Abstract
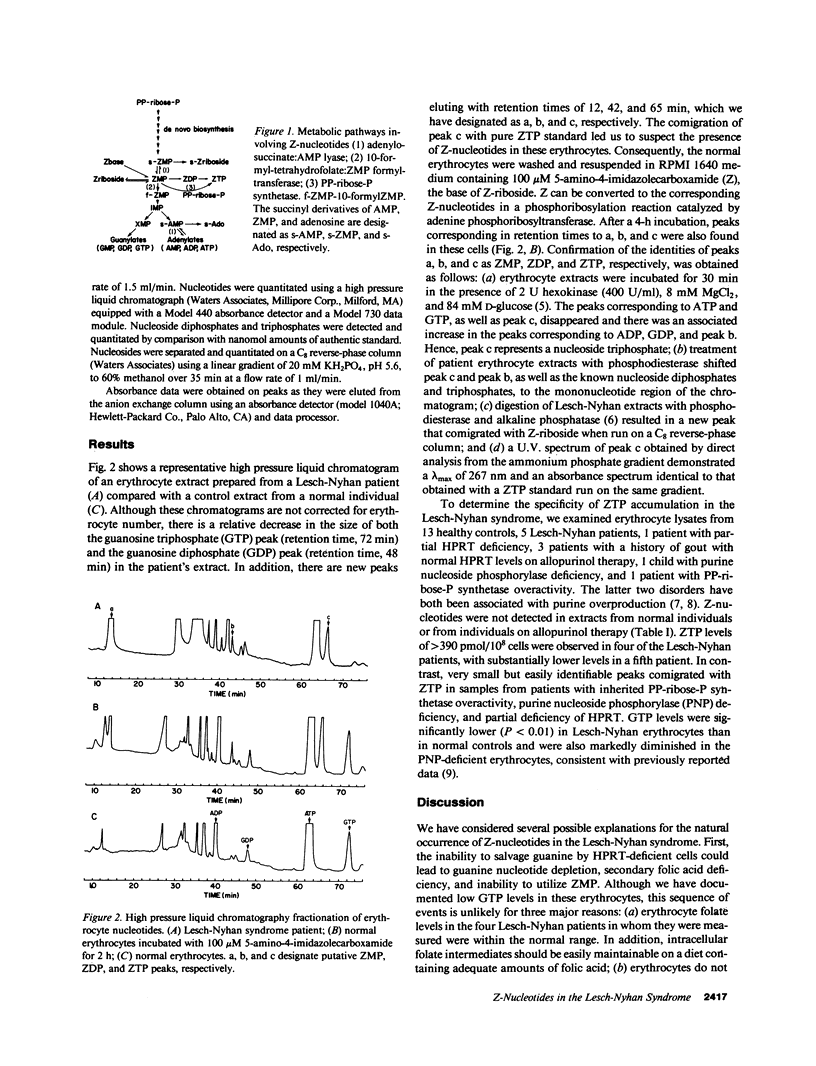
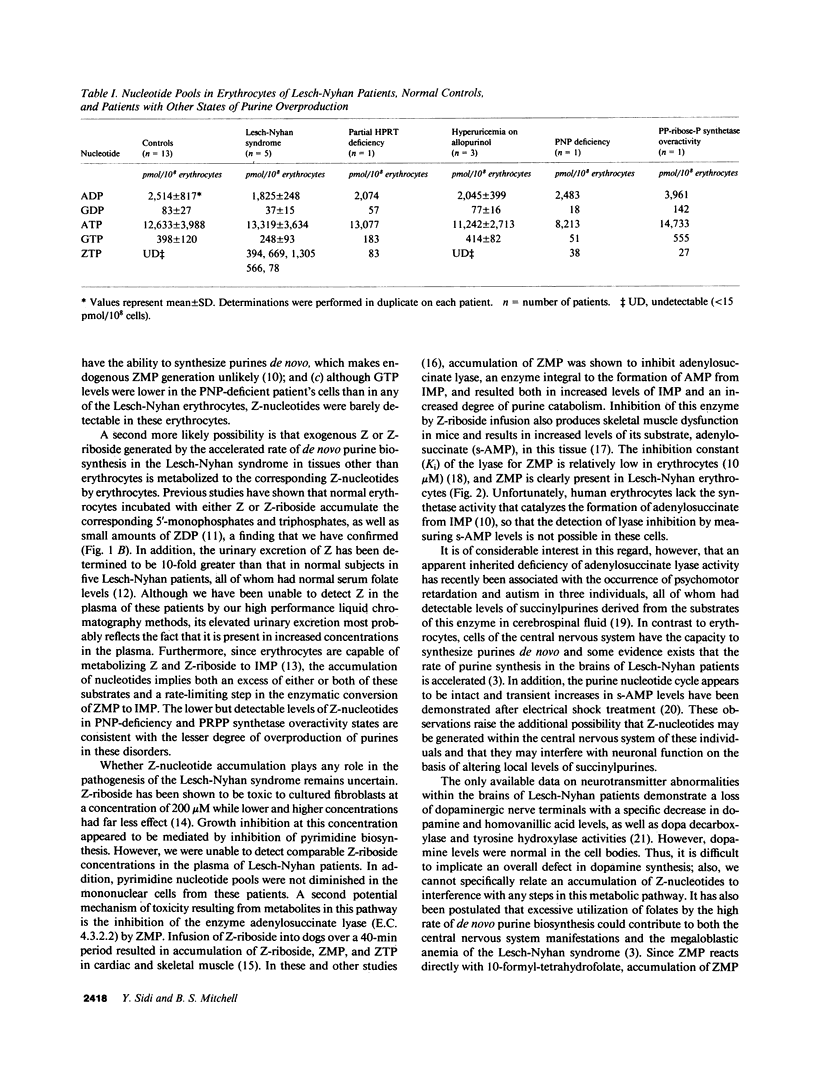
5-Amino-4-imidazolecarboxamide riboside 5'-monophosphate (ZMP) is an intermediate in the purine de novo synthetic pathway that may be further metabolized to inosine 5'-monophosphate, degraded to the corresponding nucleoside (5-amino-4-imidazole-carboxamide riboside; Z-riboside), or phosphorylated to the corresponding 5'-triphosphate (ZTP). Accumulation of ZTP in microorganisms has been associated with depletion of folate intermediates that are necessary for the conversion of ZMP to inosine 5'-monophosphate and has been postulated to play a regulatory role in cellular metabolism. We have shown the presence of Z-nucleotides in erythrocytes derived from five individuals with the Lesch-Nyhan syndrome. Erythrocyte folate levels were within the normal range, although guanosine triphosphate levels were significantly reduced below those in normal controls (P less than 0.01). A small amount of Z-nucleotide accumulation was also found in one individual with partial deficiency of the enzyme hypoxanthine guanine phosphoribosyltransferase and in two individuals with other disorders of purine overproduction. In contrast, no Z-nucleotides were detected in 13 normal controls or in three individuals with hyperuricemia on allopurinol therapy. We conclude that Z-nucleotide formation may result from markedly increased rates of de novo purine biosynthesis. It is possible that metabolites of these purine intermediates may play a role in the pathogenesis of the Lesch-Nyhan syndrome.
Full text
PDF

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bochner B. R., Ames B. N. ZTP (5-amino 4-imidazole carboxamide riboside 5'-triphosphate): a proposed alarmone for 10-formyl-tetrahydrofolate deficiency. Cell. 1982 Jul;29(3):929–937. doi: 10.1016/0092-8674(82)90455-x. [DOI] [PubMed] [Google Scholar]
- Cohen A., Doyle D., Martin D. W., Jr, Ammann A. J. Abnormal purine metabolism and purine overproduction in a patient deficient in purine nucleoside phosphorylase. N Engl J Med. 1976 Dec 23;295(26):1449–1454. doi: 10.1056/NEJM197612232952603. [DOI] [PubMed] [Google Scholar]
- Coleman M. S., Donofrio J., Hutton J. J., Hahn L., Daoud A., Lampkin B., Dyminski J. Identification and quantitation of adenine deoxynucleotides in erythrocytes of a patient with adenosine deaminase deficiency and severe combined immunodeficiency. J Biol Chem. 1978 Mar 10;253(5):1619–1626. [PubMed] [Google Scholar]
- Hershfield M. S., Fetter J. E., Small W. C., Bagnara A. S., Williams S. R., Ullman B., Martin D. W., Jr, Wasson D. B., Carson D. A. Effects of mutational loss of adenosine kinase and deoxycytidine kinase on deoxyATP accumulation and deoxyadenosine toxicity in cultured CEM human T-lymphoblastoid cells. J Biol Chem. 1982 Jun 10;257(11):6380–6386. [PubMed] [Google Scholar]
- Jaeken J., Van den Berghe G. An infantile autistic syndrome characterised by the presence of succinylpurines in body fluids. Lancet. 1984 Nov 10;2(8411):1058–1061. [PubMed] [Google Scholar]
- Lloyd K. G., Hornykiewicz O., Davidson L., Shannak K., Farley I., Goldstein M., Shibuya M., Kelley W. N., Fox I. H. Biochemical evidence of dysfunction of brain neurotransmitters in the Lesch-Nyhan syndrome. N Engl J Med. 1981 Nov 5;305(19):1106–1111. doi: 10.1056/NEJM198111053051902. [DOI] [PubMed] [Google Scholar]
- Lowy B. A., Williams M. K. Lesch-Nyhan syndrome: the synthesis of inosine 5'-phosphate in the hypoxanthine-guanine phosphoribosyltransferase-deficient erythrocyte by alternate biochemical pathways. Pediatr Res. 1977 May;11(5):691–694. doi: 10.1203/00006450-197705000-00013. [DOI] [PubMed] [Google Scholar]
- Lowy B., Dorfman B. Z. Adenylosuccinase activity in human and rabbit erythrocyte lysates. J Biol Chem. 1970 Jun;245(12):3043–3046. [PubMed] [Google Scholar]
- Newcombe D. S. The urinary excretion of aminoimidazolecarboxamide in the Lesch-Nyhan syndrome. Pediatrics. 1970 Oct;46(4):508–512. [PubMed] [Google Scholar]
- Sabina R. L., Holmes E. W., Becker M. A. The enzymatic synthesis of 5-amino-4-imidazolecarboxamide riboside triphosphate (ZTP). Science. 1984 Mar 16;223(4641):1193–1195. doi: 10.1126/science.6199843. [DOI] [PubMed] [Google Scholar]
- Sabina R. L., Kernstine K. H., Boyd R. L., Holmes E. W., Swain J. L. Metabolism of 5-amino-4-imidazolecarboxamide riboside in cardiac and skeletal muscle. Effects on purine nucleotide synthesis. J Biol Chem. 1982 Sep 10;257(17):10178–10183. [PubMed] [Google Scholar]
- Sabina R. L., Patterson D., Holmes E. W. 5-Amino-4-imidazolecarboxamide riboside (Z-riboside) metabolism in eukaryotic cells. J Biol Chem. 1985 May 25;260(10):6107–6114. [PubMed] [Google Scholar]
- Schultz V., Lowenstein J. M. The purine nucleotide cycle. Studies of ammonia production and interconversions of adenine and hypoxanthine nucleotides and nucleosides by rat brain in situ. J Biol Chem. 1978 Mar 25;253(6):1938–1943. [PubMed] [Google Scholar]
- Seegmiller J. E., Rosenbloom F. M., Kelley W. N. Enzyme defect associated with a sex-linked human neurological disorder and excessive purine synthesis. Science. 1967 Mar 31;155(3770):1682–1684. doi: 10.1126/science.155.3770.1682. [DOI] [PubMed] [Google Scholar]
- Simmonds H. A., Watson A. R., Webster D. R., Sahota A., Perrett D. GTP depletion and other erythrocyte abnormalities in inherited PNP deficiency. Biochem Pharmacol. 1982 Mar 15;31(6):941–946. doi: 10.1016/0006-2952(82)90324-0. [DOI] [PubMed] [Google Scholar]
- Sperling O., Eilam G., Sara-Persky-Brosh, De Vries A. Accelerated erythrocyte 5-phosphoribosyl-1-pyrophosphate synthesis. A familial abnormality associated with excessive uric acid production and gout. Biochem Med. 1972 Aug;6(4):310–316. doi: 10.1016/0006-2944(72)90017-8. [DOI] [PubMed] [Google Scholar]
- Swain J. L., Hines J. J., Sabina R. L., Harbury O. L., Holmes E. W. Disruption of the purine nucleotide cycle by inhibition of adenylosuccinate lyase produces skeletal muscle dysfunction. J Clin Invest. 1984 Oct;74(4):1422–1427. doi: 10.1172/JCI111553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas C. B., Meade J. C., Holmes E. W. Aminoimidazole carboxamide ribonucleoside toxicity: a model for study of pyrimidine starvation. J Cell Physiol. 1981 Jun;107(3):335–344. doi: 10.1002/jcp.1041070305. [DOI] [PubMed] [Google Scholar]
- Zimmerman T. P., Deeprose R. D. Metabolism of 5-amino-1-beta-D-ribofuranosylimidazole-4-carboxamide and related five-membered heterocycles to 5'-triphosphates in human blood and L5178Y cells. Biochem Pharmacol. 1978 Mar 1;27(5):709–716. doi: 10.1016/0006-2952(78)90508-7. [DOI] [PubMed] [Google Scholar]



