Abstract
Introduction
Dysregulation of the hedgehog signalling pathway has been linked to the development and progression of a variety of different human tumors including cancers of the skin, brain, colon, prostate, blood, and pancreas. We assessed the clinicopathological factors that are potentially related to expression of Gli1, the transcription factor that is thought to be the most reliable marker of hedgehog pathway activation in bladder cancer.
Methods
Bladder cancer cases were identified from the New Hampshire State Cancer Registry as histologically confirmed primary bladder cancer diagnosed between January 1, 2002, and July 31, 2004. Immunohistochemical analysis was performed on a tissue microarray to detect Gli1 and p53 expression in these bladder tumors. We computed odds ratios (ORs) and their 95% CIs for Gli1 positivity for pathological category using T category (from TNM), invasiveness, and grade with both the World Health Organization 1973 and World Health Organization International Society of Urological Pathology criteria. We calculated hazard ratios and their 95% CI for Gli1 positivity and recurrence for both Ta-category and invasive bladder tumors (T1+).
Results
A total of 194 men and 67 women, whose tumors were assessable for Gli1 staining, were included in the study. No appreciable differences in Gli1 staining were noted by sex, age, smoking status, or high-risk occupation. Ta-category tumors were more likely to stain for Gli1 as compared with T1-category tumors (adjusted OR = 0.38, CI: 0.17–0.87). Similarly, low-grade (grades 1–2) tumors were more likely to stain for Gli1 as compared with high-grade tumors (grade 3) (adjusted OR = 0.44, CI: 0.21–0.93). In a Cox proportional hazards regression analysis, non–muscle-invasive bladder tumors expressing Gli1 were less likely to recur (adjusted hazard ratio = 0.48; CI: 0.28–0.82; P < 0.05) than those in which Gli1 was absent.
Conclusion
Our findings indicate that Gli1 expression may be a marker of low-stage, low-grade bladder tumors and an indicator of a reduced risk of recurrence in this group.
Keywords: Gli1, Bladder neoplasm
1. Introduction
The development and progression of human cancer is a result of dysregulation of multiple genetic and epigenetic pathways [1]. Over the last decade, the hedgehog signalling pathway has been linked with several different human tumors including cancers of the skin, brain, colon, prostate, blood, and pancreas [2]. However, the role of this pathway in the development of human bladder cancer has been less well investigated and its contribution to the progression of transitional cell cancer (TCC) of the bladder is not clear.
The hedgehog pathway normally regulates cell growth, differentiation, and patterning during embryonic development. In the adult, the pathway is important in maintaining tissue homeostasis and repair and in regulating stem cell behaviour in certain epithelial tissues. Hedgehog ligands initiate signalling by inactivating the transmembrane receptor Patched1 (Ptc1), which releases the catalytic inhibitors of the protein smoothened (SMO). Active SMO triggers an intracellular signalling cascade that enables activation of Gli transcription factors. The Gli transcription factors are the primary effectors of the hedgehog pathway, and the outcome of this signalling depends on the receiving tissue type and includes genes that regulate cell proliferation (e.g., cyclin D1, N-myc, and Wnts), survival (BCL2), self-renewal (BMI1), angiogenesis (vascular endothelial growth factor), epithelial-mesenchymal transition (Snail 1 and Sip1), and invasiveness (osteopontin) [2,3]. Given the range of targets, it is not surprising that dysregulation of hedgehog signaling can be important in carcinogenesis. One of the earliest genetic alterations in urothelial cancer is loss of certain regions of chromosome 9q, and some studies have reported that the PTCH1 gene resides in this minimal deletion region [4]. However, early studies in normal urothelial cells and TCC cell lines rarely found mutations in the Ptc1 gene and little overexpression of Gli1–3, Ptc1, or Sonic Hedgehog, this lead to the conclusion that the hedgehog pathway is weakly active in normal adult urothelial cells and of limited or no importance in TCC [5]. More recent studies in urothelial cell lines and bladder tumors and genotype studies of patients with bladder cancer have led to a revaluation of the importance of hedgehog pathway signaling [6,7]. However, few of these studies have focused on the Gli1, which is thought to be the most reliable biomarker of hedgehog pathway activity [8,9], in primary human bladder tumors. Therefore, we examined the clinicopathological correlates of Gli1 expression in a population-based cohort of patients with newly diagnosed bladder cancer.
2. Methods
2.1. Study population
Data were derived from a case-control study of bladder cancer, described previously [6]. Briefly, bladder cancer cases were identified from the New Hampshire State Department of Health and Human Services Cancer Registry as histologically confirmed, primary bladder cancer diagnosed between January 1, 2002, and July 31, 2004. To be eligible for inclusion in the study, all cases had to be New Hampshire residents aged between 30 and 79 years and speak English. A total of 403 were found to be eligible and interviewed for the study.
2.2. Personal interviews
In-person interviews were conducted with consenting participants. Data were collected on participants’ sociodemo-graphic information such as education level, residence, occupation (history), medical history, lifestyle factors (including tobacco smoking), household water supply, and family history of cancer.
2.3. Pathology review
Pathology reports and slides and the original diagnostic formalin-fixed tumor block were requested from the pathology laboratories at which the initial diagnoses were made. All slides were reviewed by a single pathologist (A.S.) without knowledge of the submitting diagnosis on a standardized form. Subjects for these analyses were restricted to those with review of original pathology material(379 of 403 cases). Tumors were classified according to both 1973 World Health Organization (WHO) [10] and WHO/International Society of Urological Pathology [11] criteria. Tumors were staged according to the TNM criteria of the American Joint Commission on Cancer [12].
2.4. Immunohistochemistry
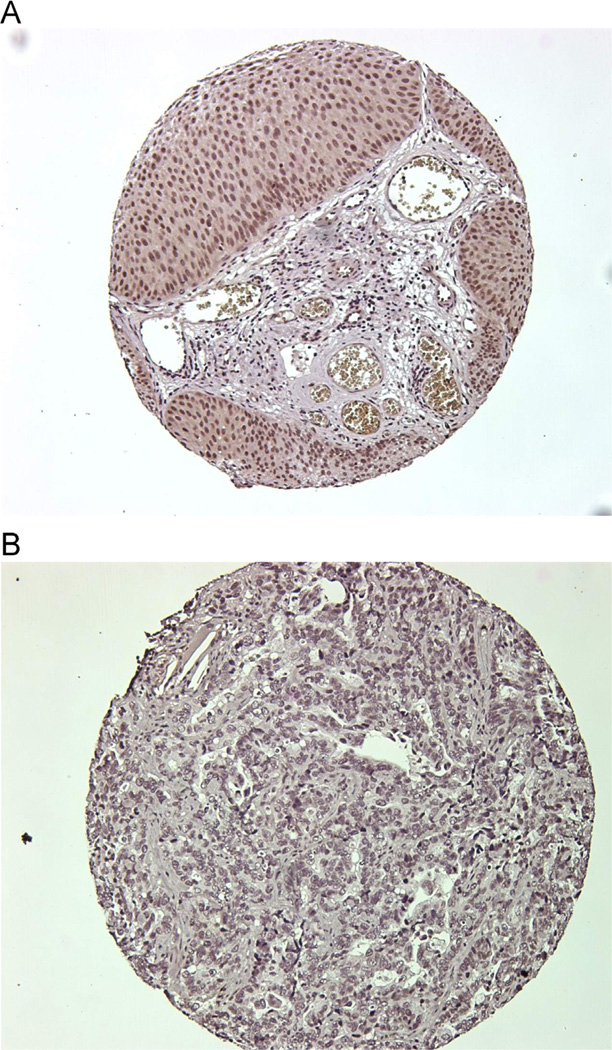
During the systematic re-review of diagnostic slides, the pathologist (A.S.) selected the tumor block and outlined regions for cutting 0.6-mm tissue cores for tissue micro-arrays (261 cases). Immunohistochemistry was done as described previously [6] using an affinity-purified rabbit polyclonal Gli1 antibody (1:300) [13]. The Gli1 score from each bladder tumor sample was examined by a research pathologist (H.L.), who was masked to patient characteristics. The Gli1 scoring criteria were based on an estimate of the percentage of tumor cells with nuclear Gli1 staining. Gli1 positivity was defined as ≥1% vs. < 1% (Fig. 1). A second Gli1 antisera (Abcam) was used to confirm these immunohistochemistry results. Immunohistochemical analysis of the tumors was also performed for TP53 and scored at 1, 2, 3+ intensity [14].
Fig. 1.
Immunohistochemistry demonstrating (A) expression of Gli1 and (B) no expression of Gli1. (Color version of figure is available online.)
2.5. Statistical analysis
We computed odds ratios (ORs) and the 95% CIs for Gli1 positivity according to pathological category using T category from the American Joint Committee on Cancer TNM staging (TIS, TA, T1, and ≥T2), invasiveness (noninvasive [Ta] and invasive [T1+]) and grade using both the WHO 1973 (grades 1 and 2 combined and grade 3) and the WHO International Society of Urological Pathology criteria (carcinoma in situ, papillary urothelial neoplasm of low malignant potential, papillary carcinoma—low grade, papillary carcinoma—high grade, and non-papillary carcinoma—high grade), and p53 immunohistochemistry staining intensity (<3 or ≥3) using unconditional logistic regression. All risk estimates were adjusted for age (<65, 65–69, and ≥70 years) and sex. When assessing the effects of tumor stage, we further adjusted for tumor grade (WHO 1973) (grades 1 and 2 combined and grade 3) Likewise, when assessing the effects of tumor grade we adjusted for tumor stage. All analyses were restricted to subjects with histology determined to be transitional cell carcinoma based on review of original pathology material (373 of 379 cases).
Information on bladder cancer recurrences was obtained from the medical records provided by the treating hospital(s) (both inpatient and outpatient records, including any pathology reports) covering the follow-up period. The records were reviewed by an experienced, certified tumor registrar to abstract the data on bladder tumors occurring subsequent to the incident tumor. Hospital registry data were used if the medical record could not be obtained. The first recurrent tumor was defined as any tumor identified after a disease-free remission period, more than 90 days after the date of initial primary bladder tumor diagnosis. Persistent primary tumors that did not have a remission period were excluded from the analysis of recurrence. The time to first event was calculated as the time between the date of initial diagnosis and the first recurrence. The diagnosis of a tumor with a greater stage or grade than the initial primary bladder tumor was defined as progression. If no events were reported, the date the patient was last seen documented in the medical record was used for censoring. Life status (alive or deceased) was determined as of January 2011 using the Social Security and the National Death Indices. Recurrence data were available on 304 of the urothelial cell carcinoma cases, with a median of 2.8 years of postdiagnosis follow-up time.
We examined the time to first bladder cancer recurrence using Kaplan-Meier plots and differences by Gli1 positivity were assessed using the log-rank test. To adjust for additional factors related to patient recurrence, Cox proportional hazards regression analysis was performed with adjustment for age, sex, smoking status (never, former, and current), and the American Joint Committee on Cancer tumor stage (0, carcinoma in situ, and I–IV), grade (1–3), size of tumor (<3 vs. ≥3 cm), multiplicity (single lesion vs. multiple lesions), treatment (transurethral resection vs. other treatments including immunotherapy, chemotherapy, radiotherapy, and cystectomy) in the model. P values represent 2-sided statistical tests with statistical significance at P < 0.05.
The statistical package SAS v9.2 was used for all the analyses.
3. Results
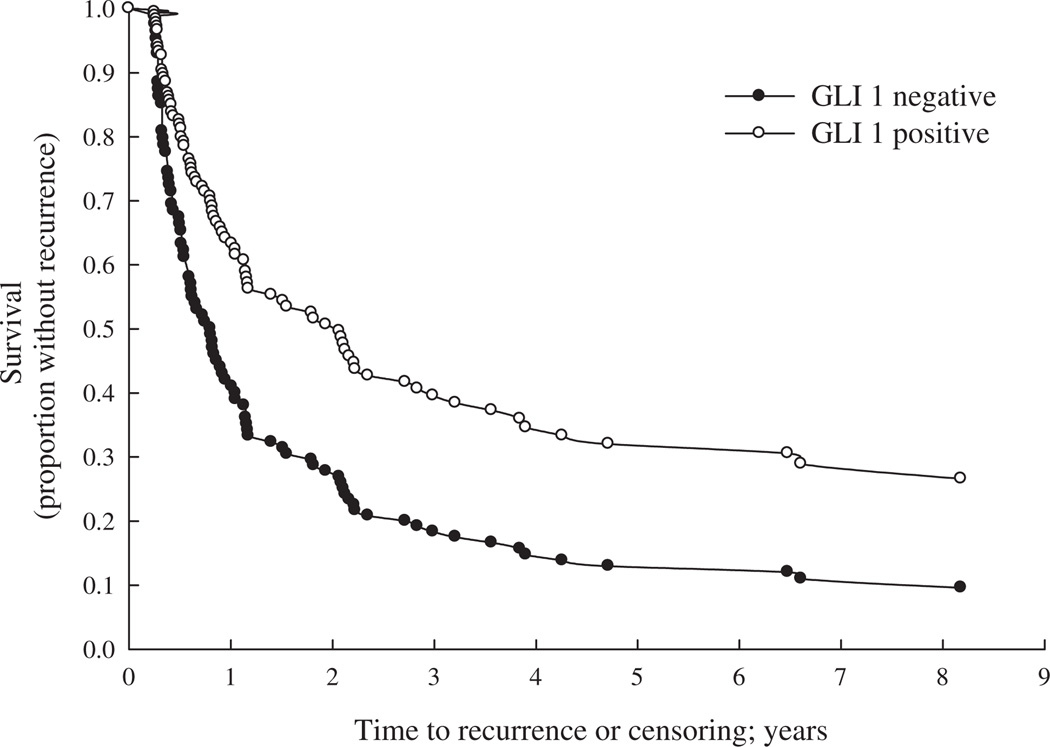
A total of 194 men and 67 women were included in the study (Table 1). No differences were noted in Gli1 expression according to sex, age, smoking status, or high-risk occupation. However, differences were observed in Gli1 staining for Ta-category vs. T1-category tumors (adjusted OR = 0.38, CI: 0.17–0.87) and low-grade tumors (grades 1–2) vs. high-grade tumors (grade 3) (adjusted OR = 0.44, CI: 0.21–0.93). Moreover, the association was even stronger when low-grade Ta-category (grades 1–2) tumors and T1/Tis/Ta (grade 3)category tumors were compared (OR = 0.29, CI: 0.15–0.57). Papillary urothelial neoplasm of low malignant potential and papillary low-grade tumors were more likely to stain positively for Gli1 than papillary high-grade tumors (OR = 0.41, CI: 0.18–0.96) and non-papillary high-grade tumors (OR = 0.18, CI: 0.06–0.54). Invasive urothelial cell carcinoma was less likely to be Gli1 positive than noninvasive tumors, although the difference was not statistically significant (OR = 0.61, CI: 0.29–1.27). Tumors staining positive for p53 were less likely to express Gli1 but again, in the multivariate analysis, the difference did not reach statistical significance (OR = 0.58, CI: 0.28–1.21) (Table 2). In subjects with an initial pathology category of Ta, the risk of bladder tumor recurrence was lower in subjects with Gli1 expression than subjects without Gli1 (hazard ratio [HR] = 0.46, CI: 0.27–0.79) (Table 3 and Fig. 2).
Table 1.
Patients demographics
| Gli1 negative, n = 60 (%) |
Gli1 positive, n = 201 (%) |
Unadjusted OR (95% CI) |
Adjusteda OR (95% CI) |
|
|---|---|---|---|---|
| Gender | ||||
| Male | 45 (75.00) | 149 (74.13) | 1.0 (ref) | 1.0 (ref) |
| Female | 15 (25.00) | 52 (25.87) | 1.05 (0.54–2.03) | 1.04 (0.54–2.03) |
| Age, y | ||||
| < 65 | 22 (36.67) | 86 (42.79) | 1.0 (ref) | 1.0 (ref) |
| 65–69 | 10 (16.67) | 39 (19.40) | 1.00 (0.43–2.31) | 1.00 (0.43–2.31) |
| ≥70 | 28 (46.67) | 76 (37.81) | 0.69 (0.37–1.31) | 0.69 (0.37–1.32) |
| Smoking status | ||||
| Never | 5 (8.62) | 32 (16.08) | 1.0 (ref) | 1.0 (ref) |
| Ever | 53 (91.38) | 167 (83.92) | 0.49 (0.18–1.33) | 0.51 (0.19–1.38) |
| Duration | ||||
| < 20 y | 11 (19.30) | 35 (17.77) | 0.50 (0.16–1.59) | 0.50 (0.15–1.60) |
| ≥ 20 y | 41 (71.93) | 130 (65.99) | 0.50 (0.18–1.35) | 0.52 (0.19–1.42) |
| High-risk occupationb | ||||
| No | 26 (44.07) | 91 (45.50) | 1.0 (ref) | 1.0 (ref) |
| Yes | 33 (55.93) | 109 (54.50) | 0.94 (0.53–1.69) | 0.95 (0.53–1.72) |
ref = reference.
Models adjusted by age at diagnosis and gender.
High-risk occupation missing 1 subject with Gli1 negative and 1 subject with Gli1 positive.
Table 2.
Tumor characteristics
| Gli1 negative, n = 60 (%) | Gli1 positive, n = 201 (%) | Unadjusteda OR (95%CI) | Adjustedb OR (95%CI) | |
|---|---|---|---|---|
| T-code staging | ||||
| TIS | 1 (1.67) | 0 (0) | *c | *c |
| Ta | 32 (53.33) | 152 (75.62) | 1.0 (ref) | 1.0 (ref) |
| T1 | 18 (30.00) | 22 (10.95) | 0.26 (0.12–0.53) | 0.38 (0.17–0.87) |
| >T2 | 9 (15.00) | 27 (13.43) | 0.63 (0.27–1.47) | 1.08 (0.40–2.90) |
| Invasiveness | ||||
| Noninvasive (TIS and TA) | 33 (55.00) | 152 (75.62) | 1.0 (ref) | 1.0 (ref) |
| Invasive (> T1) | 27 (45.00) | 49 (24.38) | 0.39 (0.22–0.72) | 0.61 (0.29–1.27) |
| Treatment | ||||
| Other treatments | 25 (41.67) | 46 (22.89) | 1.0 (ref) | 1.0 (ref) |
| TUR only | 35 (58.33) | 155 (77.11) | 2.41 (1.31–4.43) | 2.02 (0.96–4.23) |
| Grade | ||||
| Low grade (1–2) | 31 (51.67) | 152 (75.62) | 1.0 (ref) | 1.0 (ref) |
| High grade (3) | 29 (48.33) | 49 (24.38) | 0.34 (0.19–0.63) | 0.44 (0.21–0.93) |
| Noninvasive only grade | ||||
| Low grade (1–2) | 25 (75.76) | 134 (88.16) | 1.0 (ref) | 1.0 (ref) |
| High grade (3) | 8 (24.24) | 18 (11.84) | 0.42 (0.16–1.07) | 0.48 (0.18–1.30) |
| Stage grade | ||||
| Ta (G1–2) | 25 (41.67) | 134 (66.67) | 1.0 (ref) | 1.0 (ref) |
| T1, Tis, and TaG3 | 26 (43.33) | 40 (19.90) | 0.29 (0.15–0.55) | 0.29 (0.15–0.57) |
| T1 and TaG3—no TIS | 25 (42.37) | 40 (19.90) | 0.30 (0.15–0.58) | 0.31 (0.16–0.60) |
| >T2 | 9 (15.00) | 27 (13.43) | 0.56 (0.24–1.33) | 0.56 (0.23–1.35) |
| WHO/ISUP | ||||
| CIS | 1 (1.67) | 0 (.) | *c | *c |
| PUN-LMP | 8 (13.33) | 47 (23.38) | 0.98 (0.38–2.54) | 0.89 (0.33–2.36) |
| PapCa-LG | 13 (21.67) | 78 (38.81) | 1.0 (ref) | 1.0 (ref) |
| PapCa-HG | 28 (46.67) | 65 (32.34) | 0.39 (0.19–0.81) | 0.41 (0.18–0.96) |
| NoPapCa-HG | 10 (16.67) | 11 (5.47) | 0.18 (0.06–0.52) | 0.18 (0.06–0.54) |
| p53 immunohistochemistry intensity | ||||
| <3 | 38 (63.33) | 163 (81.09) | 1.0 (ref) | 1.0 (ref) |
| ≥3 | 22 (36.67) | 38 (18.91) | 0.40 (0.21–0.76) | 0.58 (0.28–1.21) |
Note: Values in italics are statistically significant.
ref = reference; TUR = transurethral resection; ISUP = International Society of Urological Pathology; CIS = carcinoma in situ; PUN-LMP = papillary urothelial neoplasm of low malignant potential; PapCa-LG = papillary carcinoma—low grade; PapCa-HG = papillary carcinoma—high grade; NoPapCa-HG = non-papillary carcinoma—high grade.
Odds ratios and 95% CI estimated from unconditional logistic regression.
Models adjusted by water arsenic concentration (µg/l) in addition to age, sex, pathological category, and grade.
Zero observations in the case group were omitted.
Table 3.
Gli1 expression associated with bladder cancer recurrence
| Stage | Gli1 expression | Recurrence, n (%) | Not recurring, n (%) | Hazard ratio (95% CI)a unadjusted | Hazard ratio (95% CI)a,b adjusted |
|---|---|---|---|---|---|
| TA | Negative | 19 (70.4) | 8 (29.6) | ||
| Positive (> 1%) | 73 (57.9) | 53 (42.1) | 0.49 (0.29–0.81) | 0.48 (0.28–0.82) | |
| T1 and ≥T2 | Negative | 7 (31.8) | 15 (68.2) | ||
| Positive (> 1%) | 17 (54.8) | 14 (45.2) | 1.34 (0.55–3.26) | 1.37 (0.53–3.55) |
Note: Values in italics are statistically significant.
TUR = transurethral resection.
Estimated from Cox proportional hazards models of time from initial diagnosis to the date of the recurring bladder cancer.
Models adjusted by age at diagnosis, sex, smoking status (never, former, and current smoker), size of tumor (<3 vs. ≥3 cm), tumor multiplicity, and treatment (TUR only vs. other treatment combinations).
Fig. 2.
Bladder cancer recurrence in subjects diagnosed with Ta-category tumors, with and without Gli1 expression. Proportion without recurrence was estimated from Cox proportional hazards model of time to first recurrence greater than 90 days from initial diagnosis, adjusted for age at diagnosis, sex, smoking status (never, former, and current smoker), size of tumor (<3 vs. ≥3 cm), tumor multiplicity, and treatment (TUR only vs. other treatment combinations). TUR = transurethral resection.
4. Discussion
The molecular pathogenesis of bladder cancer is poorly understood although several pathways including those involving cell surface receptors (epidermal growth factor receptor axis, fibroblast growth factors, and Shh pathway), transcription factors (retinoid signaling, peroxisome proliferator-activated receptor gamma and FOXA) and the p53/p63/p73 family are clearly of importance [15]. However, despite a large body of work, reliable molecular markers and reliable molecular therapeutic targets have not been identified [16]. Several recent studies have focused on the role of the hedgehog/Gli signal transduction pathway in carcinogenesis of TCC [6,7]. The hedgehog pathway operates in organogenesis, control of proliferation, and the differentiation of embryonic and adult stem cells, thus, not surprisingly, it has been linked with several different tumors. Gli proteins, which are zinc finger transcription factors, serve as the primary effectors of the pathway and regulate gene expression in different cell types. The exact mechanism of action of these proteins is not completely understood but they may have a role in the pathogenesis of bladder cancer. In our study, we chose to evaluate Gli1 expression as the marker of hedgehog pathway activation. We realize that Gli1 expression may reflect the readout of a number of noncanonical activators of Gli transcription factors. However, we believe that this is likely a small subset as previous studies support the concept that Gli1 activation is the most reliable biomarker of hedgehog pathway activity [8,9].
Recent studies have shown that arsenic, a known bladder carcinogen [17], when used to transform urothelial cells results in up-regulation of the hedgehog pathway, manifest in part by increased expression of Gli1 [6]. In addition, Gli1 knockdown studies in the muscle-invasive bladder cancer–derived cell lines, T24 and Vmcub-1, demonstrate that inhibition of Gli1 expression is associated with decreased colony formation in soft agar and a decreased tumor growth rate in a nude mouse model. A study by Mechlin etal. [18] investigated Gli expression in several different human bladder TCC cell lines (RT4, 253JP, 253BV, UMUC6, and UMUC3) and stratified them in order of increasing proliferative or invasive characteristics in an attempt to correlate the hedgehog signalling with tumor aggressiveness. The investigators tested this hypothesis by performing reverse transcription polymerase chain reaction to assess differential messenger RNA (mRNA) expression of hedgehog-related genes after 3 days of growth in cell culture. On a linear regression analysis, the expression of Gli2 mRNA was lowest in RT4 cells (highest proliferative doubling time)and highest in the UMUC3 cells (shortest doubling time). The expression of other molecules (Shh, IHH, Ptch1, SMO, SPOP, SuFu, Gli1, and Gli3) varied, and no correlation with aggressive cell behaviour was identified.
Our study of a population-based cohort of bladder tumors indicates that the expression of Gli1 is greater in low-stage (Ta), low-grade (grades 1–2) tumors and papillary bladder tumors compared with T1-category, high-grade and non-papillary, high-grade tumors. This finding of more selective activation of the hedgehog pathway in lower risk bladder cancer is supported by the work of Pignot etal. [19], who examined the expression levels of multiple mRNAs and microRNAs in this pathway in 71 bladder tumors. They found constitutive activation of the hedgehog pathway in 96% of non–muscle-invasive bladder cancer as compared with 50% of muscle-invasive bladder cancers.
Our findings are different to those of He etal. [20] who investigated the expression of 3 proteins (Shh, Ptch1, and Gli1) in the hedgehog signalling pathway in a cohort of Chinese patients with bladder cancer. These investigators reported that increased expression of Gli1 correlated with a higher pathological category, venous invasion, and lymph node metastasis. Interestingly there was no difference in protein expression between patients with high-grade (3) and low-grade (1 and 2) tumors. However, the tumors analyzed in their study differed significantly from our population-based sample in that their subjects were derived from a non-consecutive hospital-based cohort of primarily muscle-invasive tumors, as reflected in the fact that 61% of the subjects had pathological stage III disease as compared with less than 16% of such tumors in our study. In addition, different antibodies and scoring systems were used for the immunohistochemical analysis, which also may have contributed to observed differences. In essence, our results reflect an analysis of Gli1 expression in a population-based cohort, which largely consists of low-grade noninvasive tumors, whereas that of He etal. [20] reflects the results of hospital referral practice of muscle-invasive disease.
The second finding of our study was that the expression of Gli1 was a marker of a decreased risk of recurrence in the group of patients with non–muscle-invasive bladder cancer. The HR for recurrence decreased slightly from 0.49 to 0.48, with a tighter confidence interval after adjustment for multiple variables including the major risk factors for tumor recurrence including tumor multiplicity, tumor size, and treatment. To our knowledge, no other study has examined Gli1 expression as a prognostic marker specifically in non–muscle-invasive bladder cancer. He etal. [20] reported that Gli1 expression was a marker of decreased recurrence-free and overall survival in their patient population with a HR of 0.97 (95% CI: 0.33–0.94). However, as pointed out earlier, this was a non-consecutive hospital-based patient population that was heavily weighted with muscle-invasive tumors. Chen etal. [7] examined genetic variations in the hedgehog pathway in a large group of patients with bladder cancer in Texas. These investigators genotyped 177 single nucleotide polymorphisms (SNPs) in 11 hedgehog pathway genes. They identified 9 SNPs that were associated with recurrence in patients with non–muscle-invasive bladder cancer, 2 of which were replicated in a second cohort of patients from Spain. It is noteworthy that 2 independent SNP variants in Gli3 were associated with an increased recurrence rate and shorter recurrence-free survival in the group of patients receiving transurethral resection + bacillus Calmette-Guerin immunotherapy. Currently, it is unclear if the variants reflect a lesser response to the bacillus Calmette-Guerin therapy or a more aggressive phenotype in the tumor.
A robust marker of recurrence in noninvasive bladder cancer would be useful, as it would allow for selection of patients who require either less intense follow-up in the low-risk group or adjuvant intravesical therapy in the high-risk group [16,21]. Avoiding frequent cystoscopies or the side effects of intravesical chemotherapy/immunotherapy would be a quality-of-life improvement for the patient and a financial savings for the patient and society.
The strengths of our study are firstly that we report Gli1 expression in a large population-based cohort reflecting all patients with newly diagnosed bladder cancer in a single state. Secondly, because the patient cohort is so well characterized, we were able to control for multiple factors in our statistical analysis, thereby increasing the robustness of our findings.
5. Conclusion
The molecular pathways leading to the development and progression of bladder cancer are poorly understood. Our study provides further evidence that effectors of hedgehog signaling are important in the pathogenesis of bladder cancer. Specifically, we found that Gli1 expression is a marker of low-stage, low-grade bladder tumors and an indicator of a decreased risk of recurrence in patients with non–muscle-invasive bladder cancer. These findings, if confirmed in other studies, have the potential to be used clinically to stratify patients into risk groups that need either less intense follow-up or adjuvant intravesical therapy.
Acknowledgments
All grants supported by NIH, USA: CA 023108, R01 CA057594, P42 ES007373, P01ES022832, P20 GM104416, and K07 CA102327.
References
- 1.Hanahan D, Weinberg RA. Hallmarks of cancer: the next generation. Cell. 2011;144:646–674. doi: 10.1016/j.cell.2011.02.013. [DOI] [PubMed] [Google Scholar]
- 2.Briscoe J, Therond PP. The mechanisms of Hedgehog signalling and its roles in development and disease. Nat Rev Mol Cell Biol. 2013;14:416–429. doi: 10.1038/nrm3598. [DOI] [PubMed] [Google Scholar]
- 3.Stecca B, Ruiz IAA. Context-dependent regulation of the GLI code in cancer by HEDGEHOG and non-HEDGEHOG signals. J Mol Cell Biol. 2010;2:84–95. doi: 10.1093/jmcb/mjp052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Aboulkassim TO, LaRue H, Lemieux P, Rousseau F, Fradet Y. Alteration of the PATCHED locus in superficial bladder cancer. Oncogene. 2003;22:2967–2971. doi: 10.1038/sj.onc.1206513. [DOI] [PubMed] [Google Scholar]
- 5.Thievessen I, Wolter M, Prior A, Seifert HH, Schulz WA. Hedgehog signaling in normal urothelial cells and in urothelial carcinoma cell lines. J Cell Physiol. 2005;203:372–377. doi: 10.1002/jcp.20248. [DOI] [PubMed] [Google Scholar]
- 6.Fei DL, Sanchez-Mejias A, Wang Z, et al. Hedgehog signaling regulates bladder cancer growth and tumorigenicity. Cancer Res. 2012;72:4449–4458. doi: 10.1158/0008-5472.CAN-11-4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Chen M, Hildebrandt MA, Clague J, et al. Genetic variations in the sonic hedgehog pathway affect clinical outcomes in non-muscle-invasive bladder cancer. Cancer Prev Res. 2010;3:1235–1245. doi: 10.1158/1940-6207.CAPR-10-0035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee J, Platt KA, Censullo P, Ruiz i Altaba A. Gli1 is a target of Sonic hedgehog that induces ventral neural tube development. Development. 1997;124:2537–2552. doi: 10.1242/dev.124.13.2537. [DOI] [PubMed] [Google Scholar]
- 9.Kimura H, Stephen D, Joyner A, Curran T. Gli1 is important for medulloblastoma formation in Ptc1± mice. Oncogene. 2005;24:4026–4036. doi: 10.1038/sj.onc.1208567. [DOI] [PubMed] [Google Scholar]
- 10.Mostofi FK, Sobin LH, Torloni H. International histological classification of tumours. Geneva: World Health Organization; 1973. Histological typing of urinary bladder tumours. [Google Scholar]
- 11.Epstein JI, Amin MB, Reuter VR, Mostofi FK. The World Health Organization/International Society of Urological Pathology consensus classification of urothelial (transitional cell) neoplasms of the urinary bladder. Bladder Consensus Conference Committee. Am J Surg Pathol. 1998;22:1435–1448. doi: 10.1097/00000478-199812000-00001. [DOI] [PubMed] [Google Scholar]
- 12.Wittekind C, Compton CC, Greene FL, Sobin LH. TNM residual tumor classification revisited. Cancer. 2002;94:2511–2516. doi: 10.1002/cncr.10492. [DOI] [PubMed] [Google Scholar]
- 13.Fei DL, Li H, Kozul CD, et al. Activation of Hedgehog signaling by the environmental toxicant arsenic may contribute to the etiology of arsenic-induced tumors. Cancer Res. 2010;70:1981–1988. doi: 10.1158/0008-5472.CAN-09-2898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Danaee H, Nelson HH, Karagas MR, et al. Microsatellite instability at tetranucleotide repeats in skin and bladder cancer. Oncogene. 2002;21:4894–4899. doi: 10.1038/sj.onc.1205619. [DOI] [PubMed] [Google Scholar]
- 15.DeGraff DJ, Cates JM, Mauney JR, Clark PE, Matusik RJ, Adam RM. When urothelial differentiation pathways go wrong: implications for bladder cancer development and progression. Urol Oncol. 2013;31:802–811. doi: 10.1016/j.urolonc.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kamat AM, Hegarty PK, Gee JR, et al. ICUD-EAU International Consultation on Bladder Cancer 2012: screening, diagnosis, and molecular markers. Eur Urol. 2013;63:4–15. doi: 10.1016/j.eururo.2012.09.057. [DOI] [PubMed] [Google Scholar]
- 17.IARC. IARC monographs on the evaluation of carcinogenic risks to humans. France: IARC Press; 2004. Some drinking-water disinfectants and contaminants, including arsenic; pp. 37–270. [PMC free article] [PubMed] [Google Scholar]
- 18.Mechlin CW, Tanner MJ, Chen M, Buttyan R, Levin RM, Mian BM. Gli2 expression and human bladder transitional carcinoma cell invasiveness. J Urol. 2010;184:344–351. doi: 10.1016/j.juro.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 19.Pignot G, Vieillefond A, Vacher S, et al. Hedgehog pathway activation in human transitional cell carcinoma of the bladder. Br J Cancer. 2012;106:1177–1186. doi: 10.1038/bjc.2012.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.He HC, Chen JH, Chen XB, et al. Expression of hedgehog pathway components is associated with bladder cancer progression and clinical outcome. Pathol Oncol Res. 2012;18:349–355. doi: 10.1007/s12253-011-9451-2. [DOI] [PubMed] [Google Scholar]
- 21.Hall MC, Chang SS, Dalbagni G, et al. Guideline for the management of nonmuscle invasive bladder cancer (stages Ta, T1, and Tis): 2007 update. J Urol. 2007;178:2314–2330. doi: 10.1016/j.juro.2007.09.003. [DOI] [PubMed] [Google Scholar]