatran etexilate. Thromb Haemost. 2007;98:155–162. [PubMed] [Google Scholar]
- Lindahl TL, Baghaei F, Blixter IF, Gustafsson KM, Stigendal L, Sten-Linder M, Strandberg K, Hillarp A. Expert Group on Coagulation of the External Quality Assurance in Laboratory Medicine in S. Effects of the oral, direct thrombin inhibitor dabigatran on five common coagulation assays. Thromb Haemost. 2011;105:371–378. doi: 10.1160/TH10-06-0342. [DOI] [PubMed] [Google Scholar]
- Douxfils J, Mullier F, Robert S, Chatelain C, Chatelain B, Dogne JM. Impact of dabigatran on a large panel of routine or specific coagulation assays. Laboratory recommendations for monitoring of dabigatran etexilate. Thromb Haemost. 2012;107:985–997. doi: 10.1160/TH11-11-0804. [DOI] [PubMed] [Google Scholar]
- Stangier J, Feuring M. Using the HEMOCLOT direct thrombin inhibitor assay to determine plasma concentrations of dabigatran. Blood Coagul Fibrinolysis. 2012;23:138–143. doi: 10.1097/MBC.0b013e32834f1b0c. [DOI] [PubMed] [Google Scholar]
- Avecilla ST, Ferrell C, Chandler WL, Reyes M. Plasma-diluted thrombin time to measure dabigatran concentrations during dabigatran etexilate therapy. Am J Clin Pathol. 2012;137:572–574. doi: 10.1309/AJCPAU7OQM0SRPZQ. [DOI] [PubMed] [Google Scholar]
- Hawes EM, Deal AM, Funk-Adcock D, Gosselin R, Jeanneret C, Cook AM, Taylor JM, Whinna HC, Winkler AM, Moll S. Performance of coagulation tests in patients on therapeutic doses of dabigatran: a cross-sectional pharmacodynamic study based on peak and trough plasma levels. J Thromb Haemost. 2013;11:1493–1502. doi: 10.1111/jth.12308. [DOI] [PubMed] [Google Scholar]
- Douxfils J, Dogné JM, Mullier F, Chatelain B, Rönquist-Nii Y, Malmström RE, Hjemdahl P. Comparison of calibrated dilute thrombin time and aPTT tests with LC-MS/MS for the therapeutic monitoring of patients treated with dabigatran etexilate. Thromb Haemost. 2013;110:543–549. doi: 10.1160/TH13-03-0202. [DOI] [PubMed] [Google Scholar]
- Hapgood G, Butler J, Malan E, Chunilal S, Tran H. The effect of dabigatran on the activated partial thromboplastin time and thrombin time as determined by the Hemoclot thrombin inhibitor assay in patient plasma samples. Thromb Haemost. 2013;110:308–315. doi: 10.1160/TH13-04-0301. [DOI] [PubMed] [Google Scholar]
- Antovic JP, Skeppholm M, Eintrei J, Boija EE, Söderblom L, Norberg EM, Onelöv L, Rönquist-Nii Y, Pohanka A, Beck O, Hjemdahl P, Malmström RE. Evaluation of coagulation assays versus LC-MS/MS for determinations of dabigatran concentrations in plasma. Eur J Clin Pharmacol. 2013;69:1875–1881. doi: 10.1007/s00228-013-1550-4. [DOI] [PubMed] [Google Scholar]
- Lip GY, Nieuwlaat R, Pisters R, Lane DA, Crijns HJ. Refining clinical risk stratification for predicting stroke and thromboembolism in atrial fibrillation using a novel risk factor-based approach: the Euro Heart Survey on atrial fibrillation. Chest. 2010;137:263–272. doi: 10.1378/chest.09-1584. [DOI] [PubMed] [Google Scholar]
- Pisters R, Lane DA, Nieuwlaat R, de Vos CB, Crijns HJ, Lip GY. A novel user-friendly score (HAS-BLED) to assess 1-year risk of major bleeding in patients with atrial fibrillation: the Euro Heart Survey. Chest. 2010;138:1093–1100. doi: 10.1378/chest.10-0134. [DOI] [PubMed] [Google Scholar]
- Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro A. F, 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J. A new equation to estimate glomerular filtration rate. Ann Intern Med. 2009;150:604–612. doi: 10.7326/0003-4819-150-9-200905050-00006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mosteller RD. Simplified calculation of body-surface area. N Engl J Med. 1987;317:1098. doi: 10.1056/NEJM198710223171717. [DOI] [PubMed] [Google Scholar]
- Delavenne X, Moracchini J, Laporte S, Mismetti P, Basset T. UPLC MS/MS assay for routine quantification of dabigatran – a direct thrombin inhibitor – in human plasma. J Pharm Biomed Anal. 2012;58:152–156. doi: 10.1016/j.jpba.2011.09.018. [DOI] [PubMed] [Google Scholar]
- Milos M, Herak D, Kuric L, Horvat I, Zadro R. Evaluation and performance characteristics of the coagulation system: ACL TOP analyzer – HemosIL reagents. Int J Lab Hematol. 2009;31:26–35. doi: 10.1111/j.1751-553x.2007.00999.x. [DOI] [PubMed] [Google Scholar]
- IBM SPSS Statistics Information Center. Available at http://pic.dhe.ibm.com/infocenter/spssstat/v20r0m0/index.jsp (last accessed 3 February 2014)
- Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, Ezekowitz MD, Nehmiz G, Wang S, Wallentin L. The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY trial (randomized evaluation of long-term anticoagulation therapy) J Am Coll Cardiol. 2014;63:321–328. doi: 10.1016/j.jacc.2013.07.104. [DOI] [PubMed] [Google Scholar]
- Chin PK, Vella-Brincat JW, Barclay ML, Begg EJ. Perspective on dabigatran etexilate dosing: why not follow standard pharmacological principles? Br J Clin Pharmacol. 2012;74:734–740. doi: 10.1111/j.1365-2125.2012.04266.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2001. Guidance for Industry: Bioanalytical Method Validation. Available at http://www.fda.gov/downloads/Drugs/Guidances/ucm070107.pdf (last accessed 6 April 2013)
- Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron. 1976;16:31–41. doi: 10.1159/000180580. [DOI] [PubMed] [Google Scholar]
- Liesenfeld KH, Lehr T, Dansirikul C, Reilly PA, Connolly SJ, Ezekowitz MD, Yusuf S, Wallentin L, Haertter S, Staab A. Population pharmacokinetic analysis of the oral thrombin inhibitor dabigatran etexilate in patients with non-valvular atrial fibrillation from the RE-LY trial. J Thromb Haemost. 2011;9:2168–2175. doi: 10.1111/j.1538-7836.2011.04498.x. [DOI] [PubMed] [Google Scholar]
- Kaye SM, Pietiläinen KH, Kotronen A, Joutsi-Korhonen L, Kaprio J, Yki-Järvinen H, Silveira A, Hamsten A, Lassila R, Rissanen A. Obesity-related derangements of coagulation and fibrinolysis: a study of obesity-discordant monozygotic twin pairs. Obesity (Silver Spring) 2012;20:88–94. doi: 10.1038/oby.2011.287. [DOI] [PubMed] [Google Scholar]
- Ditschuneit HH, Flechtner-Mors M, Adler G. Fibrinogen in obesity before and after weight reduction. Obes Res. 1995;3:43–48. doi: 10.1002/j.1550-8528.1995.tb00119.x. [DOI] [PubMed] [Google Scholar]
- Breuer L, Ringwald J, Schwab S, Kohrmann M. Ischemic stroke in an obese patient receiving dabigatran. N Engl J Med. 2013;368:2440–2442. doi: 10.1056/NEJMc1215900. [DOI] [PubMed] [Google Scholar]
- Blech S, Ebner T, Ludwig-Schwellinger E, Stangier J, Roth W. The metabolism and disposition of the oral direct thrombin inhibitor, dabigatran, in humans. Drug Metab Dispos. 2008;36:386–399. doi: 10.1124/dmd.107.019083. [DOI] [PubMed] [Google Scholar]
- Ebner T, Wagner K, Wienen W. Dabigatran acylglucuronide, the major human metabolite of dabigatran: in vitro formation, stability, and pharmacological activity. Drug Metab Dispos. 2010;38:1567–1575. doi: 10.1124/dmd.110.033696. [DOI] [PubMed] [Google Scholar]
- Stangier J, Rathgen K, Stähle H, Mazur D. Influence of renal impairment on the pharmacokinetics and pharmacodynamics of oral dabigatran etexilate: an open-label, parallel-group, single-centre study. Clin Pharmacokinet. 2010;49:259–268. doi: 10.2165/11318170-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Dager WE, Gosselin RC, Kitchen S, Dwyre D. Dabigatran effects on the international normalized ratio, activated partial thromboplastin time, thrombin time, and fibrinogen: a multicenter, in vitro study. Ann Pharmacother. 2012;46:1627–1636. doi: 10.1345/aph.1R179. [DOI] [PubMed] [Google Scholar]
- Halbmayer WM, Weigel G, Quehenberger P, Tomasits J, Haushofer AC, Aspoeck G, Loacker L, Schnapka-Koepf M, Goebel G, Griesmacher A. Interference of the new oral anticoagulant dabigatran with frequently used coagulation tests. Clin Chem Lab Med. 2012;50:1601–1605. doi: 10.1515/cclm-2011-0888. [DOI] [PubMed] [Google Scholar]


