Abstract
A multiple-exemplar identity matching-to-sample baseline was established to encourage development of generalized IDMTS performances in three adult male capuchins. Mask (blank comparison) or Shuffled S− procedures were used to promote select (sample-S+) control in baseline relations and to assess stimulus control relations in generalized IDMTS tests. The IDMTS baseline comprised eight 3-stimulus sets or four 4-stimulus sets. Probe trials with new stimulus sets were substituted for baseline sets in successive testing sessions and subsequently converted to new baseline relations. All monkeys exhibited high accuracy on generalized IDMTS tests. A monkey who was given the Mask procedure in training and tests showed generalized IDMTS with select relations predominating. Two monkeys who were given training and testing with the Shuffled S− procedure performed somewhat better on Shuffled S− IDMTS test trials than on test trials that contained non-shuffled test IDMTS trials thus suggesting that exclusion of familiar nonmatching comparison stimuli from baseline in Shuffled S-test trials contributed to the higher accuracy scores with the former procedures. Development of select relations appeared to be a positive predictor of development of generalized IDMTS.
Keywords: generalized identity matching-to-sample, multiple-exemplar training, select and reject relations, capuchin monkeys
Relational responding based on the identity between elements is considered an abstract concept (Lazareva and Wasserman, 2008). The acquisition of such concept has been investigated in nonhumans using Identity Matching-to-Sample procedures (IDMTS). A baseline of IDMTS relations is first established via direct training and then new stimuli are substituted in that baseline to see whether new identity relations are exhibited without further training (Cumming & Berryman, 1961; Goldman & Shapiro, 1979; Souza et al. 2009; Zentall & Hogan, 1974).
Although there are many types of matching-to-sample procedures (Cumming & Berryman, 1965), their general characteristics involve presenting a sample to which the subject must exhibit an observing response. Thereafter, two or more comparison stimuli are presented. Access to reinforcers is contingent on selecting the comparison stimulus that is identical physically to the sample on each trial.
Relational learning literature suggests that different nonhuman species can exhibit generalized IDMTS when exposed to training conditions that are adapted to the needs of the species involved (Fujita, 1983, with Japanese monkeys; Herman et al. 1989, Kastak & Schusterman, 1994, with California sea lions, and Oden, Thompson, & Premack, 1988, with infant chimpanzees). Only Oden et al. (1988) reported generalized IDMTS after the minimum training history – identity conditional discriminations with only two pairs of objects before tests with new stimulus pairs. In the other cases, generalized IDMTS was obtained only after training was provided on multiple exemplars of identity relations.
In general, the literature suggests that generalized IDMTS acquisition in nonhumans is facilitated by training with large stimulus sets (e.g. 24 stimuli: Weinstein, 1941 with rhesus monkeys; Pack et al., 1991, with sea lions) which includes procedures that present a small number of trials with a large number of stimuli (e.g. the “trial-unique” procedures of Mishkin & Delacour, 1975, with rhesus monkeys; multiple exemplar training by Kastak and Schusterman, 1994, with sea lions).
In a study that directly compared IDMTS training with large stimulus sets to that with small sets using capuchin subjects, Truppa et al. (2010, p. 835) reported that the former was more efficient than the latter in promoting IDMTS transfer to new stimuli. They concluded that “the size of the training set affects the acquisition of an abstract identity concept in a MTS task in nonhuman primates” but that “methodologies used to determine many of the conditions under which this kind of ability occurs are far from straightforward” (p. 836).
One central question is why training with multiple exemplars might facilitate generalized IDMTS. Regarding small sets, Dube and colleagues (e.g. Dube & McIlvane, 1996; Dube, McIlvane, & Green, 1992) have pointed out their potential for development of unwanted stimulus control relations during training that might yield high levels of performance accuracy even if control relations are incoherent with planned performance (a case of mistaken identity matching). In such cases, they argued, tests for generalized IDMTS should be negative, because no previous identity relations had been established.
Consistent with their arguments, the literature provides clear examples of mistaken IDMTS. Cumming and Berryman (1961, 1965) with pigeons, reported control by color and by position early in their simultaneous matching experiment, Lionello and Urcuioli (1998), with pigeons reported control by sample location, and Iversen, Sidman, and Carrigan (1986) with monkeys reported that animals trained on IDMTS procedures acquired instead simple discriminations based on specific configurations of stimuli in typical three-key MTS arrangements (cf. McIlvane, 2012 for further discussion of this possibility). In the studies of Lionello and Urcuioli (1998), and Iversen and colleagues (1986), the configural nature of stimulus control was revealed when highly accurate IDMTS performances were abolished merely by changing the location of the sample stimuli.
Aiming to promote stimulus control relations consistent with true IDMTS with capuchins, Barros, Galvão, and McIlvane (2002) and Galvão et al. (2005) implemented a programmed approach that was intended to discourage mistaken IDMTS. Among the features of their procedures were: (1) using a 0-delay IDMTS procedure, with sample and comparisons stimuli presented successively to mitigate against development of control by specific trial configurations, (2) over trials, varying sample and comparison locations in a 3 × 3 matrix to avoid development of unwanted position control, (3) exposing stimuli to be used in IDMTS tests first on simple discrimination trials involving repeated discriminative function shifts (intended to minimize novelty effects and verify that all stimuli could in fact serve both positive [S+] and negative [S−] stimulus functions as they would have to do subsequently on IDMTS tests); (4) analyzing performance for evidence of adequate stimulus discriminability and replacing any potentially “confusable” stimuli; (5) reducing reinforcement probability in the baseline before test sessions, teaching the monkey to sustain IDMTS performance even when presented with a number of trials without programmed feedback, and (6) presenting test trials with partial or continuous reinforcement to prevent selective disruption due to discriminated extinction (Boren & Sidman, 1957). These procedures proved largely successful in producing test trial accuracy scores that were well above the “chance” level for the most of the tested sets: 80% or greater on three-comparison tests (chance = 33%), but there was substantial variability across the six animals that they studied. For example, one of the monkeys (M14) showed 60% of correct response in the first test session with Set E and M09 showed 90% correct in the same set (see Figure 2 from Galvão et al., 2005, p. 226).
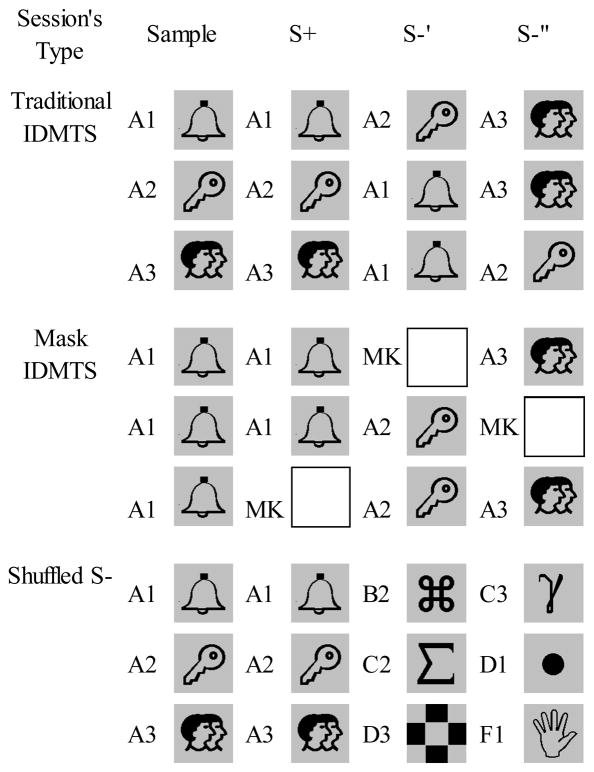
Figure 2.
Examples of trials of Traditional, Mask and Shuffled S− sessions.
The present study was conducted with the aim of producing accuracy that more nearly approached 100% scores with low inter-problem variability, documenting robust generalized IDMTS. Whereas the baseline in the Barros et al. (2002) and Galvão et al. (2005) studies consisted of the same three overtrained IDMTS relations, the present tests for generalized IDMTS were conducted in much richer baselines. Three capuchin monkeys were studied on procedures that (1) had a large number of baseline IDMTS relations (24 for two monkeys and 16 for the third), (2) a replacement-addition procedure in which newly tested IDMTS relations were added to the baseline replacing previously established relations, (3) features to promote development of select (i.e. sample-S+) and reject (i.e. sample-S−) control relations in IDMTS baseline (see Dube et al., 1992 and Lionello-DeNolf, 2009 for discussions of the importance of select and possibly reject relations in promoting generalized performance, and (4) for two monkeys, omitting reinforcement for the first trial of each new IDMTS relation tested.
Method
Subjects
Three adult male capuchin monkeys (Sapajus spp.) participated.
Esqueleto (M22) had been exposed only to IDMTS training with eight 3-stimulus sets. He had not previously been given formal tests for generalized IDMTS. Raul (M14) had experience on simultaneous simple discrimination, IDMTS training, and tests of generalized IDMTS (Brino, Galvão, & Barros, 2009; Galvão et al., 2005). Bongo (M16) had been given simple discrimination training and needed a special procedure (Rico et al., in preparation) to learn his first exemplars of identity matching relations.
The animals were housed in cages with three other capuchins. Living conditions, handling protocols, diet, veterinary care, and experimental procedures were approved by the UFPA Animal Care and Use Committee (license # CEPAE-UFPA PS001/2005) following international guidelines concerning use of animals for research purposes.
Apparatus
Sessions were carried out in an experimental chamber (0.8 × 0.8 m sides × 0.7 m high) contained within a small room (2.5 × 1.9 m sides and 2.9 m high). The floor, ceiling, and left wall of the chamber were made of steel plates perforated with circular holes. The right and front walls were solid zinc plates. Access to the chamber was provided by a 0.35 m long × 0.20 m high sliding door on the left wall. The chamber was equipped with a 15-inch touchscreen color monitor (Microtouch) accessible through a rectangular opening (0.26 m long × 0.20 m high) in the front wall. Centred 0.24 m below the opening was a receptacle for delivering 190 mg food pellets via a hose connected to a Med Associates© automatic pellet dispenser. Stimulus presentation and response recording were automatically managed by a desktop computer running custom-made software (TREL 2.1, by Iran Athaíde dos Santos or EAM 4.0.04, by Drausio Capobianco) developed specifically for research with simple and conditional discrimination tasks.
Stimuli
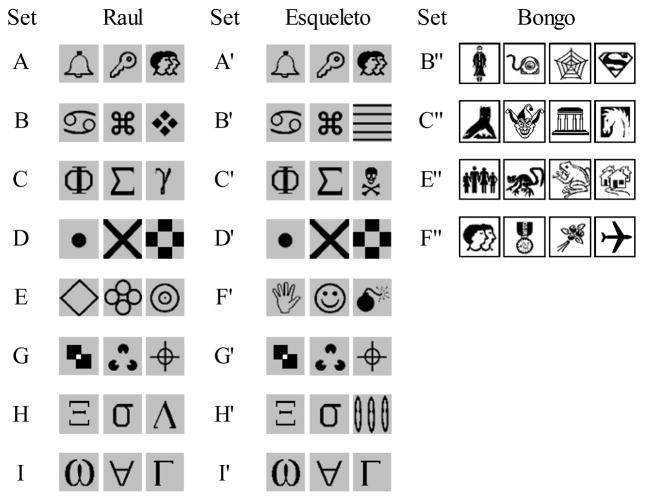
Stimuli were 2-inch squares with drawings in black, white and grey. They were presented in any of nine positions of a 3 × 3 matrix on the touch-sensitive monitor screen. Stimuli were arranged in sets of three or four.
For Esqueleto and Raul, sixteen 3-stimulus sets were used: Eight sets were used as initial baseline and eight were tested for generalized matching and added to the baseline thereafter.
For Bongo, eighteen 4-stimulus sets were used: Four sets were used in the initial baseline and fourteen in generalized IDMTS tests.
Figure 1 shows baseline sets for all monkeys (test sets will be shown with the Results).
Figure 1.
Stimulus sets used in multiple-exemplar baseline training sessions with subjects Raul, Esqueleto, and Bongo.
General Procedure
The monkeys were trained one multiple exemplar baseline, 16 (Bongo) or 24 (Raul and Esqueleto) identity relations in the same session, until criterion was achieved. Thereafter, generalized IDMTS tests with new stimulus sets were conducted. Successive testing sessions were interspersed with baseline sessions. The main difference between baseline and test sessions was that the latter presented an unfamiliar stimulus set to the monkey by substituting it for one of the former baseline sets. After a test for generalized IDMTS, the tested set replaced one of the baseline sets going forward.
IDMTS training and test trials were programmed in a zero-delay matching-to-sample procedure. A trial began with presentation of a sample stimulus; after the subject touched the sample, that stimulus was removed and comparisons stimuli were presented immediately. Choices of comparison stimuli that were identical to the samples were followed by delivery of a banana pellet and an intertrial interval (ITI); incorrect choices were followed only by the ITI. Sample and comparison stimuli – positive and negative – were equally distributed in nine positions of a 3 × 3 matrix across trials. Sessions ended when subjects completed all programmed trials or after 25 minutes had elapsed.
For each animal, there were two types of training and test sessions, Traditional IDMTS sessions and Controlling Relation IDMTS sessions. In Traditional IDMTS sessions, all comparison stimuli functioned equally often as both positive (S+) and negative (S−) stimuli, and within-trial comparison stimuli functions were determined by the sample (Dube et al. 1992). High accuracy could occur if the monkey (1) selected comparison stimuli that were identical to the sample, (2) rejected comparison stimuli that were not identical, or (3) responded to specific stimulus configurations of the trials.
In Controlling Relation sessions, the trials were designed to isolate select and reject relations. Depending on the individual monkey’s history, we used one of two techniques, Mask IDMTS sessions or Shuffled-S− sessions. In Mask (MK) sessions, a blank comparison (MK, a white square) replaced either a positive comparison (S+) or a negative comparison (S−) from trial to trial. Trials with MK substituting for an S− served to establish, when used in training, or evaluate, when used in testing, select (sample-S+) controlling relations. Those with MK substituting for the positive comparison served to establish or evaluate reject (sample-S−) controlling relations. Correct responses on both Mask trials indicated mixed control, that is, select on some trials and reject on others.
Because Raul already had a Mask history in IDMTS, his Controlling Relations sessions used such technique.
For Bongo and Esqueleto, however, we used a Shuffled S− procedure, in which different trials with the same sample-S+ relation had different combinations of S− taken from any of the baseline sets (B″– F″ for Bongo, and A′– I′ for Esqueleto; see Figure 1). The procedure was designed to discourage learning of specific reject or stimulus configuration relations (cf. Johnson & Sidman, 1993), and thus promote acquisition of select relations.
In all phases described below, Esqueleto and Bongo were given alternated sessions of Traditional and Shuffled S− trials, in both baseline training and in test. Raul was exposed to alternated sessions of Traditional and Mask IDMTS in all phases. Figure 2 shows examples of trials in each type of session.
Experimental Phases
Training and testing procedures used with the three monkeys had certain differences in the duration of intertrial intervals, number of relations presented in the same session, number of trials in a session, and so on. Those differences are presented in Table 1. Procedures used with Esqueleto will be presented first. Differences for Raul and Bongo will be indicated at the end of each phase description.
Table 1.
Procedure parameters for each subject.
| Subject | Relations per session | Trials per session
|
Choices | TS/TC | Correction | Intertrial interval | |
|---|---|---|---|---|---|---|---|
| Traditional | Shuffled S− or Mask | ||||||
| Esqueleto | 24 | 48 | 72 | 3 | 01/01 | No | 6 |
| Raul | 24 | 48 | 72 | 3 | 01/01 | No | 4 |
| Bongo | 16 | 48 | 48 | 4 | 05/03 | Yes | 10 |
Note. Choices: number of simultaneous comparisons per trial; TS: number of touches required to the sample; TC: number of touches required to the comparisons.
Multiple-exemplar baseline construction
Esqueleto’s initial baseline included Sets A′, B′, C′, D′, F′, G′, H′ and I′ (see Figure 1). The objective of this phase was merely to group trials from these formerly independent problems together. Trials from eight 3-stimulus sets constituted 24 identity relations presented in same session. Traditional IDMTS sessions had 48 trials, 2 trials for each relation. Shuffled S− sessions had 72 trials, 3 for each relation.
Raul was exposed to the same parameters of training but with Mask sessions. Bongo’s baseline (Sets B″, C″, E″ and F″, see Figure 1) presented a total of 16 relations per session. For Bongo, both IDMTS and Shuffled S− sessions had 48 trials.
For all three subjects in this phase, all correct choices were followed by pellet deliveries. Criterion for advancement from this phase to the next was > 90% of correct responses in two consecutive sessions of each type.
Preparation for tests under intermittent reinforcement
Sessions for Esqueleto were assembled with the same eight 3-stimulus sets from his baseline. In each session, the initial trials of one 3-stimulus set had no programmed reinforcement. For example, if Set A was programmed, there was no reinforcement for the first of two presentations of A1-A1, A2-A2 and A3-A3. That intermittent reinforcement was programmed in both traditional IDMTS and Shuffled S− sessions. Eight partial reinforcement sessions were successively applied with additional stimulus sets (D′, F′, A′, G′, B′, H′, C′, and I′).
Raul was exposed to a similar configuration of partial reinforcement trials in Traditional and Mask IDMTS sessions. However, Bongo was not. For him, all sessions in this and the next phase provided continuous reinforcement.
Generalized IDMTS tests
The new stimulus sets used in consecutive tests with Esqueleto were J, K, L, M, N, O, P, Q (see Table 2). In a test session, a new 3-stimulus set replaced one of the eight baseline sets. After a stimulus set was tested in the two types of sessions described above, it was incorporated to baseline before the next test session. In successive sessions, one by one, the eight baseline sets were replaced by eight new sets until a new baseline of 24 relations was formed. In each test session, intermittent reinforcement was provided for correct responses on trials with the new stimulus set and continuous reinforcement on baseline trials. Esqueleto was exposed to eight Traditional IDMTS and eight Shuffled S− test sessions, alternating for each new set. Traditional IDMTS was always followed by a Shuffled S− session for each new set tested.
Table 2.
Correct (C) and incorrect (X) choices in identity test trials for Raul and Esqueleto, in sequence, from left to right.
| Set | Relations | Stimulus | Test Trials
|
|||
|---|---|---|---|---|---|---|
| Raul | Esqueleto | |||||
|
| ||||||
| Traditional | Mask IDMTS | Traditional | Shuffled S− | |||
| J | J1-J1 |

|
CC | CCC | CC | CCC |
| J2-J2 |

|
CC | CCC | CC | CCC | |
| J3-J3 |

|
CC | CXC | CC | CCC | |
| K | K1-K1 |

|
CC | CCC | XX | CCC |
| K2-K2 |

|
CC | CCC | CC | CCC | |
| K3-K3 |

|
CC | CCC | CX | CCC | |
| L | L1-L1 |

|
CC | CXC | XC | CCC |
| L2-L2 |

|
CC | CXC | CX | CCC | |
| L3-L3 |

|
CC | XCC | XC | CCC | |
| M | M1-M1 |

|
CC | XCC | CC | CCC |
| M2-M2 |

|
CC | CCC | XX | CCC | |
| M3-M3 |

|
CC | CCX | CC | CCC | |
| N | N1-N1 |

|
CX | CCC | CC | CCC |
| N2-N2 |

|
CC | CCC | CC | CCC | |
| N3-N3 |

|
CC | CCC | CC | CXC | |
| O | O1-O1 |

|
CC | CCC | CC | CCC |
| O2-O2 |

|
CC | CCC | CC | CCC | |
| O3-O3 |

|
CC | CCC | XC | CCC | |
| P | P1-P1 |

|
CC | CXC | XC | CCC |
| P2-P2 |

|
CC | XCC | CC | XCC | |
| P3-P3 |

|
CC | XCC | CC | CCC | |
| Q | Q1-Q1 |

|
CC | CCC | CC | CCC |
| Q2-Q2 |

|
CC | CCC | CC | CCC | |
| Q3-Q3 |

|
CC | CCC | CC | CCC | |
Raul was exposed to the same sequence of Traditional and Mask IDMTS sessions, with the same sets as Esqueleto. For both subjects, baseline sessions and test sessions were alternated in the proportion 1:1.
For Bongo, fifteen 4-stimulus sets, G to V (see Table 3), were tested. Test sessions had 12 probe trials (one new 4-stimulus set) interspersed in 36 baseline trials (three familiar 4-stimulus sets). A baseline session followed each pair of test sessions with a new stimulus set (Traditional IDMTS and Shuffled S− types). During test sessions, a new 4-stimulus set replaced one of the four baseline sets. After two test sessions, the stimulus set just tested remained in the baseline. Traditional IDMTS and Shuffled S− were applied with sets G to N. For sets Q to V, only Traditional IDMTS test sessions were applied. The order of test sessions types varied across tests.
Table 3.
Correct (C) and incorrect (X) choices in identity test trials for Bongo, in sequence, from left to right.
| Set | Relation | Stimulus | Test trials
|
|
|---|---|---|---|---|
| Traditional | Shuffled S− | |||
| G | G1-G1 |

|
CCC | CCC |
| G2-G2 |

|
CCC | CCC | |
| G3-G3 |

|
CCC | CCC | |
| G4-G4 |

|
CCC | CCC | |
| H | H1-H1 |

|
XCX | CCC |
| H2-H2 |

|
CCC | CCC | |
| H3-H3 |
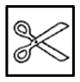
|
CCC | CCC | |
| H4-H4 |
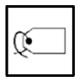
|
XCC | CCC | |
| I | I1-I1 |

|
XCC | CCC |
| I2-I2 |

|
CCC | CCC | |
| I3-I3 |

|
CXC | CCC | |
| I4-I4 |

|
CCX | CCC | |
| J | J1-J1 |
|
XCX | CCC |
| J2-J2 |

|
XCC | CCC | |
| J3-J3 |
|
XXX | CCC | |
| J4-J4 |

|
CCC | CCC | |
| K | K1-K1 |

|
XCC | CCC |
| K2-K2 |

|
CCC | CCC | |
| K3-K3 |

|
CCC | CCC | |
| K4-K4 |

|
CCC | CCC | |
| L | L1-L1 |

|
CCC | CCC |
| L2-L2 |
|
CCC | CCC | |
| L3-L3 |
|
CCC | CCC | |
| L4-L4 |

|
CCC | CCC | |
| M | M1-M1 |

|
CCC | CCC |
| M2-M2 |

|
CCC | CXC | |
| M3-M3 |

|
CCC | CCC | |
| M4-M4 |

|
CCC | CCC | |
| N | N1-N1 |
|
CCC | CCC |
| N2-N2 |

|
CCC | CCC | |
| N3-N3 |

|
XCC | CCC | |
| N4-N4 |

|
CCX | CCC | |
| Q | Q1-Q1 |

|
CCC | - |
| Q2-Q2 |
|
XCX | - | |
| Q3-Q3 |

|
CCC | - | |
| Q4-Q4 |

|
CCC | - | |
| R | R1-R1 |

|
CCC | - |
| R2-R2 |

|
CCC | - | |
| R3-R3 |
|
CXC | - | |
| R4-R4 |
|
CCX | - | |
| S | S1-S1 |

|
XXC | - |
| S2-S2 |

|
CCC | - | |
| S3-S3 |

|
CCC | - | |
| S4-S4 |

|
CCC | - | |
| T | T1-T1 |

|
CCC | - |
| T2-T2 |

|
CCX | - | |
| T3-T3 |
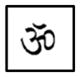
|
XXX | - | |
| T4-T4 |

|
CCC | - | |
| U | U1-U1 |

|
CCC | - |
| U2-U2 |

|
CCC | - | |
| U3-U3 |
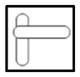
|
CCC | - | |
| U4-U4 |

|
CCC | - | |
| V | V1-V1 |

|
CCC | - |
| V2-V2 |
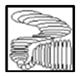
|
CCC | - | |
| V3-V3 |

|
CCC | - | |
| V4-V4 |

|
CCC | - | |
Results
For all monkeys, correct responses in sessions of multiple-exemplar baseline construction always reached > 90%. Raul, Esqueleto, and Bongo reached this criterion in two, six, and five sessions, respectively.
During preparation for tests under intermittent reinforcement, accuracy scores in each session for each monkey always exceeded 90% correct, both in Traditional and in Shuffled S− (Esqueleto) or Mask (Raul) sessions. Thus, performance accuracy continued to be very high despite the use of intermittent reinforcement to one of the eight 3-stimulus sets in each session. Esqueleto’s and Raul’s performances were highly accurate on such trials; each monkey showed only two errors in a total of 60 trials (97% correct) throughout eight successive sessions on this condition.
Table 2 shows their correct (C) and incorrect (X) choices on test trials for each new set. Esqueleto showed almost perfect performance in Shuffled S− sessions (70 of 72 correct trials, 97%). He exhibited less accurate performance on Traditional IDMTS trials, 38 correct of 48 trials (79%) overall and 75% correct on first trials with new relations (first entries in the trials in the Traditional IDMTS column). This somewhat lower accuracy may have been due to his recent experience with IDMTS tasks.
Raul responded nearly without error on Traditional IDMTS trials (47 of 48 trials, 98% correct), choosing correctly on all first presentations for each of 24 new stimulus-stimulus relations. Absence of reinforcement for correct choices on the first presentation of each new relation did not disrupt performance on the second presentation of those same relations (23 of 24 correct trials in the same session). During Mask sessions, Raul made nine errors in 72 trials of the tested relations (63/72=87.5% correct), eight occurring on trials with the mask replacing the positive comparison, i.e. trials that tested for reject control by negative stimuli. On the first presentation of each new relation in trials with MK, Raul responded correctly in 83.3% of the opportunities (20 of 24 trials).
For Bongo, performance in most testing sessions exceeded 90% correct. Like Esqueleto, Bongo’s best performances were observed during Shuffled S− tests. Table 3 shows the number of correct and incorrect trials in Traditional IDMTS and Shuffled S− test trials for Bongo. Eight Shuffled S− testing sessions were run. In the 96 trials from all tested stimulus sets, Bongo made only one error (99% correct). In fifteen Traditional-IDMTS sessions (two sessions were applied with Set H), Bongo made no errors on test trials with five of the stimulus sets (G, L, M, S and U); in two other test sessions with stimuli from Sets H and K, he made only one error in 12 test trials. In each of five other sessions, two errors occurred in 12 test trials. Thus, Bongo chose correctly on 199 of 224 (89%) trials in Traditional IDMTS sessions. Bongo responded correctly on 80% of the first presentation of each new relation tested in the Traditional-IDMTS sessions. During Shuffled S− sessions, he responded without error.
Discussion
Our procedures were successful in their primary objective: showing that capuchin monkeys can be successful at reasonably high accuracy levels on tests for generalized IDMTS. These data also suggest useful directions for developing the training methodology even further – perhaps to the point that test accuracy and inter-subject variability may come to rival that reported in well-conducted studies with verbal human children.
The multiple-exemplar training with 3 or 4 comparisons, the accumulated multiple-exemplar baseline, and perhaps the procedure of gradually replacing baseline relations with newly demonstrated relations were important variables for such demonstration.
Notably, one monkey (Esqueleto) was experimentally naïve with respect to tests of generalized IDMTS. One cannot attribute his impressive success to previous cumulative experience with IDMTS procedures as one might be tempted to do with the other monkeys. The fact that all three monkeys performed similarly increases confidence that the present procedure combination may serve its intended function of enhancing development of generalized IDMTS.
How do the present results compare to past studies of generalized IDMTS in nonhuman participants? Our monkeys performed better than did animals in past studies that have been judged highly successful (e.g., Kastak & Schusterman, 1994; Oden, Thompson, & Premack, 1988; Truppa et al. 2010). Those studies used two-choice procedures in which “chance” level scores are 50%. By contrast, chance level scores on our 3- and 4-choice tasks would be 33% and 25%, respectively. Thus, our monkeys performances exceeded chance levels by higher margins than any studies that used two-choice procedures.
Concerning the other features of the tasks presented, the Mask procedure used with Raul had somewhat different results than the Shuffled S− procedure used with the other two monkeys. While Raul’s performance on Mask trials was reasonably accurate (88% correct), his performance on Traditional IDMTS tests was virtually errorless. Notably, however, he also selected the positive comparison virtually always when the Mask was substituted for a negative comparison, indicating a predominance of sample-S+ control – a strong evidence for generalized IDMTS.
By contrast, Esqueleto and Bongo exhibited better accuracy on Shuffled S− test trials as compared with Traditional-IDMTS. We agree with Dube et al. (1992) that an S− shuffling procedure may present less of a challenge than Traditional IDMTS tests, because the former may allow the monkey merely to exclude stimuli that had been well-discriminated on prior IDMTS baseline trials. As a test for identity discrimination of matching vs. nonmatching of physical characteristics, this test is obviously different in character than the Traditional IDMTS test. The subject need only detect that the sample and positive comparison are novel. In this limited sense, therefore, the Shuffled S− procedure does demonstrate discrimination of features of previously experienced stimuli from those of the newly introduced stimuli. Moreover, this procedure may prove useful as a training feature (e.g., so-called learning by exclusion; McIlvane et al., 1981) that may assist in preparing animals for Traditional IDMTS tests. Indeed, Bongo received such a procedure in order to help him acquire his first IDMTS performances (Galvão et al., 2005).
We can only speculate about the possible beneficial effects of further preparing monkeys such as Bongo and Esqueleto for Traditional IDMTS tests by first exposing them exclusively to multiple-exemplar training using the Shuffling procedure with a large number of novel stimuli. Such a procedure would not only increase the number of IDMTS relations in the cumulative history – perhaps refining discrimination skills – but also potentially minimize problems of neophobia or neophilia (Kastak & Schusterman, 1994).
The foregoing discussion begs the question of why accuracy on many tests of Traditional IDMTS for Bongo and Esqueleto was perfect; only some relations occasioned errors. Baseline relation accuracy was always quite high, and the animals clearly did attend on those trials. We believe that such errors could be based on the similarities among newly introduced stimuli presented simultaneously as comparisons on test (i.e., primary stimulus generalization). Shuffle trials did present S− stimuli from the baseline, thus reducing the challenge of differentiating the new S+ from old S− stimuli or perhaps merely presenting stimuli with features that monkeys had previously discriminated. A future challenge for our behavioral engineering will be to develop procedures to refine the discrimination skills of our monkeys such that stimulus generalization is a less likely outcome. We suggest that such a development would help not only our own methodology, but might contribute also to the long-term search for procedures to establish and refine discrimination skills of nonverbal humans (McIlvane, 1992; Sidman & Stoddard, 1966).
Our multiple-exemplar approach also begs the question of the minimum training experience necessary to demonstrate generalized IDMTS in non-humans. As noted earlier, Oden et al. (1988) is the only report of generalized identity matching-to-sample after training with a small number of stimuli before testing; their chimpanzees performed IDMTS with new stimuli after training with only previous one pair of relations. Because they used a manual, face-to-face procedure, however, the possibility of inadvertent cuing by the experimenter could not be ruled out. Moreover, they used a two-choice procedure (chance = 50%), and thus their reported scores of 77% correct on the first presentations of new stimuli are not very impressive (cf. Sidman, 1987) nor were overall baseline scores after extended training (86%).
Perhaps the challenge should be reframed somewhat: What are the minimum procedures that (1) yield an adequate, statistically-based demonstration of generalized IDMTS capability and (2) will reliably yield convincing demonstration with a majority of animals? Although we emphasize the latter question, we do recognize that the former may relate to analysis of developmental influences. Regarding such influences, would a program of discrimination training commencing early in the lives of our capuchins render them more likely to acquire generalized IDMTS with less protracted and/or less complex training regimens?
A final methodological note: We found it possible to use partial reinforcement techniques within the context of these procedures with Raul and Esqueleto. If animals exhibit accurate IDMTS on tests despite lack of reinforcement on the same trial type(s), that outcome further bolsters an interpretation that accurate performance reflects true generalized IDMTS. Because this procedure carries the risk of actually extinguishing the very relations one is seeking to maintain and/or reveal, we proceeded cautiously in this study (partial reinforcement for a different stimulus set in each session). Although we have investigated this type of procedure to a limited extent (Brino, Galvão, & Barros, 2009), future research will be needed to establish the limits of use of such procedures, if any, or to define a training path such that these procedures can be used as successfully as they have been used with human participants with severe intellectual disabilities (e.g., McIlvane et al., 1984).
Acknowledgments
Support for this research was provided by grants from the Brazilian National Council for Scientific and Technologic Development (CNPq) to the authors. Additional support was provided by CNPq (573972/2008-7) and Research Support Foundation of São Paulo State (FAPESP 08/57705-8) to National Institute of Science and Technology on Behavior, Cognition and Education. The last author’s participation was supported by NIH grants ES15464 and MH90272.
We thank Klena Sarges for medical-veterinarian assistance and Edilson F. Pastana for managing the animals. Concerning our animals, readers of past articles from our laboratory may wonder why we have not mentioned the capuchin species Cebus apella in this text. Until recently, capuchins were broadly termed Cebus monkeys. Now, they are divided into two genera (cf. Alvaro, Silva, & Rylands, 2012): Sapajus (tufted) and Cebus (untufted). Our monkeys are of the former genus.
Contributor Information
Ana Leda F. Brino, Universidade Federal do Pará
Olavo F. Galvão, Universidade Federal do Pará
Carlos R. F. Picanço, Universidade Federal do Pará
Romariz S. Barros, Universidade Federal do Pará
Carlos B. A. Souza, Universidade Federal do Pará
Paulo R. K. Goulart, Universidade Federal do Pará
William J. McIlvane, University of Massachusetts Medical School
References
- Alfaro JWL, Silva JS, Rylands AB. How different are robust and gracile capuchin monkeys? An argument for the use of Sapajus and Cebus. American Journal of Primatology. 2012;74:273–286. doi: 10.1002/ajp.22007. [DOI] [PubMed] [Google Scholar]
- Barros RS, Galvão OF, McIlvane WJ. Generalized identity matching-to-sample in Cebus apella. The Psychological Record. 2002;52:441–460. doi: 10.1007/s40732-014-0035-x. Retrieved from http://opensiuc.lib.siu.edu/tpr/vol52/iss1/ [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boren JJ, Sidman M. A discrimination based upon repeated conditioning and extinction of avoidance behaviour. Journal of Comparative and Physiological Psychology. 1957;50(1):18–22. doi: 10.1037/h0045142. [DOI] [PubMed] [Google Scholar]
- Brino ALF, Galvão OF, Barros RS. Successive identity matching to sample tests without reinforcement in Cebus apella. Ciências & Cognição. 2009;14(2):02–11. Retrieved from http://www.cienciasecognicao.org. [Google Scholar]
- Cumming WW, Berryman R. Some data on matching behavior in the pigeon. Journal of the Experimental Analysis of Behavior. 1961;4:281–284. doi: 10.1901/jeab.1961.4-281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumming WW, Berryman R. The complex discriminated operant: Studies of matching-to-sample and related problems. In: Mostofsky DI, editor. Stimulus generalization. Stanford, CA: Stanford University Press; 1965. pp. 284–330. [Google Scholar]
- D’Amato MR, Colombo M. On the limits of the matching concept in monkeys (Cebus apella) Journal of the Experimental Analysis of Behavior. 1989;52:225–236. doi: 10.1901/jeab.1989.52-225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dube WV, McIlvane WJ. Some implications of a stimulus control topography analysis for emergent behavior and stimulus classes. In: Zentall TR, Blackman PM, editors. Stimulus class formation in humans and animals. North-Holland: Elsevier; 1996. pp. 197–218. [Google Scholar]
- Dube WV, McIlvane WJ, Green G. An analysis of generalized identity matching-to-sample test procedures. The Psychological Record. 1992;42(1):17–28. [Google Scholar]
- Fujita F. Formation of the sameness-difference concept by Japanese monkeys from a small number of color stimuli. Journal of the Experimental Analysis of Behavior. 1983;40:289–300. doi: 10.1901/jeab.1983.40-289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galvão OF, Barros RS, Santos JR, Brino ALF, Brandão S, Lavratti CM, Dube WV, McIlvane WJ. Extent and limits of matching concept in Cebus apella: A matter of experimental control? The Psychological Record. 2005;55:219–232. Retrieved from http://opensiuc.lib.siu.edu/tpr/vol55/iss2/3/ [Google Scholar]
- Galvão OF, Soares Filho PSD, Barros RS, Souza CBA. Matching-to-sample as a model of symbolic behavior for bio-behavioral investigations. Reviews in the Neurosciences. 2008;19(2–3):149–156. doi: 10.1515/revneuro.2008.19.2-3.149. [DOI] [PubMed] [Google Scholar]
- Goldman M, Shapiro S. Matching-to-sample and oddity-from-sample in goldfish. Journal of the Experimental Analysis of Behavior. 1979;31:259–266. doi: 10.1901/jeab.1979.31-259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goulart PRK, Mendonça MB, Barros RS, Galvão OF, McIlvane WJ. A note on select- and reject-controlling relations in the simple discrimination of capuchin monkeys (Cebus apella) Behavioural Processes. 2005;69:295–302. doi: 10.1016/j.beproc.2004.12.005. [DOI] [PubMed] [Google Scholar]
- Herman LM, Gory JDG, Hovancic JR, Bradshaw GL. Generalization of visual matching by bottlenosed dolphin (Tursiops truncatus): Evidence for invariance of cognitive performance with visual and auditory materials. Journal of Experimental Psychology: Animal Behavior Processes. 1989;15:124–136. doi: 10.1037/0097-7403.15.2.124. [DOI] [Google Scholar]
- Iversen IH, Sidman M, Carrigan P. Stimulus definition in conditional discriminations. Journal of the Experimental Analysis of Behavior. 1986;45:297–304. doi: 10.1901/jeab.1986.45-297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson C, Sidman M. Conditional discrimination and equivalence relations: Control by negative stimulus. Journal of the Experimental Analysis of Behavior. 1993;59:333–347. doi: 10.1901/jeab.1993.59-333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminski J, Call J, Fischer J. Word learning in a domestic dog: evidence for “fast mapping”. Science. 2004;304:1682–1683. doi: 10.1126/science.1097859. [DOI] [PubMed] [Google Scholar]
- Kastak D, Schusterman RJ. Transfer of visual identity matching-to-sample in two California sea lions (Zalophus californianus) Animal Learning & Behavior. 1994;22:427–435. doi: 10.3758/BF03209162. [DOI] [Google Scholar]
- Katz JS, Wright AA, Bachevalier J. Mechanisms of same/different abstract-concept learning by rhesus monkey (Macaca mulatta) Journal of Experimental Psychology: Animal Behavior Processes. 2002;28:358–368. doi: 10.1037//0097-7403.28.4.358. [DOI] [PubMed] [Google Scholar]
- Lazareva OF, Wasserman EA. Categories and concepts in animals. In: Menzel R, editor. Learning and memory: A comprehensive reference: Vol. 1. Learning theory and behavior. Oxford, England: Elsevier; 2008. pp. 197–226. [Google Scholar]
- Lionello KM, Urcuioli PJ. Control by sample location in pigeon’s matching to sample. Journal of the Experimental Analysis of Behavior. 1998;70:235–251. doi: 10.1901/jeab.1998.70-235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lionello-Denolf KM. The search for symmetry: 25 years in review. Learning & Behavior. 2009;37:188–203. doi: 10.3758/LB.37.2.188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIlvane WJ. Stimulus control analysis and nonverbal instructional technology for people with intellectual handicaps. In: Bray NR, editor. International review of research in mental retardation. Vol. 18. New York: Academic Press; 1992. pp. 55–109. [Google Scholar]
- McIlvane WJ. APA. Simple and complex discrimination learning. In: Madden GJ Editor-in-Chief, editor. Handbook of Behavior Analysis. Washington, DC: American Psychological Association; 2012. pp. 129–163. [Google Scholar]
- McIlvane WJ, Bass RW, O’Brien JM, Gerovac BJ, Stoddard LT. Spoken and signed naming of foods after receptive exclusion training in severe retardation. Applied Research in Mental Retardation. 1984;5:1–27. doi: 10.1016/s0270-3092(84)80016-8. [DOI] [PubMed] [Google Scholar]
- Mishkin M, Delacour J. An analysis of short-term visual memory in the monkey. Journal of Experimental Psychology: Animal Behavior Processes. 1975;1:326–334. doi: 10.1037/0097-7403.1.4.326. [DOI] [PubMed] [Google Scholar]
- Oden DL, Thompson RKR, Premack D. Spontaneous transfer of matching by infant chimpanzees (Pan troglodytes) Journal of Experimental Psychology: Animal Behavior Processes. 1988;14:140–145. doi: 10.1037/0097-7403.14.2.140. [DOI] [PubMed] [Google Scholar]
- Pack AA, Herman LM, Roitblat HL. Generalization of visual matching and delayed matching by a California sea lion (Zalophus californianus) Animal Learning & Behavior. 1991;19:37–48. doi: 10.3758/BF03197858. [DOI] [Google Scholar]
- Rico VV, Brino ALF, Goulart PRK, Galvão OF. From simple to conditional discrimination: a teaching program for capuchin monkeys. 2013 Manuscript in preparation. [Google Scholar]
- Sidman M. Two choices are not enough. Behavior Analysis. 1987;22:11–18. Retrieved from http://www.equivalence.net/pdf/Sidman_1987.pdf. [Google Scholar]
- Sidman M. Equivalence relations and behavior: A research story. Boston: Authors Cooperative; 1994. [Google Scholar]
- Sidman M, Stoddard LT. Programming perception and learning for retarded children. In: Ellis NR, editor. International review of research in mental retardation. Vol. 2. New York: Academic Press; 1966. pp. 151–208. [Google Scholar]
- Souza CBA, Borges RP, Goulart PRK, Barros RS, Galvão OF. Testes de identidade generalizada com objetos em macaco-prego (Cebus apella) Psicologia: Teoria e Pesquisa. 2009;25:169–178. Retrieved from http://www.scielo.br/pdf/ptp/v25n2/a04v25n2.pdf. [Google Scholar]
- Truppa V, Garofoli D, Castorina G, Mortari EP, Natale F, Visalbergui E. Identity concept learning in matching-to-sample tasks by tufted capuchin monkeys (Cebus apella) Animal Cognition. 2010;13:835–848. doi: 10.1007/s10071-010-0332-y. [DOI] [PubMed] [Google Scholar]
- Weinstein B. Matching-from-sample by rhesus monkeys and by children. Journal of Comparative Psychology. 1941;41:195–213. doi: 10.1037/h0063449. [DOI] [Google Scholar]
- Wilkinson KM, McIlvane WJ. Blank comparison analysis of emergent symbolic mapping by young children. Journal of Experimental Child Psychology. 1997;67:115–130. doi: 10.1006/jecp.1997.2402. [DOI] [PubMed] [Google Scholar]
- Zentall TR, Hogan DE. Abstract concept learning in the pigeon. Journal of Experimental Psychology. 1974;102:393–398. [Google Scholar]
- Wilkinson KM, Rosenquist C, McIlvane WJ. Exclusion learning and emergent symbolic category formation in individuals with severe languages impairments and intellectual disabilities. The Psychological Record. 2009;59(2):187–206. doi: 10.1007/bf03395658. Retrieved from http://www.ncbi.nlm.nih.gov/pmc/articles/PMC2818096/pdf/nihms141453.pdf. [DOI] [PMC free article] [PubMed] [Google Scholar]