Abstract
Objective
The inability to ignore irrelevant environmental noise is a common problem for people with schizophrenia. The purpose of this study was to determine if the neuronal response to distracting noise is related to mechanisms of altered attention observed in the illness.
Method
Twenty-two outpatients with schizophrenia and seventeen healthy comparison subjects performed a selective attention task in the presence or absence of distracting environmental noise while undergoing functional magnetic resonance imaging at 3T. A separate condition examining passive response to the distracting noise also was included.
Results
Group differences in neuronal response during the attention task were magnified by distracting noise, with the greatest difference being less response by patients, relative to comparison subjects, in the temporoparietal junction. Separate passive listening to distracting noise resulted in greater hippocampal response in patients, relative to comparison subjects. Across all subjects, hippocampal response to noise was inversely related to the degree to which the attention-task-related network was up-regulated to perform the task during distracting noise.
Conclusions
Given the observed hippocampal hyperactivity in response to environmental noise in patients and the inverse relationship between hippocampal response to noise and the effects of noise on the task-related network, hippocampal hyperactivity may contribute to impaired recruitment of attention networks in schizophrenia.
Keywords: Schizophrenia, Distraction, Hippocampus, fMRI, Sensory Processing
1. Introduction
“I find it hard…to concentrate…to listen to a person when there is other stuff going on…everything’s got to have my attention…I’ve got to be here, I’ve got to be there, I’ve got to be everywhere…it’s got to be quiet or I can’t think right.”
This quotation, taken a from person with schizophrenia being interviewed at the University of Colorado Medial School, illustrates two related problems commonly experienced by persons with schizophrenia. The first is an inability to ignore unimportant noises in the environment. Because this deficit in sensory filtering or sensory gating has been so consistently described over the last century, investigators have proposed that it may reflect a core feature of schizophrenia (Venables and Maher, 1967). Electrophysiological studies of the deficit suggest it may be related to elemental neuronal dysfunction of inhibitory mechanisms (Freedman et al., 1991).
Recent neuroimaging studies have begun to further elucidate the functional brain networks contributing to poor sensory filtering in schizophrenia (Hazlett et al., 2008; Kumari et al., 2007; Mathiak et al., 2011; Thoma et al., 2005; Tregellas et al., 2009; Tregellas et al., 2007). An initial functional magnetic resonance imaging (fMRI) study of auditory sensory processing deficits in schizophrenia revealed over-activity in a network of regions in persons with schizophrenia, relative to healthy comparison subjects, during poor auditory gating (Tregellas et al., 2007). The over-activity, which was observed in the hippocampus, thalamus and dorsolateral prefrontal cortex, correlated with the deficient inhibition of electroencephalographically (EEG)-recorded P50 auditory evoked activity. The observed over-activity, which has now been replicated using a more ecologically valid stimulus (Tregellas et al., 2009), was interpreted as consistent with the concept of impaired inhibitory function in schizophrenia (Lewis and Moghaddam, 2006). A recent high-density EEG study by Williams and colleagues (2011) also suggested involvement of these regions in P50 sensory gating, and abnormal function of the hippocampus and prefrontal cortex in patients (Williams et al., 2011).
It is possible that that overactivity observed during passive sensory processing tasks is related to a second problem described in the initial quotation: a deficit in attention. Attention problems, particularly deficits in selective attention, the process by which focus is maintained on goal-relevant, as opposed to goal-irrelevant information, have been widely reported in schizophrenia, and represent one of the primary cognitive deficits in the illness (Nuechterlein et al., 2009). The fundamental mechanism of selective attention deficits in schizophrenia remains unknown. Some investigators have proposed that the deficit arises from a failure of “top down” mechanisms, i.e. a stimulus-independent voluntary control, such that patients are unable to appropriately engage executive control networks to maintain focus on relevant stimuli (Luck et al., 2012) whereas others have suggested the deficit is dominated by failure of “bottom-up” mechanisms, i.e. involuntary, stimulus-dependent processes, such that impaired early processing of sensory information leads to higher-level deficits (Javitt, 2009). A failure in sensory filtering could be a mechanism that contributes to “bottom up” selective attention deficits in schizophrenia.
Studying the interaction between the neuronal response to distracting noise and the neuronal response associated with selective attention may improve our understanding of the neurobiology of schizophrenia. Towards this end, the current study used fMRI to examine the neuronal response associated with selective attention, in the presence or absence of real-world-relevant distracting noise. We hypothesized that hippocampal over-activity in response to noise would be related to a failure to appropriately engage the selective-attention-related network in schizophrenia.
2. Methods
2.1. Participants
The study included data from 39 subjects—22 outpatients with schizophrenia (4 women and 18 men; mean age = 37.6 years, SD = 11.0) and 17 healthy comparison subjects (8 women and 9 men; mean age = 34.7 years, SD = 9.4). No significant group difference in age was observed. Data from two additional subjects were excluded because of excess head motion (>1.5 mm) during scanning. Diagnoses were confirmed with the Diagnostic Interview for Genetic Studies. Of the 22 persons with schizophrenia, 17 were treated with atypical antipsychotics, three with conventional antipsychotics, and one with both conventional and atypical antipsychotics. One patient was not treated with any neuroleptics. Prior to scanning, subjects performed a hearing test ensure they did not have a substantial difference (> 10 dB) in hearing between each ear at the frequencies used in the task. No subjects were excluded based on this criterion. The study was approved by the Colorado Multiple Institution Review Board, and all participants provided written informed consent.
2.2 fMRI Methods
A high-resolution anatomical scan was first acquired for each subject. Functional images were then acquired with a gradient-echo T2* Blood Oxygenation Level Dependent (BOLD) contrast technique, with TR = 8800ms (as a clustered volume acquisition of 2000 ms scanning, plus an additional 6800 ms silent interval), TE = 30 ms, FOV=220 mm2, 642 matrix, 38 slices, 3 mm thick, 0.5 mm gap, angled parallel to the planum sphenoidale. Clustered volume acquisition was used because it minimizes the confounding effects of scanner noise on the auditory task, and improves sensitivity to the BOLD response during such tasks (Edmister et al., 1999). At the end of the session, one IR-EPI (TI=505ms) volume was acquired to improve spatial normalizaiton.
Head motion was minimized with a VacFix head-conforming vacuum cushion (Par Scientific A/S, Odense, Denmark). Auditory stimuli were presented via MR-compatible headphones (Resonance Technology, Inc., CA, USA). MR-compatible goggles (Resonance Technology, Inc, CA, USA) were used for visual stimuli. Motor responses were collected via a fiber optic response pad (Cedrus Corp, USA).
2.3 fMRI Paradigm
Subjects performed a spatial selective attention task in the presence or absence of distracting “urban white noise” (Tregellas et al., 2009). The auditory task was an oddball paradigm, in which subjects listened to a series of 1000 Hz tones (100 ms duration, 5 ms attack/delay, 80 dB), separated by 750 ms, and were asked to identify infrequent pitch deviants, which were 250 Hz higher than the standard tones. Under these relatively easy conditions, the expectation was that patients and comparisons subjects would perform similarly. A total of 336 stimuli were presented, always during the 6.8 s silent period between scans. As such, stimuli were divided into groups of 7 (a “block”). Of the 7 tones in a block, the deviant occurred with an equal probability as the 3rd, 4th or 5th tone in blocks containing a deviant. This placement was to ensure that at least 2 standard tones were heard before the deviant, and to maximize the probability that the hemodynamic response would be adequately captured by the scan occurring on average 3.5 to 5.5 seconds following the deviant. Deviants occurred in 24 of the 48 blocks psuedorandomized throughout the run, such that the overall deviant frequency was 0.071.
The task was dichotically presented, such that oddball stimuli were presented one ear (with equal right/left probability). In the opposing ear, subjects heard silence in a third of the trials, or a distracting “urban white noise” stimulus in two-thirds of trials. Spatial attention was therefore required as subjects attended to the oddball task in only one ear. The urban noise stimulus, described in Tregellas et al, 2009, consisted of a mixture of audio clips, including multiple conversations, talk and music radio stations and environmental noises blended together so that no one element was readily identifiable (Tregellas et al., 2009). The stimulus is meant to simulate what a person may experience being in a crowd in a busy urban setting. The distracting noise was presented with equal probability at a either a relatively high volume (85 db) or a low volume (75 db). Because subsequent analysis did not reveal any behavioral or neuronal effects of different noise volume, all trials with urban noise distraction were collapsed.
In addition to the 48 blocks of the oddball task with or without distracting noise, the session included 8 blocks of the urban noise alone, at 85 db, presented dichotically (equal right/left probability) during the 6.8 inter-scan period, and 8 blocks of silence. During these runs, the one-word instruction “rest” appeared on the screen in front of the subjects. During the oddball blocks, subjects were instructed to “listen.” The session totaled 64 blocks, psuedorandomized across all conditions, lasting 9.4 minutes.
2.4 Data Analysis
Data were analyzed with SPM8 (Wellcome Department of Imaging Neuroscience, London). Echo-planar images (EPI) from each subject were realigned to the first volume. The realigned images were then normalized to the Montreal Neurological Institute template using the unified segmentation algorithm (Ashburner and Friston, 2005) on the IR-EPI image and applying the estimated warp parameters to the coregistered EPI data. During normalization, data were resliced to a 3 mm3 voxel size. Finally, functional images were smoothed with an 8-mm FWHM Gaussian kernel. A 128s high pass filter was applied to remove low-frequency fluctuation in the BOLD signal.
The hemodynamic response was modeled with a double gamma function, without temporal derivatives, using the general linear model in SPM8. To account for both within-group and within-subject variance, a random effects analysis was implemented. Parameter estimates for each individual’s first level analysis (SPM contrast images) contrasting oddball blocks with deviants to blocks without deviants, all during silence (contrast 1), oddball blocks with deviants to blocks without deviants, all in the presence of the urban noise distractor (contrast 2), and urban noise alone compared to silence (contrast 3), were entered into a second-level ANOVA. Comparisons were evaluated with directional contrasts (SPM t-contrasts). The effect of task (oddball target detection) in silence and during distracting noise, both in healthy comparison subjects and in patients, was considered significant at a stringent whole-brain voxel-wise threshold of 0.05, familywise error rate (FWE) corrected. Results for all other less-powered comparisons were considered significant at a whole-brain level if they exceeded a voxel-wise threshold of p < 0.01 and a cluster extent of 69 voxels. This threshold corresponds to a whole-brain FWE cluster corrected level of p < 0.01, based on 10,000 Monte Carlo simulations using AlphaSim in AFNI (http://afni.nimh.nih.gov/afni/).
3. Results
3.1 Behavioral Results
Behavioral data collected during scanning indicates that both healthy subjects and persons with schizophrenia performed the selective attention task with a similarly high degree of accuracy. Healthy subjects performed the task with an accuracy of 98.5% (SD = 0.06) during relative silence (without distracting noise), and 96.6% (SD = 0.09) during distracting noise. Patients’ accuracy was 93.8% (SD = 0.13) during silence and 94.1% (SD = 0.13) during noise. No effects of noise on reaction times were observed. Patients’ reaction times (573 ms, SD = 148) were, however, slower than those of comparison subjects (460 ms, SD = 130) across all conditions (t = 4.3, p < 0.001).
3.2 fMRI Task Results
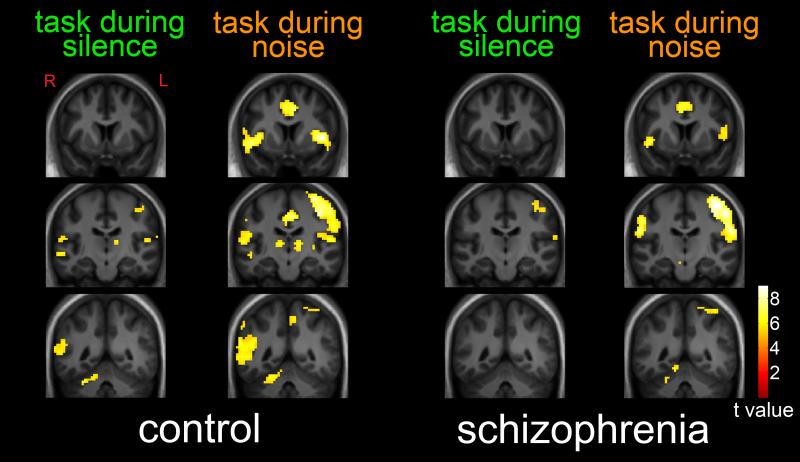
As shown in Figure 1, performance of the selective attention task in relative silence (without distracting noise) by healthy subjects was associated with neuronal response in a network of regions, including bilateral temporoparietal junction, right cerebellum, left thalamus, left precentral gyrus, bilateral superior temporal gyri and inferior frontal gyri. At the same stringent statistical threshold (p < 0.05, FWE corrected), response in patients was observed only in the left precentral gyrus and left superior temporal gyrus / temporoparietal junction. MNI coordinates and statistics for these regions are shown in Table 1. Greater task-related responses during silence in comparison subjects, relative to patients, were observed in the left superior temporal / inferior temporal gyrus, right superior temporal gyrus, right temporoparietal junction and cerebellum (Table 2).
Figure 1.
Task-Related Neuronal Response During Silence and During Distracting Noise. Statistical maps thresholded at p < 0.05, family-wise-error corrected and overlaid onto a group-average anatomical image. Data are shown in the radiological convention (R on L).
Table 1.
Task Effects During Silence and Distracting Noise
| Brain Region | MNI Coordinates | t value | cluster size | ||
|---|---|---|---|---|---|
| x | y | z | (peak) | ||
| Task During Silence | |||||
| Control | |||||
| Temporoparietal junction (L) | −66 | −31 | 19 | 7.44 | 90 |
| Temporoparietal junction (R) | 57 | −37 | 16 | 7.29 | 245 |
| Cerebellum (R) | 27 | −46 | −29 | 6.93 | 43 |
| Thalamus (L) | −15 | −13 | 7 | 6.46 | 19 |
| Precentral Gyrus (L) | −33 | −13 | 55 | 6.18 | 28 |
| Superior Temporal / Inferior Temporal Gyrus (R) | 57 | −19 | 13 | 5.76 | 16 |
| Superior Temporal / Inferior Temporal Gyrus (L) | −54 | −1 | 4 | 5.61 | 13 |
| Schizophrenia | |||||
| Precentral Gyrus (L) | −36 | −7 | 64 | 6.75 | 75 |
| Superior Temporal Gyrus / Temporoparietal junction (L) | 63 | −25 | 16 | 5.77 | 21 |
| Task During Noise | |||||
| Control | |||||
| Insula (L) | −42 | 8 | 1 | 8.92 | 286 |
| Insula (R) | 48 | 11 | −8 | 8.3 | 228 |
| Precentral Gyrus (L) | −36 | −16 | 64 | 8.28 | 1015 |
| Superior Temporal Gyrus (L) | −57 | −13 | 13 | 7.07 | |
| Cingulate Gyrus | −3 | −4 | 46 | 8.23 | 738 |
| Cerebellum (R) | 18 | −55 | −20 | 7.29 | 177 |
| Cerebellum (L) | −21 | −58 | −20 | 6.15 | 33 |
| Postcentral Gyrus (R) | 54 | −25 | 37 | 6.73 | 747 |
| Superior Temporal Gyrus (R) | 63 | −25 | 7 | 5.39 | |
| Temporoparietal junction (R) | 51 | −37 | 22 | 6.69 | |
| Thalamus (L) | −15 | −13 | 4 | 6.73 | 35 |
| Thalamus (R) | 9 | −22 | 7 | 6.08 | 26 |
| Dorsolateral Prefrontal Cortex (L) | −30 | 44 | 31 | 5.59 | 10 |
| Schizophrenia | |||||
| Precentral Gyrus (L) | −39 | −22 | 58 | 9.35 | 1043 |
| Temporoparietal junction (R) | 57 | −19 | 25 | 7.62 | |
| Postcentral Gyrus (R) | 54 | −25 | 43 | 8.95 | 292 |
| Insula / Inferior Frontal Gyrus (L) | −48 | 2 | 10 | 7.53 | 105 |
| Insula / Inferior Frontal Gyrus (R) | 45 | 8 | −5 | 6.81 | 53 |
| Cerebellum | 0 | −73 | −14 | 7.03 | 43 |
| Cerebellum (R) | 18 | −55 | −20 | 6.26 | 49 |
| Cingulate Gyrus | 6 | −1 | 52 | 6.79 | 283 |
| Parietal Cortex (L) | −18 | −67 | 58 | 6.61 | 17 |
| Thalamus (L) | −3 | −16 | −2 | 6.13 | 17 |
All listed values are significant at at voxel-wise theshold of p < 0.05, FWE corrected
Table 2.
Group Differences in Task Effects During Noise and Silence*
| Brain Region | MNI Coordinates | t value | cluster size | ||
|---|---|---|---|---|---|
| x | y | z | (peak) | ||
| Control > Schizophrenia During Silence | |||||
| Superior Temporal / Inferior Temporal Gyrus (L) | −51 | −4 | 1 | 3.52 | 72 |
| Superior Temporal Gyrus (R) | 57 | −19 | −5 | 3.37 | 101 |
| Temporoparietal junction (R) | 54 | −49 | −2 | 3.02 | |
| Cerebellum | 6 | −58 | −23 | 2.88 | 104 |
| Control > Schizophrenia During Noise | |||||
| Insula (L) | −42 | 8 | 1 | 4.49 | 455 |
| Insula (R) | 36 | 8 | 1 | 3.22 | 75 |
| Cingulate gyrus | −3 | −16 | 40 | 4.24 | 582 |
| Temporoparietal junction (R) | 48 | −52 | 19 | 4.01 | 1052 |
| Dorsolateral Prefrontal Cortex (L) | −42 | 17 | 46 | 3.14 | 202 |
| Cerebellum | 0 | −52 | −17 | 3.10 | 190 |
All listed values are significant at at voxel-wise theshold of p < 0.01 and a cluster-extent-corrected threshold of p < 0.01
Task performance in the presence of distracting noise was associated with a substantially greater spatial extent of network activation in both comparison subjects and patients (Figure 1, Table 1). Greater task-related responses during distracting noise in comparison subjects, relative to patients, were observed in the bilateral insula, cingulate gyrus, right temporoparietal junction, left dorsolateral prefrontal cortex and cerebellum (Table 2). No areas of greater task-related responses during noise were observed in patients, relative to comparison subjects. Across all subjects, a nearly significant (r = −0.31, t = 1.9, p = 0.059) inverse correlation was observed between temporoparietal junction response and reaction time during task-performance in the presence of distracting noise.
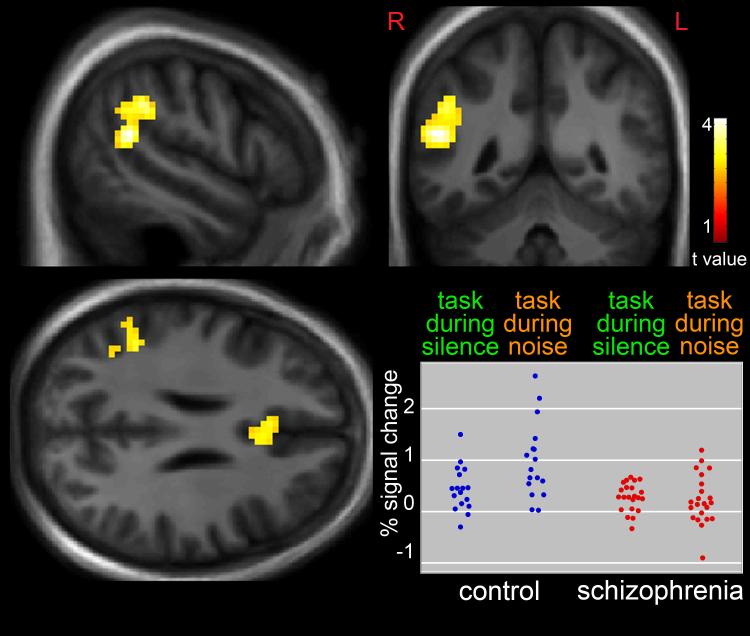
Differences in the effect of noise on task-associated neuronal response in patients, relative to comparison subjects is shown in Figure 2. The addition of distracting noise to the task was associated with significantly greater response in the right temporoparietal junction (t = 4.21, p < 0.01, corrected, x = 51, y = −49, z = 19) and, to a lesser extent, cingulate gyrus (t = 3.47, p < 0.01, corrected, x = −6, y = 23, z = 22) in comparison subjects, relative to patients. Figure 2 also shows peak temporoparietal BOLD percent signal change, relative to the global mean, for all individuals.
Figure 2.
Greater Response in the Right Temporoparietal Junction and Cingulate Gyrus in Healthy Subjects, Relative to Persons with Schizophrenia, During task Performance in the Presence of Distracting Noise, Compared to Task Performance During Silence. Statistical maps thresholded at p < 0.01, cluster-extent-corrected and overlaid onto a group-average anatomical image. Data are shown in the radiological convention (R on L). Plot shows individual responses in the temporoparietal junction during the different conditions.
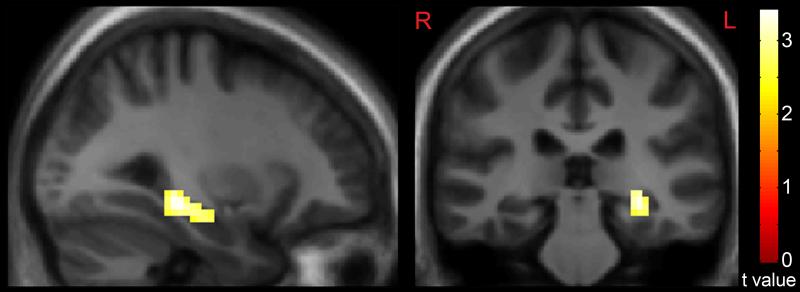
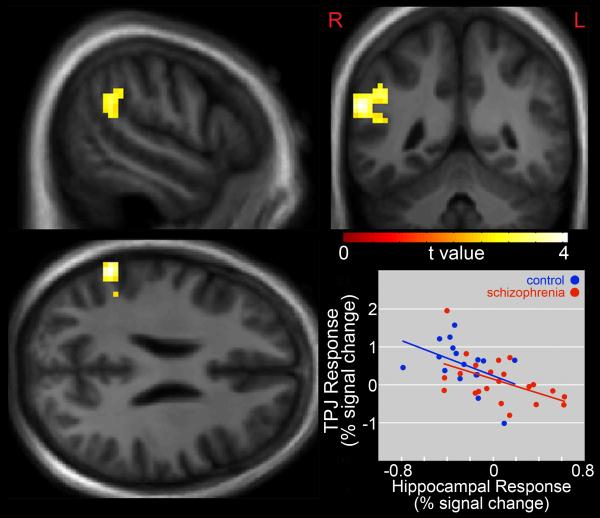
Distracting noise alone was associated with greater response in the left hippocampus (t = 3.42, p < 0.01, corrected, x = −30, y = −28, z = −11) in patients, relative to comparison subjects (Figure 3). Across all subjects, this response was positively correlated (r = 0.36, t = 2.3, p = 0.026) with reaction times from task-performance during distracting noise. A whole-brain regression analysis across all subjects examining the degree to which hippocampal response to distracting noise alone was related to noise-induced changes in the task-relevant network (contrast 3 vs (contrast 2 – contrast 1)) showed peak hippocampal responses to be inversely correlated with right temporoparietal junction response (x = 60, y = −49, z = 25, r = 0.56, t = 4.07, p < 0.01, corrected, Figure 4).
Figure 3.
Greater Hippocampal Response in Persons with Schizophrenia, Relative to Comparison Subjects, during passive listening to an “Urban White Noise” Stimulus. Statistical maps thresholded at p < 0.01, cluster-extent-corrected and overlaid onto a group-average anatomical image. Data are shown in the radiological convention (R on L).
Figure 4.
Inverse Correlation Between Hippocampal Response During Passive Noise Stimulus and Noise-Induced Changes in the Selective Attention Task-Relevant Network. Statistical maps thresholded at p < 0.01, cluster-extent-corrected and overlaid onto a group-average anatomical image. Data are shown in the radiological convention (R on L).
4. Discussion
The primary findings of this study were 1) performance of a spatial selective attention task under relatively quiet conditions was associated with a distributed network of regions in healthy subjects, 2) the network was more active when the task was performed during distracting noise, 3) relative to healthy comparison subjects, patients, who showed modestly less task-related activity during silence, showed a much more substantial failure to recruit additional task-related network in response to distracting noise, 4) distracting noise alone was associated with greater hippocampal response in patients, 5) generalized hippocampal response to noise, measured by the separate passive noise condition, was inversely related to noise effects on the task-related network.
The network of temporoparietal, temporal, cerebellar, thalamic and frontal regions shown to be associated with task performance is consistent with similar oddball-type selective attention tasks studied previously (Kiehl and Liddle, 2001; Kiehl et al., 2005; Linden et al., 1999; Witt et al., 2010; Wolf et al., 2008). Also consistent with previous studies of oddball tasks, the temporoparietal junction showed the strongest response during the task in silence in healthy subjects. This response, along with responses in other components of the task-related network, substantially expanded in spatial extent when the task was performed during distracting noise. Distracting auditory stimuli previously have been shown to increase task-related neuronal response in healthy subjects (Tomasi et al., 2005). This response has been suggested to stem from increased recruitment of attention resources to compensate for the interfering effects of the noise, consistent with the generalized phenomenon of increased cortical recruitment in response to increased processing load (Adler et al., 2001; Pugh et al., 1996; Tregellas et al., 2006).
Distracting noise alone was associated with greater hippocampal activity in patients, compared to controls. This finding replicates the largest group difference observed in our previous sensory processing study using the “urban noise” stimulus (Tregellas et al., 2009), and is in line with our prior study of sensory processing with a click-train stimulus (Tregellas et al., 2007). Hippocampal hyperactivity in schizophrenia has been shown during a variety of rest, passive, simple sensory processing, or baseline conditions using a variety of imaging techniques (Friston et al., 1992; Heckers et al., 1998; Kawasaki et al., 1992; Malaspina et al., 2004; Medoff et al., 2001; Tregellas et al., 2004). That the finding was lateralized to the left hippocampus also is consistent with previous auditory sensory processing studies (Tregellas et al., 2009; Tregellas et al., 2007) and with prior reports suggesting selective involvement of the left medial temporal cortex in schizophrenia (Friston et al., 1992). In the context in impaired sensory processing in schizophrenia, the observed hippocampal hyperactivity may represent a failure to suppress responses to unimportant stimuli, akin to the decreased suppression of evoked responses to repetitive auditory stimuli observed in the typical P50 auditory gating paradigm (Freedman et al., 1991). Given evidence for hippocampal inhibitory interneuron dysfunction in schizophrenia (Benes, 1999), the mechanism for such a suppression deficit could stem from the failure of interneurons to adequately modulate the excitation of pyramidal neurons in response to sensory input (Freedman et al., 1991).
While passive listening to distracting noise alone was associated with hippocampal overactivity in patients, playing the noise as a distractor during the oddball attention task resulted in a substantial inability of patients to up-regulate task-related response as observed in comparison subjects. The most significant group difference was observed in the right temporoparietal junction. The right temporoparietal junction has been shown to be a critical hub in the ventral attention network crucial for the detection of oddball, unexpected stimuli (Downar et al., 2000; Molholm et al., 2005; Opitz et al., 2002) as well as for assigning behavioral relevance and enabling responses to these low frequency events (Corbetta and Shulman, 2002). The present results are consistent with previous studies showing reduced engagement of this region in schizophrenia during oddball tasks (Kiehl and Liddle, 2001; Laurens et al., 2005). The observation that temporoparietal response differences between patients and controls were magnified during distracting urban noise suggests that patients may be impaired in their ability to engage this ventral attention hub during demanding task conditions. The large group difference related to noise distraction may be apparent due to our use of the clustered volume acquisition technique, which minimizes scanner noise and consequently non-task auditory distractor (i.e. non-urban noise) related activity.
A key goal of this study was to determine if response to distracting noise was related to task-associated neuronal response deficits in schizophrenia. The observed inverse correlation between hippocampal response to distracting noise and temporoparietal engagement during the oddball task, coupled with the observation of hippocampal overactivity in response to noise in patients, supports this idea. It is possible to speculate that hippocampal hyper-responsiveness to stimuli observed in the present and previous studies may lead to patients‘ inability to appropriately engage task-relevant brain networks. The idea that the hippocampus may be involved in the interplay between incoming sensory information and top-down cognitive processing also is consistent with prior work suggesting that the structure plays a role in mediating the relative balance between the strength of top-down and bottom-up inputs in functional neuronal representations (Strange et al., 2005).
In conclusion, this study found that during a selective attention task, patients with schizophrenia did not engage the task-relevant network to the same degree as comparison subjects. This difference was greatly exacerbated when distracting noise was present. The ability to up-regulate the task-related network in when distracting noise was present was inversely related to generalized hippocampal response to noise. As such, generalized hippocampal hyper-responsivity to environmental noise observed in patients may be related to their inability to appropriately engage task-relevant systems. This potential mechanism may contribute to attention and other cognitive deficits in schizophrenia and may serve as a useful tool to measure the effects of treatments to improve cognition in the illness.
References
- Adler CM, Sax KW, Holland SK, Schmithorst V, Rosenberg L, Strakowski SM. Changes in neuronal activation with increasing attention demand in healthy volunteers: an fMRI study. Synapse. 2001;42(4):266–272. doi: 10.1002/syn.1112. [DOI] [PubMed] [Google Scholar]
- Ashburner J, Friston KJ. Unified segmentation. Neuroimage. 2005;26(3):839–851. doi: 10.1016/j.neuroimage.2005.02.018. [DOI] [PubMed] [Google Scholar]
- Benes FM. Evidence for altered trisynaptic circuitry in schizophrenic hippocampus. Biol.Psychiatry. 1999;46(5):589–599. doi: 10.1016/s0006-3223(99)00136-5. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3(3):201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD. A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci. 2000;3(3):277–283. doi: 10.1038/72991. [DOI] [PubMed] [Google Scholar]
- Edmister WB, Talavage TM, Ledden PJ, Weisskoff RM. Improved auditory cortex imaging using clustered volume acquisitions. Hum.Brain Mapp. 1999;7(2):89–97. doi: 10.1002/(SICI)1097-0193(1999)7:2<89::AID-HBM2>3.0.CO;2-N. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman R, Waldo M, Bickford-Wimer P, Nagamoto H. Elementary neuronal dysfunctions in schizophrenia. Schizophr.Res. 1991;4(2):233–243. doi: 10.1016/0920-9964(91)90035-p. [DOI] [PubMed] [Google Scholar]
- Friston KJ, Liddle PF, Frith CD, Hirsch SR, Frackowiak RS. The left medial temporal region and schizophrenia. A PET study. Brain : a journal of neurology. 1992;115(Pt 2):367–382. doi: 10.1093/brain/115.2.367. [DOI] [PubMed] [Google Scholar]
- Hazlett EA, Buchsbaum MS, Zhang J, Newmark RE, Glanton CF, Zelmanova Y, Haznedar MM, Chu KW, Nenadic I, Kemether EM, Tang CY, New AS, Siever LJ. Frontal-striatal-thalamic mediodorsal nucleus dysfunction in schizophrenia-spectrum patients during sensorimotor gating. Neuroimage. 2008;2(3):1164–1177. doi: 10.1016/j.neuroimage.2008.05.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heckers S, Rauch SL, Goff D, Savage CR, Schacter DL, Fischman AJ, Alpert NM. Impaired recruitment of the hippocampus during conscious recollection in schizophrenia. Nat.Neurosci. 1998;1(4):318–323. doi: 10.1038/1137. [DOI] [PubMed] [Google Scholar]
- Javitt DC. When doors of perception close: bottom-up models of disrupted cognition in schizophrenia. Annu Rev Clin Psychol. 2009;5:249–275. doi: 10.1146/annurev.clinpsy.032408.153502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawasaki Y, Suzuki M, Maeda Y, Urata K, Yamaguchi N, Matsuda H, Hisada K, Takashima T. Regional cerebral blood flow in patients with schizophrenia. A preliminary report. European Archives of Psychiatry and Clinical Neuroscience. 1992;241(4):195–200. doi: 10.1007/BF02190252. [DOI] [PubMed] [Google Scholar]
- Kiehl K, Liddle P. An event-related functional magnetic resonance imaging study of an auditory oddball task in schizophrenia. Schizophrenia Research. 2001;48(2-3):159–171. doi: 10.1016/s0920-9964(00)00117-1. [DOI] [PubMed] [Google Scholar]
- Kiehl KA, Stevens MC, Laurens KR, Pearlson G, Calhoun VD, Liddle PF. An adaptive reflexive processing model of neurocognitive function: supporting evidence from a large scale (n = 100) fMRI study of an auditory oddball task. Neuroimage. 2005;25(3):899–915. doi: 10.1016/j.neuroimage.2004.12.035. [DOI] [PubMed] [Google Scholar]
- Kumari V, Antonova E, Geyer MA, Ffytche D, Williams SC, Sharma T. A fMRI investigation of startle gating deficits in schizophrenia patients treated with typical or atypical antipsychotics. Int J Neuropsychopharmacol. 2007;10(4):463–477. doi: 10.1017/S1461145706007139. [DOI] [PubMed] [Google Scholar]
- Laurens KR, Kiehl KA, Ngan ET, Liddle PF. Attention orienting dysfunction during salient novel stimulus processing in schizophrenia. Schizophrenia Research. 2005;75(2-3):159–171. doi: 10.1016/j.schres.2004.12.010. [DOI] [PubMed] [Google Scholar]
- Lewis DA, Moghaddam B. Cognitive dysfunction in schizophrenia: convergence of gamma-aminobutyric acid and glutamate alterations. Arch.Neurol. 2006;63(10):1372–1376. doi: 10.1001/archneur.63.10.1372. [DOI] [PubMed] [Google Scholar]
- Linden DE, Prvulovic D, Formisano E, Vollinger M, Zanella FE, Goebel R, Dierks T. The functional neuroanatomy of target detection: an fMRI study of visual and auditory oddball tasks. Cerebral Cortex. 1999;9(8):815–823. doi: 10.1093/cercor/9.8.815. [DOI] [PubMed] [Google Scholar]
- Luck SJ, Ford JM, Sarter M, Lustig C. CNTRICS final biomarker selection: Control of attention. Schizophrenia Bulletin. 2012;38(1):53–61. doi: 10.1093/schbul/sbr065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malaspina D, Harkavy-Friedman J, Corcoran C, Mujica-Parodi L, Printz D, Gorman JM, Van Heertum R. Resting neural activity distinguishes subgroups of schizophrenia patients. Biological Psychiatry. 2004;56(12):931–937. doi: 10.1016/j.biopsych.2004.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathiak K, Ackermann H, Rapp A, Mathiak KA, Shergill S, Riecker A, Kircher TT. Neuromagnetic oscillations and hemodynamic correlates of P50 suppression in schizophrenia. Psychiatry research. 2011;194(1):95–104. doi: 10.1016/j.pscychresns.2011.01.001. [DOI] [PubMed] [Google Scholar]
- Medoff DR, Holcomb HH, Lahti AC, Tamminga CA. Probing the human hippocampus using rCBF: contrasts in schizophrenia. Hippocampus. 2001;11(5):543–550. doi: 10.1002/hipo.1070. [DOI] [PubMed] [Google Scholar]
- Molholm S, Martinez A, Ritter W, Javitt DC, Foxe JJ. The neural circuitry of pre-attentive auditory change-detection: an fMRI study of pitch and duration mismatch negativity generators. Cerebral Cortex. 2005;15(5):545–551. doi: 10.1093/cercor/bhh155. [DOI] [PubMed] [Google Scholar]
- Nuechterlein KH, Luck SJ, Lustig C, Sarter M. CNTRICS final task selection: control of attention. Schizophrenia Bulletin. 2009;35(1):182–196. doi: 10.1093/schbul/sbn158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Opitz B, Rinne T, Mecklinger A, von Cramon DY, Schroger E. Differential contribution of frontal and temporal cortices to auditory change detection: fMRI and ERP results. Neuroimage. 2002;15(1):167–174. doi: 10.1006/nimg.2001.0970. [DOI] [PubMed] [Google Scholar]
- Pugh KR, offywitz BA, Shaywitz SE, Fulbright RK, Byrd D, Skudlarski P, Shankweiler DP, Katz L, Constable RT, Fletcher J, Lacadie C, Marchione K, Gore JC. Auditory selective attention: an fMRI investigation. Neuroimage. 1996;4(3 Pt 1):159–173. doi: 10.1006/nimg.1996.0067. [DOI] [PubMed] [Google Scholar]
- Strange BA, Duggins A, Penny W, Dolan RJ, Friston KJ. Information theory, novelty and hippocampal responses: unpredicted or unpredictable? Neural Netw. 2005;18(3):225–230. doi: 10.1016/j.neunet.2004.12.004. [DOI] [PubMed] [Google Scholar]
- Thoma RJ, Hanlon FM, Moses SN, Ricker D, Huang M, Edgar C, Irwin J, Torres F, Weisend MP, Adler LE, Miller GA, Canive JM. M50 sensory gating predicts negative symptoms in schizophrenia. Schizophr.Res. 2005;73(2-3):311–318. doi: 10.1016/j.schres.2004.07.001. [DOI] [PubMed] [Google Scholar]
- Tomasi D, Caparelli EC, Chang L, Ernst T. fMRI-acoustic noise alters brain activation during working memory tasks. Neuroimage. 2005;27(2):377–386. doi: 10.1016/j.neuroimage.2005.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas J, Ellis J, Shatti S, Du Y, Rojas D. Increased Hippocampal, Thalamic, and Prefrontal Hemodynamic Response to an Urban Noise Stimulus in Schizophrenia. American Journal of Psychiatry. 2009;166(3):354. doi: 10.1176/appi.ajp.2008.08030411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Davalos DB, Rojas DC. Effect of task difficulty on the functional anatomy of temporal processing. Neuroimage. 2006;32(1):307–315. doi: 10.1016/j.neuroimage.2006.02.036. [DOI] [PubMed] [Google Scholar]
- Tregellas JR, Davalos DB, Rojas DC, Waldo MC, Gibson L, Wylie K, Du YP, Freedman R. Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr Res. 2007;92(1-3):262–272. doi: 10.1016/j.schres.2006.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tregellas JR, Tanabe JL, Miller DE, Ross RG, Olincy A, Freedman R. Neurobiology of smooth pursuit eye movement deficits in schizophrenia: an fMRI study. Am.J Psychiatry. 2004;161(2):315–321. doi: 10.1176/appi.ajp.161.2.315. [DOI] [PubMed] [Google Scholar]
- Venables PH, Maher BA. Input Dysfunction in Schizophrenia, Progress in Experimental Personality Research. Academic PRess; Orlando, FL: 1967. pp. 1–64. [PubMed] [Google Scholar]
- Williams TJ, Nuechterlein KH, Subotnik KL, Yee CM. Distinct neural generators of sensory gating in schizophrenia. Psychophysiology. 2011;48(4):470–478. doi: 10.1111/j.1469-8986.2010.01119.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Witt ST, Lovejoy DW, Pearlson GD, Stevens MC. Decreased prefrontal cortex activity in mild traumatic brain injury during performance of an auditory oddball task. Brain Imaging Behav. 2010;4(3-4):232–247. doi: 10.1007/s11682-010-9102-3. [DOI] [PubMed] [Google Scholar]
- Wolf DH, Turetsky BI, Loughead J, Elliott MA, Pratiwadi R, Gur RE, Gur RC. Auditory Oddball fMRI in Schizophrenia: Association of Negative Symptoms with Regional Hypoactivation to Novel Distractors. Brain Imaging Behav. 2008;2(2):132–145. doi: 10.1007/s11682-008-9022-7. [DOI] [PMC free article] [PubMed] [Google Scholar]