Abstract
Background
The vast majority of AIS patients who require surgical intervention are women. Blood loss is a major concern during the operation.
Methods
The medical records of all female AIS patients who underwent posterior correction and fusion operations using the all-pedicle screw system from January 2012 to January 2014 were reviewed. Patients with irregular menstruation; underwent osteotomy; use coagulants were excluded from the study. The remaining patients were divided into 4 groups according to the operation date in the menstrual cycle (A: premenstrual group, 24–30 d; B: follicle group, 6–11 d; C: ovulatory group, 12–17 d; D: luteal group, 18–23 d). The information of patients from the 4 groups was reviewed. The data was analyzed using analysis of variance, the Student-Newman-Keels test and Kruskal-Wallis Test.
Results
A total of 161 patients were included in this study. There were 40 patients included in group A, 38 patients in group B, 41 patients in group C and 42 patients in group D. The 4 groups were matched in age (P = 0.238), body height (P = 0.291), body weight (P = 0.756), Risser sign (P = 0.576), mean curve Cobb angle (P = 0.520), and bending flexibility index (P = 0.547), the number of levels fused (P = 0.397). The activated partial thromboplastin time (P = 0.235) and prothrombin time (P = 0.074) tended to be higher in group A, but the difference was not statistically significant. The fibrinogen level was lower in group B than the other 3 groups (P = 0.039). Blood loss and normalized intraoperative blood loss (NBL) was significantly higher in group A than the other 3 groups (P<0.01).
Conclusions
The hemostatic function tended to be lower in the premenstrual phase. The fibrinogen level was lowest in the mid-follicle phase. Female AIS patients tended to endure more intraoperative blood loss when the operation was performed in the premenstrual phase during the menstrual cycle.
Introduction
Adolescent idiopathic scoliosis (AIS) is a structural, lateral, rotated curvature of the spine that arises in otherwise healthy children at or around puberty. AIS may affect 1–3% of the at-risk population when defined as a Cobb angle>10° [1]. Surgical intervention may be required when the Cobb angle is greater than 40°. For patients with a major curve greater than 30°, female/male ratio could be as high as 10∶1. The likelihood of curve progression in female patients is even higher [2]–[4]. The vast majority of AIS patients who require surgical intervention are women.
Intraoperative blood loss (IOBL) is a major concern during the operation and may lead to many complications such as hypotension, anemia, coagulopathy, infection, and the need for transfusion of large volume of blood products with associated risks [5]. It is also associated with a higher prevalence of non-neurologic complications [6]. Numerous hemostatic agents have been used to reduce the IOBL during scoliosis surgery and have demonstrated variable efficacy; however, mortality, morbidity (need for blood transfusion, rebleeding, or other complications), and medico-economic criteria have not been appropriately evaluated [5], [7]–[9].
Sex hormone variations may affect the level of blood coagulation factors, thus, influence the hemostatic function in female adolescents [10], [11]. Some studies showed that treatment with contraceptive medication may affect the hemostatic potential in women [12]–[14]. Sex hormone variations were also thought to be related to the hypercoagulable status during pregnancy [15], [16]. However, studies of hemostatic status during the different phases of the normal physiological menstrual cycle show contradictory results [10]. The current study aimed to determine if clinical hemostatic indicators and IOBL vary among female AIS patients operated in different phases of the menstrual cycle. To the best of our knowledge, no studies have addressed this issue to date.
Methods
Objectives
In this retrospective analysis, we investigated the effect of operation time during different phases of menstrual cycle on IOBL in female adolescent idiopathic scoliosis patients who underwent posterior correction and all pedicle screw fixation. Previous studies have shown that increase in blood sex hormone could cause blood coagulation changes. We speculate that natural hormone variation during normal menstrual cycle may affect blood coagulation too. This variation is especially meaningful considering that the majority of AIS patients who need surgical intervention are female and the large IOBL during posterior correction and fusion surgery for AIS patients.
Participants
Female AIS Patients, who sought medical help and underwent posterior correction and all pedicle screw fixation and fusion surgery in Chinghai hospital between January 2012 and January 2014, were retrospectively reviewed. The diagnosis of AIS followed the description of Weinstein et al [1]. Other subtypes of scoliosis were excluded by medical history, physical and radiological examination. Diagnosis was made independently by three attending clinicians, and only patients who were diagnosed with AIS by all the three doctors were included in this study. Details of the patients' menstruation history including the date of last menstruation, duration of menstruation, duration of menstrual cycle and rhythm of menstrual cycle were attained from the medical record (this information was record on the medical record according to the medical record writing norm issued by Ministry of Health of China), Patients with irregular menstruation, underwent osteotomy, using coagulants or combined with other diseases that may affect the operation were excluded. The remaining patients were divided into 4 groups according to the operation date during the different phases of the menstrual cycle (A: premenstrual group, 24–30 d; B: follicle group, 6–11 d; C: ovulatory group, 12–17 d; and D: luteal group, 18–23 d).
Follow-up Outcome Measures
Parameters, including: patient age; body weight; body height; the number of levels fused; major curve Cobb angle; major curve bending flexibility index; blood type; albumin (Alb); hemoglobin (Hg); fibrinogen; platelet count (PLT); activated partial thromboplastin time (APTT); prothrombin time (PT); thrombin time (TT); IOBL; operation date and menstrual cycle data were reviewed. Normalized intraoperative blood loss (intraoperative blood loss per level fused per kilogram, NBL) was calculated using the following equation:
Patients and Anthropometric Measurements
The study protocol was approved by the Institutional Review Board of the Second Military Medical University, Shanghai, China. Written informed consent was obtained from every participant. The protocols of this study have been approved by the institutional review board of Changhai Hospital.
Statistical analysis
Data from different groups was analyzed using analysis of variance (ANOVA), the number of levels fused was analyzed by Kruskal-Wallis Test. Differences between the groups were further analyzed using the Student-Newman-Keuls (SNK) test. The data was checked for normality and equal variances. A P value less than 0.05 was considered significant for each individual test in this study. Statistical Package for Social Science software 18.0 (SPSS Inc., Chicago, IL, USA) was used to perform the statistical analysis. Graphs were drawn using GraphPad Prism 5.0 (GraphPad Software Inc., San Diego, CA, USA).
Results
Baseline and Perioperative Characteristics of Patients
A total of 276 female AIS patients underwent posterior correction and spinal fusion were screened. Among them, 115 patients were excluded because of: Ponte Osteotomy during the surgery (38); combined anterior approach (17); diagnosed coagulopathies (9); menstrual cycle problems (44, irregular menstrual cycle, menstrual cycle longer than 30 days or shorter than 28 days, before menarche); or intraoperative hemostasis (43). The remaining 161 patients were reviewed. There were 40 patients included in group A, 38 patients in group B, 41 patients in group C, 42 patients in group D. ANOVA showed that the 4 groups were matched in age (P = 0.246), body height (P = 0.359), body weight (P = 0.348), Risser sign (P = 0.628), mean curve Cobb angle (P = 0.596), bending Cobb (P = 0.993), bending flexibility index (P = 0.849). Kruskal-Wallis Test showed there was no difference in the number of levels fused (P = 0.497). The APTT (P = 0.168) and PT (P = 0.107) tended to be higher in group A than the other groups; however, the difference was not statistically significant. There were no significant differences in Hg (P = 0.875), PLT (P = 0.517), Alb (P = 0.247), and TT (P = 0.949) between the groups. There were significant differences in fibrinogen (P = 0.039), IOBL (P<0.01), and NBL (P<0.01) between the groups. The details of the 4 groups analyzed by ANOVA are shown in Table 1.
Table 1. Clinical characteristics of the patients.
| Variable | Group A | Group B | Group C | Group D | Mean | P |
| Number | 40 | 38 | 41 | 42 | ||
| Age (y) | 15.22±2.03 | 14.57±1.66 | 14.66±1.53 | 15.13±1.74 | 14.90 | 0.246 |
| Height (cm) | 158.55±4.71 | 159.42±4.18 | 157.71±3.67 | 158.80±4.64 | 158.60 | 0.359 |
| Weight (kg) | 47.04±6.37 | 48.95±5.66 | 46.88±5.86 | 48.27±5.88 | 47.76 | 0.348 |
| Risser sign | 3.60±1.19 | 3.45±1.03 | 3.38±1.19 | 3.29±1.23 | 3.43 | 0.682 |
| Major curve (°) | 51.60±11.99 | 49.34±10.27 | 48.48±8.14 | 49.54±11.67 | 49.73 | 0.596 |
| Bending cobb (°) | 13.28±7.46 | 13.39±6.74 | 13.10±6.55 | 13.00±5.59 | 13.19 | 0.993 |
| B-Flexibility index | 0.75±0.10 | 0.73±0.12 | 0.73±0.12 | 0.74±0.08 | 0.74 | 0.849 |
| Alb (g/L) | 41.45±2.09 | 41.50±2.05 | 40.83±2.19 | 40.78±1.97 | 41.13 | 0.247 |
| Hg (g/L) | 123.98±7.04 | 125.08±8.67 | 125.31±8.72 | 125.17±7.89 | 124.89 | 0.875 |
| PLT | 215.85±35.67 | 221.37±38.53 | 222.21±52.09 | 230.46±46.79 | 222.53 | 0.517 |
| APTT (s) | 38.34±1.90 | 37.14±2.39 | 37.55±2.97 | 37.54±2.19 | 37.65 | 0.168 |
| PT (s) | 13.58±0.41 | 13.39±0.37 | 13.37±0.47 | 13.36±0.51 | 13.42 | 0.107 |
| Fibrinogen (g/L) | 2.62±0.44 | 2.35±0.47 | 2.57±0.47 | 2.60±0.43 | 2.54 | 0.039 |
| Thrombin time (s) | 16.90±1.67 | 16.97±1.70 | 16.87±1.30 | 16.77±1.50 | 16.88 | 0.949 |
| The number of levels fused | 10.65±1.67 | 10.79±1.70 | 10.64±2.08 | 10.15±2.22 | 10.55 | 0.497 |
| IOBL (ml) | 1078.75±295.28 | 952.11±194.27 | 863.81±201.77 | 847.80±266.03 | 933.98 | <0.01 |
| NBL (ml/f*kg) | 2.17±0.46 | 1.83±0.34 | 1.77±0.34 | 1.76±0.40 | 1.87 | <0.01 |
The SNK test (fibrinogen, blood loss, NBL) showed that the IOBL and NBL were significantly higher in group A than in other groups; there was no observed difference between groups B, C, and D. Fibrinogen was significantly lower in group B than groups A, C, and D; there was no observed difference between groups A, C, and D. Tables 2, 3, and 4 show data analyzed using the SNK test regarding fibrinogen, blood loss, and NBL. The fluctuations of the clinical coagulation test are shown in Figures 1(A–E). Figure 2 shows the differences in NBL between each group and the scatter point of NBL in group A according to the day of the menstrual cycle; the NBL tended to be higher among the subjects who underwent surgery in the last days of the menstrual cycle.
Table 2. Student-Newman-Keuls test: Fibrinogen.
| Group | N | Subset for alpha = 0.05 | |
| 1 | 2 | ||
| B | 38 | 2.354211 | |
| C | 42 | 2.569048 | |
| D | 41 | 2.602927 | |
| A | 40 | 2.618750 | |
| Sig. | 1.000 | .876 | |
Table 2 shows the Fibrinogen level was NBLsignificant lower in group B.
Table 3. Student-Newman-Keuls test: NBL.
| Team | N | Subset for alpha = 0.05 | |
| 1 | 2 | ||
| D | 42 | 1.755241 | |
| C | 41 | 1.769632 | |
| B | 38 | 1.829504 | |
| A | 40 | 2.167856 | |
| Sig. | 0.666477 | 1.000 | |
Table 3 shows the NBL was significant higher in group A.
Table 4. Student-Newman-Keuls test: Blood loss.
| Team | N | Subset for alpha = 0.05 | |
| 1 | 2 | ||
| D | 41 | 847.80 | |
| C | 42 | 863.81 | |
| B | 38 | 952.11 | |
| A | 40 | 1078.75 | |
| Sig. | 0.136 | 1.000 | |
The means for groups in homogeneous subsets are displayed.
Uses harmonic mean sample size = 40.195.
The group sizes are unequal. The harmonic mean of the group sizes is used. Type I error levels are not guaranteed.
Table 4 shows the Blood loss was significant higher in group A.
Figure 1. Variations of clinical coagulate indicators between groups.
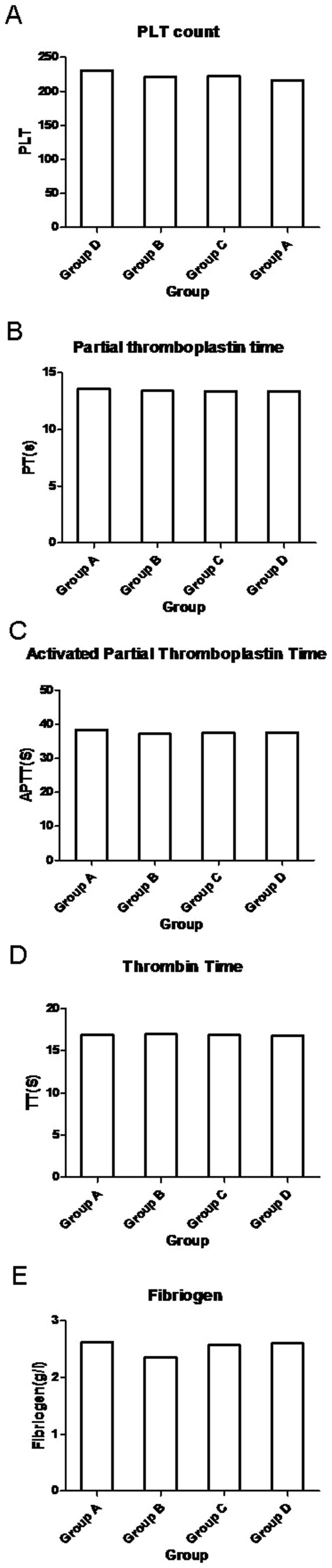
There were no difference between groups in PLT count(A), PT(B), APTT(C), TT(D); The Fibrigen level in group B was significant lower than other 3 groups(E).
Figure 2. Average NBL between different groups and scatter diagram of NBL in group A.
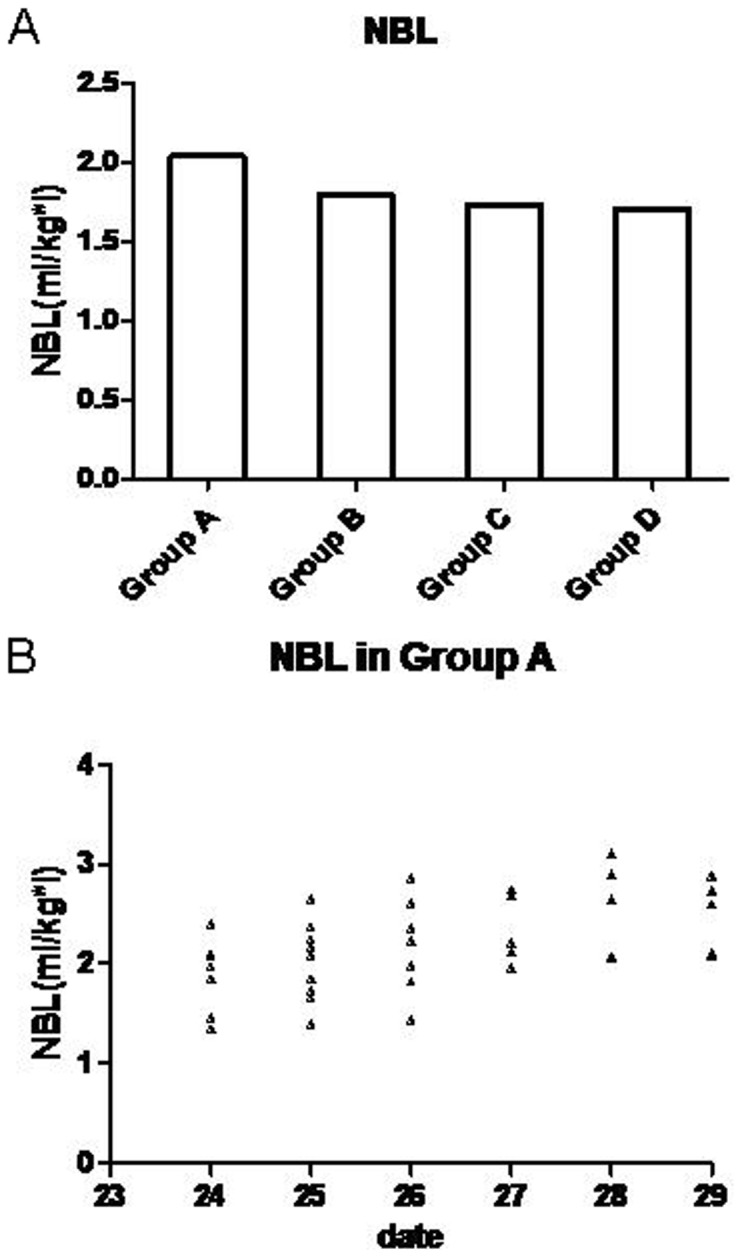
NBL between groups (A) show NBL was significantly higher in group A than the other 3 groups, There was no difference between other 3 groups. Scatter diagram of NBL (B) in group A show the NBL has a tendency to increase during the last days of the menstrual cycle and achieve a peak during 1–2 days before Menstruation.
Discussion
Posterior correction with multilevel spinal fusion (PCSF) has been proven to be the most effective way to treat AIS [1], [17], [18]. With a long fusion range, PCSF is associated with significant IOBL normally ranging from 750 to 1500 mL [19]. This was confirmed by our study, with a mean blood loss of 933.73 mL (500–2000 mL). Both increased IOBL and blood transfusion are associated with hypotension, anemia, coagulopathy, infection, allergic reactions, acute or delayed immune hemolytic reactions, iron overload, and graft-versus-host disease [5], [6], [20]. Numerous hemostatic agents have been used to reduce the IOBL during scoliosis surgery and have demonstrated variable efficacy without enough consideration about complications [5], [7]–[9].
A significant finding in our study was that patients in group A experienced significantly higher IOBL (total: 1078.25±295.28 mL; NBL: 2.04±0.35 mL/kg) than those in group B (total: 952.11±194.27 mL; NBL: 1.80±0.30 (mL/kg*l), group C (total: 864.81±201.77 mL; NBL: 1.73±0.27 mL/kg*l), and group D (total: 846.83±266.63 mL; NBL: 1.71±0.34 (mL/kg*l). Even in group A, the NBL of patients tended to increase as the operation date approached menstruation. This indicates that patients who undergo surgery before menstruation may experience more blood loss. The differences between the groups may be attributed to the hormone-induced hemostatic function change. Van Roojen et al [21] studied the blood hemostatic factors of 11 women before and after taking emergency hormonal contraception (which could rapidly increase the blood estrogen level). They found the plasma concentrations of free protein S, F1+2, and Factor VIIa and the APTT-based APCsr changed toward a more precoagulant state. Ibrahimi et al [11] studied 49 women who received oral contraceptive and found increased concentrations of fibrinogen and factor VIII as well as decreased PT and APTT after treatment. Most of the studies show that women taking estrogen/progesterone will have an increased level of factor VII, factor X, factor XII, and fibrinogen. Furthermore, both the level and activity of antithrombin antigen were decreased while the levels of factor V and factor VIII remained unchanged [22]. These studies show a positive association between oral estrogen and hemostatic ability.
However, studies on variations in hemostatic factors during the normal menstrual cycle are rare and show contradictory results. Miller et al [23] conducted a cross-sectional study of 175 women and found that the levels of Von Willebrand factor (VWF) and factor VIII were lowest during menstruation. Similar results were confirmed by some studies [24]–[26], while other studies reported that there were no cyclic variations in VWF, factor VIII, and factor XI during the menstrual cycle [24], [27]–[30]. Cederblad et al [31] studied 30 normal women whose blood samples were taken on 6 occasions: day 1, 2 and 3 of menstruation; day 5–9 (follicular phase); day 12–16 (around ovulation); and day 19–23 (luteal phase). They found that the concentration of factor II–VII–X was lowest during menstruation. Bolis et al [32] reported a significant decrease of factor XIII during the periovulatory phase in a study of 10 women. The present study shows that both APTT and PT were higher in group A than in the other 3 groups (Table 1; Figure 1, B and C), but the difference was not statistically significant (P = 0.168, P = 0.107) and still confined within the normal range. However, this difference may have been induced by the changes of hemostatic factors, which then caused the difference in IOBL between the groups.
Another finding of this study was that the fibrinogen level was lowest in the follicle phase (group B: follicle group, Figure 1 E, P = 0.039). Several studies reported on the variations of fibrinogen levels during the menstrual cycle. Six of these studies reported the lowest levels of fibrinogen during the follicular or mid-cycle phase [24], [27], [29], [33]–[35], 2 reported the lowest levels during luteal phase [36], [37], while other studies reported no cyclic variation [28], [38]–[40]. We speculate that the reason for this may be the consumption during menstruation and the relatively low level of sex hormone during menstruation, since oral estrogen increases fibrinogen concentration in blood [41], [42].
We normalized the IOBL by dividing blood loss by number of levels fused and by patients' weight. The number of levels fused has been reported to be a predictor of IOBL in scoliosis surgery [43], [44]. In the present study, body weight significantly varied between patients; this was caused by the individual differences in the development stages during puberty. This method was also used by Amit et al [45] when comparing the blood loss between different types of scoliosis surgery; the method proved to be an effective way to eliminate the effect of the number of levels fused and body weight.
One of the concerns of our study was its retrospective design. We relied on the IOBL noted on the medical records when collecting the data. However, the estimation of IOBL in scoliosis surgery was performed by 2 senior anesthesiologists in our hospital, while following strict criteria. In other words, all the AIS patients were evaluated by the same group of physicians using the same criteria, thus, individual error was minimized. Another concern of the study might be lack the data of patients from 1st–5th days of menstrual cycle. Actually, we rarely perform the posterior correction and fusion surgery during menstruation. There were two reasons that we did not perform the surgery during menstruation. Firstly, the posterior correction and fusion surgery was featured by its large IOBL, but during menstruation the patients were experiencing fundamental blood loss. Secondly, we think menstruation was a kind of injury to the endometrium of the female AIS patient. We want to do our best to avoid these shortcomings since the surgery we are going to perform is a selective operation.
Our study had several strengths. First, our data was collected from a single institution. All the treatments followed a uniform procedure, and all the evaluations were performed using the same criteria. We confined our review time to 2 years to avoid confounding factors such as changes in the surgeon's surgical skills, improvement of equipment, etc. This minimized the system error of this study. Second, we eliminated any factors that may have affected our study, such as age over 18 years (patients may have a rigid curve as their age increases thus increase the IOBL), combined anterior and posterior approach, use of antifibrinolytic medication before or during surgery, and use of osteotomy during surgery. Finally, rather than only dividing the patients into follicle and luteal groups as in previous clinical and basic studies related to this field [25], [27], [29], [38], [39], [46], [47], we divided our subjects into 4 groups which included perimenstrual days; this made our study more comprehensive and allowed us to find that patients who underwent surgery during the premenstrual phase tended to endure more IOBL. As a selective operation with relatively high IOBL, in female AIS patients, it may be more plausible to perform the correction and fusion surgery at a different time than premenstrual days during the menstrual cycle.
Conclusions
Female AIS patients tended to endure more IOBL when the operation was performed in the premenstrual phase of the menstrual cycle. The hemostatic function tended to be lower in the premenstrual phase; however, statistical significance was not reached. The fibrinogen level was lowest during the mid-follicle phase.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. The data are included in the paper.
Funding Statement
These authors have no support or funding to report.
References
- 1. Weinstein SL, Dolan LA, Cheng JC, Danielsson A, Morcuende JA (2008) Adolescent idiopathic scoliosis. The Lancet 371:1527–1537. [DOI] [PubMed] [Google Scholar]
- 2. Luk KD, Lee CF, Cheung KM, Cheng JC, Ng BK, et al. (2010) Clinical effectiveness of school screening for adolescent idiopathic scoliosis: a large population-based retrospective cohort study. Spine (Phila Pa 1976) 35:1607–1614. [DOI] [PubMed] [Google Scholar]
- 3. Raggio CL (2006) Sexual dimorphism in adolescent idiopathic scoliosis. Orthop Clin North Am 37:555–558. [DOI] [PubMed] [Google Scholar]
- 4. Hresko MT (2013) Clinical practice. Idiopathic scoliosis in adolescents. N Engl J Med 368:834–841. [DOI] [PubMed] [Google Scholar]
- 5. Elgafy H, Bransford RJ, McGuire RA, Dettori JR, Fischer D (2010) Blood loss in major spine surgery: are there effective measures to decrease massive hemorrhage in major spine fusion surgery? Spine 35:S47. [DOI] [PubMed] [Google Scholar]
- 6. Carreon LY, Puno RM, Lenke LG, Richards BS, Sucato DJ, et al. (2007) Non-neurologic complications following surgery for adolescent idiopathic scoliosis. The Journal of Bone & Joint Surgery 89:2427–2432. [DOI] [PubMed] [Google Scholar]
- 7. Gill JB, Chin Y, Levin A, Feng D (2008) The Use of Antifibrinolytic Agents in Spine SurgeryA Meta-Analysis. The Journal of Bone & Joint Surgery 90:2399–2407. [DOI] [PubMed] [Google Scholar]
- 8.Tzortzopoulou A, Cepeda MS, Schumann R, Carr DB (2008) Antifibrinolytic agents for reducing blood loss in scoliosis surgery in children. Cochrane Database Syst Rev 3. [DOI] [PubMed]
- 9. Aubourg R, Putzolu J, Bouche S, Galmiche H, Denis C, et al. (2011) Surgical hemostatic agents: Assessment of drugs and medical devices. Journal of Visceral Surgery 148:e405–e408. [DOI] [PubMed] [Google Scholar]
- 10. Knol HM, Kemperman RF, Kluin-Nelemans HC, Mulder AB, Meijer K (2008) Haemostatic variables during normal menstrual cycle. Thromb Haemost 99:1019–1029. [DOI] [PubMed] [Google Scholar]
- 11. Ibrahimi E, Koni M (2014) Effects of estrogens and progestagens on the primary variables of haemostasis. International Journal of Reproduction, Contraception, Obstetrics and Gynecology 3:31–33. [Google Scholar]
- 12. Daly L, Bonnar J (1990) Comparative studies of 30 µg ethinyl estradiol combined with gestodene and desogestrel on blood coagulation, fibrinolysis, and platelets. American journal of obstetrics and gynecology 163:430–437. [DOI] [PubMed] [Google Scholar]
- 13. Luyer M, Khosla S, Owen W, Miller V (2001) Prospective randomized study of effects of unopposed estrogen replacement therapy on markers of coagulation and inflammation in postmenopausal women. Journal of Clinical Endocrinology & Metabolism 86:3629–3634. [DOI] [PubMed] [Google Scholar]
- 14. Fruzzetti F, Bitzer J (2010) Review of clinical experience with estradiol in combined oral contraceptives. Contraception 81:8–15. [DOI] [PubMed] [Google Scholar]
- 15. Prisco D, Ciuti G, Falciani M (2005) Hemostatic changes in normal pregnancy; haematologica reports. 1(10):1–5. [Google Scholar]
- 16. Kujovich JL (2004) Hormones and pregnancy: thromboembolic risks for women. British journal of haematology 126:443–454. [DOI] [PubMed] [Google Scholar]
- 17. Bjerkreim I, Steen H, Brox JI (2007) Idiopathic scoliosis treated with Cotrel-Dubousset instrumentation: evaluation 10 years after surgery. Spine 32:2103–2110. [DOI] [PubMed] [Google Scholar]
- 18. Wang Y, Fei Q, Qiu G, Lee CI, Shen J, et al. (2008) Anterior spinal fusion versus posterior spinal fusion for moderate lumbar/thoracolumbar adolescent idiopathic scoliosis: a prospective study. Spine 33:2166–2172. [DOI] [PubMed] [Google Scholar]
- 19. Ialenti MN, Lonner BS, Verma K, Dean L, Valdevit A, et al. (2013) Predicting Operative Blood Loss During Spinal Fusion for Adolescent Idiopathic Scoliosis. Journal of Pediatric Orthopaedics 33:372–376. [DOI] [PubMed] [Google Scholar]
- 20.Klein HG, Anstee DJ (2013) Mollison's blood transfusion in clinical medicine: John Wiley & Sons.
- 21. van Rooijen M, Silveira A, Thomassen S, Hansson L, Rosing J, et al. (2007) Rapid activation of haemostasis after hormonal emergency contraception. Thrombosis and Haemostasis-Stuttgart- 97:15. [PubMed] [Google Scholar]
- 22. Norris LA, Bonnar J (1997) 9 Haemostatic changes and the oral contraceptive pill. Baillière's clinical obstetrics and gynaecology 11:545–564. [DOI] [PubMed] [Google Scholar]
- 23. Miller CH, Dilley AB, Drews C, Richardson L, Evatt B (2002) Changes in von Willebrand factor and factor VIII levels during the menstrual cycle. Thromb Haemost 87:1082–1083. [PubMed] [Google Scholar]
- 24. Kadir RA, Economides DL, Sabin CA, Owens D, Lee CA (1999) Variations in coagulation factors in women: effects of age, ethnicity, menstrual cycle and combined oral contraceptive. Thrombosis and Haemostasis-Stuttgart- 82:1456–1461. [PubMed] [Google Scholar]
- 25. Blombäck M, Landgren B, Stiernholm Y, Andersson O (1997) The effect of progesterone on the haemostatic mechanism. Thrombosis and haemostasis 77:105–108. [PubMed] [Google Scholar]
- 26. Mandalaki T, Louizou C, Dimitriadou C, Symeonidis P (1980) Variations in factor VIII during the menstrual cycle in normal women. The New England journal of medicine 302:1093–1094. [DOI] [PubMed] [Google Scholar]
- 27. Feuring M, Christ M, Roell A, Schueller P, Losel R, et al. (2002) Alterations in platelet function during the ovarian cycle. Blood coagulation & fibrinolysis 13:443–447. [DOI] [PubMed] [Google Scholar]
- 28. Giardina E-GV, Chen HJ, Sciacca RR, Rabbani LE (2004) Dynamic variability of hemostatic and fibrinolytic factors in young women. Journal of Clinical Endocrinology & Metabolism 89:6179–6184. [DOI] [PubMed] [Google Scholar]
- 29. Koh SC, Prasad R, Fong Y (2005) Hemostatic status and fibrinolytic response potential at different phases of the menstrual cycle. Clinical and applied thrombosis/hemostasis 11:295–301. [DOI] [PubMed] [Google Scholar]
- 30. Onundarson P, Gudmundsdottir B, Arnfinnsdottir A, Kjeld M, Olafsson O (2001) Von Willebrand factor does not vary during normal menstrual cycle. Thrombosis and haemostasis 85:183–184. [PubMed] [Google Scholar]
- 31. Cederblad G, Hahn L, Korsan-Bengtsen K, Pehrsson N, Rybo G (1977) Variations in blood coagulation, fibrinolysis, platelet function and various plasma proteins during the menstrual cycle. Pathophysiology of Haemostasis and Thrombosis 6:294–302. [DOI] [PubMed] [Google Scholar]
- 32. Bolis P, Franchi M, Marino L, Paganelli A, Sampaolo P (1982) Serial detection of plasma-factor XIII levels during the ovulatory cycle and estroprogestative contraception. Clinical and Experimental Obstetrics and Gynecology 9:22–25. [PubMed] [Google Scholar]
- 33. Larsen L, Andersen H, Hansen A, Andersen O (1996) Variation in risk indicators of cardiovascular disease during the menstrual cycle: an investigation of within-subject variations in glutathione peroxidase, haemostatic variables, lipids and lipoproteins in healthy young women. Scandinavian journal of clinical & laboratory investigation 56:241–249. [DOI] [PubMed] [Google Scholar]
- 34. Dapper D, Didia B (2002) Haemorheological changes during the menstrual cycle. East African medical journal 79:181–183. [DOI] [PubMed] [Google Scholar]
- 35. Lebech A-M, Kjaer A (1989) Lipid metabolism and coagulation during the normal menstrual cycle. Hormone and metabolic research 21:445–448. [DOI] [PubMed] [Google Scholar]
- 36. Ricci G, Cerneca F, Simeone R, Pozzobon C, Guarnieri S, et al. (2004) Impact of highly purified urinary FSH and recombinant FSH on haemostasis: an open-label, randomized, controlled trial. Human Reproduction 19:838–848. [DOI] [PubMed] [Google Scholar]
- 37. Solerte SB, Fioravanti M, Spinillo A, Ferrari E, Guaschino S (1988) Association between hormonal and haemorheological changes during the menstrual cycle in healthy women. BJOG: An International Journal of Obstetrics & Gynaecology 95:1305–1308. [DOI] [PubMed] [Google Scholar]
- 38. Repina MA, Korzo M, Zinina TA (2002) Effect of hormone replacement therapy with femoston on hemostasis in peri-and postmenopausal women. Medical Science Monitor 8:PI78–PI84. [PubMed] [Google Scholar]
- 39. Roell A, Schueller P, Schultz A, Losel R, Wehling M, et al. (2007) Effect of oral contraceptives and ovarian cycle on platelet function. Platelets 18:165–170. [DOI] [PubMed] [Google Scholar]
- 40. Blombäck M, Eneroth P, Landgren B, Lagerström M, Anderson O (1992) On the intraindividual and gender variability of haemostatic components. Thrombosis and haemostasis 67:70–75. [PubMed] [Google Scholar]
- 41. Sitruk-Ware R, Nath A (2013) Characteristics and metabolic effects of estrogen and progestins contained in oral contraceptive pills. Best Practice & Research Clinical Endocrinology & Metabolism 27:13–24. [DOI] [PubMed] [Google Scholar]
- 42. Cleuren AC, van der Linden IK, de Visser YP, Wagenaar GT, Reitsma PH, et al. (2010) 17α-Ethinylestradiol rapidly alters transcript levels of murine coagulation genes via estrogen receptor α. Journal of Thrombosis and Haemostasis 8:1838–1846. [DOI] [PubMed] [Google Scholar]
- 43. Guay J, Haig M, Lortie L, Guertin M-C, Poitras B (1994) Predicting blood loss in surgery for idiopathic scoliosis. Canadian journal of anaesthesia 41:775–781. [DOI] [PubMed] [Google Scholar]
- 44. Nuttall GA, Horlocker TT, Santrach PJ, Oliver Jr WC, Dekutoski MB, et al. (2000) Predictors of blood transfusions in spinal instrumentation and fusion surgery. Spine 25:596–601. [DOI] [PubMed] [Google Scholar]
- 45. Jain A, Njoku DB, Sponseller PD (2012) Does patient diagnosis predict blood loss during posterior spinal fusion in children? Spine 37:1683–1687. [DOI] [PubMed] [Google Scholar]
- 46. Paraskevaidis E, Davidson EJ, Koliopoulos G, Alamanos Y, Lolis E, et al. (2002) Bleeding after loop electrosurgical excision procedure performed in either the follicular or luteal phase of the menstrual cycle: a randomized trial. Obstetrics & Gynecology 99:997–1000. [DOI] [PubMed] [Google Scholar]
- 47. He S, Silveira A, Hamsten A, Blombäck M, Bremme K (1999) Haemostatic, endothelial and lipoprotein parameters and blood pressure levels in women with a history of preeclampsia. Thrombosis And Haemostasis-Stuttgart- 81:538–542. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. The data are included in the paper.


