Abstract
Most theories predict that macromolecular crowding stabilizes globular proteins, but recent studies show that weak attractive interactions can result in crowding-induced destabilization. Osmolytes are ubiquitous in biology and help protect cells against stress. Given that dehydration stress adds to the crowded nature of the cytoplasm, we speculated that cells might use osmolytes to overcome the destabilization caused by the increased weak interactions that accompany desiccation. We used NMR-detected amide proton exchange experiments to measure the stability of the test protein chymotrypsin inhibitor 2 under physiologically relevant crowded conditions in the presence and absence of the osmolyte glycine betaine. The osmolyte overcame the destabilizing effect of the cytosol. This result provides a physiologically relevant explanation for the accumulation of osmolytes by dehydration-stressed cells.
Keywords: macromolecular crowding, nonspecific interaction, osmolytes, protein stability, amide proton exchange
Introduction
Globular proteins have been called the robots of the cell.1 Despite their essential role, globular proteins are only marginally stable, possessing denaturation free energies ( ) of 10 kcal/mol or less in simple buffered solutions.2 The intracellular environment, however, is far from simple. For instance, macromolecules can occupy more than 30% of a cell's volume, reaching concentrations exceeding 300 g/L.3 Even the bacterium Escherichia coli contains ∼4000 different proteins.4,5
) of 10 kcal/mol or less in simple buffered solutions.2 The intracellular environment, however, is far from simple. For instance, macromolecules can occupy more than 30% of a cell's volume, reaching concentrations exceeding 300 g/L.3 Even the bacterium Escherichia coli contains ∼4000 different proteins.4,5
For many years this crowded environment was thought to only stabilize globular proteins. Evidence came from studies of protein stability in synthetic polymer crowders such as Ficoll and polyvinylpyrrolidone.6–8 The stabilization was attributed to crowding-induced steric repulsions that favor the more compact native state over the ensemble of less compact denatured states.
Recently, it was shown that crowding is not always stabilizing.9–19 For instance, crowding by both individual globular proteins (e.g., bovine serum albumin, lysozyme), by cell lysates and crowding in living cells can actually destabilize globular proteins.13–19
One reason for the difference between the expectation of stabilization and the observations of destabilization arises from a chemical difference between synthetic polymers and more biologically relevant crowders. The simple polymers are relatively inert with respect to protein surfaces11 such that crowding effects are dominated by steric repulsions. Protein crowders, on the other hand, can sometimes interact favorably with the surface of proteins being studied. Unfolding a structured protein leads to exposure of additional sites for favorable crowder–protein interactions, lowering the free energy of the denatured state, and destabilizing the protein.12 This is the same way urea denatures proteins,20 which is consistent with the presence of similar functional groups (hydrogen bond donating-nitrogens and -accepting carbonyl oxygens, on urea and the protein backbone.
However, cells contain more than macromolecules. Naturally occurring osmolytes,21–28 for instance, can reach nearly M concentrations in E. coli.22 These small molecules protect cells against stress.21,22 One such stress, desiccation, further increases the concentration of macromolecules in the cytoplasm, making the intracellular environment even more crowded.23 One role of osmolytes in relieving dehydration stress is to counteract the loss of cellular water. Here, we consider the idea that osmolytes also act by overcoming the destabilizing effect of the increased weak attractive interactions in the cytoplasm that accompany desiccation.
We tested this explanation by examining the stability of chymotrypsin inhibitor 2 (CI2) in the presence and absence of the osmolyte, glycine betaine (N,N,N-trimethylglycine), and in the presence and absence of a physiologically relevant model of the E. coli proteome.15 We chose glycine betaine for three reasons. First, it is ubiquitous in biology. Second, it is the key osmolyte of E. coli.24 Third, it provides a sensitive test for the effects of osmolytes under crowded conditions because glycine betaine has only a modest stabilizing effect in buffer.25 We prepared the model cytoplasm from E. coli lysate by removing membranes, nucleic acids, nucleic acid binding proteins, and metabolites.15 Mass spectroscopic analysis indicates that our total protein lysate is representative of the proteome.15
We measured the stability of CI2 by using NMR-detected amide H/D exchange.29 The studies were conducted at a physiologically relevant glycine betaine concentration of 0.4 M.22 The H/D exchange rates of individual backbone amide protons can be converted to free energies of opening (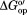 ) if the test protein is stable and the intrinsic exchange is rate determining.29,30 These conditions are met for CI2 in buffer and lysates.18,31
) if the test protein is stable and the intrinsic exchange is rate determining.29,30 These conditions are met for CI2 in buffer and lysates.18,31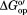 values were quantified under four conditions: in buffer (50 mM sodium phosphate buffer, 20°C, pH 7.0), in buffered 100 g/L protein lysate, in buffer containing 0.4 M glycine betaine, and in buffered 100 g/L protein lysate containing 0.4 M glycine betaine. The complete datasets are given in Supporting Information Table S1 and in our previous study.15
values were quantified under four conditions: in buffer (50 mM sodium phosphate buffer, 20°C, pH 7.0), in buffered 100 g/L protein lysate, in buffer containing 0.4 M glycine betaine, and in buffered 100 g/L protein lysate containing 0.4 M glycine betaine. The complete datasets are given in Supporting Information Table S1 and in our previous study.15
The protein lysate decreases relative to buffer at every residue we can measure [Fig. 1(a)]. This result indicates that weak, nonspecific protein–protein interactions can overcome the stabilizing effect of hard core repulsions.15 Adding glycine betaine to the lysate leads to a striking effect [Fig. 1(b)]; despite the presence of the lysate, the osmolyte increases
relative to buffer at every residue we can measure [Fig. 1(a)]. This result indicates that weak, nonspecific protein–protein interactions can overcome the stabilizing effect of hard core repulsions.15 Adding glycine betaine to the lysate leads to a striking effect [Fig. 1(b)]; despite the presence of the lysate, the osmolyte increases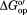 at every quantifiable residue, except the C-terminus.
at every quantifiable residue, except the C-terminus.
Figure 1.
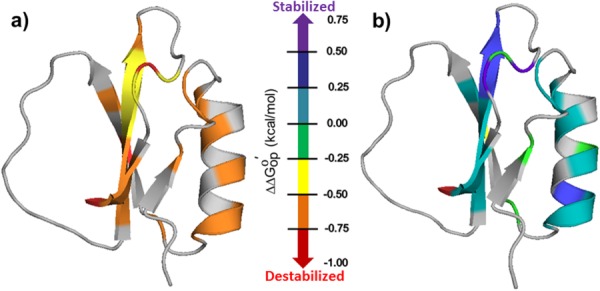
Backbone of CI2 colored by stability changes in kcal/mol. (a)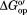 in buffered 100.0 g/L protein lysate minus
in buffered 100.0 g/L protein lysate minus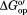 in buffer alone. (b)
in buffer alone. (b) in buffered 100.0 g/L protein lysate with 0.4 M glycine betaine minus
in buffered 100.0 g/L protein lysate with 0.4 M glycine betaine minus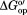 in buffered 0.4 M glycine betaine.
in buffered 0.4 M glycine betaine.
Stabilization is also observed for globally exchanging residues (Table I), whose average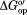 value equals the free energy of denaturation as determined by, for example, calorimetry.32 Adding glycine betaine to the protein lysate stabilizes CI2 by 0.8 kcal/mol compared to buffer alone. The lysate destabilizes CI2 by 0.6 kcal/mol compared to buffer. Adding glycine betaine to the lysate increased the stability compared to buffer by 0.2 kcal/mol, and adding the osmolyte to buffer increased the stability by the same amount.
value equals the free energy of denaturation as determined by, for example, calorimetry.32 Adding glycine betaine to the protein lysate stabilizes CI2 by 0.8 kcal/mol compared to buffer alone. The lysate destabilizes CI2 by 0.6 kcal/mol compared to buffer. Adding glycine betaine to the lysate increased the stability compared to buffer by 0.2 kcal/mol, and adding the osmolyte to buffer increased the stability by the same amount.
Table I.
Average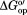 , in kcal/mol, for Amide Protons Exposed on Global Unfolding of the I29A;I37H Variant of CI2
, in kcal/mol, for Amide Protons Exposed on Global Unfolding of the I29A;I37H Variant of CI2
| Solutiona |
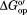 b b
|
 c c
|
|---|---|---|
| Buffer | 6.9 ± 0.1 | — |
| Lysate | 6.3 ± 0.1 | −0.6 ± 0.1 |
| GB+lysate | 7.08 ± 0.09 | +0.2 ± 0.1 |
| GB | 7.1 ± 0.1 | +0.2 ± 0.1 |
100 g/L protein lysate, 0.4 M glycine betaine (GB).
Uncertainties are the standard errors of the mean from the data in Supporting Information Table S1.
Error propagation on .
.
The straightforward interpretation is that the attractive interactions between the proteins in the lysate and CI2 are mitigated by the osmolyte. This interpretation is the same one used long ago to explain how osmolytes overcome the destabilizing effect of urea in shark bladder.21 The parallel between urea and cytoplasmic proteins also highlights the fact that urea and the surface of globular proteins possess the same functional groups.
In dilute solution, osmolytes stabilize globular proteins because the backbone prefers to interact with water rather than osmolyte, favoring the compact native state of the protein.25–28 The physicochemical mechanism by which glycine betaine mitigates the attractive interactions between the proteins in the lysate and CI2 remains to be determined. However, the stabilizing effect of osmolytes is compatible with mitigation of protein–protein interactions, because it is well known that osmolytes can mitigate aggregation33,34 and help prevent protein-fouling of materials used for implanted devices.35
Empirically, the fact that the osmolyte has the same stabilizing effect in both protein lysate and buffer suggests that glycine betaine causes proteins to be “invisible” to one another. This results in crowding effects similar to those exhibited by synthetic polymers, including polyvinylpyrrolidone (Fig. 2).8 However, we cannot draw a firm parallel between the two systems because synthetic polymers stabilize proteins due to lack of net attractive interactions.11 We know that this absence of interaction does not hold completely in lysate because analysis of CI2 backbone chemical shifts (Fig. 3) shows that although glycine betaine decreases the interactions between CI2 and the protein lysate, some remain.
Figure 2.
Stability changes brought about by buffered (50 mM sodium phosphate) 100.0 g/L protein lysate containing 0.4 M GB (red; 20°C, pH 7.0) and buffered 100.0 g/L polyvinylpyrrolidone (black, 37°C, pH 5.4, 50 mM sodium acetate) compared to their respective buffers. Positive values denote increased stability. Experiments with only GB were performed once. Bars represent standard errors of the mean for solutions containing 100.0 g/L protein. The PVP data have been published.8
Figure 3.
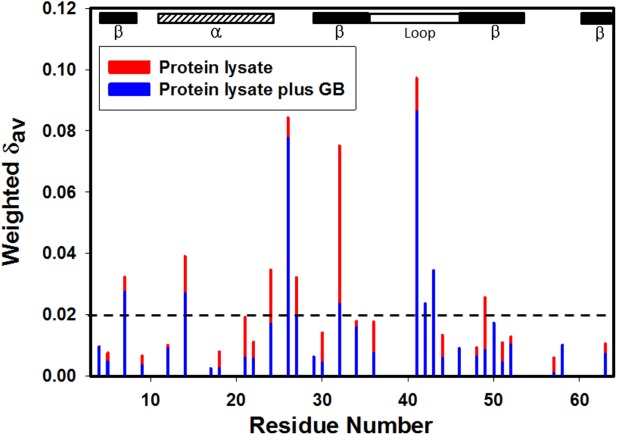
Weighted chemical shift changes (Δδav)36 of CI2 compared to buffer [red, 100.0 g/L protein lysate; blue, 100.0 g/L protein lysate plus 0.4 M GB]. Δδav is the shift in lysate minus that in buffer. Values greater than 0.02 ppm are significant as shown from replicate experiments.7
In summary, if crowding were always stabilizing, it could not provide a rationale for the existence of osmolytes as relievers of dehydration stress, because dehydration increases the concentration of macromolecules in cells. Thus, the observation that an osmolyte overcomes the protein-destabilizing effect of crowding provides an explanation for the ubiquity of osmolytes in biology.21
Materials and Methods
The protein lysate was prepared from saturated E. coli cultures. Membranes, nucleic acids, and nucleic-acid-bound proteins were removed as described.15
15N-enriched CI2 was expressed and purified as described.6–8,13,15,18,31 Stock solutions of 0.4 M glycine betaine were made in deuterated, 50 mM sodium phosphate buffer, and pHread adjusted to 7.0. Experiments were performed with solutions containing 100.0 mg pre-exchanged, deuterated, total protein lysate resuspended in 1.0 mL of 0.4 M glycine betaine phosphate buffer. The 100.0 g/L lysate contained 92 ±3 g of proteins (modified Lowry Assay).15 NMR experiments were performed as described.15,18 The concentration of CI2 was 1 mM. Experiments in buffered 100.0 g/L lysate plus 0.4 M glycine betaine were performed in triplicate. values are tabulated in Supporting Information Table S1.
values are tabulated in Supporting Information Table S1.
Acknowledgments
Authors thank Elizabeth Pielak and William Monteith for helpful comments.
Supporting Information
Additional Supporting Information may be found in the online version of this article.
Supplementary Information
References
- 1.Tanford C, Reynolds J. Nature's robots: a history of proteins. Oxford, UK: Oxford University Press; 2001. [Google Scholar]
- 2.Creighton TE. 2010. The biophysical chemistry of nucleic acids and proteins. Eastbourne, UK: Helvetian Press.
- 3.Zimmerman SB, Trach SO. Estimation of macromolecule concentrations and excluded volume effects for the cytoplasm of Escherichia coli. J Mol Biol. 1991;222:599–620. doi: 10.1016/0022-2836(91)90499-v. [DOI] [PubMed] [Google Scholar]
- 4.Blattner F. The complete genome sequence of Escherichia coli K-12. Science. 1997;277:1453–1462. doi: 10.1126/science.277.5331.1453. [DOI] [PubMed] [Google Scholar]
- 5.Neidhardt FC. Chemical composition of Escherichia coli. Vol. 1. Washington, D.C: American Society of Microbiology; 1987. [Google Scholar]
- 6.Benton LA, Smith AE, Young GB, Pielak GJ. Unexpected effects of macromolecular crowding on protein stability. Biochemistry. 2012;51:9773–9775. doi: 10.1021/bi300909q. [DOI] [PubMed] [Google Scholar]
- 7.Charlton LM, Barnes CO, Li C, Orans J, Young GB, Pielak GJ. Residue-level interrogation of macromolecular crowding effects on protein stability. J Am Chem Soc. 2008;130:6826–6830. doi: 10.1021/ja8005995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miklos AC, Li C, Sharaf NG, Pielak GJ. Volume exclusion and soft interaction effects on protein stability under crowded conditions. Biochemistry. 2010;49:6984–6991. doi: 10.1021/bi100727y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.McGuffee SR, Elcock AH. Diffusion, crowding & protein stability in a dynamic molecular model of the bacterial cytoplasm. PLoS Comput Biol. 2010;6:e1000694. doi: 10.1371/journal.pcbi.1000694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Feig M, Sugita Y. Variable interactions between protein crowders and biomolecular solutes are important in understanding cellular crowding. J Phys Chem B. 2012;116:599–605. doi: 10.1021/jp209302e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Li C, Pielak GJ. Using NMR to distinguish viscosity effects from nonspecific protein binding under crowded conditions. J Am Chem Soc. 2009;131:1368–1369. doi: 10.1021/ja808428d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sarkar M, Li C, Pielak GJ. Soft interactions and crowding. Biophys Rev. 2013;5:187–194. doi: 10.1007/s12551-013-0104-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Miklos AC, Sarkar M, Wang Y, Pielak GJ. Protein crowding tunes protein stability. J Am Chem Soc. 2011;133:7116–7120. doi: 10.1021/ja200067p. [DOI] [PubMed] [Google Scholar]
- 14.Wang Y, Sarkar M, Smith AE, Krois AS, Pielak GJ. Macromolecular crowding and protein stability. J Am Chem Soc. 2012;134:16614–16618. doi: 10.1021/ja305300m. [DOI] [PubMed] [Google Scholar]
- 15.Sarkar M, Lu J, Pielak GJ. Protein-crowder charge and protein stability. Biochemistry. 2014;53:1601–1606. doi: 10.1021/bi4016346. [DOI] [PubMed] [Google Scholar]
- 16.Ignatova Z, Krishnan B, Bombardier JP, Marcelino AMC, Hong J, Gierasch LM. From the test tube to the cell: exploring the folding and aggregation of a β-clam protein. J Pept Sci. 2007;88:157–163. doi: 10.1002/bip.20665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schlesinger AP, Wang Y, Tadeo X, Millet O, Pielak GJ. Macromolecular crowding fails to fold a globular protein in cells. J Am Chem Soc. 2011;133:8082–8085. doi: 10.1021/ja201206t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Sarkar M, Smith AE, Pielak GJ. Impact of reconstituted cytosol on protein stability. Proc Natl Acad Sci USA. 2013;110:19342–19347. doi: 10.1073/pnas.1312678110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Guzman I, Gelman H, Tai J, Gruebele M. The extracellular protein VlsE is destabilized inside cells. J Mol Biol. 2014;426:11–20. doi: 10.1016/j.jmb.2013.08.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Makhatadze GI, Privalov PL. Protein interactions with urea and guanidinium chloride: a calorimetric study. J Mol Biol. 1992;226:491–505. doi: 10.1016/0022-2836(92)90963-k. [DOI] [PubMed] [Google Scholar]
- 21.Yancey PH, Clark ME, Hand SC, Bowlus RD, Somero GN. Living with water stress: evolution of osmolyte systems. Science. 1982;217:1214–1222. doi: 10.1126/science.7112124. [DOI] [PubMed] [Google Scholar]
- 22.Larsen PI, Sydnes LK, Landfald B, Strøm AR. Osmoregulation in Escherichia coli by accumulation of organic osmolytes: betaines, glutamic acid, and trehalose. Arch Microbiol. 1987;147:1–7. doi: 10.1007/BF00492896. [DOI] [PubMed] [Google Scholar]
- 23.Konopka MC, Weisshaar JC, Record MT., Jr Methods of changing biopolymer volume fraction and cytoplasmic solute concentrations for in vivo biophysical studies. Methods Enzymol. 2007;428:487–504. doi: 10.1016/S0076-6879(07)28027-9. [DOI] [PubMed] [Google Scholar]
- 24.Cayley S, Record MT., Jr Roles of cytoplasmic osmolytes, water, and crowding in the response of Escherichia coli to osmotic stress: biophysical basis of osmoprotection by glycine betaine. Biochemistry. 2003;42:12596–12609. doi: 10.1021/bi0347297. [DOI] [PubMed] [Google Scholar]
- 25.Felitsky DJ, Cannon JG, Capp MW, Hong J, Van Wynsberghe AW, Anderson CF, Record MT. The exclusion of glycine betaine from anionic biopolymer surface: why glycine betaine Is an effective osmoprotectant but also a compatible solute. Biochemistry. 2004;43:14732–14743. doi: 10.1021/bi049115w. [DOI] [PubMed] [Google Scholar]
- 26.Capp MW, Pegram LM, Saecker RM, Kratz M, Riccardi D, Wendorff T, Cannon JG, Record MT. Interactions of the osmolyte glycine betaine with molecular surfaces in water: thermodynamics, structural interpretation, and prediction of m-values. Biochemistry. 2009;48:10372–10379. doi: 10.1021/bi901273r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Rosgen J, Pettitt BM, Bolen DW. An analysis of the molecular origin of osmolyte-dependent protein stability. Protein Sci. 2007;16:733–743. doi: 10.1110/ps.062671607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Street TO, Bolen DW, Rose GD. A molecular mechanism for osmolyte-induced protein stability. Proc Natl Acad Sci USA. 2006;103:13997–14002. doi: 10.1073/pnas.0606236103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miklos AC, Li C, Pielak GJ. Using NMR-detected backbone amide 1H exchange to assess macromolecular crowding effects on globular-protein stability. Methods Enzymol. 2009;466:1–18. doi: 10.1016/S0076-6879(09)66001-8. [DOI] [PubMed] [Google Scholar]
- 30.Englander SW, Kallenbach NR. Hydrogen exchange and structural dynamics of proteins and nucleic acids. Q Rev Biophys. 1983;16:521–655. doi: 10.1017/s0033583500005217. [DOI] [PubMed] [Google Scholar]
- 31.Smith AE, Sarkar M, Young GB, Pielak GJ. Amide proton exchange of a dynamic loop in cell extracts. Protein Sci. 2013;22:1313–1319. doi: 10.1002/pro.2318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Neira JL, Itzhaki LS, Otzen DE, Davis B, Fersht AR. Hydrogen exchange in chymotrypsin inhibitor 2 probed by mutagenesis. J Mol Biol. 1997;270:99–110. doi: 10.1006/jmbi.1997.1088. [DOI] [PubMed] [Google Scholar]
- 33.Collins KD. Ions from the Hofmeister series and osmolytes: effects on proteins in solution and in the crystallization process. Methods. 2004;34:300–311. doi: 10.1016/j.ymeth.2004.03.021. [DOI] [PubMed] [Google Scholar]
- 34.Gong B, Zhang L-Y, Pang C-P, Lam DS-C, Yam GH-F. Trimethylamine N-oxide alleviates the severe aggregation and ER stress caused by G98R α-crystallin. Mol Vision. 2009;15:2829–2840. [PMC free article] [PubMed] [Google Scholar]
- 35.Yang W, Xue H, Li W, Zhang J, Jiang S. Pursuing “Zero” Protein Adsorption of Poly(carboxybetaine) from Undiluted Blood Serum and Plasma. Langmuir. 2009;25:11911–11916. doi: 10.1021/la9015788. [DOI] [PubMed] [Google Scholar]
- 36.Davison T, Nie X, Ma W, Lin Y, Kay C, Benchimol S, Arrowsmith C. Structure and functionality of a designed p53 dimer. J Mol Biol. 2001;307:605–617. doi: 10.1006/jmbi.2001.4450. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Information


