Abstract
Neuroimaging biomarkers have potential role in the early diagnosis as well as periodic follow-up of neurodegenerative diseases such as Alzheimer’s disease (AD). Structural imaging biomarkers can be used to predict those who are at risk or in preclinical stages of AD. It could possibly be useful even in predicting the conversion of Mild Cognitive Impairment (MCI) an early stage of AD to AD. In addition there has been a lot of progress in molecular imaging in AD. This article presents a review of recent progress in selected imaging biomarkers for early diagnosis, classification, and progression, of AD. A comprehensive integrative strategy initiated early in the cognitive decline is perhaps the most effective method of controlling progression to Alzheimer’s disease.
INTRODUCTION
Alzheimer’s disease (AD) is the most common cause of dementia among people aged 60 years and older.1 The prevalence of AD ranges from 6.44% in south India2 to 4.86% in Shanghai,3 China to 3.92% in Sri Lanka for populations above 65 years.4 The main clinical feature of AD is increasing memory impairment followed by impairment of other cognitive domains, a characteristic pathological cortical and hippocampal atrophy, histological feature of senile plaques of amyloid deposits and neurofibrillary tangles consisting of intraneuronal tau fibrillary tangles.5
The prevalence of AD is expected to increase dramatically as the population around the globe continues to age. Better understanding of this dementing disease, therefore, is essential, and early diagnosis combined with a comprehensive management strategy initiated early in the course of the cognitive decline will likely be the most effective method of controlling the progression of AD.6–8 Currently one of the major handicaps towards achieving this is the difficulty in early and definitive diagnosis of AD. Over the past decade there has been a tremendous amount of research output in the field of biomarkers of AD. In this article, we review the current knowledge on structural imaging changes associated with AD. Structural MRI studies and functional studies such as PET and SPECT are being widely researched in the diagnosis of AD.9–11 Structural and functional imaging may be useful for the early diagnosis of AD.12,13 With increasing research in disease modifying therapy in AD and recognition of mild cognitive impairment (MCI) as a very incipient stage of AD, early diagnosis of AD will assist in early initiation of disease modifiying therapy. This in turn will aid in improving the quality of life of patients with AD.
Mild cognitive impairement
MCI is a predementia condition that has been shown to have a high likelihood of progression to AD.14,15 It is characterized by impairment in one domain of cognition with relatively preserved other cognitive domains in the presence of unimpaired functional abilities.16,17 MCI can be categorized as amnestic MCI (aMCI) and non-amnestic MCI (naMCI). aMCI refers to patients who are functionally independent but with impairment in the memory domain. Whereas naMCI includes functionally independent patients with impairment in one or more non-memory domains of cognition such as attention, executive functioning, language and visuospatial processing etc. Some studies suggests that patients with the aMCI subtype have a higher risk of progression to AD.18
NEUROIMAGING BIOMARKERS FOR EARLY DETECTION OF ALZHEIMER’S DISEASE
Neuroimaging biomarkers
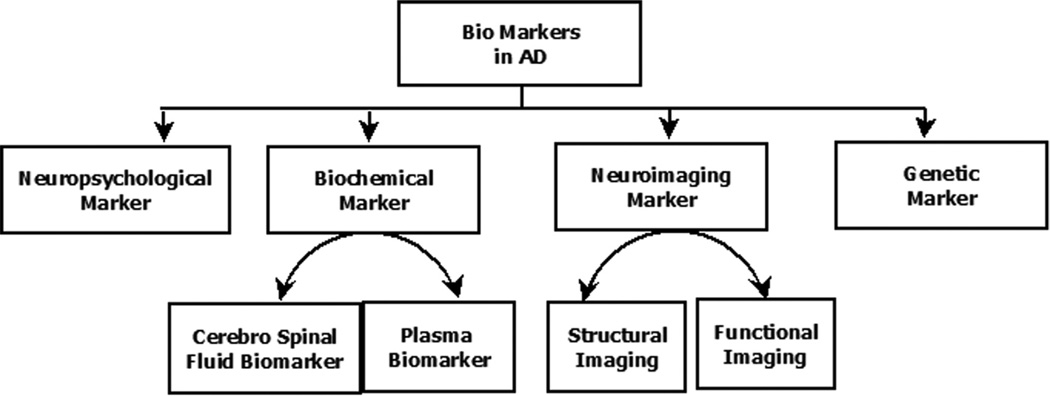
Biomarkers have diagnostic and prognostic value in the early detection of AD. Research is ongoing in a wide spectrum of biomarkers (Figure 1) associated with AD. Among these, possibly neuroimaging has the potential for predicting the transition from MCI to AD.19 Several brain-imaging techniques are often used for studying the neuropathological processes and morphological and functional changes occurring in AD. Neuroimaging methods are helpful not only in the early diagnosis but also in differentiating AD from other neurodegenerative diseases.20 Studies have revealed that imaging techniques can be used for the prediction of conversion of MCI to AD. Neuroimaging techniques can be mainly classified as structural and functional. The main structural imaging techniques are computed tomography (CT) and magnetic resonance imaging (MRI). The CT imaging technique provides high resolution and has the ability to distinguish two structures from each other as separate. However, MRI imaging technique, due to its high spatial resolution can be used to distinguish the difference between two arbitrarily similar but not identical tissues.21,22 Other techniques such as positron emission tomography (PET), single photon emission computed tomography (SPECT), and functional MRI (fMRI) are examples of functional neuroimaging techniques.23 Even though functional imaging technique provides some structural information; their spatial resolution is lower than structural imaging technique.
Figure 1.
A flow chart of different biomarkers that are being researched in the realms of Alzheimers’s disease.
STRUCTURAL IMAGING TECHNIQUES
Computed tomography (CT)
CT is not used as a standard technique for early diagnosis of AD. CT is mainly used to rule out potentially surgically treatable causes of dementia such as tumors and sub-dural hemorrhage, etc. In AD, the CT scan analysis may reveal diffuse cerebral atrophy with enlargement of the cortical sulci and increased size of ventricles. However, these are late changes in AD. Some studies have suggested that, medial temporal lobe atrophy could predict the earlier detection of AD.24–26 The main advantages of this imaging technique is that, it may help in the differential diagnosis of dementia, such as ruling out a paramedian tumor or a normal pressure hydrocephalus. In developing resource constrained nations, it is also less expensive, faster and more widely available than MRI.27 Other than the afore mentioned, CT does not have any role in the early diagnosis of AD.
Structural magnetic resonance imaging
MRI is one of the non-invasive imaging techniques for the structural analysis of AD brains.28 Frisoni and colleagues demonstrated convincingly the phenomenon of medial temporal lobe atrophy as an early marker in AD.29 The decline from normal to MCI and to AD has been investigated mainly using MRI studies.30 Most of the MRI studies demonstrated that atrophy of the medial temporal lobe structures (hippocampus, and entorhinal cortex) is common in AD.31 Structural MRI analysis has demonstrated that medial temporal atrophy is associated with increased risk of developing AD and can predict future memory decline in healthy adults.32 Current research focuses on some of the volumetric analysis techniques for the early detection of AD.33,34 Earliest technique was the visual impression which evolved to manual volumetry and later into automated volumetry. Volumetric analysis of MRI can detect significant changes in the size of brain regions. Regional atrophy measurement during the progress of AD is a potentially promising diagnostics indicator.35,36 The various volumetry assessment tools and methods are listed in Table-1.
Table 1.
Currently available volumetry assessment tools and image analysis methods for Alzheirmer’s disease
| Volumetric method | Specifications |
|---|---|
| Manual tracing | It is used for image processing analysis and visualization. |
| Voxel based morphometry (VBM) | Automated technique for investigation of focal differences in brain anatomy. |
| Individual brain atlases using statistical parametric mapping (IBASPM) | Automatically segmenting cerebral structures and computing the volume of gross anatomical structures |
| The insight segmentation and registration toolkit. - SNake automatic partitioning (ITK – SNAP) | Semi-automated 3-D brain segmentation technique |
| Free surfer | To automatically identify and measure brain regions. |
| Functional magnetic resonance imaging of the brain (FMRIB) software library (FSL) | Analysis tools for fMRI MRI, and DTI brain imaging data |
| Statistical parametric mapping (SPM) | Automated analysis of brain imaging data |
| MarsBar | It is used for ROI analysis and statistical analysis of ROI data |
| Medical image processing analysis and visualization (MIPAV) | It is used for image processing analysis and visualization. |
MRI, Magnetic resonance imaging; fMRI, Functional MRI; ROI, Region of interest; DTI, Diffusion tensor imaging.
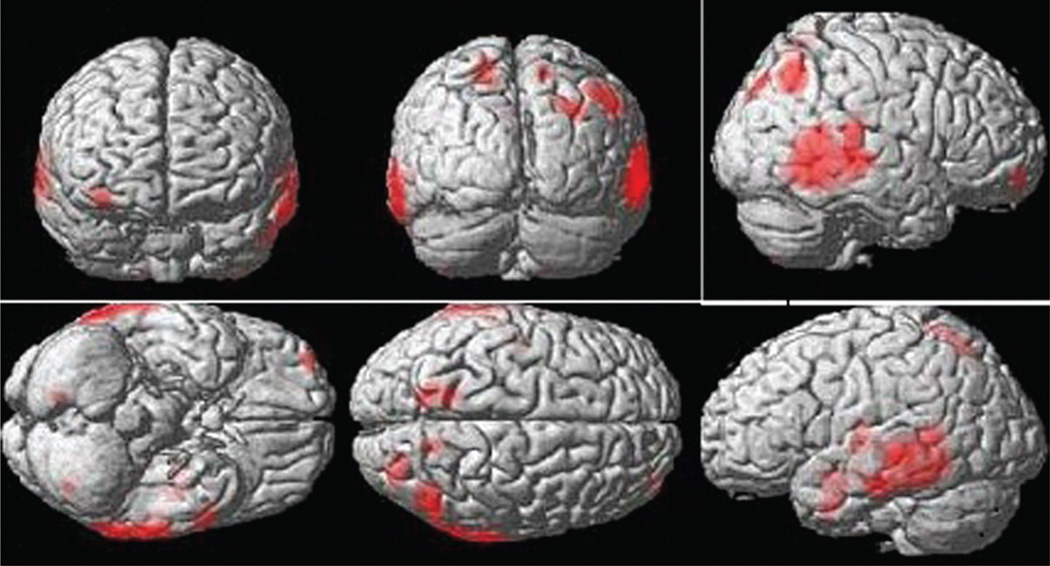
Volumetric methods are quantitative and have been used to demonstrate significant differences in volumes of specific medial temporal lobe structures, such as the hippocampus and amygdala among cognitive diagnostic groups.27,37,38 The manual volumetric technique requires an excellent working knowledge of neuroanatomy as well as good skill in delineating regions of interest (ROI). Manual volumetry is a very time consuming process compared to automated volumetric methods. Voxel based morphometry (VBM) is an automatic volumetric approach, which allows distinguishing between healthy controls and patients (Figure 2) based on the volume of brain and region of interest.39–41 IBASPM and MarsBar are the toolboxes of SPM to distinguish an AD group from a normal control group. Free surfer and FMRIB Software Library (FSL) (http://www.fmrib.ox.ac.uk) make available brain region templates for the automated methods that can detect hippocampal atrophy in patients and provide information about the shape, position or volume of brain tissue.40
Figure 2.
Results of voxel based morphometry (VBM) showing atrophic areas in patients with Alzheimers’s disease.
Recent VBM techniques have demonstrated that in subjects who converted from MCI to clinical AD, atrophy not only involved the MTL but also widespread neocortical regions when compared to the cognitively stable MCI subjects.37
Diffusion tensor imaging
Diffusion tensor imaging (DTI) is the advanced MR Imaging technique, which uses MRI to measure non-random movement of water molecules. It can thus track the fibers of tracts within the brain. There are reports that DTI can detect early microstructural alterations in AD patients before gross anatomic alterations become visible, alterations, which are generally not detected by conventional MRI.42 In DTI, the main parameters measured are Fractional Anisotropy (FA), apparent diffusion coefficient (ADC) and mean diffusivity (MD). Fiber tract integrity provides a direct assessment of white matter fibers and could be potentially used as a biomarker for AD.43 Anisotropy means the movement of water molecules is greater lengthwise along neural tract relative to their movement across tract width. In any disruption in the axon, water molecules moves more randomly through the tissues causes a reduction in anisotropy that characterizes AD. ADC describes the interaction of the diffusing molecules with cellular structures and to detect the overall diffusivity. The characteristic of mean diffusivity is same as that of ADC characteristics. The importance of DTI in neuroimaging is to be able to provide ROI analysis, three dimensional visualization, color mapping, fiber tracking and computerized approach for tensor calculation.44,45 A clinical application of DTI could be to assess the differential diagnosis of dementia (AD and vascular dementia) using the DTI based tensor maps.46 Region based DTI analysis results shows that hippocampal microstructural changes could be a better predictor of risk of progression of MCI to AD.47 In DTI it is possible to analyze the connectivity and plastic organization of the human brain. Some reports suggest that the main DTI parameters FA and ADC values could be possibly used as AD biomarkers.48,49
FUNCTIONAL IMAGING TEACHNIQUES
Functional MRI (fMRI)
Functional MRI (fMRI) is a noninvasive imaging technique for functional analysis. This neuroimaging technique could be used to monitor AD patient’s treatment.50 fMRI is a useful method to characterize the functional abnormalities in dementia subjects. It measures the oxygen concentration of certain specific brain areas corresponding to particular stimuli or cognitive tasks.51,52 This technique has high spatial and temporal resolutions. In brief the principle of fMRI consists of acquisition of brain images during a specific brain activity and in a basal state. During analysis, the basal state activity is subtracted from the specific task activity to yield the specific brain areas where blood flow had increased due to the blood oxygen level dependent (BOLD) response53 during the performance of the specific task by the brain. Resting and activation state of fMRI studies show that AD patients have lesser-coordinated activity in the hippocampus, inferior parietal lobes and cingulate cortex compared with healthy controls.50,54 Recent advances in fMRI have helped to identify the neural substrate for cognitive behavioral functions in early phases of neurodegenerative disorder and correlated them with neuroanatomical network.55
Molecular and beta-amyloid imaging
Molecular imaging technique aims to diagnose AD in its earliest stages. This technique determines the disease changes at molecular level in the brain The results of several clinical trials indicates that PET imaging of amyloid plaques can identify patients destined to develop AD several years before the development of dementia.56,57 Recently, radiotracers for amyloid plaques have shown the presence and extent of plaques in the brain.58 This could allow for early detection of AD. Direct imaging of amyloid load in a brain with AD would be useful for the early diagnosis of AD and the development and assessment of new treatment.59 Currently used molecular imaging compounds in AD research include the following.60
Pittsburgh compound B
Pittsburgh compound B (PIB) was the first radiotracer used by Klunk and colleagues for highlighting deposits of beta amyloid plaques.61 It helps to visualize the pathological hallmark of AD in living individuals during a PET scan. PIB is a fluorescent analog of thioflavin T, which can be used in PET scans to image beta amyloid plaques in neuronal tissue. PIB may be used in investigational studies of AD.62,63
Florbetapir F 18
Florbetapir F 18 (18F-AV-45) is a novel radiotracer. It is a novel tracer for PET imaging of β amyloid plaques in the brain of AD patients.64
Florbetaben
Florbetaben (BAY 94-9172) is a radiotracer designed to detect beta amyloid during PET scan.
18F flutemetamol
18F flutemetamol (Flute) is a radiotracer for PET scan that is structurally identical to PIB except for one fluorine atom in place of a carbon atom.
PIB-PET technique is the most extensively and best validated tracer. Negative amyloid scans indicate absence of AD with high accuracy, but healthy elderly controls have positive amyloid scans whose predictive value in isolation is less clear. PIB-PET is a powerful imaging technique, to examine the relationship between clinical symptoms, amyloid deposition and structural and functional brain changes between the controls and AD patients.65,66 Accumulation of the β amyloid peptide in the brain is a hallmark of AD. Amyloid imaging can identify patients with MCI who have A-beta aggregation in the early stage (Figure 3). Amyloid imaging consists of an injection of a radiolabel led ligand targeting amyloid aggrates and use of PET technology to acquire images of the brain in order to display foci of abnormal amyloid accumulation. This technique will possibly provide an increase in the diagnostic accuracy of AD in the near future. In future, amyloid imaging will possibly be considered as a biomarker for AD and this can be useful for drug development and to clarify their prognostic and diagnostic power in relation to age, risk factors, and AD subtypes.58,67 There is evidence, however, from the Australian Imaging Biomarkers and Lifestyle (AIBL) study, that the total amyloid burden alone does not alone play a role in hippocampal atrophy and cognitive decline68, just as there is evidence that that the amyloid burden in the temporal neocortex is related to hippocampal atrophy even in nondemented elderly subjects.69
Figure 3.
FDG and PIB PET images for Alzheimers’s disease patient and normal controls (Modified from Mistur et al.73)
Positron emission tomography
Positron emission tomography (PET) is a powerful imaging technique, has been used to investigate functional alterations of the brain both in healthy and ill subjects. PET scanning is a molecular imaging technique that allows physicians to obtain three- dimensional images of what is happening in a patient’s brain at the molecular and cellular level.70 Studies have suggested that PET is very accurate at diagnosing AD and differentiating it from other dementias. Regional cerebral metabolism studies with PET have used 18F-2fluoro-2-deoxy-D-glucose (FDG) as a metabolic marker in early diagnosis and preclinical detection of dementia. FDG PET measures the glucose metabolism in different regions of the brain.71 This technique can be used to identify the causes of dementia and to have a differential diagnosis, as well as, predict conversion to AD in patients with MCI.72 On FDG-PET examinations, severe reductions in the rate of brain glucose consumptions in AD patients as compared to normal elderly have been shown.73 FDG –PET studies report that AD patients show regional metabolic reductions involving the parietal-temporal and posterior cingulate cortices and the frontal areas in advanced disease.74 It has been assumed that metabolic change associated with neocortical dysfunctions may be detectable by FDG-PET before atrophy appears.23 FDG-PET provides closer relation to clinical symptoms, but it is less sensitive in preclinical disease PIB-PET imaging seems more capable to detect early changes in progression of AD than FDG-PET imaging given its correlation with measures of cognitive testing and hippocampal atrophy by MRI. In future a combination of FDG and PIB-PET technique may be more useful in predicting short-term conversion to AD.75 In AD, FDG-PET imaging techniques often showed the reduced use of glucose in brain areas which are important in memory, learning and problem solving.
Magnetic resonance spectroscopy (MRS)
Magnetic resonance spectroscopy (MRS) is a non-invasive imaging technique for assessing metabolic and molecular correlates of dementia. It provides vital biological information at the molecular level. MRS technique can predict future progression to AD in patients with MCI and tracking disease progression. In future H1 MRS has a potential role in early and differential diagnosis of dementia.76 Proton MRS detect normal metabolic pattern in patients with mild neurological impairment and severe brain abnormalities. However quantitative measurement of regional brain volume can be useful in the diagnosis of dementia. Proton MRS can provide useful information to differentiate between AD and other dementias. Magnetic resonance spectroscopy has proven useful to monitor disease progression in early AD.77 MRS of the hippocampus and other cortical areas could predict conversion from amnestic mild cognitive impairment to probable AD. Pedro and colleagues suggests that H-MRS of the occipital cortex may be valuable tool in predicting conversion from MCI to probable AD.
Single photon emission computed tomography (SPECT)
SPECT is a molecular imaging technique; It uses highly targeted radiotracers to detect cellular or chemical changes linked to specific disease. The technique for evaluation of brain perfusion uses a rotating gamma camera. SPECT imaging aids in the differential diagnosis of patient with dementia.78 SPECT imaging could serve as a tool to make accurate diagnosis and measure the progression of changes in the brain. Beta amyloid imaging technology can discriminate individuals with AD. The amount of beta amyloid protein detected in the brain reflect the severity of symptoms of memory loss.79 The combination of SPECT and PET Imaging studies could help to identify and evaluate patients from early to late in the disease.80,81 The development of specific imaging agents for direct mapping of A-beta plaques in the living brain is a great challenge. Currently the use of PIB in combination with PET allows the imaging of beta amyloid plaques in the brain that are indicative of AD.82 18Fflutemetamol combined with PET imaging can detect probable early stage AD and MCI. Amyloid imaging may help in the early detection of the disease. SPECT is a cheaper method than PET but less specific.
Magnetic encephalography (MEG)
Magnetic encephalography (MEG) is a noninvasive neuroimaging technique for measuring the magnetic field patterns generated by the brain. Hence, it may help to understand the relationship between brain function and cognition.83 It is possible that future research could reveal that in AD, MEG could serve as a potential biomarker and could be used for the differential diagnosis. Moreover, MEG shows particular promise in predicting the development of MCI from normal cognition. Some studies have reported that individuals with subjective memory complaints showed higher MEG activation than controls.84 Spontaneous MEG activity shows increased slow rhythms and reduced fast activity in AD patients compared with healthy subjects.85 Some studies suggest that the presence of low frequency magnetic activity associated with AD pathology. Studies suggested that low frequency in temporoparietal regions plays a key role in the transition of MCI to AD. Studies estimated the MEG oscillations to detect changes in subject with MCI in the earliest preclinical stages of dementia. MEG is capable of detecting alterations in the functional organizations of the central nervous system. MCI subject has showed decreased mean frequency MEG power spectrum as compared to healthy controls and AD patients.86–88
CONCLUSION
The development of neuroimaging technique for AD has the ability to detect clinical or pathological change overtime. Neuroimaging techniques have important role in research and clinical practice. Advances in structural and functional neuroimaging techniques allow detection of AD, years before the symptoms of dementia develop. A recent major advance is the development of amyloid imaging techniques that allows in vivo identification of amyloid deposition in the brain. Longitudinal structural and functional imaging studies seem currently most robust to evaluate progressive impairment in MCI and AD. However, from the perspective of developing countries of the many technologies available, CT head scan and structural MRI imaging are the most useful, widely available and affordable imaging modalities.
ACKNOWLEDGEMENTS
Funding: National Institute on Aging (NIA), USA (grant no. R21AG029799 and R01AG039330-01) to PSM.
Footnotes
DISCLOSURE
Conflict of interest: None.
REFERENCES
- 1.Bischkopf J, Busse A, Angermeyer MC. Mild cognitive impairment – a review of prevalence, incidence and outcome according to current approaches. Acta Psychiatr Scand. 2002;106:403–414. doi: 10.1034/j.1600-0447.2002.01417.x. [DOI] [PubMed] [Google Scholar]
- 2.Mathuranath PS, Cherian PJ, Mathew R, et al. Dementia in Kerala, South India: prevalence and influence of age, education and gender. Int J Geriatr Psychiatry. 2010;25:290–297. doi: 10.1002/gps.2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zhang MY, Katzman R, Salmon D, et al. The prevalence of dementia and Alzheimer’s disease in Shanghai, China: impact of age, gender, and education. Ann Neurol. 1990;27:428–437. doi: 10.1002/ana.410270412. [DOI] [PubMed] [Google Scholar]
- 4.de Silva HA, Gunatilake SB, Smith AD. Prevalence of dementia in a semi-urban population in Sri Lanka: report from a regional survey. Int J Geriatr Psychiatry. 2003;18:711–715. doi: 10.1002/gps.909. [DOI] [PubMed] [Google Scholar]
- 5.Humpel C. Identifying and validating biomarkers for Alzheimer’s disease. Trends Biotechnol. 2011;29:26–32. doi: 10.1016/j.tibtech.2010.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Wattamwar PR, Mathuranath PS. An overview of biomarkers in Alzheimer’s disease. Ann Indian Acad Neurol. 2010;13:S116–S123. doi: 10.4103/0972-2327.74256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Landau SM, Harvey D, Madison CM, et al. Comparing predictors of conversion and decline in mild cognitive impairment. Neurology. 2010;75(3):230–238. doi: 10.1212/WNL.0b013e3181e8e8b8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Buckner RL, Snyder AZ, Shannon BJ, et al. Molecular, structural, and functional characterization of Alzheimer’s disease: evidence for a relationship between default activity, amyloid, and memory. J Neurosci. 2005;25:7709–7717. doi: 10.1523/JNEUROSCI.2177-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ortiz-Teran L, Santos JMR, Cabrera Martin MDL, Ortiz Alonso T. Currently available neuroimaging approaches in Alzheimers disease early diagonosis. In: De La Monte S, editor. The clinical spectrum of Alzheimer’s disease - The charge toward comprehensive diagnostic and therapeutic strategies. New York: InTech; 2011. pp. 147–180. [Google Scholar]
- 10.Barber RC. Biomarkers for early detection of Alzheimers disease. J Am Osteopath Assoc. 2010;8:110–119. [PubMed] [Google Scholar]
- 11.Zhang L, Chang R, Chu L-W, Mak K-F. Current neuroimaging techniques in Alzheimer’s disease and applications in animal models. Am J Nucl Med Mol Imaging. 2012;2:386–404. [PMC free article] [PubMed] [Google Scholar]
- 12.Ferreira LK, Busatto GF. Neuroimaging in Alzheimer’s disease: current role in clinical practice and potential future applications. Clinics (Sao Paulo) 2011;66(Suppl 1):19–24. doi: 10.1590/S1807-59322011001300003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Masdeua JC, Zubietab JL, Javier A. Neuroimaging as a marker of the onset and progression of AD. J Neurol Sci. 2005;236:55–64. doi: 10.1016/j.jns.2005.05.001. [DOI] [PubMed] [Google Scholar]
- 14.Karas GB, Scheltens P, Rombouts SA, et al. Global and local gray matter loss in mild cognitive impairment and Alzheimer’s disease. Neuroimage. 2004;23:708–716. doi: 10.1016/j.neuroimage.2004.07.006. [DOI] [PubMed] [Google Scholar]
- 15.Burns A, Zaudig M. Mild cognitive impairment in older people. Lancet. 2002;360:1963–1965. doi: 10.1016/S0140-6736(02)11920-9. [DOI] [PubMed] [Google Scholar]
- 16.Petersen RC. Mild cognitive impairment: Aging to Alzheimers disease. N Engl J Med. 2003;349:15–39. [Google Scholar]
- 17.Petersen RC, Smith GE, Waring SC, Ivnik RJ, Tangelos E, Kokmen E. Mild cognitive impairment: clinical characterization and outcome. Arch Neurol. 1999;56:303–308. doi: 10.1001/archneur.56.3.303. [DOI] [PubMed] [Google Scholar]
- 18.McEvoy LK, Fennema-Notestine C, Roddey JC, et al. Alzheimers disease: Quantitative structural neuroimaging for detection and prediction of clinical and structural changes in MCI. Radiology. 2009;251:195–205. doi: 10.1148/radiol.2511080924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hampel H, Frank R, Broich K, et al. Biomarkers for Alzheimer’s disease: academic, industry and regulatory perspectives. Nat Rev Drug Discov. 2010;9:560–574. doi: 10.1038/nrd3115. [DOI] [PubMed] [Google Scholar]
- 20.Westman E, Simmons A, Zhang Y, et al. Multivariate analysis of MRI data for Alzheimer’s disease, mild cognitive impairment and healthy controls. Neuroimage. 2011;54(2):1178–1187. doi: 10.1016/j.neuroimage.2010.08.044. [DOI] [PubMed] [Google Scholar]
- 21.Wolz R, Julkunen V, Koikkalainen J, et al. Multi-method analysis of MRI images in early diagnostics of Alzheimer’s disease. PLoS One. 2011;6:e25446. doi: 10.1371/journal.pone.0025446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Perrin RJ, Fagan AM, Holtzman DM. Multimodal techniques for diagnosis and prognosis of Alzheimer’s disease. Nature. 2009;461:916–922. doi: 10.1038/nature08538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Walhovd KB, Fjell AM, Brewer J, et al. Combining MR imaging, positron-emission tomography, and CSF biomarkers in the diagnosis and prognosis of Alzheimer disease. Am J Neuroradiol. 2010;31:347–354. doi: 10.3174/ajnr.A1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Desikan RS, Cabral HJ, Christopher P, et al. Automated MRI measures identify individuals with MCI and AD. Brain. 2009;132:2048–2057. doi: 10.1093/brain/awp123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Devanand DP, Bensal R, Liu J, Hao X, Pradhaban G, Peterson BS. MRI hippocampal and entorhinal cortex mapping in predicting conversion to AD. Neuroimage. 2012;60:1622–1629. doi: 10.1016/j.neuroimage.2012.01.075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Devanand DP, Pradhaban G, Liu X, et al. Hippocampal and entorhinal atrophy in mild cognitive impairment: prediction of Alzheimer disease. Neurology. 2007;68:828–836. doi: 10.1212/01.wnl.0000256697.20968.d7. [DOI] [PubMed] [Google Scholar]
- 27.Duchesne S, Caroli A, Geroldi C, Barillot C, Frisoni GB, Collins DL. MRI-based automated computer classification of probable AD versus normal controls. IEEE Trans Med Imaging. 2008;27:509–520. doi: 10.1109/TMI.2007.908685. [DOI] [PubMed] [Google Scholar]
- 28.Jack CR, Jr, Bernstein MA, Fox NC, et al. The Alzheimer’s Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging. 2008;27:685–691. doi: 10.1002/jmri.21049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Frisoni GB, Fox NC, Clifford R, Jack CR, Jr, Scheltens P, Thompson PM. The clinical use of structural MRI in AD. Nat Rev Neurol. 2010;6(2):67–77. doi: 10.1038/nrneurol.2009.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Vemuri P, Gunter JL, Senjem ML, et al. Alzheimer’s disease diagnosis in individual subjects using structural MR images: validation studies. Neuroimage. 2008;39:1186–1197. doi: 10.1016/j.neuroimage.2007.09.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Colliot O, Chetelat G, Chupin M, et al. Discrimination between Alzheimer disease, mild cognitive impairment, and normal aging by using automated segmentation of the hippocampus. Radiology. 2008;248:194–201. doi: 10.1148/radiol.2481070876. [DOI] [PubMed] [Google Scholar]
- 32.Dickerson BC, Stoub TR, Shah RC, et al. Alzheimer-signature MRI biomarker predicts AD dementia in cognitively normal adults. Neurology. 2011;76:1395–1402. doi: 10.1212/WNL.0b013e3182166e96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fleisher AS, Sun S, Taylor C, et al. Volumetric MRI vs clinical predictors of Alzheimer disease in mild cognitive impairment. Neurology. 2008;70:191–199. doi: 10.1212/01.wnl.0000287091.57376.65. [DOI] [PubMed] [Google Scholar]
- 34.Smith AD, Jobst KA. Use of structural imaging to study the progression of AD. Br Med Bull. 1996;52:575–586. doi: 10.1093/oxfordjournals.bmb.a011568. [DOI] [PubMed] [Google Scholar]
- 35.Fennema-Notestine C, Hagler DJ, Jr, McEvoy LK, et al. Structural MRI biomarker for Preclinical and mild Alzheimers disease. Hum Brain Mapp. 2009;30(10):3238–3253. doi: 10.1002/hbm.20744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Robertsson B, Nordström M, Wijk H. Investigating poor insight in AD: A survey research approaches. Dementia. 2007;6:44–61. [Google Scholar]
- 37.Risacher SL, Shen L, West JD, et al. Longitudinal MRI atrophy biomarkers: relationship to conversion in the ADNI cohort. Neurobiol Aging. 2009;31:1401–1418. doi: 10.1016/j.neurobiolaging.2010.04.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Gerardin E, Chetelat G, Chupin M, et al. Multidimensional classification of hippocampal shape features discriminates Alzheimer’s disease and mild cognitive impairment from normal aging. Neuroimage. 2009;47:1476–1486. doi: 10.1016/j.neuroimage.2009.05.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mechelli A, Price CJ, Friston KJ, Ashburner J. Voxel-based morphometry of the human brain: Methods and applications. Current Medical Imaging Reviews. 2005;1:1–9. [Google Scholar]
- 40.Ashburner J, Friston KJ. Voxel-based morphometry--the methods. Neuroimage. 2000;11:805–821. doi: 10.1006/nimg.2000.0582. [DOI] [PubMed] [Google Scholar]
- 41.Ishii K, Kawachi T, Sasaki H, et al. Voxel-based morphometric comparison between early- and late-onset mild Alzheimer’s disease and assessment of diagnostic performance of z score images. Am J Neuroradiol. 2005;26:333–340. [PMC free article] [PubMed] [Google Scholar]
- 42.Masutani Y, Aoki S, Abe O, Hayashi N, Otomo K. MR diffusion tensor imaging: recent advance and new techniques for diffusion tensor visualization. Eur J Radiol. 2003;46:53–66. doi: 10.1016/s0720-048x(02)00328-5. [DOI] [PubMed] [Google Scholar]
- 43.Vasconcelos LdG, Brucki SMD, Jackowiski AP, Bueno OFA. Diffusion tensor imaging for Alzheimers disease. Dement Neuropsychol. 2009;3:268–274. doi: 10.1590/S1980-57642009DN30400002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chua TC, Wen W, Slavin MJ, Sachdev PS. Diffusion tensor imaging in mild cognitive impairment and Alzheimer’s disease: a review. Curr Opin Neurol. 2008;21:83–92. doi: 10.1097/WCO.0b013e3282f4594b. [DOI] [PubMed] [Google Scholar]
- 45.Sundgren PC, Dong Q, Gomez-Hassan D, Mukherji SK, Maly P, Welsh R. Diffusion tensor imaging of the brain: review of clinical applications. Neuroradiology. 2004;46(5):339–350. doi: 10.1007/s00234-003-1114-x. [DOI] [PubMed] [Google Scholar]
- 46.Alves GS, Sudo FK, Alves CEO, et al. Diffusion tensor imaging studies in vascular disease. A review of the literature. Dement Neuropsychol. 2012;6:158–163. doi: 10.1590/S1980-57642012DN06030008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Clerx L, Visser PJ, Verhey F, Aalten P. New MRI markers for Alzheimer’s disease: A meta-analysis of diffusion tensor imaging and a comparison with medial temporal lobe measurements. J Alzheimer Dis. 2012;29:1–9. doi: 10.3233/JAD-2011-110797. [DOI] [PubMed] [Google Scholar]
- 48.Medina D, DeToledo-Morrell L, Urresta F, et al. White matter changes in mild cognitive impairment and AD: A diffusion tensor imaging study. Neurobiol Aging. 2006;27(5):663–672. doi: 10.1016/j.neurobiolaging.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 49.Le Bihan D, Mangin JF, Poupon C, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 50.Wang K, Liang M, Wang L, et al. Altered functional connectivity in early Alzheimer’s disease: a resting-state fMRI study. Hum Brain Mapp. 2007;28:967–978. doi: 10.1002/hbm.20324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Tripoliti EE, Fotiadis DI, Argyropoulou M, Manis G. A six stage approach for the diagnosis of the Alzheimer’s disease based on fMRI data. J Biomed Inform. 2010;43:307–320. doi: 10.1016/j.jbi.2009.10.004. [DOI] [PubMed] [Google Scholar]
- 52.Wagner AD. Early detection of Alzheimer’s disease: an fMRI marker for people at risk? Nat Neurosci. 2000;3:973–974. doi: 10.1038/79904. [DOI] [PubMed] [Google Scholar]
- 53.Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer’s disease. Ann NY Acad Sci. 2007;1097:146–155. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- 54.Tripoliti EE, Fotiadis DI, Argyropoulou M. A supervised method to assist the diagnosis and classification of the status of Alzheimer’s disease using data from an fMRI experiment. 30th Annual International Conference of the IEEE; 2008; 20-24 August, 2008; Vancouver, Canada: IEEE; 2008. pp. 4419–4422. [DOI] [PubMed] [Google Scholar]
- 55.He Y, Wang L, Zang Y, et al. Regional coherence changes in the early stages of Alzheimer’s disease: a combined structural and resting-state functional MRI study. Neuroimage. 2007;35:488–500. doi: 10.1016/j.neuroimage.2006.11.042. [DOI] [PubMed] [Google Scholar]
- 56.Ellis KA, Rowe CC, Szoeke CE, et al. Advances in structural and molecular neuroimaging in Alzheimer’s disease. Med J Aust. 2011;194:S20–S23. doi: 10.5694/j.1326-5377.2011.tb02938.x. [DOI] [PubMed] [Google Scholar]
- 57.Bolmont T, Haiss F, Eicke D, et al. Dynamics of the microglial/amyloid interaction indicate a role in plaque maintenance. J Neurosci. 2008;28:4283–4292. doi: 10.1523/JNEUROSCI.4814-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Mosconi L, Tsui WH, Herholz K, et al. Multicenter standardized 18F-FDG PET diagnosis of mild cognitive impairment, Alzheimer’s disease, and other dementias. J Nucl Med. 2008;49:390–398. doi: 10.2967/jnumed.107.045385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Phelps ME. Positron emission tomography provides molecular imaging of biological processes. Proc Natl Acad Sci. 2000;97:9226–9233. doi: 10.1073/pnas.97.16.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Hefendehl JK, Milford D, Eicke D, et al. Repeatable target localization for long-term in vivo imaging of mice with 2-photon microscopy. J Neurosci Methods. 2012;205:357–363. doi: 10.1016/j.jneumeth.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 61.Klunk WE, Engler H, Nordberg A, et al. Imaging brain amyloid in Alzheimer’s disease with Pittsburgh Compound-B. Ann Neurol. 2004;55:306–319. doi: 10.1002/ana.20009. [DOI] [PubMed] [Google Scholar]
- 62.Jack CR, Lowe VJ, Senjem ML, et al. 11 C PiB and structural MRI provide complementary information in imaging of AD and MCI. Brain. 2008;131:665–680. doi: 10.1093/brain/awm336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Silverman DH, Gambhir SS, Huang HW, et al. Evaluating early dementia with and without assessment of regional cerebral metabolism by PET: a comparison of predicted costs and benefits. J Nucl Med. 2002;43:253–266. [PubMed] [Google Scholar]
- 64.Villemagne VL, Ong K, Mulligan RS, et al. Amyloid imaging with (18)F-florbetaben in Alzheimer disease and other dementias. J Nucl Med. 2011;52:1210–1217. doi: 10.2967/jnumed.111.089730. [DOI] [PubMed] [Google Scholar]
- 65.Rowe CC, Ackerman U, Browne W, et al. Imaging of amyloid beta in Alzheimer’s disease with 18F-BAY94-9172, a novel PET tracer: proof of mechanism. Lancet Neurol. 2008;7:129–135. doi: 10.1016/S1474-4422(08)70001-2. [DOI] [PubMed] [Google Scholar]
- 66.Illan IA, Gorriz JM, Ramirez J, et al. Machine learning for very early Alzheimer’s Disease diagnosis; a 18F-FDG and PiB PET comparison. Nuclear Science Symposium Conference Record (NSS/MIC), 2010 IEEE; 2010; Oct. 30 2010-Nov. 6, 2010; Knoxville, Tennessee, USA: IEEE; 2010. pp. 2334–2337. [Google Scholar]
- 67.Rabinovici GD, Rosen HJ, Alkalay A, et al. Amyloid vs FDG-PET in the differential diagnosis of AD and FTLD. Neurology. 2011;77(23):2034–2042. doi: 10.1212/WNL.0b013e31823b9c5e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Rowe CC, Ellis KA, Rimajova M, et al. Amyloid imaging results from the Australian Imaging, Biomarkers and Lifestyle (AIBL) study of aging. Neurobiol Aging. 2010;31:1275–1283. doi: 10.1016/j.neurobiolaging.2010.04.007. [DOI] [PubMed] [Google Scholar]
- 69.Bourgeat P, Chetelat G, Villemagne VL, et al. Beta-amyloid burden in the temporal neocortex is related to hippocampal atrophy in elderly subjects without dementia. Neurology. 2010;74:121–127. doi: 10.1212/WNL.0b013e3181c918b5. [DOI] [PubMed] [Google Scholar]
- 70.Phelps ME. PET: the merging of biology and imaging into molecular imaging. J Nucl Med. 2000;41:661–681. [PubMed] [Google Scholar]
- 71.Yanase D, Matsunari I, Yajima K, et al. Brain FDG PET study of normal aging in Japanese: effect of atrophy correction. Eur J Nucl Med Mol Imaging. 2005;32:794–805. doi: 10.1007/s00259-005-1767-2. [DOI] [PubMed] [Google Scholar]
- 72.Xiong KL, Yang QW, Gong SG, Zhang WG. The role of positron emission tomography imaging of beta-amyloid in patients with Alzheimer’s disease. Nucl Med Commun. 2010;31:4–11. doi: 10.1097/mnm.0b013e32833019f3. [DOI] [PubMed] [Google Scholar]
- 73.Mistur R, Mosconi L, De Santi S, et al. Current Challenges for the Early Detection of Alzheimer’s Disease: Brain Imaging and CSF Studies. J Clin Neurol. 2009;5:153–166. doi: 10.3988/jcn.2009.5.4.153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Nobili F, Morbelli S. [18F]FDG-PET as a biomarker for early Alzheimer’s disease. The Open Nuclear Medicine Journal. 2010;2:46–52. [Google Scholar]
- 75.Chetelat G, Desgranges B, de la Sayette V, Viader F, Eustache F, Baron JC. Mild cognitive impairment: Can FDG-PET predict who is to rapidly convert to Alzheimer’s disease? Neurology. 2003;60:1374–1377. doi: 10.1212/01.wnl.0000055847.17752.e6. [DOI] [PubMed] [Google Scholar]
- 76.Kantarci K, Knopman DS, Dickson DW, et al. Alzheimer disease: Postmortem neuropathologic correlates of antemortem 1H MR spectroscopic metabolite measurements in Alzheimer disease. Radiology. 2008;248:210–220. doi: 10.1148/radiol.2481071590. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Dixon RMBK, Budge MM, Styles P, Smith AD. Longitudinal quantitative proton magnetic resonance spectroscopy of the hippocampus in AD. Brain. 2002;125:2332–2341. doi: 10.1093/brain/awf226. [DOI] [PubMed] [Google Scholar]
- 78.Stuhler E, Platsch G, Weih M, Kornhuber J, Kuwert T, Merhof D. Multiple discriminant analysis of SPECT data for alzheimer’s disease, frontotemporal dementia and asymptomatic controls. Nuclear Science Symposium and Medical Imaging Conference (NSS/MIC), 2011 IEEE; 2011; Oct. 27 2012-Nov. 3, 2012; Anaheim, California, USA: IEEE; 2011. pp. 4398–43401. [Google Scholar]
- 79.Matsuda H. Role of neuroimaging in Alzheimer’s disease, with emphasis on brain perfusion SPECT. J Nucl Med. 2007;48:1289–1300. doi: 10.2967/jnumed.106.037218. [DOI] [PubMed] [Google Scholar]
- 80.Huang C, Eidelberg D, Habeck C, et al. Imaging markers of mild cognitive impairment: multivariate analysis of CBF SPECT. Neurobiol Aging. 2007;28:1062–1069. doi: 10.1016/j.neurobiolaging.2006.05.017. [DOI] [PubMed] [Google Scholar]
- 81.Herholz K, Schopphoff H, Schmidt M, et al. Direct comparison of spatially normalized PET and SPECT scans in Alzheimer’s disease. J Nucl Med. 2002;43:21–26. [PubMed] [Google Scholar]
- 82.Matsuda H, Mizumura S, Nagao T, et al. Automated discrimination between very early Alzheimer disease and controls using an easy Z-score imaging system for multicenter brain perfusion single-photon emission tomography. Am J Neuroradiol. 2007;28:731–736. [PMC free article] [PubMed] [Google Scholar]
- 83.Berendse HW, Verbunt JP, Scheltens P, van Dijk BW, Jonkman EJ. Magnetoencephalographic analysis of cortical activity in Alzheimer’s disease: a pilot study. Clin Neurophysiol. 2000;111:604–612. doi: 10.1016/s1388-2457(99)00309-0. [DOI] [PubMed] [Google Scholar]
- 84.Zamrini E, Maestu F, Pekkonen E, et al. Magnetoencephalography as a putative biomarker for Alzheimer’s disease. Int J Alzheimers Dis 2011. 2011 doi: 10.4061/2011/280289. Article ID 280289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Maestu F, Baykova E, Ruiz JM, et al. Increased biomagnetic activity in healthy elderly with subjective memory complaints. Clin Neurophysiol. 2011;122:499–505. doi: 10.1016/j.clinph.2010.08.004. [DOI] [PubMed] [Google Scholar]
- 86.Fernandez A, Hornero R, Mayo A, Poza J, Gil-Gregorio P, Ortiz T. MEG spectral profile in Alzheimer’s disease and mild cognitive impairment. Clin Neurophysiol. 2006;117:306–314. doi: 10.1016/j.clinph.2005.10.017. [DOI] [PubMed] [Google Scholar]
- 87.Osipova D, Ahveninen J, Jensen O, Ylikoski A, Pekkonen E. Altered generation of spontaneous oscillations in Alzheimer’s disease. Neuroimage. 2005;27:835–841. doi: 10.1016/j.neuroimage.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 88.Poza J, Hornero R, Abásolo D, Fernández A, García M. Extraction of spectral based measures from MEG background oscillations in Alzheimer’s disease. Med Eng Phys. 2007;29:1073–1083. doi: 10.1016/j.medengphy.2006.11.006. [DOI] [PubMed] [Google Scholar]