Abstract
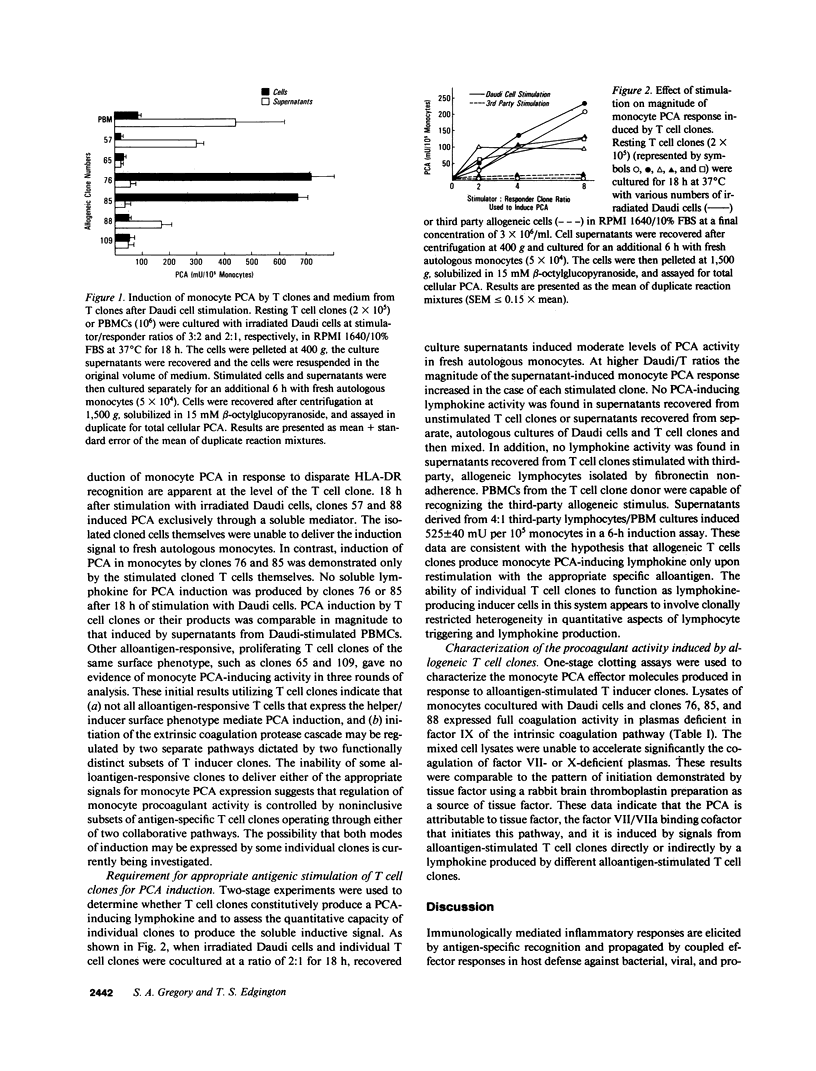
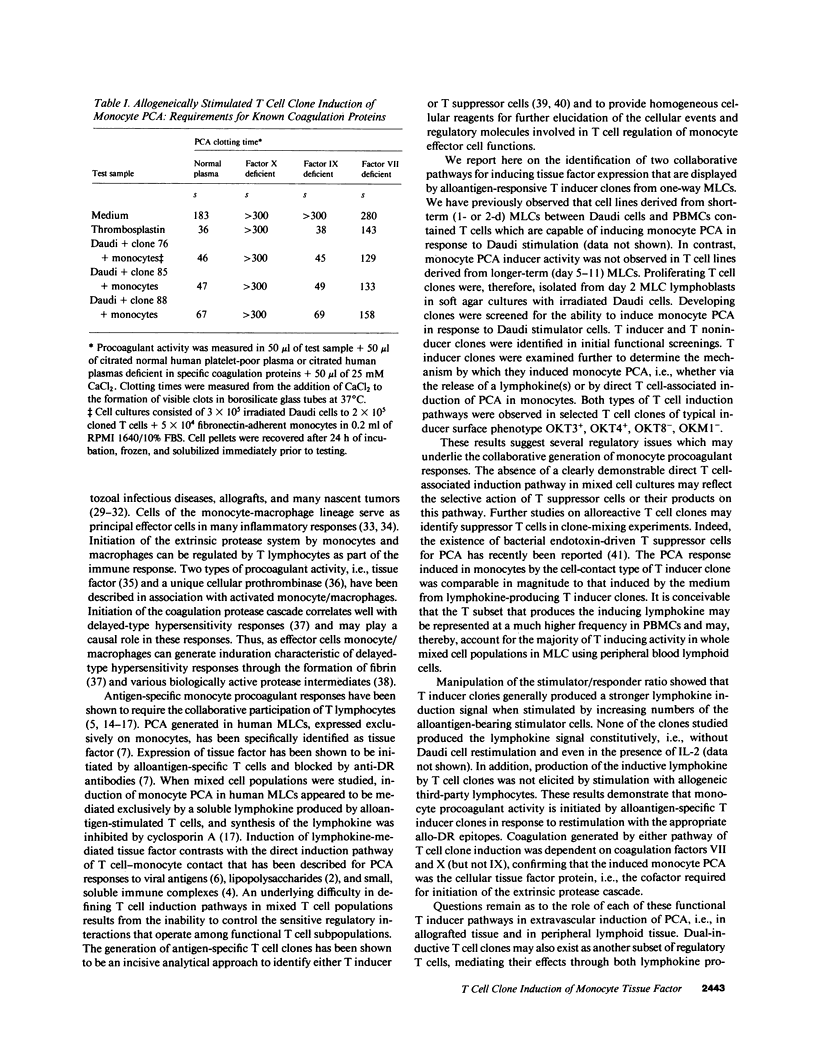
One component of the cellular immune response to antigens is the expression of procoagulant activity (PCA) by monocytes and macrophages. Induction of human monocyte PCA in response to alloantigenic stimulation requires the collaboration of HLA-DR-responsive T cells. In mixed lymphocyte cultures (MLCs), the induction of monocyte tissue factor appears to be mediated exclusively by a T cell-derived lymphokine. We have used a soft agar cloning method to generate alloantigen-responsive T cell clones from MLCs between irradiated Daudi lymphoblastoid cells and human peripheral blood mononuclear cells. Developing clones were screened for the ability to induce PCA in fresh autologous monocytes in response to Daudi stimulator cells. PCA induction was observed with some, but not all, proliferating T cell clones and two modes of induction were apparent. Some T cell clones mediated PCA induction exclusively by lymphokine production, whereas other clones delivered induction signals by direct cellular collaboration with the monocyte effector cells. These two inductive pathways were represented in distinct, non-inclusive functional subsets of T cell clones. Constitutive production of soluble inducer signals was not observed in T inducer clones. The magnitude of the monocyte PCA response increased in response to an increase in the allogeneic stimulator/T clone responder ratio, and third-party allogeneic cells were unable to elicit the PCA-inducing lymphokine signals from T inducer clones. Both modes of induction were shown to generate tissue factor protein activity in monocytes. Collectively, these results suggest that PCA induction can be initiated in response to alloantigens through collaboration with certain OKT3+, OKT4+, OKT8-, OKM1- T inducer clones, and that induction can be mediated by at least two different functional subsets of human T cells. Stimulation with the appropriate alloantigen may elicit both lymphokine and T cell-contact pathways of induction of tissue factor in human monocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Altman A., Sferruzza A., Weiner R. G., Katz D. H. Constitutive and mitogen-induced production of T cell growth factor by stable T cell hybridoma lines. J Immunol. 1982 Mar;128(3):1365–1371. [PubMed] [Google Scholar]
- Bar-Shavit R., Kahn A., Wilner G. D., Fenton J. W., 2nd Monocyte chemotaxis: stimulation by specific exosite region in thrombin. Science. 1983 May 13;220(4598):728–731. doi: 10.1126/science.6836310. [DOI] [PubMed] [Google Scholar]
- Bevilacqua M. P., Amrani D., Mosesson M. W., Bianco C. Receptors for cold-insoluble globulin (plasma fibronectin) on human monocytes. J Exp Med. 1981 Jan 1;153(1):42–60. doi: 10.1084/jem.153.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevilacqua M. P., Pober J. S., Majeau G. R., Cotran R. S., Gimbrone M. A., Jr Interleukin 1 (IL-1) induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984 Aug 1;160(2):618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgington T. S. Recognition coupled responses of the monocyte: activation of coagulation pathways. Nouv Rev Fr Hematol. 1983;25(1):1–6. [PubMed] [Google Scholar]
- Edwards R. L., Rickles F. R. The role of human T cells (and T cell products) for monocyte tissue factor generation. J Immunol. 1980 Aug;125(2):606–609. [PubMed] [Google Scholar]
- Farram E., Geczy C. L., Moon D. K., Hopper K. The ability of lymphokine and lipopolysaccharide to induce procoagulant activity in mouse macrophage cell lines. J Immunol. 1983 Jun;130(6):2750–2756. [PubMed] [Google Scholar]
- Freundlich B., Avdalovic N. Use of gelatin/plasma coated flasks for isolating human peripheral blood monocytes. J Immunol Methods. 1983 Aug 12;62(1):31–37. doi: 10.1016/0022-1759(83)90107-2. [DOI] [PubMed] [Google Scholar]
- Geczy C. L., Farram E., Moon D. K., Meyer P. A., McKenzie I. F. Macrophage procoagulant activity as a measure of cell-mediated immunity in the mouse. J Immunol. 1983 Jun;130(6):2743–2749. [PubMed] [Google Scholar]
- Geczy C. L., Hopper K. E. A mechanism of migration inhibition in delayed-type hypersensitivity reactions. II. Lymphokines promote procoagulant activity of macrophages in vitro. J Immunol. 1981 Mar;126(3):1059–1065. [PubMed] [Google Scholar]
- Gillis S., Ferm M. M., Ou W., Smith K. A. T cell growth factor: parameters of production and a quantitative microassay for activity. J Immunol. 1978 Jun;120(6):2027–2032. [PubMed] [Google Scholar]
- Glasebrook A. L., Fitch F. W. Alloreactive cloned T cell lines. I. Interactions between cloned amplifier and cytolytic T cell lines. J Exp Med. 1980 Apr 1;151(4):876–895. doi: 10.1084/jem.151.4.876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hattler B. G., Jr, Rocklin R. E., Ward P. A., Rickles F. R. Functional features of lymphocytes recovered from a human renal allograft. Cell Immunol. 1973 Nov;9(2):289–296. doi: 10.1016/0008-8749(73)90080-4. [DOI] [PubMed] [Google Scholar]
- Helin H. J., Edgington T. S. Cyclosporin A regulates monocyte/macrophage effector functions by affecting instructor T cells: inhibition of monocyte procoagulant response to allogeneic stimulation. J Immunol. 1984 Mar;132(3):1074–1076. [PubMed] [Google Scholar]
- Helin H. J., Fox R. I., Edgington T. S. The instructor cell for the human procoagulant monocyte response to bacterial lipopolysaccharide is a Leu-3a+ T cell by fluorescence-activated cell sorting. J Immunol. 1983 Aug;131(2):749–752. [PubMed] [Google Scholar]
- Helin H., Edgington T. S. A distinct "slow" cellular pathway involving soluble mediators for the T cell-instructed induction of monocyte tissue factor activity in an allogeneic immune response. J Immunol. 1984 May;132(5):2457–2463. [PubMed] [Google Scholar]
- Helin H., Edgington T. S. Allogeneic induction of the human T cell-instructed monocyte procoagulant response is rapid and is elicited by HLA-DR. J Exp Med. 1983 Sep 1;158(3):962–975. doi: 10.1084/jem.158.3.962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hopper K. E., Geczy C. L., Davies W. A. A mechanism of migration inhibition in delayed-type hypersensitivity reactions. I. Fibrin deposition on the surface of elicited peritoneal macrophages on vivo. J Immunol. 1981 Mar;126(3):1052–1058. [PubMed] [Google Scholar]
- Kornbluth J., Silver D. M., Dupont B. Cloning and characterization of primary alloreactive human T-lymphocytes. Immunol Rev. 1981;54:111–155. doi: 10.1111/j.1600-065x.1981.tb00436.x. [DOI] [PubMed] [Google Scholar]
- Kurt-Jones E. A., Beller D. I., Mizel S. B., Unanue E. R. Identification of a membrane-associated interleukin 1 in macrophages. Proc Natl Acad Sci U S A. 1985 Feb;82(4):1204–1208. doi: 10.1073/pnas.82.4.1204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G. A., Edgington T. S. Lymphocyte cooperation is required for amplification of macrophage procoagulant activity. J Exp Med. 1980 May 1;151(5):1232–1244. doi: 10.1084/jem.151.5.1232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G. A., Leibowitz J. L., Edgington T. S. Induction of monocyte procoagulant activity by murine hepatitis virus type 3 parallels disease susceptibility in mice. J Exp Med. 1981 Oct 1;154(4):1150–1163. doi: 10.1084/jem.154.4.1150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levy G. A., Schwartz B. S., Edgington T. S. The kinetics and metabolic requirements for direct lymphocyte induction of human procoagulant monokines by bacterial lipopolysaccharide. J Immunol. 1981 Jul;127(1):357–363. [PubMed] [Google Scholar]
- MACKANESS G. B. Cellular resistance to infection. J Exp Med. 1962 Sep 1;116:381–406. doi: 10.1084/jem.116.3.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meuer S. C., Cooper D. A., Hodgdon J. C., Hussey R. E., Morimoto C., Schlossman S. F., Reinherz E. L. Immunoregulatory human T lymphocytes triggered as a consequence of viral infection: clonal analysis of helper, suppressor inducer and suppressor effector cell populations. J Immunol. 1983 Sep;131(3):1167–1172. [PubMed] [Google Scholar]
- Meuer S. C., Schlossman S. F., Reinherz E. L. Clonal analysis of human cytotoxic T lymphocytes: T4+ and T8+ effector T cells recognize products of different major histocompatibility complex regions. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4395–4399. doi: 10.1073/pnas.79.14.4395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nash A. A., Phelan J., Wildy P. Cell-mediated immunity in herpes simplex virus-infected mice: H-2 mapping of the delayed-type hypersensitivity response and the antiviral T cell response. J Immunol. 1981 Apr;126(4):1260–1262. [PubMed] [Google Scholar]
- Niemetz J. Coagulant activity of leukocytes. Tissue factor activity. J Clin Invest. 1972 Feb;51(2):307–313. doi: 10.1172/JCI106815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niemetz J., Fani K. Role of leukocytes in blood coagulation and the generalized Shwartzman reaction. Nat New Biol. 1971 Aug 25;232(34):247–248. doi: 10.1038/newbio232247a0. [DOI] [PubMed] [Google Scholar]
- Rickles F. R., Edwards R. L. Activation of blood coagulation in cancer: Trousseau's syndrome revisited. Blood. 1983 Jul;62(1):14–31. [PubMed] [Google Scholar]
- Rivers R. P., Hathaway W. E., Weston W. L. The endotoxin-induced coagulant activity of human monocytes. Br J Haematol. 1975 Jul;30(3):311–316. doi: 10.1111/j.1365-2141.1975.tb00547.x. [DOI] [PubMed] [Google Scholar]
- Rozenszajn L. A., Shoham D., Kalechman I. Clonal proliferation of PHA-stimulated human lymphocytes in soft agar culture. Immunology. 1975 Dec;29(6):1041–1055. [PMC free article] [PubMed] [Google Scholar]
- Schwartz B. S., Edgington T. S. Immune complex-induced human monocyte procoagulant activity. I. a rapid unidirectional lymphocyte-instructed pathway. J Exp Med. 1981 Sep 1;154(3):892–906. doi: 10.1084/jem.154.3.892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz B. S., Levy G. A., Fair D. S., Edgington T. S. Murine lymphoid procoagulant activity induced by bacterial lipopolysaccharide and immune complexes is a monocyte prothrombinase. J Exp Med. 1982 May 1;155(5):1464–1479. doi: 10.1084/jem.155.5.1464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira A. R., Teixeira G., Macêdo V., Prata A. Acquired cell-mediated immunodepression in acute Chagas' disease. J Clin Invest. 1978 Dec;62(6):1132–1141. doi: 10.1172/JCI109232. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsao B. P., Fair D. S., Curtiss L. K., Edgington T. S. Monocytes can be induced by lipopolysaccharide-triggered T lymphocytes to express functional factor VII/VIIa protease activity. J Exp Med. 1984 Apr 1;159(4):1042–1057. doi: 10.1084/jem.159.4.1042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkman A. The origin and turnover of mononuclear cells in peritoneal exudates in rats. J Exp Med. 1966 Aug 1;124(2):241–254. doi: 10.1084/jem.124.2.241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Furth R., Cohn Z. A. The origin and kinetics of mononuclear phagocytes. J Exp Med. 1968 Sep 1;128(3):415–435. doi: 10.1084/jem.128.3.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Ginkel C. J., Zeijlemaker W. P., Stricker L. A., Oh J. I., van Aken W. G. Enhancement of monocyte thromboplastin activity by antigenically stimulated lymphocytes: a link between immune reactivity and blood coagulation. Eur J Immunol. 1981 Jul;11(7):579–583. doi: 10.1002/eji.1830110711. [DOI] [PubMed] [Google Scholar]



