Abstract
Aim
The aim was to evaluate clinical risk factors associated with myotoxicity in statin users.
Methods
This was a cohort study of patients prescribed a statin in UK primary care practices contributing to the Clinical Practice Research Datalink. Outcomes of interest were creatine phosphokinase (CPK) concentrations and clinical records of rhabdomyolysis.
Results
The cohort comprised 641 703 statin users. Simvastatin was most frequently prescribed (66.3%), followed by atorvastatin (24.4%). CPK was measured in 127 209 patients: 81.4% within normal range and 0.7% above <four times the upper limit of normal (ULN). Rhabdomyolysis was recorded in 59 patients. Patients with concomitant prescribing of CYP3A4-interacting drugs had an increased odds ratio (OR) of rhabdomyolysis compared with controls (OR 3.71, 95% CI 1.18, 11.61) and >four times ULN CPK compared with normal CPK (OR 1.28, 95% CI 1.01, 1.60). Rosuvastatin users had higher risk of >four times ULN CPK (OR 1.62, 95% CI 1.22, 2.15) as did patients with larger daily doses of other statin types. A recent clinical record of myalgia was associated with an increased OR of >four times ULN CPK (OR 1.73, 95% CI 1.37, 2.18). In patients who were rechallenged to statins and had repeat CPK measurements after >four times ULN CPK abnormalities, 54.8% of the repeat CPK values were within normal range, 32.1% between one to three times and 13.0% >four times ULN.
Conclusions
The frequencies of substantive CPK increases and rhabdomyolysis during statin treatment were low, with highest risks seen in those on large daily doses or interacting drugs and on rosuvastatin. CPK measurements appeared to have been done in a haphazard manner and better guidance is needed.
Keywords: cardiovascular disease, creatinine, phosphokinase, rhabdomyolysis, statins
What is Already Known on this Subject —
Use of statins is associated with raised creatine phosphokinase (CPK) and rarely with rhabdomyolysis.
What this Study Adds —
Substantive CPK increases and rhabdomyolysis with statin use were particularly seen in patients starting treatment, those on large daily doses or interacting drugs or with larger numbers of concomitant drugs.
Comparative data regarding the safety profile of all statins in clinical use are presented.
The frequency of recurrent large CPK abnormalities was low in those rechallenged with statins.
Better, more evidence-based guidance on measurement of CPK concentrations in patients taking statins is needed.
Introduction
Statins are now amongst the most widely used group of drugs. Large clinical trials have established that statins are effective in the primary and secondary prevention of coronary heart disease 1,2. However, their use is associated with muscle pains, raised creatine phosphokinase (CPK) and rarely with rhabdomyolysis, which can be fatal 3. The importance of statin-induced muscle toxicity is also evidenced by the withdrawal of cerivastatin in 2001 after it caused approximately 100 deaths related to rhabdomyolysis 4.
The potential mechanisms underlying statin induced myotoxicity are complex and poorly understood 5–9. There are many hypotheses. It has been suggested that increased cytotoxicity may be due to intracellular depletion of essential metabolites and/or destabilization of membranes. Serum ubiquinone concemtrations decrease with statin treatment and thus ubiquinone depletion has been suggested as an alternative mechanism. A recent study has suggested that atrogin-1, a key gene involved in skeletal muscle atrophy, may be a critical mediator of skeletal muscle damage 10, but this remains to be tested in patients with differing degrees of muscle damage. A genome-wide association study has found that variation in the statin transporter gene SLCO1B1 was associated with a higher risk of myopathy in patients on simvastatin 11.
Although the frequency of muscle toxicity has not been shown to be high in clinical trials, it may actually be higher in real-world settings, but there is very little population based data. This is further complicated by the heterogeneity in presentation. For example, many patients actually complain of myalgia without having an increase in CPK concentrations 3. As the use of statins is set to increase further in the future, muscle toxicity will become an increasingly important clinical problem, and it is important we define mechanisms and predisposing factors. The objective of this study, as specified in the protocol, was to evaluate the clinical risk factors associated with myotoxicity and rhabdomyolysis in a large population of statin users. Cases with myotoxicity as defined by CPK increases were identified and risk factors were analyzed.
Methods
Data source
This study used data from the UK Clinical Practice Research Datalink (CPRD), formerly known as the General Practice Research Database. CPRD comprises computerized medical records maintained by general practitioners (GPs). GPs play a key role in the UK health care system, as they are responsible for primary health care and specialist referrals. Patients are affiliated with a practice, which centralizes the medical information from the GPs, specialist referrals and hospitalizations. The data recorded in the CPRD since 1987 include demographic information, prescription details, clinical events, preventive care provided, specialist referrals, laboratory results, hospital admissions and their major outcomes 12. A recent review of all validation studies found that medical data in the CPRD were generally of high quality 13. Most UK practices now receive laboratory results electronically directly from the laboratory. The study formed the basis of a pharmacogenetic evaluation of risk factors 14.
Study population
The study population included individuals aged 18 years or older who were prescribed a statin, with the first-ever statin prescription at least 1 year after start of CPRD data collection. The date of the first recorded statin prescription constituted the cohort entry date. Within this population, the first laboratory record of CPK measurement or the first medical record of rhabdomyolysis in the 3 months following a statin prescription and after 1 January 2000 were identified (i.e. all cases were considered to be current users of statins at the date of the CPK measurement or rhabomyolysis). The index date was the date of the CPK measurement or rhabdomyolysis. The cases of rhabdomyolysis were based on records as recorded by GPs into the electronic health record (based on their or hospital diagnosis). The CPK values were classified according to the upper limit of normal (ULN). ULN was defined as 19 U l–1 for men and 170 U l–1 for women (http://www.gpnotebook.co.uk/simplepage.cfm?ID=1886060577, accessed 12 October 2009). Three sets of case-control analyses were conducted. The first analysis set concerned a comparison of different CPK concentrations in patients with a CPK measurement. Patients with normal CPK concentrations were considered the controls. The second analysis compared cases with rhabdomyolysis or >10 times ULN CPK concentrations with matched statin users without rhabdomyolysis who did not have a CPK measurement or had a CPK concentration below >10 times ULN. The matching variables were age (±5 years), gender, general practice and number of prior statin prescriptions at the index date. The index date of the controls was that of the matched case. Six controls were randomly selected for each case with rhabdomyolysis or >10 times ULN CPK. The third analysis were conducted to measure the likelihood of CPK measurement for different patient characteristics. Each patient with a CPK measurement that was found to be within the normal range was matched to one control without a CPRD measurement. In all analyses, cases and controls were required to have received a statin prescription in the 91 days before. Cases with myocardial infarction, trauma or falls recorded in the period of time from 1 month before to 2 weeks after the CPK measurement or rhabomyolysis were excluded.
The following clinical risk factors were measured at the date of CPK measurement or rhabdomyolysis: age, gender, body mass index, smoking status, number of non-statin prescriptions issued in the 3 months before, prescribing in the 6 months before of antihypertensives, drugs interacting with statins through CYP3A4 (amiodarone, fibrates, ciclosporin, azole antifungals, macrolide antibiotics, protease inhibitors, calcium channel blockers), drugs interacting through mechanisms other than a CYP3A4 interaction (such as digoxin, warfarin, fenofibrate, gemfibrozil, nicotinic acid), oral corticosteroids, medical history of diabetes mellitus, hypothyroidism, hyperthyroidism, chronic obstructive pulmonary disease and records in the 1 month before of myalgia. Furthermore, type and strength of the most recently issued statin prescriptions and the extent of switching between different types of statins was determined.
The predictors of CPK concentration or rhabdomyolysis were analyzed using logistic regression that compared cases with controls (conditional regression was used in the matched case-control analyses). Crude and adjusted odds ratios (ORs) and 95% confidence intervals (95% CI) were estimated for each of the measured characteristics. In the case of absence of recording of body mass index and smoking, an indicator of missingness was included in the regression models. An explorative analysis was also conducted comparing cases and controls for the types of drugs issued in the previous 3 months. Drugs were classified by substance and logistic regression was conducted for those drugs that had been used by at least 1% of the cases and controls. The false discovery rate (FDR) adjusted P values were estimated in order to minimize the effects of multiple testing and the finding of false positive statistical associations.
The extent of repeat statin prescribing and changes in statin type or dose was measured after the CPK measurement or rhabdomyolysis. It was evaluated whether a repeat statin prescription was issued in the time period from 2 weeks to 6 months after the CPK measurement or rhabdomyolysis. In this analysis, the type and dose of the first repeat statin prescription was compared with those of the statin prescription issued most recently prior to CPK measurement or rhabdomyolysis. Furthermore, life tables were created in order to estimate the persistence of statin use for patients who received a repeat statin prescription. Persistence was based on the statin prescribing data. If a patient did not receive another statin prescription within the duration of use (mean of 30 days) plus a washout period of 91 days, this patient was considered to have discontinued statin treatment. These analyses were stratified by concentration of CPK.
Results
A total of 641 703 patients who were prescribed a statin were identified. The mean duration of follow-up (from start to end of statin treatment) was 4.1 years and the total follow-up was 2.6 million person-years. The total number of statin prescriptions issued to the study population exceeded 22.7 million. Simvastatin was the most frequently prescribed statin (66.3% of all prescriptions) followed by atorvastatin (24.4%), pravastatin (4.9%), rosuvastatin (3.6%), fluvastatin (0.6%) and cerivastatin (0.1%). The most frequently prescribed daily dose was 40 mg for simvastatin (53.6% of prescriptions for this type), 10 mg for atorvastatin (44.3%) and 10 mg for rosuvastatin (68.2%). The 80 mg dose of simvastatin was issued in 1.1% of the simvastatin prescriptions.
One hundred and twenty-seven thousand two hundred and nine patients (19.8%) had a record of a CPK measurement during statin treatment. Most of the patients were found to have CPK within the normal range (n = 103 610; 81.4%), 894 patients (0.7%) had CPK concentrations at least four times the ULN and 182 patients (0.1%) at least 10 times the ULN. Rhabdomyolysis was recorded in 59 patients (incidence rate of 1.8 per 100000 person-years in women and 2.6 in men). Table 1 shows the characteristics of the patients at the date of the CPK measurement or rhabdomyolysis. Women were less likely to have high CPK values during statin treatment. The mean number of prescriptions in the 3 months before increased with higher CPK values. Of the 7043 patients with a GP-recorded symptom of myalgia, 5675 patients (80.6%) had normal CPK concentrations and 80 patients (1.1%) had CPK concentrations at least four times the ULN.
Table 1.
Characteristics of statins users with a CPK measurement or rhabomyolysis and matched statin users without CPK measurement
| Characteristics | Normal CPK (n = 103 610) | Matched controls to normal CPK patients (n = 103 610) | One to two timwa ULN CPK (n = 18 892) | Two to three times ULN CPK (n = 2950) | Three to four times ULN CPK (n = 863) | 4-5x ULN CPK (n = 333) | 5-10x CPK (n = 379) | 10+x ULN CPK (n=182) | Rhabdomyolysis (n = 59) |
|---|---|---|---|---|---|---|---|---|---|
| Women (%) | 50735 (49.0%) | 50735 (49.0%) | 6482 (34.3%) | 798 (27.1%) | 253 (29.3%) | 94 (28.2%) | 108 (28.5%) | 72 (39.6%) | 22 (37.3%) |
| Mean age (years) (SD) | 65.8 (11.2) | 65.8 (11.3) | 64.2 (11.2) | 63.7 (11.7) | 63.4 (11.7) | 64 (12.8) | 62.6 (13.3) | 64.3 (14.6) | 69.6 (15.9) |
| Number of non-statin prescriptions in three months before (standard deviation) | 11.2 (12.3) | 11.9 (13.4) | 10.5 (11.7) | 11.1 (11.4) | 11.4 (11) | 12.3 (12.6) | 13.4 (17.7) | 15.4 (17.8) | 25.8 (26.9) |
| Recent prescribing of | |||||||||
| Antihypertensives (%) | 73708 (71.1%) | 75911 (73.3%) | 13279 (70.3%) | 2087 (70.7%) | 624 (72.3%) | 244 (73.3%) | 280 (73.9%) | 136 (74.7%) | 47 (79.7%) |
| CYP3A4 interacting drugs (%) | 8117 (7.8%) | 8003 (7.7%) | 1293 (6.8%) | 218 (7.4%) | 74 (8.6%) | 39 (11.7%) | 39 (10.3%) | 17 (9.3%) | 9 (15.3%) |
| Non- CYP3A4 interacting drugs (%) | 5990 (5.8%) | 6206 (6.0%) | 1129 (6%) | 190 (6.4%) | 59 (6.8%) | 22 (6.6%) | 33 (8.7%) | 17 (9.3%) | 9 (15.3%) |
| Oral corticosteroids (%) | 3998 (3.9%) | 3826 (3.7%) | 481 (2.5%) | 99 (3.4%) | 29 (3.4%) | 10 (3%) | 21 (5.5%) | 12 (6.6%) | 5 (8.5%) |
| Recent myalgia (%) | 5675 (5.5%) | 138 (0.1%) | 1057 (5.6%) | 180 (6.1%) | 51 (5.9%) | 25 (7.5%) | 30 (7.9%) | 25 (13.7%) | 4 (6.8%) |
| Recent renal failure (%) | 1792 (1.7%) | 839 (0.8%) | 334 (1.8%) | 69 (2.3%) | 19 (2.2%) | 5 (1.5%) | 8 (2.1%) | 8 (4.4%) | 32 (54.2%) |
| Medical history of | |||||||||
| Hyperthyroidism (%) | 1823 (1.8%) | 1845 (1.8%) | 276 (1.5%) | 38 (1.3%) | 16 (1.9%) | 10 (3%) | 10 (2.6%) | 7 (3.8%) | 0 (0%) |
| Hypothyroidism (%) | 8920 (8.6%) | 8666 (8.4%) | 1461 (7.7%) | 235 (8%) | 82 (9.5%) | 38 (11.4%) | 28 (7.4%) | 23 (12.6%) | 5 (8.5%) |
| Chronic obstructive pulmonary disease (%) | 5743 (5.5%) | 6434 (6.2%) | 975 (5.2%) | 157 (5.3%) | 50 (5.8%) | 23 (6.9%) | 24 (6.3%) | 16 (8.8%) | 3 (5.1%) |
| Diabetes mellitus (%) | 23237 (22.4%) | 24793 (23.9%) | 4391 (23.2%) | 720 (24.4%) | 223 (25.8%) | 95 (28.5%) | 83 (21.9%) | 43 (23.6%) | 22 (37.3%) |
| Type of statin | |||||||||
| Simvastatin (%) | 69463 (67.0%) | 70413 (68.0%) | 12482 (66.1%) | 1881 (63.8%) | 579 (67.1%) | 216 (64.9%) | 248 (65.4%) | 107 (58.8%) | 45 (76.3%) |
| Atorvastatin (%) | 24599 (23.7%) | 24514 (23.7%) | 4629 (24.5%) | 744 (25.2%) | 196 (22.7%) | 76 (22.8%) | 87 (23.0%) | 54 (29.7%) | 8 (13.6%) |
| Cerivastatin (%) | 225 (0.2%) | 183 (0.2%) | 31 (0.2%) | 5 (0.2%) | 0 (0%) | 1 (0.3%) | 2 (0.5%) | 0 (0%) | 0 (0%) |
| Fluvastatin (%) | 735 (0.7%) | 727 (0.7%) | 123 (0.7%) | 24 (0.8%) | 8 (0.9%) | 4 (1.2%) | 4 (1.1%) | 1 (0.5%) | 1 (1.7%) |
| Pravastatin (%) | 4855 (4.7%) | 4799 (4.6%) | 795 (4.2%) | 142 (4.8%) | 42 (4.9%) | 14 (4.2%) | 17 (4.5%) | 10 (5.5%) | 2 (3.4%) |
| Rosuvastatin (%) | 3733 (3.6%) | 2974 (2.9%) | 832 (4.4%) | 154 (5.2%) | 38 (4.4%) | 22 (6.6%) | 21 (5.5%) | 10 (5.5%) | 3 (5.1%) |
Patients with a recent GP visit for myalgia were substantially more likely to have a CPK test with values within the normal range (adjusted OR of 43.79, 95% CI 36.75, 52.17), indicating the higher likelihood of CPK testing in patients with myopathy. Rosuvastatin users were also more likely to have a CPK measurement within the normal range compared with simvastatin users (adjusted OR 1.32, 95% CI 1.25, 1.39). Patients who received 50 or more non-statin prescriptions in 3 months were less likely to be tested (adjusted OR for normal CPK 0.68, adjusted 95% CI 0.62, 0.75).
Table 2 shows the associations between concentrations of CPK or rhabdomyolysis and risk factors. Patients with GP-recorded myalgia had an increased odds of >four times ULN CPK concentrations compared with patients without myalgia and with a normal CPK test (adjusted OR 1.73, 95% CI 1.37, 2.18). Patients with concomitant prescribing of CYP3A4 interacting drugs also had an increased odds of >four times ULN CPK concentrations (adjusted OR 1.28, 95% CI 1.02, 1.60). The odds of rhabdomyolysis were increased in patients with concomitant prescribing of CYP3A4 interacting drugs (adjusted OR 3.71, 95% CI 1.18, 11.61) and in those who received more than 50 recent non-statin prescriptions (adjusted OR 13.52, 95% CI 1.03, 177.3).
Table 2.
OR of increased CPK concentrations (compared with statin users with normal CPK concentrations) and of rhabdomyolysis (compared with statin users without rhabdomyolysis) stratified by clinical risk factors
| Risk factor§ | One to two times ULN* | Two to three times ULN* | Three to four times ULN* | >four times ULN* | Rhabdomyolysis† | Rhabdomyolysis or >10 times ULN† |
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI)‡ | Adjusted OR (95% CI)‡ | Adjusted OR (95% CI)‡ | Adjusted OR (95% CI)‡ | Adjusted OR (95% CI)‡ | Adjusted OR (95% CI)‡ | |
| Recent record of myalgia | 1.04 (0.97, 1.11) | 1.15 (0.99, 1.35) | 1.12 (0.84, 1.49) | 1.73 (1.37, 2.18) | 23.90 (2.03, 281.50) | 47.02 (15.76, 140.20) |
| Recent prescribing history of | ||||||
| Antihypertensives | 1.00 (0.96, 1.04) | 1.00 (0.92, 1.09) | 1.10 (0.94, 1.29) | 1.13 (0.96, 1.32) | 0.95 (0.43, 2.13) | 0.90 (0.63, 1.28) |
| CYP3A4 interacting drugs | 0.93 (0.87, 0.99) | 0.98 (0.85, 1.13) | 1.13 (0.88, 1.45) | 1.28 (1.02, 1.60) | 3.71 (1.18, 11.61) | 1.83 (1.10, 3.04) |
| Non-CYP3A4 interacting drugs | 1.14 (1.05, 1.24) | 1.12 (0.93, 1.35) | 1.24 (0.89, 1.73) | 1.31 (0.97, 1.79) | 0.72 (0.21, 2.54) | 1.08 (0.58, 2.02) |
| Oral corticosteroids | 0.72 (0.65, 0.80) | 0.95 (0.77, 1.17) | 0.90 (0.625, 1.32) | 1.12 (0.81, 1.55) | 2.40 (0.63, 9.09) | 1.73 (0.92, 3.22) |
| Number of non-statin prescriptions in previous 3 months | ||||||
| 1–3 | 0.94 (0.87, 1.01) | 0.90 (0.75, 1.08) | 0.87 (0.61, 1.24) | 0.80 (0.57, 1.12) | 0.37 (0.02, 5.41) | 0.84 (0.33, 2.16) |
| 4–8 | 0.87 (0.80, 0.94) | 0.83 (0.69, 0.99) | 1.06 (0.76, 1.49) | 0.79 (0.56, 1.12) | 1.24 (0.12, 12.77) | 1.05 (0.42, 2.61) |
| 9–18 | 0.85 (0.79, 0.92) | 0.85 (0.71, 1.03) | 1.03 (0.72, 1.48) | 0.96 (0.68, 1.36) | 1.79 (0.16, 19.96) | 1.64 (0.66, 4.06) |
| 19–49 | 0.87 (0.80, 0.95) | 1.04 (0.84, 1.27) | 1.20 (0.81, 1.77) | 1.31 (0.90, 1.91) | 3.04 (0.25, 36.69) | 2.03 (0.78, 5.28) |
| 50+ | 0.90 (0.75, 1.06) | 0.88 (0.58, 1.33) | 1.28 (0.63, 2.57) | 1.44 (0.77, 2.69) | 13.52 (1.03, 177.3) | 3.68 (1.18, 11.51) |
| Medical history of | ||||||
| Hyperthyroidism | 1.00 (0.87, 1.14) | 0.90 (0.64, 1.25) | 1.18 (0.72, 1.97) | 1.87 (1.24, 2.80) | – | 1.81 (0.71, 4.64) |
| Hypothyroidism | 1.13 (1.06, 1.20) | 1.33 (1.15, 1.53) | 1.54 (1.20, 1.96) | 1.41 (1.11, 1.79) | 0.74 (0.21, 2.62) | 1.51 (0.92, 2.48) |
| Diabetes mellitus | 0.97 (0.93, 1.00) | 0.99 (0.90, 1.08) | 1.10 (0.94, 1.29) | 1.01 (0.86, 1.18) | 1.31 (0.68, 2.55) | 1.03 (0.73, 1.44) |
| Chronic obstructive pulmonary disease | 1.10 (1.02. 1.18) | 1.09 (0.91, 1.29) | 1.20 (0.88, 1.62) | 1.27 (0.96, 1.67) | 0.25 (0.05, 1.22) | 1.26 (0.70, 2.26) |
Patients with increased CPK concentrations were compared with unmatched statin users with normal CPK concentrations.
Patients with rhabdomyolysis (or with >10 times ULN CPK concentrations) were compared with matched statin users without rhabdomyolysis who did not have a CPK measurement or a CPK concentratrion below >10 times ULN.
Adjusted for body mass index, smoking status, number of non-statin prescriptions in the previous 3 months, recent prescribing of antihypertensives, CYP3A4 interacting drugs, non-CYP3A4 interacting drugs, oral corticosteroids, history of atrial fibrillation, hypothyroidism, hyperthyroidism, chronic obstructive pulmonary disease or diabetes mellitus; in the comparisons with normal CPK, the analyses were also adjusted for age and gender.
Reference group are patients without the risk factor (or no non-statin prescriptions in the previous 3 months).
As shown in Table 3, rosuvastatin users had a higher risk of rhabdomyolysis compared with simvastatin users, although this difference did not reach statistical significance (adjusted OR 2.92, 95% CI 0.59, 14.47). Rosuvastatin users had an increased odds of >four times ULN CPK concentrations compared with simvastatin users with a normal CPK test (adjusted OR 1.62, 95% CI 1.22, 2.15). The mean daily dose of rosuvastatin remained stable over calendar time (about 11 mg). The prescribing of rosuvastatin with a lower daily dose increased over time (in 2003, 0% was 5 mg daily; 2007, 7.9%; 2011, 24.6%) as did prescribing of higher doses (in 2003, 9.1% was 20 mg daily; 2007, 15.9%; 2011, 20.1%). There were no statistically significant differences in the odds of CPK abnormalities and rhabdomyolysis between atorvastatin and pravastatin compared with simvastatin. The risks of rhabdomyolysis and >four times ULN CPK abnormalities tended to be highest with the largest daily doses (irrespective of any type of statin).
Table 3.
OR of increased CPK concentrations (compared with statin users with normal CPK concentrations) and of rhabdomyolysis (compared with statin users without rhabdomyolysis) stratified by type and dose of statin exposure
| Risk factor | One to two ULN* | Two to three times ULN* | Three to four times ULN* | >four times ULN* | Rhabdomyolysis† | Rhabdomyolysis or 10+x ULN† |
|---|---|---|---|---|---|---|
| Adjusted OR (95% CI)‡ | Adjusted OR (95% CI)‡ | Adjusted OR (95% CI)‡ | Adjusted OR (95% CI)‡ | Adjusted OR (95% CI)‡ | Adjusted OR (95% CI)‡ | |
| Type of statin | ||||||
| Simvastatin | Reference | Reference | Reference | Reference | Reference | Reference |
| Atorvastatin | 1.05 (1.01, 1.09) | 1.11 (1.02, 1.21) | 0.93 (0.79,1.10) | 1.03 (0.88, 1.20) | 1.18 (0.47, 3.01) | 1.31 (0.91–1.88) |
| Pravastatin | 0.94 (0.87, 1.02) | 1.12 (0.94, 1.34) | 1.05 (0.77, 1.44) | 0.99 (0.72, 1.36) | 3.28 (0.56, 19.27) | 1.19 (0.58–2.45) |
| Rosuvastatin | 1.22 (1.13, 1.32) | 1.49 (1.25, 1.76) | 1.16 (0.84, 1.62) | 1.62 (1.22, 2.15) | 2.92 (0.59, 14.47) | 2.90 (1.40–6.01) |
| Type and dose of statin | ||||||
| Simvastatin 10 mg | Reference | Reference | Reference | Reference | Reference | Reference |
| Atorvastatin 10 mg | 1.05 (0.98, 1.13) | 1.00 (0.85, 1.18) | 0.94 (0.68, 1.29) | 1.09 (0.79, 1.49) | 3.04 (0.39, 23.37) | 1.55 (0.70–3.42) |
| Rosuvastatin 5 mg | 1.34 (1.12, 1.61) | 1.65 (1.13, 2.41) | 2.00 (1.03, 3.91) | 2.07 (1.06, 4.05) | – | 3.72 (0.76–18.27) |
| Simvastatin 20 mg | 1.08 (1.02, 1.15) | 0.97 (0.84, 1.12) | 1.19 (0.90, 1.56) | 1.16 (0.88, 1.54) | 0.63 (0.14, 2.95) | 0.82 (0.39–1.70) |
| Atorvastatin 20 mg | 1.19 (1.10, 1.29) | 1.18 (0.98, 1.42) | 1.35 (0.96, 1.90) | 1.29 (0.90, 1.84) | 0.51 (0.04, 6.10) | 0.94 (0.38–2.32) |
| Rosuvastatin 10 mg | 1.21 (1.08, 1.35) | 1.41 (1.12, 1.79) | 1.37 (0.86, 2.17) | 1.76 (1.15, 2.70) | – | 1.87 (0.51–6.82) |
| Simvastatin 40 mg | 1.10 (1.03, 1.16) | 1.01 (0.88, 1.15) | 1.21 (0.93, 1.58) | 1.33 (1.02, 1.73) | 1.84 (0.46, 7.33) | 1.53 (0.77–3.05) |
| Atorvastatin 40 mg | 1.31 (1.20, 1.43) | 1.30 (1.06, 1.60) | 1.29 (0.87, 1.92) | 1.52 (1.04, 2.23) | 1.74 (0.25, 12.02) | 1.86 (0.80–4.33) |
| Rosuvastatin 20–40 mg | 1.82 (1.52, 2.18) | 1.52 (0.99, 2.33) | 0.71 (0.22, 2.80) | 2.95 (1.58, 5.51) | 6.37 (0.66, 61.48) | 7.64 (1.92–30.40) |
| Simvastatin 80 mg | 1.41 (1.17, 1.71) | 1.13 (0.71, 1.80) | 2.79 (1.53, 5.08) | 2.21 (1.13, 4.33) | 17.32 (0.46, 647.3) | 2.59 (0.69–9.68) |
| Atorvastatin 80 mg | 1.11 (0.96, 1.29) | 1.09 (0.78, 1.52) | 0.93 (0.48, 1.82) | 1.87 (1.13, 3.12) | 5.23 (0.21, 132.8) | 4.71 (1.51–14.69) |
Patients with increased CPK concentrations were compared with unmatched statin users with normal CPK concentrations.
Patients with rhabdomyolysis (or with >10 times ULN CPK concentrations) were compared with matched statin users without rhabdomyolysis who did not have a CPK measurement or a CPK concentration below >10 times ULN.
Adjusted for body mass index, smoking status, number of non-statin prescriptions in the previous 3 months, recent prescribing of antihypertensives, CYP3A4 interacting drugs, non-CYP3A4 interacting drugs, oral corticosteroids, history of atrial fibrillation, hypothyroidism, hyperthyroidism, chronic obstructive pulmonary disease or diabetes mellitus; in the comparisons with normal CPK, the analyses were also adjusted for age and gender.
The explorative analysis found that concomitant users of allopurinol, ezetimibe, clopidogrel, furosemide, bisoprolol fumarate and ramipril were more likely to have CPK concentrations of >four times ULN (compared with normal CPK concentrations) with false discovery rate-adjusted values of 0.05 or less (Table 4). The crude OR for CPK concentrations of >four times ULN in statin users concomitantly using allopurinol was 2.01 (95% CI 1.46, 2.75). For ezetimibe and clopidogrel, these numbers were 1.99 (95% CI 1.27, 3.12) and 1.49 (95% CI 1.17, 1.89), respectively.
Table 4.
Explorative analysis of OR of four times ULN CPK concentrations (compared with statin users with normal CPK concentrations) and of rhabdomyolysis (compared with statin users without rhabdomyolysis) stratified by concomitant drugs (results are shown for the drugs with the 20 highest ORs)
| >four times ULN CPK | Rhabdomyolysis | ||||||
|---|---|---|---|---|---|---|---|
| Crude OR (95% CI) | Crude P value | False discovery rate-adjusted P value | Crude OR (95% CI) | Crude P value | False discover rate-adjusted P value | ||
| Allopurinol | 2.01 (1.46, 2.75) | <0.001 | <0.001 | Bumetanide | 9.00 (1.50, 53.84) | 0.02 | 0.24 |
| Ezetimibe | 1.99 (1.27, 3.12) | 0.002 | 0.03 | Trimethoprim | 7.14 (1.57, 32.36) | 0.01 | 0.24 |
| Sildenafil citrate | 1.80 (1.17, 2.75) | 0.01 | 0.09 | Erythromycin | 6.00 (1.21, 29.73) | 0.03 | 0.29 |
| Hydrocortisone acetate/miconazole nitrate | 1.70 (1.00, 2.90) | 0.05 | 0.24 | Isopropyl myristate/liquid paraffin | 5.21 (1.03, 26.35) | 0.05 | 0.38 |
| Indapamide hemihydrate | 1.70 (1.14, 2.53) | 0.01 | 0.11 | Diazepam | 4.80 (1.29, 17.88) | 0.02 | 0.24 |
| Spironolactone | 1.68 (1.02, 2.77) | 0.04 | 0.22 | Spironolactone | 4.50 (1.01, 20.11) | 0.05 | 0.38 |
| Ipratropium bromide | 1.61 (0.95, 2.74) | 0.08 | 0.32 | Ferrous sulphate | 4.26 (1.28, 14.21) | 0.02 | 0.24 |
| Flucloxacillin sodium | 1.60 (1.08, 2.37) | 0.02 | 0.14 | Timolol maleate/dorzolamide hydrochloride | 4.00 (0.67, 23.94) | 0.13 | 0.76 |
| Emulsifying wax/liquid paraffin/white soft paraffin | 1.59 (0.95, 2.66) | 0.08 | 0.32 | Citalopram hydrobromide | 3.63 (1.40, 9.41) | 0.01 | 0.24 |
| Esomeprazole magnesium | 1.59 (0.95, 2.65) | 0.08 | 0.32 | Flucloxacillin sodium | 3.33 (1.07, 10.42) | 0.04 | 0.35 |
| Nicorandil | 1.55 (1.03, 2.33) | 0.04 | 0.22 | Cefalexin | 3.33 (0.54, 20.52) | 0.19 | 0.96 |
| Clopidogrel | 1.49 (1.17, 1.89) | <0.001 | 0.02 | Lactulose | 3.27 (1.21, 8.82) | 0.02 | 0.24 |
| Furosemide | 1.48 (1.19,1.85) | <0.001 | 0.01 | Betahistine dihydrochloride | 3.00 (0.55, 16.38) | 0.20 | 0.96 |
| Bisoprolol fumarate | 1.47 (1.18, 1.84) | <0.001 | 0.01 | Potassium bicarbonate/sodium alginate | 2.89 (0.83, 10.06) | 0.09 | 0.63 |
| Chloramphenicol | 1.44 (0.84, 2.44) | 0.18 | 0.59 | Dosulepin hydrochloride | 2.77 (0.44, 17.29) | 0.27 | 0.98 |
| Candesartan cilexetil | 1.41 (1.02, 1.96) | 0.04 | 0.22 | Rosiglitazone maleate | 2.77 (0.44, 17.29) | 0.27 | 0.98 |
| Diltiazem hydrochloride | 1.40 (1.02, 1.93) | 0.04 | 0.22 | Fluoxetine hydrochloride | 2.70 (0.67, 10.93) | 0.16 | 0.91 |
| Ramipril | 1.37 (1.15, 1.62) | <0.001 | 0.01 | Folic acid | 2.40 (0.47, 12.37) | 0.30 | 0.98 |
| Hydrocortisone | 1.37 (0.79, 2.37) | 0.27 | 0.67 | Ispaghula husk | 2.40 (0.47, 12.37) | 0.30 | 0.98 |
| Erythromycin | 1.37 (0.79, 2.37) | 0.27 | 0.67 | Lisinopril | 2.32 (1.09, 4.93) | 0.03 | 0.29 |
Patients with rhabomyolyisis were least likely to continue statin treatment (Table 5). Of the rhabdomyolysis cases, 58.0% did not receive a statin prescription in the 6 months after. In the control patients, this figure was 2.9%. Switching of type and dose occurred infrequently in patients with CPK abnormalities or rhabdomyolysis. In the patients who continued statin treatment, the duration of statin treatment (i.e. persistence) was lowest in patients with rhabdomyolysis (Figure 1).
Table 5.
Extent of statin exposure and changes in statin type or dose in the 6 months after the CPK measurement or rhabdomyolysis (for patients who were alive at month 6)
| Statin exposure afterwards | Normal CPK | One to two times ULN | Two to three times ULN | Three to four times ULN | >four times ULN | Rhabdomyolysis | Rhabdomyolysis/>10 times ULN |
|---|---|---|---|---|---|---|---|
| No statin | 8.6% | 12.9% | 26.0% | 35.9% | 44.9% | 58.0% | 55.9% |
| Same statin same dose | 82.7% | 75.6% | 60.5% | 48.1% | 38.8% | 34.0% | 31.9% |
| Same statin different dose | 2.4% | 3.8% | 5.0% | 6.5% | 3.9% | 2.0% | 1.9% |
| Different statin | 6.4% | 7.7% | 8.5% | 9.5% | 12.4% | 6.0% | 10.3% |
| Controls | Controls | Controls | Controls | Controls | Controls | Controls | |
|---|---|---|---|---|---|---|---|
| No statin | 10.3% | 5.1% | 4.0% | 3.8% | 3.5% | 2.9% | 4.5% |
| Same statin same dose | 86.5% | 91.5% | 92.7% | 93.1% | 92.6% | 94.9% | 91.7% |
| Same statin different dose | 0.8% | 0.8% | 0.7% | 0.7% | 1.1% | 1.0% | 1.7% |
| Different statin | 2.4% | 2.5% | 2.6% | 2.4% | 2.8% | 1.3% | 2.1% |
Figure 1.
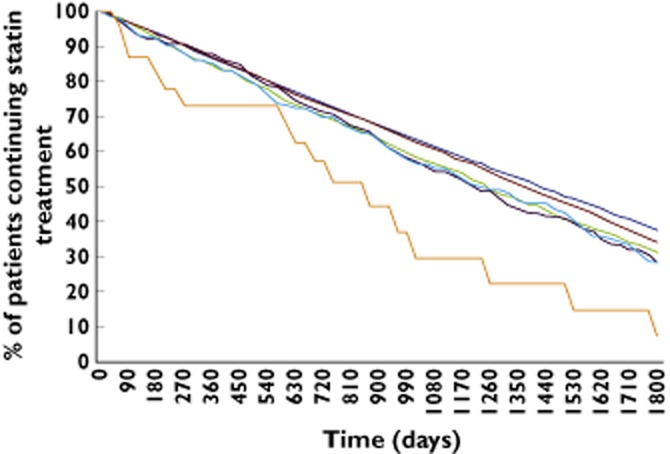
Persistence to statin treatment after the CPK measurement or rhabomyolysis. x axis: Time in days of duration of statin treatment from the first statin prescription after the CPK measurement or rhabomyolysis. y axis: Percentage of patients continuing statin treatment at a time point.  , Normal CPK;
, Normal CPK;  , one to two times ULN;
, one to two times ULN;  , two to three times ULN;
, two to three times ULN;  , three to four times ULN;
, three to four times ULN;  , >four times ULN;
, >four times ULN;  , rhabdomyolysis
, rhabdomyolysis
Table 6 shows the distribution of CPK values after rechallenge to statins in patients with repeat CPK measurements. Of the patients with >four times ULN CPK abnormalities, the repeat CPK measurement during continued statin treatment was within normal range for 54.8% of the patients, increased between one to three times ULN for 32.1% and increased >four times ULN for 13.0%.
Table 6.
CPK values after rechallenge to statins in patients with repeat CPK measurements
| Repeat CPK value | |||
|---|---|---|---|
| Normal CPK | One to three times ULN | >four times ULN | |
| First CPK value | n patients (%) | n patients (%) | n patients (%) |
| Normal CPK | 36822 (96.3%) | 1405 (3.7%) | 14 (0.04%) |
| One to three times ULN | 4838 (57.6%) | 3538 (42.1%) | 28 (0.3%) |
| >four times ULN | 181 (54.8%) | 106 (32.1%) | 43 (13.0%) |
Discussion
This study found that CPK was measured in about 20% of statin users. The frequency of major CPK increases and incidence rate of rhabdomyolysis were found to be low. Statin users with concomitant prescribing of interacting drugs had higher risks of rhabdomyolysis and major CPK increases. Increased risks of these outcomes were also found in rosuvastatin users and patients using higher daily doses (irrespective of type). The frequency of recurrent large CPK abnormalities was also low in patients who were rechallenged with statins.
This study focused on CPK abnormalities rather on GP-recorded symptoms of skeletal muscle complaints. The literature on skeletal muscle complaints is confusing, in part because of a lack of consistent definitions across studies 15. Four different syndromes have been defined: statin myopathy (any muscle complaints related to these drugs, myalgia (muscle complaints) without serum CPK elevations), myositis (muscle symptoms with CPK elevations) and rhabdomyolysis 15. The challenge with the myopathy syndrome is that its frequency may vary with the method for data collection. A study that used data from two different primary care databases in the UK reported a 10-fold difference in the incidence of myopathy in statin users 16. Clinical trials have also reported varying rates. In the Heart Protection Study, 32.9% of statin users and 33.2% of patients on placebo reported muscle pain, while the WOSCOPS study reported frequencies of 3.5% and 3.7%, respectively 17,18. Given that the results in our study were to be used for pharmacogenetic testing, the focus was on laboratory measurements of CPK. Our study is the first large observational study to report on the distribution of different concentrations of CPK in statin users. Few clinical trials have reported on the frequencies of CPK abnormalities below 10 times ULN 15. The frequency of substantially raised CPK was found to be low in the present study and most CPK concentrations were within the normal range or concerned small increases, which is consistent with a previous report 19. Other studies have reported an absolute risk of rhabdomyolysis during statin treatment between 5 and 10 per 100 000 person-years 20–22. In the present study, the incidence of rhabdomyolysis and >10 times CPK abnormalities was 9.1 per 100 000 person-years (the other studies used CPK concentrations in their case assessment 20–22).
A recent review by the FDA found that the incidence of myopathy (as defined by >10 times ULN CPK concentrations with or without unexplained muscle weakness or pain) was approximately three times as high with the 80 mg dose of simvastatin compared with superior LDL cholesterol-lowering doses of rosuvastatin or atorvastatin 23. The recommendation of the FDA was that the 80 mg dose of simvastatin should only be used in patients who have been taking this dose ‘chronically’ (e.g. for 12 months or more) without signs or symptoms of clinically significant muscle toxicity 23. In the UK, 80 mg simvastatin is only prescribed infrequently. We did find higher risks of rhabdomyolysis or >10 times ULN CPK in patients prescribed a daily dose of 80 mg atorvastatin and 20–40 mg rosuvastatin. We did not find major changes in the prescribed dosages of rosuvastatin despite changes in the recommendations for dosing. Further, our data show that the risks of rhabdomyolysis or larger CPK abnormalities were greatest in patients who recently started statin treatment and in patients with a substantive prescribing of other drugs (presumably reflecting a substantial co-morbidity). Our findings of repeat CPK in patients rechallenged to statins are consistent with a recent study that found that most patients who had statin-related adverse events but were rechallenged were still taking a statin 1 year later 24.
It is well known that statins can interact with drugs that are metabolized by the CYP3A4 isoenzyme increasing the risk of myotoxicity. About half of the currently available drugs are metabolized by this enzyme and it is believed that over half of the rhabdomyolysis cases are related to drug interactions 25. The present study confirmed this increased risk of myotoxicity with CYP3A4 interacting drugs. It was also found that the risk of larger CPK abnormalities and rhabdomyolysis was most pronounced in patients taking several drug treatments, and no increased risk in those with few concomitant treatments. With respect to the type of drug, it is interesting to note that concomitant users of allopurinol, clopidogrel and furosemide had substantial risks of myotoxicity. An interaction with clopidogrel and cerivastatin has been reported previously 26. A recent study found increased risks of myotoxicity with concomitant use of erythromycin and statins 27. In this study, we also observed this association although it did not reach statistical significance after adjustment for multiple testing. It has been shown that screening for statin related muscle toxicity is not useful in a primary care setting 19 and this is evidenced by the large number of normal CPK measurements in our study. However, some patients do develop large increases in CPK, which does indicate the need for better targeting of CPK testing and to identify the kinds of patients who would most benefit from testing.
There are several limitations to this study. One limitation is that patients in this study were not screened systematically for CPK but rather tested based on either a clinical indication or usual practice of a GP. It is also possible that patients had CPK checked by the GP when they complained of muscle symptoms, but the GP did not record the complaint in the casenotes. Consistent with this, the likelihood of being tested was found to be substantially higher in statin users with a recent GP record of myopathy and rosuvastatin users (as indicated by the higher odds of having a test with normal CPK concentrations). However these different levels of testing in patients with myopathy and using rosuvastatin were not done with prior knowledge by GPs of the outcome of the CPK test. Our comparison of normal with increased CPK concentrations may be less affected by this bias of differential likelihood of testing. The reporting of laboratory results is typically done electronically, with the results loaded automatically into the electronic health records after review by the GP. Another limitation of this study was that information on alternative causes of CPK abnormalities was incomplete. As an example, there was no information on the level of exercise, which may cause increases in CPK. Lack of information on the actual drug intake and patients’ compliance was a further limitation. Finally, our study only looked at patients on statins with CPK rises, and does not provide any indication of how frequently patients with statins develop muscle pains without any rise in CPK concentrations.
In conclusion, substantive CPK increases and rhabdomyolysis with statin use were particularly seen in patients starting treatment, those on large daily doses or interacting drugs (reflecting higher systemic exposure), in patients on larger numbers of concomitant drugs and on rosuvastatin. However, the overall risk of CPK rise with statin use was low reflecting clinical trial data, while CPK measurements appeared to have been done in a haphazard manner. There is a need to develop better, more evidence-based guidance on measurement of CPK concentrations in patients taking statins.
Competing Interests
All authors have completed the ICMJE uniform disclosure form at http://www.icmje.org/coi_disclosure.pdf and declare no other support from any organization for the submitted work, no financial relationships with any organizations that might have an interest in the submitted work in the previous 3 years; no other relationships or activities that could appear to have influenced the submitted work.
The views expressed in this paper are those of the authors and do not reflect the official policy or position of the MHRA. CPRD is owned by the UK Department of Health and operates within the Medicines and Healthcare products Regulatory Agency (MHRA). CPRD has received funding from the MHRA, Wellcome Trust, Medical Research Council, NIHR Health Technology Assessment programme, Innovative Medicine Initiative, UK Department of Health, Technology Strategy Board, Seventh Framework Programme EU, various universities, contract research organisations and pharmaceutical companies. The Department of Pharmacoepidemiology & Pharmacotherapy, Utrecht Institute for Pharmaceutical Sciences has received unrestricted funding for pharmacoepidemiological research from GlaxoSmithKline, Novo Nordisk, the private-public funded Top Institute Pharma (http://www.tipharma.nl, includes co-funding from universities, government, and industry), the Dutch Medicines Evaluation Board, and the Dutch Ministry of Health.
This study was funded by a grant from the e-Health Initiative funded jointly by the Medical Research Council (reference: MC_qA137929), Wellcome Trust, EPSRC and ESRC. MP is a NIHR Senior Investigator.
References
- Taylor F, Huffman MD, Macedo AF, Moore THM, Burke M, Davey Smith G, Ward K, Ebrahim S. Statins for the primary prevention of cardiovascular disease. Cochrane Database Syst Rev. 2013;(1) doi: 10.1002/14651858.CD004816.pub5. CD004816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez J, Ramirez G, Rundek T, Sacco RL. Statin therapy in the prevention of recurrent cardiovascular events: a sex-based meta-analysis. Arch Intern Med. 2012;172:909–919. doi: 10.1001/archinternmed.2012.2145. [DOI] [PubMed] [Google Scholar]
- Harper CR, Jacobson TA. The broad spectrum of statin myopathy: from myalgia to rhabdomyolysis. Curr Opin Lipidol. 2007;18:401–408. doi: 10.1097/MOL.0b013e32825a6773. [DOI] [PubMed] [Google Scholar]
- Jamal SM, Eisenberg MJ, Christopoulos S. Rhabdomyolysis associated with hydroxymethylglutaryl-coenzyme A reductase inhibitors. Am Heart J. 2004;147:956–965. doi: 10.1016/j.ahj.2003.12.037. [DOI] [PubMed] [Google Scholar]
- Sewright KA, Clarkson PM, Thompson PD. Statin myopathy: incidence, risk factors, and pathophysiology. Curr Atheroscler Rep. 2007;9:389–396. doi: 10.1007/s11883-007-0050-3. [DOI] [PubMed] [Google Scholar]
- Laaksonen R. On the mechanisms of statin-induced myopathy. Clin Pharmacol Ther. 2006;79:529–531. doi: 10.1016/j.clpt.2006.02.013. [DOI] [PubMed] [Google Scholar]
- Chapman MJ, Carrie A. Mechanisms of statin-induced myopathy: a role for the ubiquitin-proteasome pathway? Arterioscler Thromb Vasc Biol. 2005;25:2441–2444. doi: 10.1161/10.1161/01.ATV.0000194548.11901.a4. [DOI] [PubMed] [Google Scholar]
- Owczarek J, Jasińska M, Orszulak-Michalak D. Drug-induced myopathies. An overview of the possible mechanisms. Pharmacol Rep. 2013;57:23–34. [PubMed] [Google Scholar]
- Baker SK. Molecular clues into the pathogenesis of statin-mediated muscle toxicity. Muscle Nerve. 2005;31:572–580. doi: 10.1002/mus.20291. [DOI] [PubMed] [Google Scholar]
- Hanai J, Cao P, Tanksale P, Imamura S, Koshimizu E, Zhao J, Kishi S, Yamashita M, Phillips PS, Sukhatme VP, Lecker SH. The muscle-specific ubiquitin ligase atrogin-1/MAFbx mediates statin-induced muscle toxicity. J Clin Invest. 2007;117:3940–3951. doi: 10.1172/JCI32741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link E, Parish S, Armitage J, Bowman L, Heath S, Matsuda F, Gut I, Lathrop M, Collins R. SLCO1B1 variants and statin-induced myopathy – a genomewide study. N Engl J Med. 2008;359:789–799. doi: 10.1056/NEJMoa0801936. [DOI] [PubMed] [Google Scholar]
- Williams T, van Staa TP, Puri S, Eaton S. Recent advances in the utility and use of the General Practice Research Database as an example of a UK Primary Care Data resource. Ther Adv Drug Saf. 2012;3:89–99. doi: 10.1177/2042098611435911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herrett E, Thomas SL, Schoonen WM, Smeeth L, Hall AJ. Validation and validity of diagnoses in the General Practice Research Database: a systematic review. Br J Clin Pharmacol. 2010;69:4–14. doi: 10.1111/j.1365-2125.2009.03537.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carr DF, O’Meara H, Jorgensen AL, Campbell J, Hobbs M, McCann G, van Staa T, Pirmohamed M. SLCO1B1 genetic variant associated with statin-induced myopathy: a proof of concept study using the clinical practice research datalink (CPRD) Clin Pharmacol Ther. 2013;94:695–701. doi: 10.1038/clpt.2013.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson PD, Clarkson P, Karas RH. Statin-associated myopathy. JAMA. 2003;289:1681–1690. doi: 10.1001/jama.289.13.1681. [DOI] [PubMed] [Google Scholar]
- Molokhia M, McKeigue P, Curcin V, Majeed A. Statin induced myopathy and myalgia: time trend analysis and comparison of risk associated with statin class from 1991–2006. PLoS ONE. 2008;3:e2522. doi: 10.1371/journal.pone.0002522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MRC/BHF Heart Protection Study of cholesterol lowering with simvastatin in 20,536 high-risk individuals: a randomised placebo-controlled trial. Lancet. 2002;360:7–22. doi: 10.1016/S0140-6736(02)09327-3. [DOI] [PubMed] [Google Scholar]
- West of Scotland Coronary Prevention Study: identification of high-risk groups and comparison with other cardiovascular intervention trials. Lancet. 1996;348:1339–1342. [PubMed] [Google Scholar]
- Smith CC, Bernstein LI, Davis RB, Rind DM, Shmerling RH. Screening for statin-related toxicity: the yield of transaminase and creatine kinase measurements in a primary care setting. Arch Intern Med. 2003;163:688–692. doi: 10.1001/archinte.163.6.688. [DOI] [PubMed] [Google Scholar]
- Graham DJ, Staffa JA, Shatin D, Andrade SE, Schech SD, La Grenade L, Gurwitz JH, Chan KA, Goodman MJ, Platt R. Incidence of hospitalized rhabdomyolysis in patients treated with lipid-lowering drugs. JAMA. 2004;292:2585–2590. doi: 10.1001/jama.292.21.2585. [DOI] [PubMed] [Google Scholar]
- Floyd JS, Heckbert SR, Weiss NS, Carrell DS, Psaty BM. Use of administrative data to estimate the incidence of statin-related rhabdomyolysis. JAMA. 2012;307:1580–1582. doi: 10.1001/jama.2012.489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang C-H, Kusama M, Ono S, Sugiyama Y, Orii T, Akazawa M. Assessment of statin-associated muscle toxicity in Japan: a cohort study conducted using claims database and laboratory information. BMJ Open. 2013;3 doi: 10.1136/bmjopen-2012-002040. e002040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Egan A, Colman E. Weighing the benefits of high-dose simvastatin against the risk of myopathy. N Engl J Med. 2011;365:285–287. doi: 10.1056/NEJMp1106689. [DOI] [PubMed] [Google Scholar]
- Zhang H, Plutzky J, Skentzos S, Morrison F, Mar P, Shubina M, Turchin A. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158:526–534. doi: 10.7326/0003-4819-158-7-201304020-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatzizisis YS, Koskinas KC, Misirli G, Vaklavas C, Hatzitolios A, Giannoglou GD. Risk factors and drug interactions predisposing to statin-induced myopathy: implications for risk assessment, prevention and treatment. Drug Saf. 2010;33:171–187. doi: 10.2165/11319380-000000000-00000. [DOI] [PubMed] [Google Scholar]
- Floyd JS, Kaspera R, Marciante KD, Weiss NS, Heckbert SR, Lumley T, Wiggins KL, Tamraz B, Kwok P-Y, Totah RA, Psaty BM. A screening study of drug-drug interactions in cerivastatin users: an adverse effect of clopidogrel. Clin Pharmacol Ther. 2012;91:896–904. doi: 10.1038/clpt.2011.295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patel AM, Shariff S, Bailey DG, Juurlink DN, Gandhi S, Mamdani M, Gomes T, Fleet J, Hwang YJ, Garg AX. Statin toxicity from macrolide antibiotic coprescription: a population-based cohort study. Ann Intern Med. 2013;158:869–876. doi: 10.7326/0003-4819-158-12-201306180-00004. [DOI] [PubMed] [Google Scholar]


