Abstract
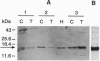
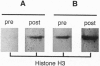
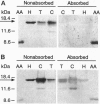
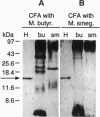
A 15-kDa protein detected initially in amyloidotic ileum from a transgenic mouse and subsequently in control (nontransgenic) ileum by various polyclonal rabbit antiserums applied to electroblots of extracts derived from these tissues was identified by partial sequence analysis as histone H3. Antiserums were made against immunogens unrelated to the histone, but they recognized calf thymus histone H3 (14.7 kDa) on Western blots. The bacterial component of the Freund's medium used as an adjuvant for the immunogens was either Mycobacterium butyricum or Mycobacterium smegmatis. Absorption tests with histone H3 and sonicated M. butyricum substantiated the presence of anti-histone H3 activity in the antiserums. These findings indicate that the two mycobacterium species make a protein with epitopes perceived as nonself by recipient rabbits but sufficiently similar to epitopes of mammalian histone H3 that the rabbits produced antibodies cross-reactive with the histone.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlan H., Gersten M. J., Salk P. L., Salk J. Mechanisms of autoimmunity and AIDS: prospects for therapeutic intervention. Res Immunol. 1994 Mar-Apr;145(3):165–183. doi: 10.1016/S0923-2494(94)80181-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Charbonneau H., Kumar S., Novack J. P., Blumenthal D. K., Griffin P. R., Shabanowitz J., Hunt D. F., Beavo J. A., Walsh K. A. Evidence for domain organization within the 61-kDa calmodulin-dependent cyclic nucleotide phosphodiesterase from bovine brain. Biochemistry. 1991 Aug 13;30(32):7931–7940. doi: 10.1021/bi00246a009. [DOI] [PubMed] [Google Scholar]
- Drlica K., Rouviere-Yaniv J. Histonelike proteins of bacteria. Microbiol Rev. 1987 Sep;51(3):301–319. doi: 10.1128/mr.51.3.301-319.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ericsson L. H., Eriksen N., Walsh K. A., Benditt E. P. Primary structure of duck amyloid protein A. The form deposited in tissues may be identical to its serum precursor. FEBS Lett. 1987 Jun 22;218(1):11–16. doi: 10.1016/0014-5793(87)81008-6. [DOI] [PubMed] [Google Scholar]
- Eriksen N., Benditt E. P. Isolation and characterization of the amyloid-related apoprotein (SAA) from human high density lipoprotein. Proc Natl Acad Sci U S A. 1980 Nov;77(11):6860–6864. doi: 10.1073/pnas.77.11.6860. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eto K., Suzuki S., Singh V. K., Shinohara T. Immunization with recombinant Escherichia coli expressing retinal S-antigen-induced experimental autoimmune uveitis (EAU) in Lewis rats. Cell Immunol. 1993 Mar;147(1):203–214. doi: 10.1006/cimm.1993.1060. [DOI] [PubMed] [Google Scholar]
- Fukuchi K., Ogburn C. E., Smith A. C., Kunkel D. D., Furlong C. E., Deeb S. S., Nochlin D., Sumi S. M., Martin G. M. Transgenic animal models for Alzheimer's disease. Ann N Y Acad Sci. 1993 Sep 24;695:217–223. doi: 10.1111/j.1749-6632.1993.tb23055.x. [DOI] [PubMed] [Google Scholar]
- Hermodson M. A., Kuhn R. W., Walsh K. A., Neurath H., Eriksen N., Benditt E. P. Amino acid sequence of monkey amyloid protein A. Biochemistry. 1972 Aug 1;11(16):2934–2938. doi: 10.1021/bi00766a002. [DOI] [PubMed] [Google Scholar]
- Hoffman J. S., Benditt E. P. Changes in high density lipoprotein content following endotoxin administration in the mouse. Formation of serum amyloid protein-rich subfractions. J Biol Chem. 1982 Sep 10;257(17):10510–10517. [PubMed] [Google Scholar]
- Isenberg I. Histones. Annu Rev Biochem. 1979;48:159–191. doi: 10.1146/annurev.bi.48.070179.001111. [DOI] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Nickerson C., Luthra H., David C. Antigenic mimicry and autoimmune diseases. Int Rev Immunol. 1991;7(3):205–224. doi: 10.3109/08830189109061775. [DOI] [PubMed] [Google Scholar]
- Oldstone M. B. Molecular mimicry and autoimmune disease. Cell. 1987 Sep 11;50(6):819–820. doi: 10.1016/0092-8674(87)90507-1. [DOI] [PubMed] [Google Scholar]
- Pras M., Schubert M., Zucker-Franklin D., Rimon A., Franklin E. C. The characterization of soluble amyloid prepared in water. J Clin Invest. 1968 Apr;47(4):924–933. doi: 10.1172/JCI105784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenfeld J., Capdevielle J., Guillemot J. C., Ferrara P. In-gel digestion of proteins for internal sequence analysis after one- or two-dimensional gel electrophoresis. Anal Biochem. 1992 May 15;203(1):173–179. doi: 10.1016/0003-2697(92)90061-b. [DOI] [PubMed] [Google Scholar]
- Shinohara T., Singh V. K., Tsuda M., Yamaki K., Abe T., Suzuki S. S-antigen: from gene to autoimmune uveitis. Exp Eye Res. 1990 Jun;50(6):751–757. doi: 10.1016/0014-4835(90)90125-e. [DOI] [PubMed] [Google Scholar]
- Sittman D. B., Chiu I. M., Pan C. J., Cohn R. H., Kedes L. H., Marzluff W. F. Isolation of two clusters of mouse histone genes. Proc Natl Acad Sci U S A. 1981 Jul;78(7):4078–4082. doi: 10.1073/pnas.78.7.4078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Snell G. D. The H-2 locus of the mouse: observations and speculations concerning its comparative genetics and its polymorphism. Folia Biol (Praha) 1968;14(5):335–358. [PubMed] [Google Scholar]