Abstract
Background
The DNA methylating agent temozolomide was developed primarily for treatment of glioblastoma. However, preclinical data have suggested a broader application for treatment of childhood cancer. Temozolomide was tested against the PPTP solid tumor and ALL models.
Procedures
Temozolomide was tested against the PPTP in vitro panel at concentrations ranging from 0.1 to 1,000 μM and was tested against the PPTP in vivo panels at doses from 22 to 100 mg/kg administered orally daily for 5 days, repeated at day 21.
Results
In vitro temozolomide showed cytotoxicity with a median relative IC50 (rIC50) value of 380 μM against the PPTP cell lines (range 1 to > 1,000 μM). The three lines with rIC50 values lesser than 10 μM had low MGMT expression compared to the remaining cell lines. In vivo temozolomide demonstrated significant toxicity at 100 mg/kg, but induced tumor regressions in 15 of 23 evaluable solid tumor models (13 maintained CR [MCR], 2 CR) and 5 of 8 ALL models (3 MCR, 2 CR). There was a steep dose response curve, with lower activity at 66 mg/kg temozolomide and with tumor regressions at 22 and 44 mg/kg restricted to models with low MGMT expression.
Conclusions
Temozolomide demonstrated high level antitumor activity against both solid tumor and leukemia models, but also elicited significant toxicity at the highest dose level. Lowering the dose of TMZ to more closely match clinical exposures markedly reduced the antitumor activity for many xenograft lines with responsiveness at lower doses closely related to low MGMT expression.
Keywords: developmental therapeutics, preclinical testing, temodar
INTRODUCTION
Temozolomide (TMZ) is an oral DNA methylating agent with proven antitumor activity in both preclinical and clinical studies. Originally synthesized in 1987 as a treatment for melanoma, TMZ is an imidazotetrazine derivative of the alkylating agent dacarbazine [1–3]. Unlike other chemotherapeutic agents in this class that are metabolized in the liver, TMZ undergoes spontaneous breakdown to the short-lived monomethyl triazeno imidazole carboximide (MITC) at all sites. TMZ readily crosses the blood–brain barrier (BBB), and it is currently approved for the treatment of newly diagnosed glioblastoma multiforme (GBM) and is also approved for the treatment of refractory anaplastic astrocytoma.
The main cytotoxic action of TMZ is through the methylation of DNA at the O6 position of guanine. The active metabolite of TMZ is MTIC that decomposes to the reactive methyldiazonium cation that methylates DNA at the O6 position of guanine as well as at the N7 and N3 positions of guanine and adenine, respectively [4]. The cellular toxicity of temozolomide-induced DNA damage at clinically relevant concentrations is not immediate and requires at least two cycles of DNA replication. The cytotoxic effect of temozolomide results from the inability of O6-methyl guanine (O6-meG) to correctly pair with cytosine during DNA replication, resulting in a single nucleotide mismatch. Futile cycles of mismatch repair with removal and reinsertion of thymine opposite O6-meG eventually result in an apoptosis-inducing double strand DNA break at the next cycle of DNA replication [5–7]. Documented resistance mechanisms to TMZ include expression of O6-methylguanine-DNA methyltransferase (MGMT) [8] and loss of DNA mismatch repair activity [5,9].
Preclinical studies have demonstrated the broad activity of TMZ against cancer xenografts [lung cancer [10], neoplastic meningitis [11], adenocarcinoma [12], pediatric solid tumors [13], and melanoma [14,15]]. In vivo studies have documented that the efficacy of TMZ is dependent on the schedule of delivery, with an improved response when the drug is given at equal doses for a period of five consecutive days [14,16]. The antitumor activity of TMZ has been demonstrated in orthotopic intracranial tumors, further demonstrating its ability to cross the BBB [17]. In addition, preclinical studies have also demonstrated that TMZ works synergistically with O6-benzylguanine [14] and can improve efficacy of other chemotherapeutics [18].
Clinical trials have investigated the efficacy of TMZ treatment against many types of cancer such as lymphomas [19,20]—including T-cell lymphomas [21] and low-grade non-Hodgkin’s lymphoma [22]—leukemia [23–25], pancreatic cancer [26], renal cell carcinoma [27], nasopharyngeal carcinoma [28], sarcoma [29], breast cancer [30,31], melanoma [32–34], brain metastases in metastatic malignant melanoma [35], and brain tumors [36]. TMZ was granted FDA approval in the treatment of recurrent anaplastic astrocytoma in 1999, with subsequent approval for the first-line therapy of glioblastoma multiforme [37].
TMZ was selected for systematic testing by the NCI-supported Pediatric Preclinical Testing Program’s (PPTP) because of its substantial preclinical and clinical activity against a wide range of cancer types. Its evaluation also provides an opportunity to compare clinical activity for temozolomide to activity observed against preclinical models. This report describes the testing of TMZ against the PPTP’s in vitro panel and also describes testing against its in vivo panel at a range of dose levels.
MATERIALS AND METHODS
In vitro testing
Testing was performed using DIMSCAN, a semiautomatic fluorescence-based digital image microscopy system that quantifies viable (using fluorescein diacetate [FDA]) cell numbers in tissue culture multiwell plates [38]. Cells were incubated in the presence of drug for 96 hours at concentrations from 0.1 to 1,000 μM with replicates of 6–12 for each concentration evaluated. Mean fluorescence values were determined for each concentration tested and then normalized to the mean control fluorescence for the line to determine relative mean fluorescence values. For analysis of in vitro testing results, a non-linear regression, sigmoidal dose-response model was fitted using GraphPad Prism 5.03 to the relative mean fluorescence values vs. the log-transformed concentration (X) for the in vitro PPTP study data:
The terms are defined as follows: rIC50 (relative IC50) is the concentration of agent that gives a response half way between Bottom and Top; HillSlope describes the steepness of the dose-response curve; and Top and Bottom are the plateaus in the T/C% values at low and high concentrations, respectively. The F-test was used to determine whether there is statistical evidence for a plateau at higher concentrations. The null hypothesis is that the simpler model (bottom T/C% = 0) is correct and the alternative is that not constraining the bottom T/C% value is correct. If the F-test P-value is <0.05, then the conclusion is that the more complicated model with the bottom unconstrained fits significantly better and that the agent therefore has a non-zero plateau effect at higher concentrations. Absolute IC50 values represent the concentration at which the agent reduces cell survival to 50% of the control value [39]. To compare activity between cell lines, the ratio of the median rIC50 to individual cell line’s rIC50 value is used (larger values connote greater sensitivity).
In vivo tumor growth inhibition studies
CB17SC scid−/− female mice (Taconic Farms, Germantown NY), were used to propagate subcutaneously implanted kidney/rhabdoid tumors, sarcomas (Ewing, osteosarcoma, rhabdomyosarcoma), neuroblastoma, and non-glioblastoma brain tumors, while BALB/c nu/nu mice were used for glioma models, as previously described [40]. Human leukemia cells were propagated by intravenous inoculation in female non-obese diabetic (NOD)/scid−/− mice as described previously [41]. Female mice were used irrespective of the patient gender from which the original tumor was derived. All mice were maintained under barrier conditions and experiments were conducted using protocols and conditions approved by the institutional animal care and use committee of the appropriate consortium member. Ten mice (solid tumors) or eight mice (leukemias) were used in each control or treatment group. Tumor volumes (cm3) [solid tumor xenografts] or percentages of human CD45-positive [hCD45] cells [ALL xenografts] were determined as previously described [42] and responses were determined using three activity measures as previously described [42]. An in-depth description of the analysis methods is included in the Supplemental Response Definitions section.
Analysis for MGMT Expression
Western blot analysis was as described previously [13]. Antibodies against MGMT and GAPDH (loading control) were from Cell Signaling (Danvers, MA). Immunoreactive bands were visualized by using Western Lightning Plus ECL (Perkin Elmer, Inc., Waltham, MA) and HyBlot CL film (Denville Scientific, Inc., Metuchen, NJ). Image Quant (GE Healthcare Life Sciences, Piscataway, NJ) was used to integrate immunoreactive bands for quantitation of MGMT:GAPDH.
Statistical Methods
The exact log-rank test, as implemented using Proc StatXact for SAS®, was used to compare event-free survival distributions between treatment and control groups. P-values were two-sided and were not adjusted for multiple comparisons given the exploratory nature of the studies. The Mann–Whitney test was used to test the difference of medians of rIC50 values between the groups of lines with similar tumor types to the remaining lines of the panel and to compare differences in MGMT expression levels between different histology- or response-defined groups.
Drugs and Formulation
TMZ was provided to the PPTP by the Drug Repository at NIH, through the Cancer Therapy Evaluation Program (NCI). TMZ was dissolved in sterile water, and was administered at 100 mg/kg P.O. daily for 5 days with the cycle repeated at day 21. For repeat testing TMZ was administered at 66, 44, or 22 mg/kg on the same schedule. Drug was provided to each consortium investigator in coded vials for blinded testing.
RESULTS
Temozolomide In Vitro Testing
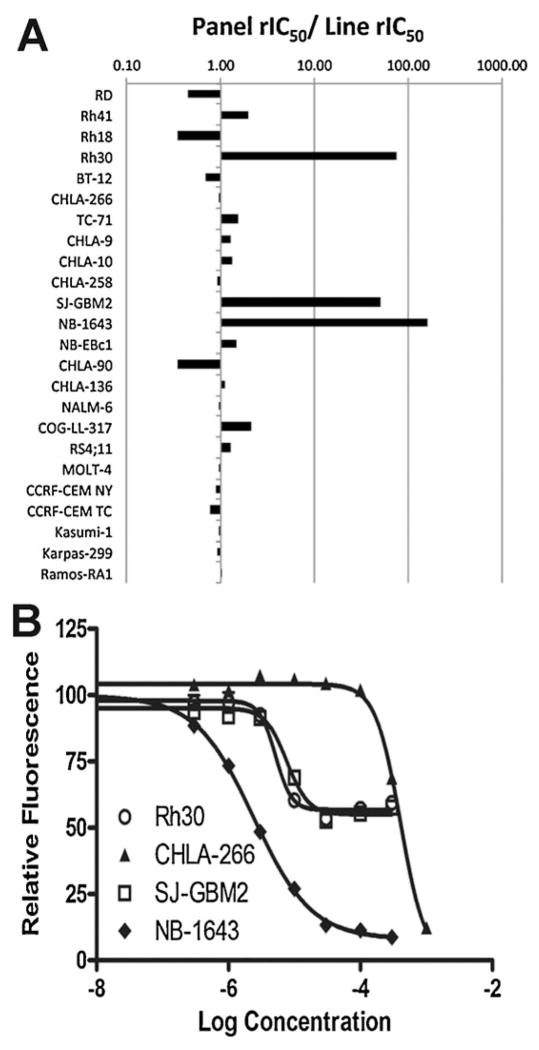
TMZ was evaluated against the 23 cell lines in the PPTP in vitro panel using 96-hour exposures to concentrations ranging from 0.1–1,000 μM. The median rIC50 for the in vitro panel was 380 μM (Table I). There was an approximately 150-fold range in rIC50 values, with the most sensitive cell lines being the rhabdomyosarcoma cell line Rh30, the neuroblastoma cell line NB-1643, and the glioblastoma cell line GBM2, which all had rIC50 values < 10 μM (Fig. 1A). The least sensitive cell line (Rh18) had a rIC50 of >1,000 μM. Concentration response curves for the three sensitive cell lines and a typical non-sensitive cell line (CHLA-266, rIC50 of 405 μM) are shown in Figure 1B. Each of the sensitive cell lines shows a non-zero plateau at concentrations up to 300 μM, consistent with prior results showing that the primary response to temozolomide in the first 48–72 hours of exposure for MGMT negative lines is G2-M cell cycle arrest with apoptosis initiating between 72 and 120 hours [43].
TABLE I.
In Vitro Sensitivity of Cell Lines to Temozolomide
| Cell line | Histotype | rIC50 (μM) | Panel rIC50/line rIC50 | MGMT relative expression |
|---|---|---|---|---|
| RD | Rhabdomyosarcoma | 858 | 0.44 | 1.15 |
| Rh41 | Rhabdomyosarcoma | 191 | 1.99 | 0.79 |
| Rh18 | Rhabdomyosarcoma | >1,000 | 0.35 | 1.04 |
| Rh30 | Rhabdomyosarcoma | 5 | 74.91 | 0.37 |
| BT-12 | Rhabdoid | 558 | 0.68 | 1.00 |
| CHLA-266 | Rhabdoid | 405 | 0.94 | 1.22 |
| TC-71 | Ewing sarcoma | 245 | 1.55 | 0.82 |
| CHLA-9 | Ewing sarcoma | 294 | 1.30 | 1.19 |
| CHLA-10 | Ewing sarcoma | 290 | 1.31 | 0.67 |
| CHLA-258 | Ewing sarcoma | 406 | 0.94 | 1.76 |
| SJ-GBM2 | Glioblastoma | 8 | 49.71 | 0.34 |
| NB-1643 | Neuroblastoma | 2 | 157.7 | 0.44 |
| NB-EBc1 | Neuroblastoma | 256 | 1.49 | 0.80 |
| CHLA-90 | Neuroblastoma | 1,098 | 0.35 | 1.06 |
| CHLA-136 | Neuroblastoma | 345 | 1.10 | 0.92 |
| NALM-6 | ALL | 392 | 0.97 | 1.37 |
| COG-LL-317 | ALL | 181 | 2.11 | 1.39 |
| RS4;11 | ALL | 300 | 1.27 | 1.92 |
| MOLT-4 | ALL | 399 | 0.95 | 1.12 |
| CCRF-CEM (1) | ALL | 424 | 0.90 | 1.10 |
| CCRF-CEM (2) | ALL | 502 | 0.76 | 1.10 |
| Kasumi-1 | AML | 389 | 0.98 | 1.86 |
| Karpas-299 | ALCL | 407 | 0.93 | 0.79 |
| Ramos-RA1 | NHL | 372 | 1.02 | 1.37 |
| Median | 380 | 1.00 | 1.08 | |
| Minimum | 2 | 0.35 | 0.34 | |
| Maximum | >1,000 | 150.20 | 1.92 |
Fig. 1.
A: Temozolomide in vitro activity. The figure shows the ratio of the median rIC50 of the entire panel to that of each cell line. Higher ratios are indicative of greater sensitivity to temozolomide and are shown in the figure by bars to the right of the midpoint line (ratio = 1.0). B: Concentration-response curves for sensitive cell lines and a typical non-sensitive cell line.
Temozolomide In Vivo Testing
TMZ was initially evaluated at 100 mg/kg daily ×5 q 21 days in 41 xenograft models. Eighty-five of 77 mice died during the study (10.9%), with 4 of 388 in the control arms (1.0%) and 81 of 389 in the TMZ treatment arms (20.8%). Toxicity was greatest in the panels of soft tissue sarcoma and kidney tumors, and toxicity was distributed evenly between cycle 1 and cycle 2 of treatment. Ten solid tumor xenografts were inevaluable because of toxicity while no toxicity was observed in the ALL models. A complete summary of results is provided in Supplemental Table I, including total numbers of mice, number of mice that died (or were otherwise excluded), numbers of mice with events and average times to event, tumor growth delay, as well as numbers of responses and T/C values.
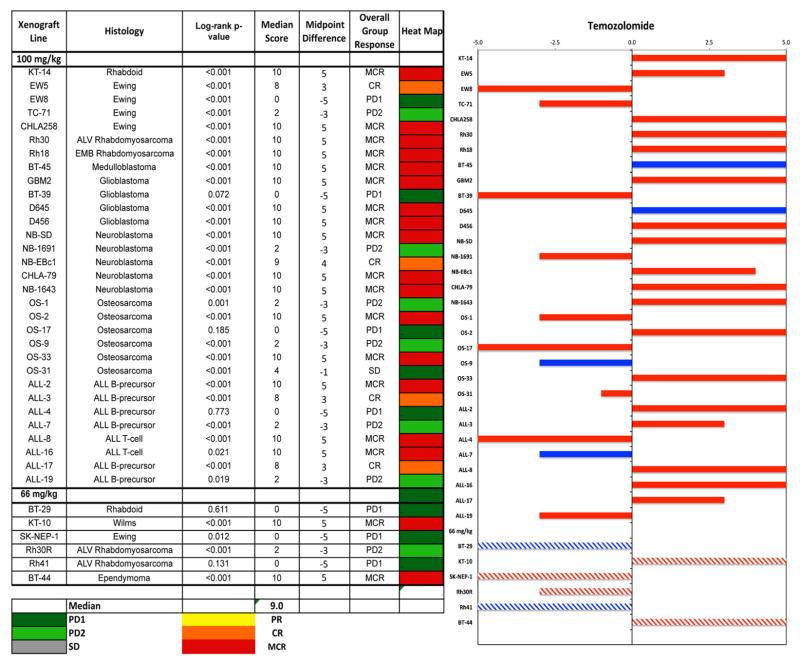
Antitumor effects were evaluated using the PPTP activity measures for time to event (EFS T/C), tumor growth delay (tumor volume T/C), and objective response. TMZ induced significant growth delay in 21 of 23 solid tumor lines (91.3%) at the 100 mg/kg dose, and demonstrated high or intermediate activity in most tumors, Table II. The in vivo testing results for the objective response measure of activity are presented in Figure 2 in a “heat-map” format as well as a “COMPARE”-like format, based on the scoring criteria described in the Material and Methods and the Supplemental Response Definitions Section. The latter analysis demonstrates relative tumor sensitivities around the midpoint score of five (stable disease). Objective responses were seen in 15 of 23 solid tumor models (maintained complete regressions (MCR) in 13 lines and CR in 2 lines) with examples of typical solid tumor response shown in Supplemental Figure 1. Complete responses were seen in five of eight ALL models with three models maintaining their response throughout the observation period.
TABLE II.
In Vivo Sensitivity of PPTP Xenografts to Temozolomide (100 and 66 mg/kg)
| Line description | Tumor type | Median time to event | P-value | EFS T/C | Median RTV at end of study | Tumor volume T/Ca | Median group response | EFS activityb | Relative MGMT expression |
|---|---|---|---|---|---|---|---|---|---|
| 100 mg/kg | |||||||||
| KT-14 | Rhabdoid | >EP | <0.001 | >4.2 | 0.0 | 0.12 | MCR | High | 0.99 |
| EW5 | Ewing | >EP | <0.001 | >3.9 | 2.3 | 0.04 | CR | Int | 1.21 |
| EW8 | Ewing | 12.7 | <0.001 | 1.5 | >4 | 0.37 | PD1 | Low | 1.33 |
| TC-71 | Ewing | 25.6 | <0.001 | 2.5 | >4 | 0.30 | PD2 | Int | 1.02 |
| CHLA258 | Ewing | >EP | <0.001 | >3.8 | 0.0 | 0.01 | MCR | High | 1.64 |
| Rh30 | ALV RMS | >EP | <0.001 | >3.3 | 0.0 | 0.00 | MCR | High | 0.99 |
| Rh18 | EMB RMS | >EP | <0.001 | >3.5 | 0.0 | 0.11 | MCR | High | 1.12 |
| BT-45 | Medulloblastoma | >EP | <0.001 | >2.7 | 0.0 | 0.24 | MCR | High | 1.20 |
| GBM2 | Glioblastoma | >EP | <0.001 | >3.4 | 0.0 | 0.11 | MCR | High | 0.37 |
| BT-39 | Glioblastoma | 11.0 | 0.072 | 1.1 | >4 | 0.83 | PD1 | Low | 0.77 |
| D645 | Glioblastoma | >EP | <0.001 | >4.7 | 0.0 | 0.21 | MCR | High | 0.37 |
| D456 | Glioblastoma | >EP | <0.001 | >5.8 | 0.0 | 0.12 | MCR | High | 0.37 |
| NB-SD | Neuroblastoma | >EP | <0.001 | >3.5 | 0.0 | 0.06 | MCR | High | 0.74 |
| NB-1691 | Neuroblastoma | 41.0 | <0.001 | 6.5 | >4 | 0.42 | PD2 | Int | 0.98 |
| NB-EBc1 | Neuroblastoma | >EP | <0.001 | >2.4 | 0.4 | 0.52 | CR | High | 1.07 |
| CHLA-79 | Neuroblastoma | >EP | <0.001 | >4.2 | 0.2 | 0.08 | MCR | High | 0.38 |
| NB-1643 | Neuroblastoma | >EP | <0.001 | >5.7 | 0.1 | 0.11 | MCR | High | 0.45 |
| OS-1 | Osteosarcoma | >EP | 0.001 | >1.4 | 3.4 | 0.65 | PD2 | NE | 0.83 |
| OS-2 | Osteosarcoma | >EP | <0.001 | >2.0 | 0.2 | 0.46 | MCR | High | 0.52 |
| OS-17 | Osteosarcoma | 16.1 | 0.185 | 1.2 | >4 | 0.87 | PD1 | Low | 0.93 |
| OS-9 | Osteosarcoma | 41.4 | <0.001 | 1.6 | >4 | 0.80 | PD2 | Low | 0.84 |
| OS-33 | Osteosarcoma | >EP | <0.001 | >3.8 | 0.1 | 0.33 | MCR | High | 0.35 |
| OS-31 | Osteosarcoma | >EP | <0.001 | >2.5 | 1.0 | 0.61 | SD | Int | 0.68 |
| ALL-2 | ALL B-precursor | >EP | <0.001 | >2.5 | 0.1 | MCR | High | 1.66 | |
| ALL-3 | ALL B-precursor | >EP | <0.001 | >4.4 | 1.7 | CR | Int | 1.64 | |
| ALL-4 | ALL B-precursor | 5.8 | 0.773 | 1.2 | >25 | PD1 | Low | 2.30 | |
| ALL-7 | ALL B-precursor | 12.6 | <0.001 | 1.9 | >25 | PD2 | Low | 2.04 | |
| ALL-8 | ALL T-cell | >EP | <0.001 | >4.6 | 0.3 | MCR | High | 1.29 | |
| ALL-16 | ALL T-cell | >EP | 0.021 | >2.7 | 0.2 | MCR | High | 1.42 | |
| ALL-17 | ALL B-precursor | 38.5 | <0.001 | 4.9 | >25 | CR | Int | 1.69 | |
| ALL-19 | ALL B-precursor | 9.6 | 0.019 | 3.2 | >25 | PD2 | Int | 3.27 | |
| 66 mg/kg | |||||||||
| BT-29 | Rhabdoid | 14.0 | 0.611 | 1.0 | >4 | 0.83 | PD1 | Low | 0.98 |
| KT-10 | Wilms | >EP | <0.001 | >3.9 | 0.0 | 0.01 | MCR | High | 1.74 |
| SK-NEP-1 | Ewing | 18.3 | 0.012 | 1.4 | >4 | 0.70 | PD1 | Low | 1.08 |
| Rh30R | ALV RMS | 23.9 | <0.001 | 2.1 | >4 | 0.32 | PD2 | Int | 0.86 |
| Rh41 | ALV RMS | 12.5 | 0.131 | 1.4 | >4 | 0.72 | PD1 | Low | 0.92 |
| BT-44 | Ependymoma | >EP | <0.001 | >1.7 | 0.0 | 0.44 | MCR | NE | 1.01 |
NE, not evaluable.
Tumor volume T/C value: Relative tumor volumes (RTV) for control (C) and treatment (T) mice were calculated at day 21 or when all mice in the control and treated groups still had measurable tumor volumes (if less than 21 days). The T/C value is the mean RTV for the treatment group divided by the mean RTV for the control group. High activity = T/C ≤ 0.15; Intermediate activity = T/C ≤ 0.45 but >0.15; and Low activity = T/C > 0.45.
Objective response measures are described in detail in the Supplemental Response Definitions. PD1 = Progressive disease with EFS T/C ≤ 1.5, and PD2 = Progressive disease with EFS T/C >1.5.
Fig. 2.
Temozolomide in vivo objective response activity. Left: The colored heat map depicts group response scores. A high level of activity is indicated by a score of six or more, intermediate activity by a score of ≥2 but <6, and low activity by a score of <2. Right: Representation of tumor sensitivity based on the difference of individual tumor lines from the midpoint response (stable disease). Bars to the right of the median represent lines that are more sensitive, and to the left are tumor models that are less sensitive. Red bars indicate lines with a significant difference in EFS distribution between treatment and control groups, while blue bars indicate lines for which the EFS distributions were not significantly different. For results from re-testing at 66 mg/kg (hatched bars) significant and not significant are color coded as pink and light blue, respectively.
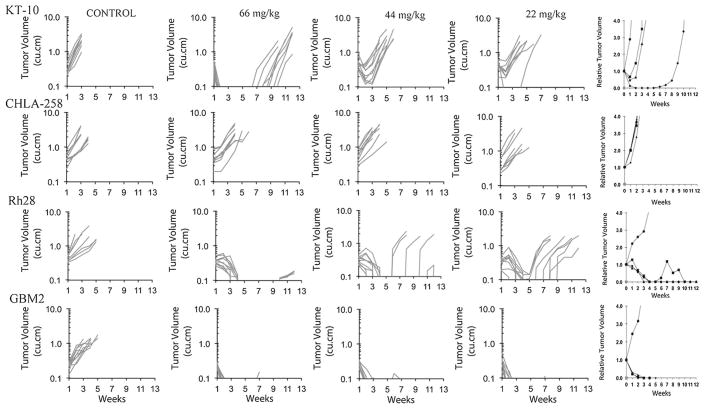
Because of the excessive toxicity in some tumor panels when TMZ was administered at 100 mg/kg, TMZ was re-evaluated against eight tumor lines for which there was excessive toxicity, at a dose of 66 mg/kg on the same schedule of administration. Among these eight models, the toxicity rate for treated animals was reduced to 8.0%, with two lines excluded because of excessive toxicity. TMZ induced significant growth delay in four of six evaluable models, and induced MCR in two lines (Table II and Supplemental Table II). Tumors in surviving mice from the excluded groups were also MCR (Supplemental Table II). However, these data suggested a steep dose-response relationship, as several tumors (BT-29, SKNEP1, Rh30R, Rh41) exhibiting MCR at 100 mg/kg exhibited progressive disease at the 66 mg/kg dose level. To further define the dose response relationship, we examined responses of five models to TMZ at 66, 44, and 22 mg/kg using the same schedule of administration (Table III and Supplemental Table III). The Wilms tumor, KT-10 demonstrated CR, SD, and PD2 responses at 66, 44, and 22 mg/kg, respectively. For the ALL-3 model, reduction in temozolomide dose from 100–66 mg/kg reduced the response from CR to PD1. Similarly CHLA-258 did not respond at any dose level below 100 mg/kg. The MGMT-deficient glioblastoma (GBM2) and rhabdomyosarcoma (Rh28) lines were highly responsive with MCR and CR, respectively, at the lowest dose tested. Dose-response tumor growth curves and relative tumor volume curves are shown in Figure 3.
TABLE III.
In Vivo Dose Response of PPTP Xenografts to Temozolomide
| Line description | Tumor type | Dose (mg/kg) | Median time to event | P-value | EFS T/C | Median RTV at end of study | Tumor volume T/Ca | Median group response | EFS activityb |
|---|---|---|---|---|---|---|---|---|---|
| KT-10 | Wilms | 66 | 70.9 | <0.001 | 7.6 | >4 | 0.00 | CR | Int |
| 44 | 25.5 | <0.001 | 2.7 | >4 | 0.13 | SD | Int | ||
| 22 | 23.1 | <0.001 | 2.5 | >4 | 0.22 | PD2 | Int | ||
| CHLA258 | Ewing | 66 | 18.1 | 0.156 | 1.2 | >4 | 0.72 | PD1 | Low |
| 44 | 14.6 | 0.871 | 0.9 | >4 | 1.03 | PD1 | Low | ||
| 22 | 15.6 | 0.551 | 1.0 | >4 | 1.00 | PD1 | Low | ||
| Rh28 | ALV RMS | 66 | >EP | <0.001 | >6.8 | 0.0 | 0.36 | MCR | High |
| 44 | >EP | <0.001 | >6.8 | 1.2 | 0.36 | CR | Int | ||
| 22 | 69.9 | <0.001 | 5.6 | >4 | 0.50 | CR | Int | ||
| GBM2 | Glioblastoma | 66 | >EP | 0.008 | >2.4 | 0.2 | 0.12 | MCR | High |
| 44 | >EP | 0.034 | >2.4 | 0.3 | 0.14 | MCR | High | ||
| 22 | >EP | 0.046 | >2.4 | 0.1 | 0.11 | MCR | High |
Tumor volume T/C value: Relative tumor volumes (RTV) for control (C) and treatment (T) mice were calculated at day 21 or when all mice in the control and treated groups still had measurable tumor volumes (if less than 21 days). The T/C value is the mean RTV for the treatment group divided by the mean RTV for the control group. High activity = T/C ≤ 0.15; Intermediate activity = T/C ≤ 0.45 but >0.15; and Low activity = T/C > 45.
Objective response measures are described in detail in the Supplemental Response Definitions. PD1 = Progressive disease with EFS T/C ≤ 1.5, and PD2 = Progressive disease with EFS T/C > 1.5.
Fig. 3.
Temozolomide dose-response activity against solid tumor xenografts. Individual tumor volume graphs are shown for KT-10, CHLA-258, Rh28, and GBM2 for control (untreated) and treated tumor groups. Relative tumor volume graphs are shown in the right panels: (➂) Control; (➄) 22 mg/kg; (➉) 44 mg/kg; (➀) 66 mg/kg.
MGMT and Sensitivity to Temozolomide
MGMT expression at the RNA level was strongly correlated with expression at the protein level (Supplemental Figure 2A). The low values for MGMT expression at the RNA level correspond to undetectable MGMT expression at the protein level. For the in vitro panel, MGMT expression at the RNA level was significantly lower (P = .007) for the three cell lines with rIC50 values <10 μM compared to the remaining PPTP cell lines (Supplemental Figure 2B). For the in vivo panel, MGMT RNA expression levels were significantly higher in the ALL xenografts compared to the solid tumors (P < 0.001). For the solid tumor panel MGMT expression was comparable for xenografts achieving CR/MCR to 100 mg/kg temozolomide compared to those with PD1/PD2 responses to the same dose, Supplemental Figure 3. However, when testing was done at reduced doses among four xenografts with MCRs to 100 mg/kg, sensitivity to temozolomide as assessed by objective response was maintained down to 22 mg/kg in two xenografts with low MGMT expression (Rh28 and GBM2), but was lost at 44 mg/kg in two xenografts with high MGMT expression (KT-10 and CHLA258). For the ALL xenografts the efficacy at the 100 mg/kg dose stratified according to MGMT expression, with models attaining CR/MCR having lower MGMT expression than lines with PD1/PD2 response (P = 0.036). However, the MGMT-expressing ALL-3 xenograft lost responsiveness to temozolomide at the more clinically relevant doses below 100 mg/kg.
DISCUSSION
TMZ has been shown to be efficacious in preclinical models of various tumor xenografts, including malignant glioma [44]. The drug is particularly useful in adult patients with brain tumors due to its excellent penetration into the CNS and almost 100% bioavailability and linear pharmacokinetics after oral administration [45], and it is now a component of standard frontline therapy for adults with high grade glioma [37]. Phase I studies in adults and children have shown that the drug has an excellent safety profile with myelosuppression being the dose-limiting toxicity [46–48], however there are reports of therapy-related leukemia after temozolomide treatment (recently reviewed by Ogura et al. [49]).
TMZ showed limited activity in a phase 2 study against recurrent pediatric brain tumors, including objective responses for high-grade glioma (1 of 23), low grade glioma (1 of 21), and medulloblastoma (3 of 25) [50]. Another phase 2 study in children with recurrent high grade gliomas produced an objective response rate of 20% (4 of 20) [51]. However, temozolomide failed to improve outcome in children with high-grade astrocytomas when given in combination with radiation therapy and then used as maintenance therapy [52]. The same TMZ regimen was no more effective than previously reported regimens for the treatment of children with diffuse intrinsic pontine gliomas [53]. Temozolomide induced occasional objective responses in a phase 2 study that included children with non-CNS solid tumors [54], and it induced a 20% objective response rate (5 of 25) in a phase 2 study for children with recurrent neuroblastoma [55]. The current focus of pediatric clinical research for TMZ is its use in combination with other cytotoxic agents with an emphasis on topoisomerase I inhibitors based on preclinical data for the irinotecan plus TMZ combination [56]. Phase 1 and 2 studies of the irinotecan plus TMZ combination have shown objective responses for both neuroblastoma [57–59] and for Ewing sarcoma [60,61].
A key question in interpreting TMZ preclinical results is the relative systemic exposures in mice at the tested TMZ doses compared to the exposures achievable in humans. Stevens et al. [16] administered TMZ orally to mice at a dose of 20 mg/kg, and the TMZ area-under-the-curve (as interpolated by Middlemas et al. [13]) was approximately 38 μg/ml hour. Murine systemic exposure for TMZ and MTIC at a TMZ dose of 66 mg/kg administered orally and given with irinotecan were 47 and 1.3 μg/ml hour, respectively [56]. These results were similar to previous results for TMZ administered to mice as a single agent at 66 mg/kg 40 and 1.9 μg/ml hour for TMZ and MTIC, respectively [13]. In children with leukemia receiving TMZ at 200 mg/m2, the TMZ systemic exposure was 30 μg/ml hour [25]. Phase 1 studies in children with solid tumors support a TMZ systemic exposure of approximately 40 μg/ml hour at the 200 mg/m2 dose [47,62,63]. In adults, a systemic exposure of ~30 μg/ml hour was observed in a phase 1 study at the 200 mg/m2 dose [64], while a second study observed systemic exposures in the 30–35 μg/ml hour range at this dose [65], with similar results observed for TMZ given in combination with cisplatin [66]. The totality of these results are consistent with a clinically relevant dose of TMZ in mice being lower than 66 mg/kg.
TMZ produced CRs or MCRs across 15 of 23 evaluable solid tumor xenografts in the PPTP in vivo testing panel at the 100 mg/kg dose (Table II). Nonresponders represented four of six osteosarcoma lines and two of four Ewing sarcoma lines. Due to toxicity issues at the 100 mg/kg dose level, 8 xenograft lines that were highly responsive at 100 mg/kg (MCR) despite toxicity were again tested at 66 mg/kg. Of the six evaluable studies, two (33%) produced the same median group response (MCR) while the remaining four xenografts had progressive disease (PD) when treated at the lower dose, indicating a steep dose response relationship. To further define the relationship between dose and tumor response, four solid tumor models and one ALL model were treated with TMZ at doses between 66 and 22 mg/kg. Only the two xenografts known to be deficient in MGMT demonstrated regressions at the two lower doses tested (22 and 44 mg/kg).
The most sensitive cell lines in vitro (NB-1643, Rh30, and GBM2) all had rIC50 values <10 μM, while the median IC50 value was 380 μM and the least sensitive cell line (Rh18) had a rIC50 > 1,000 μM. The three hypersensitive cell lines had low MGMT expression, supporting MGMT expression status as a primary mechanism defining high level sensitivity to temozolomide. The in vivo results are similar, with high TMZ doses producing MCRs in most models tested, but with responsiveness at lower dose levels tracking with low MGMT expression. One of the alternative mechanisms of resistance to temozolomide, loss of mismatch repair, was not observed within the MGMT low expressing PPTP xenografts. The significantly higher levels MGMT expression in the ALL versus the solid tumor xenografts point to potential differences between regulation of MGMT expression between pediatric ALL and pediatric solid tumors.
In conclusion, TMZ showed high-level activity across the PPTP panel at high doses that produce systemic exposures not achievable in humans. There was a steep dose-response curve, and lowering the dose of TMZ to more closely match clinical exposures markedly reduced the antitumor activity for many xenograft lines with responsiveness closely related to low MGMT expression. These results suggest that targeting patients whose tumors have low MGMT expression may be an effective strategy for optimizing use of temozolomide in the pediatric setting. Additional work is needed to further address the potential for enhancing efficacy by combination with other agents.
Supplementary Material
Acknowledgments
Grant sponsor: National Cancer Institute; Grant numbers: NO1-CM-42216, CA21765, CA108786.
This work was supported by NO1-CM-42216, CA21765, and CA108786 from the National Cancer Institute and used TMZ supplied by the NIH Drug Repository. In addition to the authors this paper represents work contributed by the following: Denise Alexander, Sherry Ansher, Catherine A. Billups, Ingrid Boehm, Joshua Courtright, Mila Dolotin, Kathryn Evans, Edward Favours, Danuta Gasinski, Henry S. Friedman, Debbie Payne-Turner, Doris A. Phelps, Jennifer Richmond, Chandra Tucker, Amy E. Watkins, Joe Zeidner, Ellen Zhang, and Jian Zhang. Children’s Cancer Institute Australia is affiliated with the University of New South Wales and Sydney Children’s Hospitals Network.
Footnotes
Conflict of interest: Nothing to declare.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Villano JL, Seery TE, Bressler LR. Temozolomide in malignant gliomas: Current use and future targets. Cancer Chemother Pharmacol. 2009;64:647–655. doi: 10.1007/s00280-009-1050-5. Epub 2009/06/23. [DOI] [PubMed] [Google Scholar]
- 2.Newlands ES, Blackledge GR, Slack JA, et al. Phase I trial of temozolomide (CCRG 81045: M&B 39831: NSC 362856) Br J Cancer. 1992;65:287–291. doi: 10.1038/bjc.1992.57. Epub 1992/02/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Syro LV, Ortiz LD, Scheithauer BW, et al. Treatment of pituitary neoplasms with temozolomide: A review. Cancer. 2010:454–462. doi: 10.1002/cncr.25413. Epub 2010/09/17. [DOI] [PubMed] [Google Scholar]
- 4.Tisdale MJ. Antitumor imidazotetrazines–XV. Role of guanine O6 alkylation in the mechanism of cytotoxicity of imidazotetrazinones. Biochem Pharmacol. 1987;36:457–462. doi: 10.1016/0006-2952(87)90351-0. Epub 1987/02/15. [DOI] [PubMed] [Google Scholar]
- 5.D’Atri S, Tentori L, Lacal PM, et al. Involvement of the mismatch repair system in temozolomide-induced apoptosis. Mol Pharmacol. 1998;54:334–341. doi: 10.1124/mol.54.2.334. Epub 1998/08/04. [DOI] [PubMed] [Google Scholar]
- 6.Quiros S, Roos WP, Kaina B. Processing of O6-methylguanine into DNA double-strand breaks requires two rounds of replication whereas apoptosis is also induced in subsequent cell cycles. Cell Cycle (Georgetown, Tex) 2010;9:168–178. doi: 10.4161/cc.9.1.10363. Epub 2009/12/18. [DOI] [PubMed] [Google Scholar]
- 7.Roos WP, Batista LF, Naumann SC, et al. Apoptosis in malignant glioma cells triggered by the temozolomide-induced DNA lesion O6-methylguanine. Oncogene. 2007;26:186–197. doi: 10.1038/sj.onc.1209785. Epub 2006/07/05. [DOI] [PubMed] [Google Scholar]
- 8.Gerson SL. MGMT: Its role in cancer aetiology and cancer therapeutics. Nat Rev. 2004;4:296–307. doi: 10.1038/nrc1319. Epub 2004/04/02. [DOI] [PubMed] [Google Scholar]
- 9.Pollack IF, Hamilton RL, Sobol RW, et al. Mismatch repair deficiency is an uncommon mechanism of alkylator resistance in pediatric malignant gliomas: A report from the Children’s Oncology Group. Pediatr Blood Cancer. 2010;55:1066–1071. doi: 10.1002/pbc.22634. Epub 2010/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tentori L, Leonetti C, Aquino A. Temozolomide reduces the metastatic potential of Lewis lung carcinoma (3LL) in mice: Role of alpha-6 integrin phosphorylation. Eur J Cancer. 1995;31A:746–754. doi: 10.1016/0959-8049(94)00521-6. Epub 1995/01/01. [DOI] [PubMed] [Google Scholar]
- 11.Sampson JH, Archer GE, Villavicencio AT, et al. Treatment of neoplastic meningitis with intrathecal temozolomide. Clin Cancer Res. 1999;5:1183–1188. Epub 1999/06/03. [PubMed] [Google Scholar]
- 12.Koc ON, Reese JS, Davis BM, et al. DeltaMGMT-transduced bone marrow infusion increases tolerance to O6-benzylguanine and 1,3-bis(2-chloroethyl)-1-nitrosourea and allows intensive therapy of 1,3-bis(2-chloroethyl)-1-nitrosourea-resistant human colon cancer xenografts. Hum Gene Ther. 1999;10:1021–1030. doi: 10.1089/10430349950018418. Epub 1999/05/01. [DOI] [PubMed] [Google Scholar]
- 13.Middlemas DS, Stewart CF, Kirstein MN, et al. Biochemical correlates of temozolomide sensitivity in pediatric solid tumor xenograft models. Clin Cancer Res. 2000;6:998–1007. Epub 2000/03/31. [PubMed] [Google Scholar]
- 14.Wedge SR, Porteous JK, Newlands ES. Effect of single and multiple administration of an O6-benzyl-guanine/temozolomide combination: An evaluation in a human melanoma xenograft model. Cancer Chemother Pharmacol. 1997;40:266–272. doi: 10.1007/s002800050657. Epub 1997/01/01. [DOI] [PubMed] [Google Scholar]
- 15.Middleton MR, Kelly J, Thatcher N, et al. O(6)-(4-bromothenyl)guanine improves the therapeutic index of temozolomide against A375M melanoma xenografts. Int J Cancer. 2000;85:248–252. doi: 10.1002/(sici)1097-0215(20000115)85:2<248::aid-ijc16>3.0.co;2-v. Epub 2000/01/11. [DOI] [PubMed] [Google Scholar]
- 16.Stevens MF, Hickman JA, Langdon SP, et al. Antitumor activity and pharmacokinetics in mice of 8-carbamoyl-3-methyl-imidazo[5,1-d]-1,2,3,5-tetrazin-4(3H)-one (CCRG 81045; M & B 39831), a novel drug with potential as an alternative to dacarbazine. Cancer Res. 1987;47:5846–5852. Epub 1987/11/15. [PubMed] [Google Scholar]
- 17.Friedman HS, Johnson SP, Dong Q, et al. Methylator resistance mediated by mismatch repair deficiency in a glioblastoma multiforme xenograft. Cancer Res. 1997;57:2933–2936. Epub 1997/07/15. [PubMed] [Google Scholar]
- 18.Plowman J, Waud WR, Koutsoukos AD, et al. Preclinical antitumor activity of temozolomide in mice: efficacy against human brain tumor xenografts and synergism with 1,3-bis(2-chloroethyl)-1-nitrosourea. Cancer Res. 1994;54:3793–3799. Epub 1994/07/15. [PubMed] [Google Scholar]
- 19.Dolan ME, McRae BL, Ferries-Rowe E, et al. O6-alkylguanine-DNA alkyltransferase in cutaneous T-cell lymphoma: implications for treatment with alkylating agents. Clin Cancer Res. 1999;5:2059–2064. Epub 1999/09/03. [PubMed] [Google Scholar]
- 20.Reni M, Ferreri AJ, Landoni C, et al. Salvage therapy with temozolomide in an immunocompetent patient with primary brain lymphoma. J Natl Cancer Inst. 2000;92:575–576. doi: 10.1093/jnci/92.7.575. Epub 2000/04/06. [DOI] [PubMed] [Google Scholar]
- 21.Bunn PA, Jr, Hoffman SJ, Norris D, et al. Systemic therapy of cutaneous T-cell lymphomas (mycosis fungoides and the Sezary syndrome) Ann Intern Med. 1994;121:592–602. doi: 10.7326/0003-4819-121-8-199410150-00007. Epub 1994/10/15. [DOI] [PubMed] [Google Scholar]
- 22.Woll PJ, Crowther D, Johnson PW, et al. Phase II trial of temozolomide in low-grade non-Hodgkin’s lymphoma. Br J Cancer. 1995;72:183–184. doi: 10.1038/bjc.1995.299. Epub 1995/07/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Piccioni D, D’Atri S, Papa G, et al. Cisplatin increases sensitivity of human leukemic blasts to triazene compounds. J Chemother. 1995;7:224–229. doi: 10.1179/joc.1995.7.3.224. Epub 1995/06/01. [DOI] [PubMed] [Google Scholar]
- 24.D’Atri S, Piccioni D, Castellano A, et al. Chemosensitivity to triazene compounds and O6-alkylguanine-DNA alkyltransferase levels: Studies with blasts of leukaemic patients. Ann Oncol. 1995;6:389–393. doi: 10.1093/oxfordjournals.annonc.a059189. Epub 1995/04/01. [DOI] [PubMed] [Google Scholar]
- 25.Horton TM, Thompson PA, Berg SL, et al. Phase I pharmacokinetic and pharmacodynamic study of temozolomide in pediatric patients with refractory or recurrent leukemia: A Children’s Oncology Group Study. J Clin Oncol. 2007;25:4922–4928. doi: 10.1200/JCO.2007.12.0667. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Moore MJ, Feld R, Hedley D, et al. A phase II study of temozolomide in advanced untreated pancreatic cancer. Invest New Drugs. 1998;16:77–79. doi: 10.1023/a:1006043332368. Epub 1998/09/18. [DOI] [PubMed] [Google Scholar]
- 27.Sunkara U, Walczak JR, Summerson L, et al. A phase II trial of temozolomide and IFN-alpha in patients with advanced renal cell carcinoma. J Interferon Cytokine Res. 2004;24:37–41. doi: 10.1089/107999004772719891. Epub 2004/02/26. [DOI] [PubMed] [Google Scholar]
- 28.Chan AT, Leung TW, Kwan WH, et al. Phase II study of Temodal in the treatment of patients with advanced nasopharyngeal carcinoma. Cancer Chemother Pharmacol. 1998;42:247–249. doi: 10.1007/s002800050812. Epub 1998/07/31. [DOI] [PubMed] [Google Scholar]
- 29.Woll PJ, Judson I, Lee SM, et al. Temozolomide in adult patients with advanced soft tissue sarcoma: A phase II study of the EORTC Soft Tissue and Bone Sarcoma Group. Eur J Cancer. 1999;35:410–412. doi: 10.1016/s0959-8049(98)00403-1. Epub 1999/08/17. [DOI] [PubMed] [Google Scholar]
- 30.O’Shaughnessy JA. Oral alkylating agents for breast cancer therapy. Drugs. 1999;58:1–9. doi: 10.2165/00003495-199958003-00001. Epub 2000/03/11. [DOI] [PubMed] [Google Scholar]
- 31.Trudeau ME, Crump M, Charpentier D, et al. Temozolomide in metastatic breast cancer (MBC): A phase II trial of the National Cancer Institute of Canada-Clinical Trials Group (NCIC-CTG) Ann Oncol. 2006;17:952–956. doi: 10.1093/annonc/mdl056. Epub 2006/03/28. [DOI] [PubMed] [Google Scholar]
- 32.Myatt N, Cree IA, Kurbacher CM, et al. The ex vivo chemosensitivity profile of choroidal melanoma. Anticancer Drugs. 1997;8:756–762. doi: 10.1097/00001813-199709000-00004. Epub 1997/12/13. [DOI] [PubMed] [Google Scholar]
- 33.Anderson CM, Buzaid AC, Legha SS. Systemic treatments for advanced cutaneous melanoma. Oncology (Williston Park) 1995;9:1149–1158. discussion 63–64, 67–68; Epub 1995/11/01. [PubMed] [Google Scholar]
- 34.Agarwala SS, Kirkwood JM. Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist. 2000;5:144–151. doi: 10.1634/theoncologist.5-2-144. Epub 2000/05/05. [DOI] [PubMed] [Google Scholar]
- 35.Danson S, Lorigan P, Arance A, et al. Randomized phase II study of temozolomide given every 8hours or daily with either interferon alfa-2b or thalidomide in metastatic malignant melanoma. J Clin Oncol. 2003;21:2551–2557. doi: 10.1200/JCO.2003.10.039. Epub 2003/06/28. [DOI] [PubMed] [Google Scholar]
- 36.Mrugala MM, Adair J, Kiem HP. Temozolomide: Expanding its role in brain cancer. Drugs Today (Barc) 2010;46:833–846. doi: 10.1358/dot.2010.46.11.1549024. Epub 2011/01/13. [DOI] [PubMed] [Google Scholar]
- 37.Hegi ME, Diserens AC, Gorlia T, et al. MGMT gene silencing and benefit from temozolomide in glioblastoma. N Engl J Med. 2005;352:997–1003. doi: 10.1056/NEJMoa043331. Epub 2005/03/11. [DOI] [PubMed] [Google Scholar]
- 38.Keshelava N, Frgala T, Krejsa J, et al. DIMSCAN: A microcomputer fluorescence-based cytotoxicity assay for preclinical testing of combination chemotherapy. Methods Mol Med. 2005;110:139–153. doi: 10.1385/1-59259-869-2:139. [DOI] [PubMed] [Google Scholar]
- 39.Sebaugh JL. Guidelines for accurate EC50/IC50 estimation. Pharmaceut Stat. 2011;10:128–134. doi: 10.1002/pst.426. [DOI] [PubMed] [Google Scholar]
- 40.Friedman HS, Colvin OM, Skapek SX, et al. Experimental chemotherapy of human medulloblastoma cell lines and transplantable xenografts with bifunctional alkylating agents. Cancer Res. 1988;48:4189–4195. [PubMed] [Google Scholar]
- 41.Liem NL, Papa RA, Milross CG, et al. Characterization of childhood acute lymphoblastic leukemia xenograft models for the preclinical evaluation of new therapies. Blood. 2004;103:3905–3914. doi: 10.1182/blood-2003-08-2911. [DOI] [PubMed] [Google Scholar]
- 42.Houghton PJ, Morton CL, Tucker C, et al. The pediatric preclinical testing program: Description of models and early testing results. Pediatr Blood Cancer. 2007;49:928–940. doi: 10.1002/pbc.21078. [DOI] [PubMed] [Google Scholar]
- 43.Zhang J, Stevens MF, Laughton CA, et al. Acquired resistance to temozolomide in glioma cell lines: Molecular mechanisms and potential translational applications. Oncology. 2010;78:103–114. doi: 10.1159/000306139. Epub 2010/04/02. [DOI] [PubMed] [Google Scholar]
- 44.Friedman HS, Kerby T, Calvert H. Temozolomide and treatment of malignant glioma. Clin Cancer Res. 2000;6:2585–2597. Epub 2000/07/29. [PubMed] [Google Scholar]
- 45.Ostermann S, Csajka C, Buclin T, et al. Plasma and cerebrospinal fluid population pharmacokinetics of temozolomide in malignant glioma patients. Clin Cancer Res. 2004;10:3728–3736. doi: 10.1158/1078-0432.CCR-03-0807. Epub 2004/06/03. [DOI] [PubMed] [Google Scholar]
- 46.Brada M, Judson I, Beale P, et al. Phase I dose-escalation and pharmacokinetic study of temozolomide (SCH 52365) for refractory or relapsing malignancies. British J Cancer. 1999;81:1022–1030. doi: 10.1038/sj.bjc.6690802. Epub 1999/11/27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Estlin EJ, Lashford L, Ablett S, et al. Phase I study of temozolomide in paediatric patients with advanced cancer. United Kingdom Children’s Cancer Study Group. Br J Cancer. 1998;78:652–661. doi: 10.1038/bjc.1998.555. Epub 1998/09/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Nicholson HS, Krailo M, Ames MM, et al. Phase I study of temozolomide in children and adolescents with recurrent solid tumors: A report from the Children’s Cancer Group. J Clin Oncol. 1998;16:3037–3043. doi: 10.1200/JCO.1998.16.9.3037. Epub 1998/09/17. [DOI] [PubMed] [Google Scholar]
- 49.Ogura M, Todo T, Tanaka M, et al. Temozolomide may induce therapy-related acute lymphoblastic leukaemia. Br J Haematol. 2011;154:663–665. doi: 10.1111/j.1365-2141.2011.08641.x. Epub 2011/04/27. [DOI] [PubMed] [Google Scholar]
- 50.Nicholson HS, Kretschmar CS, Krailo M, et al. Phase 2 study of temozolomide in children and adolescents with recurrent central nervous system tumors: A report from the Children’s Oncology Group. Cancer. 2007;110:1542–1550. doi: 10.1002/cncr.22961. Epub 2007/08/21. [DOI] [PubMed] [Google Scholar]
- 51.Verschuur AC, Grill J, Lelouch-Tubiana A, et al. Temozolomide in paediatric high-grade glioma: A key for combination therapy? Br J Cancer. 2004;91:425–429. doi: 10.1038/sj.bjc.6601997. Epub 2004/07/22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Cohen KJ, Pollack IF, Zhou T, et al. Temozolomide in the treatment of high-grade gliomas in children: A report from the Children’s Oncology Group. Neuro Oncol. 2011;13:317–323. doi: 10.1093/neuonc/noq191. Epub 2011/02/23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cohen KJ, Heideman RL, Zhou T, et al. Temozolomide in the treatment of children with newly diagnosed diffuse intrinsic pontine gliomas: A report from the Children’s Oncology Group. Neuro Oncol. 2011;13:410–416. doi: 10.1093/neuonc/noq205. Epub 2011/02/25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.De Sio L, Milano GM, Castellano A, et al. Temozolomide in resistant or relapsed pediatric solid tumors. Pediatr Blood Cancer. 2006;47:30–36. doi: 10.1002/pbc.20516. Epub 2005/07/28. [DOI] [PubMed] [Google Scholar]
- 55.Rubie H, Chisholm J, Defachelles AS, et al. Phase II study of temozolomide in relapsed or refractory high-risk neuroblastoma: A joint Societe Francaise des Cancers de l’Enfant and United Kingdom Children Cancer Study Group-New Agents Group Study. J Clin Oncol. 2006;24:5259–5264. doi: 10.1200/JCO.2006.06.1572. Epub 2006/11/23. [DOI] [PubMed] [Google Scholar]
- 56.Houghton PJ, Stewart CF, Cheshire PJ, et al. Antitumor activity of temozolomide combined with irinotecan is partly independent of O6-methylguanine-DNA methyltransferase and mismatch repair phenotypes in xenograft models. Clin Cancer Res. 2000;6:4110–4118. Epub 2000/10/29. [PubMed] [Google Scholar]
- 57.Bagatell R, London WB, Wagner LM, et al. Phase II study of irinotecan and temozolomide in children with relapsed or refractory neuroblastoma: A Children’s Oncology Group study. J Clin Oncol. 2011;29:208–213. doi: 10.1200/JCO.2010.31.7107. Epub 2010/12/01. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kushner BH, Kramer K, Modak S, et al. Irinotecan plus temozolomide for relapsed or refractory neuroblastoma. J Clin Oncol. 2006;24:5271–5276. doi: 10.1200/JCO.2006.06.7272. Epub 2006/11/23. [DOI] [PubMed] [Google Scholar]
- 59.Wagner LM, Villablanca JG, Stewart CF, et al. Phase I trial of oral irinotecan and temozolomide for children with relapsed high-risk neuroblastoma: A new approach to neuroblastoma therapy consortium study. J Clin Oncol. 2009;27:1290–1296. doi: 10.1200/JCO.2008.18.5918. Epub 2009/01/28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Casey DA, Wexler LH, Merchant MS, et al. Irinotecan and temozolomide for Ewing sarcoma: The Memorial Sloan-Kettering experience. Pediatr Blood Cancer. 2009;53:1029–1034. doi: 10.1002/pbc.22206. Epub 2009/07/29. [DOI] [PubMed] [Google Scholar]
- 61.Wagner LM, McAllister N, Goldsby RE, et al. Temozolomide and intravenous irinotecan for treatment of advanced Ewing sarcoma. Pediatr Blood Cancer. 2007;48:132–139. doi: 10.1002/pbc.20697. Epub 2005/12/01. [DOI] [PubMed] [Google Scholar]
- 62.Panetta JC, Kirstein MN, Gajjar A, et al. Population pharmacokinetics of temozolomide and metabolites in infants and children with primary central nervous system tumors. Cancer Chemother Pharmacol. 2003;52:435–441. doi: 10.1007/s00280-003-0670-4. Epub 2003/09/19. [DOI] [PubMed] [Google Scholar]
- 63.Meany HJ, Warren KE, Fox E, et al. Pharmacokinetics of temozolomide administered in combination with O6-benzylguanine in children and adolescents with refractory solid tumors. Cancer Chemother Pharmacol. 2009;65:137–142. doi: 10.1007/s00280-009-1015-8. Epub 2009/05/12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dhodapkar M, Rubin J, Reid JM, et al. Phase I trial of temozolomide (NSC 362856) in patients with advanced cancer. Clin Cancer Res. 1997;3:1093–1100. Epub 1997/07/01. [PubMed] [Google Scholar]
- 65.Hammond LA, Eckardt JR, Baker SD, et al. Phase I and pharmacokinetic study of temozolomide on a daily-for-5-days schedule in patients with advanced solid malignancies. J Clin Oncol. 1999;17:2604–2613. doi: 10.1200/JCO.1999.17.8.2604. Epub 1999/11/24. [DOI] [PubMed] [Google Scholar]
- 66.Britten CD, Rowinsky EK, Baker SD, et al. A Phase I and pharmacokinetic study of temozolomide and cisplatin in patients with advanced solid malignancies. Clin Cancer Res. 1999;5:1629–1637. Epub 1999/08/03. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.