To the Editor:
Early studies showed that up to 80% of children with food allergy demonstrate high spontaneous basophil histamine release (SBHR).1 An association with IgE was suggested because basophils from food-allergic (FA) children additionally released histamine to the IgE-dependent histamine-releasing factor.2 However, the exact mechanism(s) responsible for SBHR, including the dependency for IgE, remains poorly understood. We recently reported that SBHR significantly decreased in children undergoing sublingual immunotherapy/oral immunotherapy (SLIT/OIT) for cow's milk allergy,3 prompting further investigations of this response and other markers of basophil hyperresponsiveness.
Basophils were isolated from the blood of 38 FA (all to cow's milk) and 18 nonallergic children, as described in the Methods section of this article's Online Repository available at www.jacionline.org. Twenty-four children had been followed at baseline and during the SLIT/OIT protocol.3 Thus, baseline values for several basophil parameters investigated in this subgroup are restated herein to demonstrate correlative associations not previously reported. Supporting demographic and clinical information regarding all subjects is summarized in Table E1 of this article's Online Repository available at www.jacionline.org. Diagnosis of milk allergy was based on a convincing history of acute reactivity to cow's milk (rxn+) and a milk-specific IgE level of more than 0.10 KUA/L (cap+). All FA subjects were strictly avoiding milk at the time blood was drawn. Control subjects had no history of acute reaction to any food (rxn−), had never avoided any foods, and were currently tolerating milk in their diet. Although, 3 of the 18 control subjects did have low levels of milk-specific IgE (1.52 KUA/L being the highest), these were less than those in the FA group (median, 52.5 KUA/L; range, 1.9-1108.0 KUA/L).
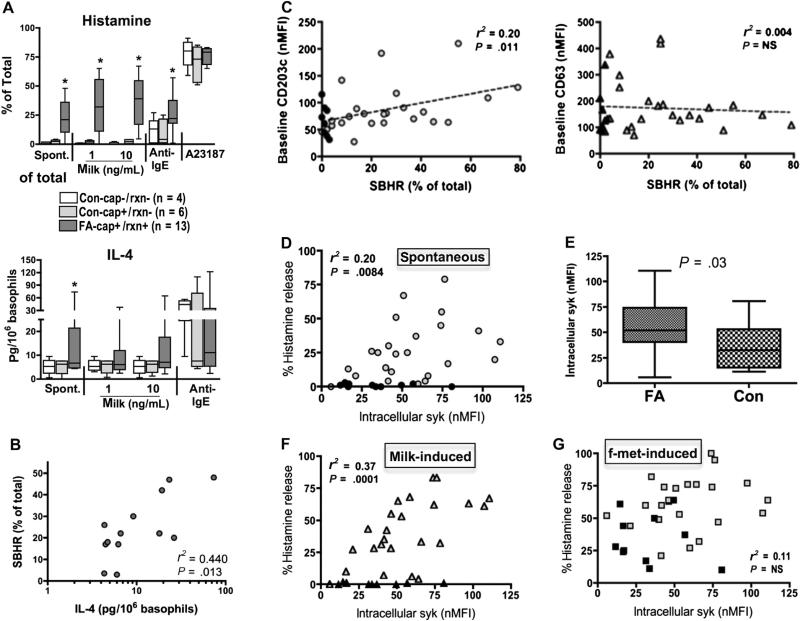
Basophils from 13 FA and 10 control subjects were investigated for histamine and IL-4 responses as shown in Fig 1, A. Eleven of the 13 FA subjects demonstrated SBHR (17%-48%, median of 22%), while this phenotype was not evident in the 10 control subjects, including those testing IgE-positive to milk but having no reaction history (Con−cap+/rxn−). Basophil histamine release (BHR) to milk occurred among the FA subjects but was absent among the controls. All subjects demonstrated BHR to anti-IgE and calcium ionophore (positive controls). Basophils from FA subjects additionally secreted IL-4 spontaneously, with response patterns that mirrored those for histamine. SBHR and spontaneous IL-4 significantly correlated (Fig 1, B; r = 0.66, P = .01), yet neither correlated with total or milk-specific IgE levels (data not shown).
FIG 1.
A, SBHR and IL-4 (spont.), compared with induced, among FA and control (Con) subjects (Cap+-milk-specific IgE; rxn+-clinical reactivity to milk; anti-IgE—an activating anti-IgE; A23187—calcium ionophore). SBHR correlates with IL-4 (B), baselineCD203c (C), and intracellular syk (D) but not CD63 (C). E, Baseline syk also higher in basophils of FA vs Con subjects. F and G, Relationship between syk levels and milk-induced and f-met-induced BHR, respectively. nMFI, Net mean fluorescence intensity; NS, not significant.
We previously found that constitutive expression of CD203c and SBHR decreased in subjects who had undergone SLIT/OIT.3 This prompted our analysis and subsequent discovery that SBHR significantly correlated (although modestly) with constitutive expression of CD203c, but not with CD63 (Fig 1, C). In addition, protein levels of spleen tyrosine kinase (syk)—a marker of IgE-dependent activation,4 showed a similar correlation with SBHR (Fig 1, D) and were approximately 2-fold greater in basophils of FA subjects than in basophils of controls (Fig 1, E). As expected, syk correlated with BHR to milk extract, but not to release induced by f-met—an IgE-independent stimulus (Fig 1, F and G).
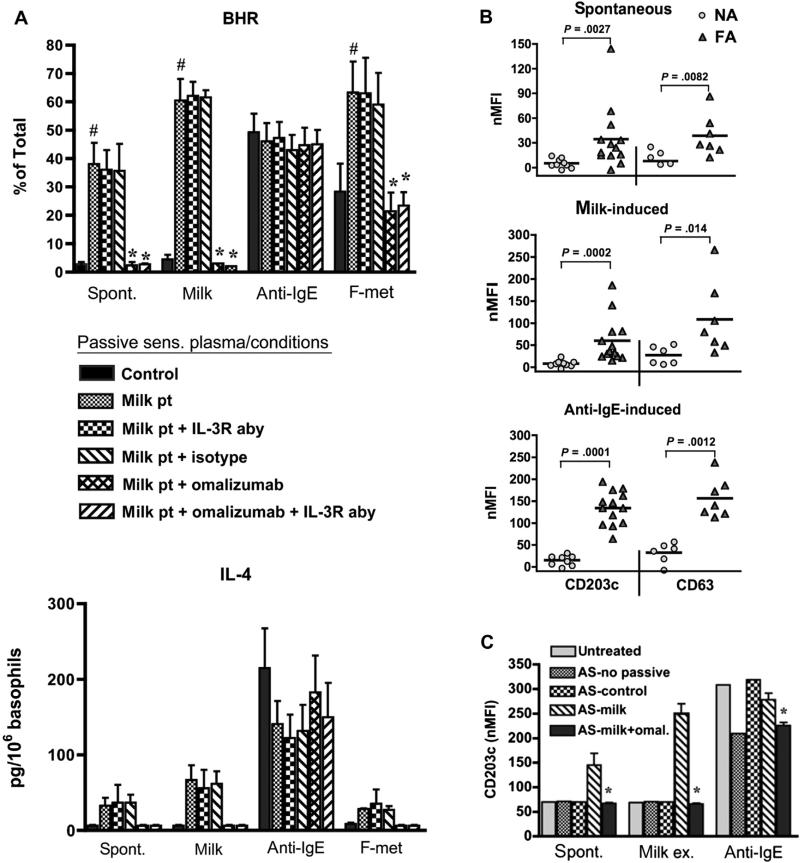
We next asked whether SBHR was a property intrinsic to allergic basophils, or whether this phenotype could be passively transferred to basophils from nonallergic subjects. Remarkably, several markers of basophil hyperresponsiveness were exhibited by normal basophils following passive sensitization using plasma from FA subjects, but not with control plasma spiked with matching amounts (500 ng/mL) of myeloma IgE. Respective values for SBHR (35% ± 8% vs 2.5% ± 0.5% total histamine) and IL-4 (45 ± 5 vs 6 ± 2 pg/106 basophil) are shown in Fig 2, A. Transfer of both responses was ablated by neutralizing IgE with omalizumab during the passive sensitization step whereas neutralizing IL-3 activity—a potent enhancer of basophil responses—did not. As expected, reactivity to milk was likewise transferred to nonallergic basophils and prevented by neutralizing IgE. Responsiveness to goat anti-human IgE antibody was not inhibited by omalizumab, indicating the retention of residual receptor-bound IgE despite lactic acid treatment.
FIG 2.
Markers of hyperresponsiveness are transferred to normal basophils via plasma from FA (milk pt) vs control (NA) subjects and are inhibited by omalizumab. A, Values (mean ± SEM) for BHR (n = 3) and IL-4 secretion (n = 2). #P < .05 (vs control). *P < .05 (vs milk pt + isotype). B and C, Spontaneous and induced CD203c/CD63. *P < .05 (vs AS-milk). AS, Treatment with lactic acid before passive sensitization; NA, nonallergic; nMFI, net mean fluorescence intensity.
We further found that CD203c and CD63 expression on nonallergic basophils spontaneously increased after passively sensitizing with plasma from FA subjects, but not with plasma from nonallergic control subjects (Fig 2, B). Reactivity to milk and anti-IgE was also transferred as expected. This phenomenon was IgE-dependent because the addition of omalizumab during passive sensitization inhibited both the spontaneous induction of CD203 (and CD63—data not shown) and the reactivity to milk (Fig 2, C).
In conclusion, we confirm herein the findings of May,1 who showed high SBHR among FA subjects, and further demonstrate that this phenotype correlates with spontaneous IL-4 secretion, higher constitutive expression of CD203c, and increased levels of intracellular syk. Most significantly, we demonstrate that SBHR and IL-4 secretion, along with increased CD203c expression, are all transferable to basophils of nonallergic subjects via passive sensitization using plasma from FA individuals. Transfer of these markers is completely suppressed by omalizumab, pointing to the requirement of IgE (an extrinsic factor) for these responses. However, SBHR did not correlate with total or milk-specific IgE (data not shown), reflecting evidence that intracellular syk levels are rate limiting in determining basophil sensitivity to IgE-dependent stimulation, not the overall amount of IgE.4
Whether the transfer of SBHR (or other markers) has diagnostic value by predicting whether one is at risk for adverse reactions to milk (or other hypersensitivities) remains to be evaluated, but recent studies suggest that this may be the case. For instance, higher expression of CD203c has been associated with asthma exacerbations,5 and was greater among patients with more severe clinical reactivity to milk.6 OIT and anti-IgE therapy have been shown to reduce CD203c, suggesting that therapeutic interventions can modulate its expression.3,7
IgE/allergen complexes have been implicated in SBHR,8 even though in vitro responses (often exceeding 50% of total) seem incompatible with survival if the same intensity was to be achieved in vivo. In addition, higher expression of CD203c by basophils from allergic subjects may indicate a subthreshold level of activation (or priming), perhaps reflecting histamine release in a manner (eg, piecemeal) distinct from anaphylactic degranulation.9 We hypothesize that an unknown secondary mechanism(s) in vivo prevents full-blown anaphylactic degranulation of basophils, possibly explaining, in part, why such reactions are not more common. This suppressive activity seems lost once the cells are processed ex vivo and cultured, thus allowing subthreshold activation to proceed to full-blown degranulation, with induction of CD63 and SBHR. If so, the exact mechanism(s) preventing this progression in vivo requires further investigation. The findings presented herein could help provide the momentum, and newfound tools, for pursuing this hypothesis.
METHODS
Subjects
All blood specimens were obtained by venipuncture with informed consent by using protocols approved by the institutional review board. Basophils isolated from a total of 38 FA and 18 nonallergic children were investigated for histamine release, with additional markers (eg, IL-4, syk, and CD203c/CD63) also investigated when sufficient numbers of cells were isolated. In particular, basophil responses from 13 of 14 subjects, who were part of a previous study exploring dendritic cell response in children allergic to cow's milk,E1 were investigated for SBHR and IL-4. The remaining subjects consisted of 24 children who were followed at baseline and during a protocol involving SLIT/OIT.E1 Baseline values for several of the basophil parameters investigated in this SLIT/OIT subgroup are restated herein to demonstrate correlative associations not previously reported. Basophils from this group were used to investigate SBHR, CD203c/CD63, and intracellular syk levels. Supporting information regarding all subjects has been previously reported,E1 with pertinent information summarized in Table E1. As described later, plasma specimens were also collected from many of these subjects and were used in the passive sensitization experiments described herein.
For all subjects, the diagnosis of food allergy was based on a convincing history of reaction following exposure to cow's milk and a milk-specific IgE level of more than 0.10 KUA/L (UniCAP; Phadia, Uppsala, Sweden). Milk was strictly being avoided by each subject at the time blood was drawn. Sensitization to foods (defined by food-specific IgE level of >0.35 KUA/L) currently being ingested on a regular basis, current medications, and presence of other allergic diseases (eczema, allergic rhinitis, asthma) were determined by patient and parental interviews and review of medical records. Control subjects had no history of acute reaction to any food, had never avoided any foods, and were currently tolerating milk in their diet.
Cell preparation and culture
Blood specimens anticoagulated with ethylenediaminetetraacetic acid were subjected to double Percoll density centrifugation, as described in detail elsewhere.E2 Briefly, plasma was saved and stored at –20°C. Cells accumulating on the 61% Percoll density consisted of 3% to 15% basophils. These basophil-enriched cells (BECs) were cultured in media alone or stimulated in medium containing crude milk extract (1-10 μg/mL), goat anti-human IgE antibody (1-100 ng/mL), calcium ionophore A23187 (500 ng/mL), or f-Met peptide (10−6 mol/L). Culture medium consisted of Iscove's modified Dulbecco's medium supplemented with 5% FCS, nonessential amino acids, and 10 μg/mL gentamicin (pH, 7.2-7.4). This conditioned medium was chosen over several others (that also supported SBHR) because of its capacity to support cytokine secretion by basophils.E2 Histamine release was measured after 30 minutes and IL-4 release after 4 hours of culture. For the passive transfer experiments using plasma, BEC suspensions from nonallergic donors were used as is (ie, for the CD203c assays), or additionally underwent negative selection by using the Stemsep basophil enrichment cocktail (StemCell Technologies, Vancouver, British Columbia, Canada) to purify basophils (>97%) for the SBHR and IL-4 assays. In both instances, cells were treated with lactic acid solution (pH, 3.9)E3 for 30 seconds on ice to partially remove cell surface–bound IgE, washed, and then passively sensitized for 30 minutes with plasma containing up to 500 ng/mL of total IgE (determined by the Uni-Cap measurements). Control plasma from nonallergic subjects was used in some experiments, and was supplemented with in-house JK myeloma IgE to bring the final concentration to 500 ng/mL. For some conditions, omalizumab (10 μg/mL) and/or IL-3 receptor (α and β subunits)-blocking antibodies (2 μg/mL), or isotype control antibodies were added during the passive sensitization step.
Histamine and cytokine measurements
A portion of each culture supernatant was assessed for histamine by using automated fluorimetry, with values reported as a percentage of total histamine content determined by lysing an equivalent number of basophils, as originally described.E4 The remaining supernatant was assayed for IL-4 by using ELISA (eBioscience, San Diego, Calif).
Flow cytometry
The measurement of intracellular syk protein in basophils was performed as previously described.E5 Surface staining of CD203c and CD63 was performed as previously reported.E6 The expression of all 3 markers is reported as normalized net mean fluorescence intensity defined as the difference between staining with specific antibody and isotype-labeled cells corrected for instrument variability by using CaliBRITE allophycocyanin calibration beads (BD Biosciences, Franklin Lakes, NJ). Basophils within BEC suspensions were identified as IL-3 receptor (CD123)+, blood dendritic cell antigen-2− cells.E5,E7
Serologic measurements
Measurements of total IgE, milk-specific IgE, Fx5 multitest, and the Phadiatop multiallergen screen were performed on plasma by the Johns Hopkins University Dermatology Allergy and Clinical Immunology Reference Laboratory by using a fluorescent-based enzyme immunoassay performed on the ImmunoCAP 250 (Phadia, Kalamazoo, Mich). Phadiatop is a single measurement that detects IgE antibody specific for the 10 most common aeroallergens, and the Fx5 is a single measurement that detects IgE to the 6 most common food allergens (egg, milk, soy, peanut, wheat, and fish).
Statistics
Analyses were performed by using Prism Software (GraphPad Software, San Diego, Calif). Comparisons were by done by using t tests when compared data were normally distributed. Otherwise, comparisons were by done by using the Mann-Whitney U test. Pearson correlations were performed (all data were normally distributed) as indicated. Significant P values defined as less than .05 are shown.
Supplementary Material
Acknowledgments
This study was supported in part by grants R21AI079853 (J.T.S.), R21AI092443 (J.T.S.), and K23 AI091869 (P.A.F.-G.) from the National Institute of Allergy and Infectious Diseases, National Institutes of Health, as well as by a Johns Hopkins Clinician Scientist Award (P.A.F.-G.) and an ARTrust Faculty Development Award (P.A.F.-G.).
J. T. Schroeder has received a grant and travel support from the National Institute of Allergy and Infectious Disease, National Institutes of Health (NIH), and has received other grants from the NIH. D.W. MacGlashan has received grants from the NIH. R. Wood has consultant arrangements with the Asthma and Allergy Foundation of America, is employed by Johns Hopkins University, has received grants from the NIH, and has received royalties from UpToDate. P. A. Frischmeyer-Guerrerio has received grants from the NIH, ARTrust/American Academy of Allergy, Asthma & Immunology, Johns Hopkins University, and the American Lung Association.
Footnotes
Disclosure of potential conflict of interest: The rest of the authors declare that they have no relevant conflicts of interest.
REFERENCES
- 1.May CD. High spontaneous release of histamine in vitro from leukocytes of persons hypersensitive to food. J Allergy Clin Immunol. 1976;58:432–7. doi: 10.1016/0091-6749(76)90124-x. [DOI] [PubMed] [Google Scholar]
- 2.Sampson HA, Broadbent KR, Bernhisel-Broadbent J. Spontaneous release of histamine from basophils and histamine-releasing factor in patients with atopic dermatitis and food hypersensitivity. N Engl J Med. 1989;321:228–32. doi: 10.1056/NEJM198907273210405. [DOI] [PubMed] [Google Scholar]
- 3.Keet CA, Frischmeyer-Guerrerio PA, Thyagarajan A, Schroeder JT, Hamilton RG, Boden S, et al. The safety and efficacy of sublingual and oral immunotherapy for milk allergy. J Allergy Clin Immunol. 2012;129:448–55. 455, e1–5. doi: 10.1016/j.jaci.2011.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.MacGlashan DW., Jr Relationship between spleen tyrosine kinase and phosphatidylinositol 59 phosphatase expression and secretion from human basophils in the general population. J Allergy Clin Immunol. 2007;119:626–33. doi: 10.1016/j.jaci.2006.09.040. [DOI] [PubMed] [Google Scholar]
- 5.Ono E, Taniguchi M, Higashi N, Mita H, Kajiwara K, Yamaguchi H, et al. CD203c expression on human basophils is associated with asthma exacerbation. J Allergy Clin Immunol. 2010;125:483–9. e3. doi: 10.1016/j.jaci.2009.10.074. [DOI] [PubMed] [Google Scholar]
- 6.Ford LS, Bloom KA, Nowak-Wegrzyn AH, Shreffler WG, Masilamani M, Sampson HA. Basophil reactivity, wheal size, and immunoglobulin levels distinguish degrees of cow's milk tolerance. J Allergy Clin Immunol. 2013;131:180–6. e1–3. doi: 10.1016/j.jaci.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gernez Y, Tirouvanziam R, Yu G, Ghosn EE, Reshamwala N, Nguyen T, et al. Baso-phil CD203c levels are increased at baseline and can be used to monitor omalizumab treatment in subjects with nut allergy. Int Arch Allergy Immunol. 2011;154:318–27. doi: 10.1159/000321824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Paganelli R, Levinsky RJ, Brostoff J, Wraith DG. Immune complexes containing food proteins in normal and atopic subjects after oral challenge and effect of sodium cromoglycate on antigen absorption. Lancet. 1979;1:1270–2. doi: 10.1016/s0140-6736(79)92230-x. [DOI] [PubMed] [Google Scholar]
- 9.Macglashan D., Jr Marked differences in the signaling requirements for expression of CD203c and CD11b versus CD63 expression and histamine release in human basophils. Int Arch Allergy Immunol. 2012;159:243–52. doi: 10.1159/000332150. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.