Abstract
Background
The value of family history as a risk factor for kidney failure has not been determined in a nationwide setting.
Aim
This nationwide family study aimed to determine familial risks for kidney failure in Sweden.
Methods
The Swedish multi-generation register on 0–78-year-old subjects were linked to the Swedish patient register and the Cause of death register for 1987–2010. Individuals diagnosed with acute kidney failure (n = 10063), chronic kidney failure (n = 18668), or unspecified kidney failure (n = 3731) were included. Kidney failure patients with cystic kidney disease, congenital kidney and urinary tract malformations, urolithiasis, and rare inherited kidney syndromes, and hyperoxaluria were excluded. Standardized incidence ratios (SIRs) were calculated for individuals whose parents/siblings were diagnosed with kidney failure compared to those whose parents or siblings were not.
Results
The concordant (same disease) familial risks (sibling/parent history) were increased for chronic kidney failure SIR = 2.02 (95% confidence interval, CI 1.90–2.14) but not for acute kidney failure SIR = 1.08 (95% CI 0.94–1.22) and for unspecified kidney failure SIR = 1.25 (95% CI 0.94–1.63). However, the discordant (different disease) familial risk for acute kidney failure SIR = 1.19 (95% CI 1.06–1.32) and unspecified kidney failure SIR = 1.63 (95% CI 1.40–1.90) was significantly increased in individuals with a family history of chronic kidney failure. The familial risk for chronic kidney failure was similar for males SIR = 2.04 (95% CI 1.90–2.20) and females SIR = 1.97 (95% CI 1.78–2.17). Familial risks for chronic kidney failure were highest at age of 10–19 years SIR = 6.33 (95% CI 4.16–9.22).
Conclusions
The present study shows that family history is an important risk factor for chronic kidney failure but to a lower degree for acute kidney failure and unspecified kidney failure.
Introduction
Chronic kidney disease (CKD) is a worldwide medical problem with poor outcomes, high costs, and increased risk of cardiovascular comorbidities and all-cause mortality [1]–[3]. In developed countries, it is associated with old age, diabetes, hypertension, obesity, and cardiovascular disease [1]. Diabetic glomerulosclerosis and hypertensive nephrosclerosis are the presumed pathological entities but exact diagnosis is often difficult [1]. Familial and genetic factors are increasingly recognized as important for the development of CKD and end-stage renal disease (ESRD) [4]–[8]. Ferguson et al. first reported that family history of ESRD is common among African Americans with ESRD [9]. Several case-series and case-control studies have confirmed the importance of family history of kidney disease in different populations of patients with CKD and/or ERSD [10]–[18]. However, few follow-up studies have determined the importance of family history of CKD and/or ESRD [19]. In one such study, Hsu et al. found a modest effect of self-reported family history of kidney disease (hazard ratio (HR) = 1.40) [19]. No study has determined whether familial factors influence the risk of acute kidney failure, which is an increasing global problem [20].
Though multiple genetic loci have been associated with progressive kidney failure and function, [21]–[23] heritability estimates suggests that only a small proportion of the total heritable contribution to the phenotypic variation of CKD have been identified. Large-scale follow-up studies to determine the importance of family history of CKD may therefore be of clinical value for risk assessment, and may help for planning of genetic studies. Clustering of a disease in families may be caused both by both genetic and non-genetic factors [24]. Increased familial risks may indicate shared environmental and lifestyle factors are of importance for disease development, and not only inherited biological factors [25]. However, without familial clustering a genetic cause is unlikely [24].
To our knowledge, there has not been any nationwide follow-up study whose aim was to determine familial risks of kidney failure among offspring/siblings. This nationwide follow-up study determined the familial risks of different forms of kidney failure – chronic kidney failure, acute kidney failure and unspecified kidney failure (i.e., not specified whether it is acute or chronic) – in the offspring/siblings of individuals with kidney failure. The present study underlines the importance of familial factors in kidney failure.
Materials and Methods
The dataset used in this study was constructed by linking several national Swedish registers provided by the Swedish government-owned statistics bureau Statistics Sweden and the National Board of Health and Welfare [26]. The Swedish multigenerational register contains information on family relationships for index persons born in Sweden in 1932 and later. Individuals born in 1932 or later and who were alive 1987 constituted the present study population. Linkages were made to National Census data in order to ascertain individual-level socioeconomic status, to the Swedish cause of death register (1987–2010), to the Swedish outpatient care register (2001–2010), and to the Swedish hospital discharge register (1987–2010), the last of which records nationwide dates of hospitalization and hospital diagnoses since 1987. All linkages were performed using the individual national identification number that is assigned to each resident in Sweden for their lifetime. This number was replaced by a serial number in order to preserve anonymity. The serial numbers were used to check that each individual was entered only once (for his or her first main or secondary diagnosis of kidney failure). Over 8.1 million individuals and their biological parents (3.8 million families) were included in the database; the oldest (born in 1932) were 78 years at the end of follow-up period, which ran from 1987–2010.
Predictor and outcome variables
The predictor variable was family history (in a sibling or parent) of kidney failure (defined below) between 1987 and 2010. Separate risks were also determined for parental and sibling history of kidney failure. The outcome variable was first main or secondary event of kidney failure (acute kidney failure, chronic kidney failure, unspecified kidney failure) in the Swedish hospital discharge register, the Swedish outpatient care register, or the Swedish cause of death register. Acute kidney failure was defined by the following ICD codes: 584 (ICD-9) and N17 (ICD-10). Unspecified kidney failure was defined by the following ICD codes: 586 (ICD-9) and N19 (ICD-10). Chronic kidney failure was defined by the following ICD and surgical codes: 585,V45B, and V56 (ICD-9); N18, N26, T82.4, Y84.1, Z49, Z94.0, and Z99.2 (ICD-10); 6070, 6071, 6072, 6073, 6077, 6079, 9211, 9212, 9213, 9314, and 9200 (dialysis or kidney transplantation related surgical codes for 1987–1996); and V9211, V9212, V9200, V9531, V9532, V9507, KAS00, KAS10, KAS20, KAS40, KAS50, KAS60, KAS96, KAS97, and JAK10, TJA33, TJA35, TKA20 (dialysis or kidney transplantation related procedure and surgical codes for 1997–2010). Only main and secondary diagnoses were considered to ensure high validity. Kidney failure patients with cystic kidney disease (Q61, ICD-10; and 753B, ICD-9), congenital kidney and urinary tract malformations (Q60, Q62, Q63, Q64, ICD-10; and 753A, 753C, 753D, 753E, 753F, 753G, 753H, 753W, 753X, ICD-9), urolithiasis (N20-N23, ICD-10; and 592, ICD-9), rare inherited kidney diseases such as Alports syndrome and Laurence Moon-Biedl-Bardet syndrome (Q87.8A, Q87.8B, ICD-10), and hyperoxaluria (E74.8B, ICD-10; and 271W, ICD-9) were excluded.
Individual variables included in the analysis
The following variables were included in the analysis: 1) Sex: males or female; 2) Age: Age at diagnosis was categorized into 5-year groups; 3) Time period: The follow-up period was divided into 5-year intervals in order to adjust for changes in incidence rates over time; 4) Socioeconomic status: For both males and females, socioeconomic status was defined by occupation, which was divided into six groups: (1) farmers, (2) blue-collar workers, (3) white-collar workers, (4) professionals, (5) self-employed workers, and (6) others (economically inactive individuals including unemployed individuals and homemakers); 5) Geographic region of residence: To allow adjustment for regional differences in incidence rates, geographic region of residence was divided into three groups: (1) large city, i.e., Stockholm, Gothenburg, or Malmo; (2) Southern Sweden (excluding the large cities, all of which lie in Southern Sweden); and (3) Northern Sweden; and 6) Comorbidity. Comorbidity was defined as a main or secondary diagnosis at follow-up between 1987 and 2010 with the following ICD-codes in the Swedish hospital discharge register or the Swedish outpatient care register: 1) chronic obstructive pulmonary disease (490–496 (ICD-9) and J40–J47 (ICD-10)); 2) obesity (278A and 278B (ICD-9) and E65 and E66 (ICD-10)); 3) alcoholism and alcohol-related liver disease (291, 303, 571A, 571B, 571C, and 571D (ICD-9) and F10 and K70 (ICD-10)); 4) diabetes mellitus (250 (ICD-9) and E10-E14 (ICD-10)); 5) hypertension (401–405 (ICD-9) and I10-I15 (ICD-10)); 6) coronary heart disease (410–414 (ICD-9) and I20-I25 (ICD-10)); 7) heart failure (428 (ICD-9) and I50 (ICD-10)); 8) hyperlipidaemia (272A, 272B, 272C, 272D, and 272E (ICD-9) and E78.0, E78.1, E78.2, E78.3, E78.4, and E78.5 (ICD-10)); and 9) stroke (430–438 (ICD-9) and I60-I69 (ICD-10)).
Statistical Analysis
For the analysis of familial risks of kidney failure, a previously described method was used [27]. The method is described in detail by Hemminki et al [28] and takes into account clustering within families, since it is based on complete ascertainment of sib ships in affected individuals. Person-years at risk (i.e., the number of persons at risk multiplied by the time at risk) were calculated from the start of the follow-up on 1 January 1987 until diagnosis for kidney failure, death, emigration, or the end of the follow-up (31 December 2010) [29]. Age-adjusted incidence rates were calculated for the whole follow-up period, divided into 5-year periods [29]. Standardized incidence ratios (SIRs) were used to measure the relative risk of kidney failure in individuals with one or more parents with a history of kidney failure compared with individuals with parents without a history of kidney failure. Similar calculations were performed separately for siblings.
The familial SIRs were calculated as the ratio of observed (O) and expected (E) numbers of kidney failure cases using the indirect standardization method:
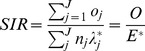 |
where 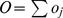 denotes the total observed number of cases in the study group; E
* (the expected number of cases) is calculated by applying stratum-specific standard incidence rates (λ*
j) obtained from the reference group to the stratum-specific person-years of risk (n
j) for the study group; o
j represents the observed number of cases that the cohort subjects contribute to the jth stratum; and J represents the strata defined by cross-classification of the following adjustment variables: age (5-year groups), sex, socioeconomic status, time period (5-year groups), geographic region of residence, and comorbidities. 95% confidence intervals (95% CIs) were calculated assuming a Poisson distribution [29].
denotes the total observed number of cases in the study group; E
* (the expected number of cases) is calculated by applying stratum-specific standard incidence rates (λ*
j) obtained from the reference group to the stratum-specific person-years of risk (n
j) for the study group; o
j represents the observed number of cases that the cohort subjects contribute to the jth stratum; and J represents the strata defined by cross-classification of the following adjustment variables: age (5-year groups), sex, socioeconomic status, time period (5-year groups), geographic region of residence, and comorbidities. 95% confidence intervals (95% CIs) were calculated assuming a Poisson distribution [29].
Data values are accurate to two decimals places. All analyses were performed using SAS version 9.2 (Institute, Cary, NC, USA).
Ethical Considerations
Statistics Sweden and the National Board of Health and Welfare maintain the nationwide registers used in the present study. This study was approved by the Ethics Committee at Lund University (approval number 409/2008 Lund with complementary approvals dated September 1, 2009, and January 22, 2010) and recommendations of the Declaration of Helsinki were complied with. The ethics committee waived informed consent as a requirement.
Results
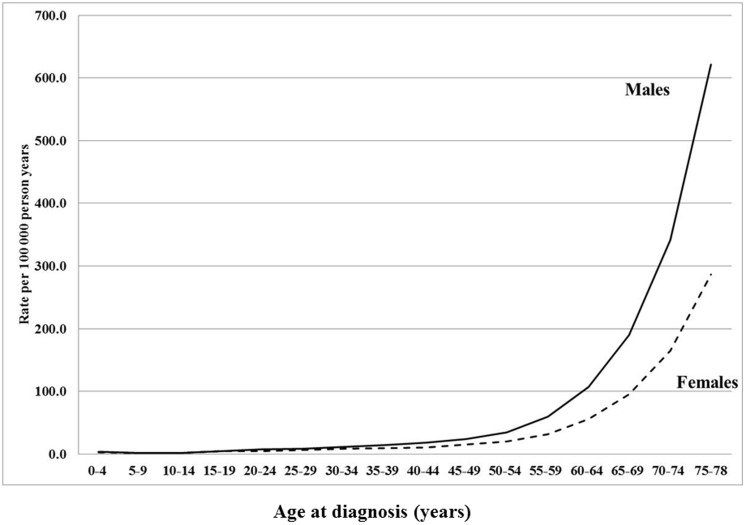
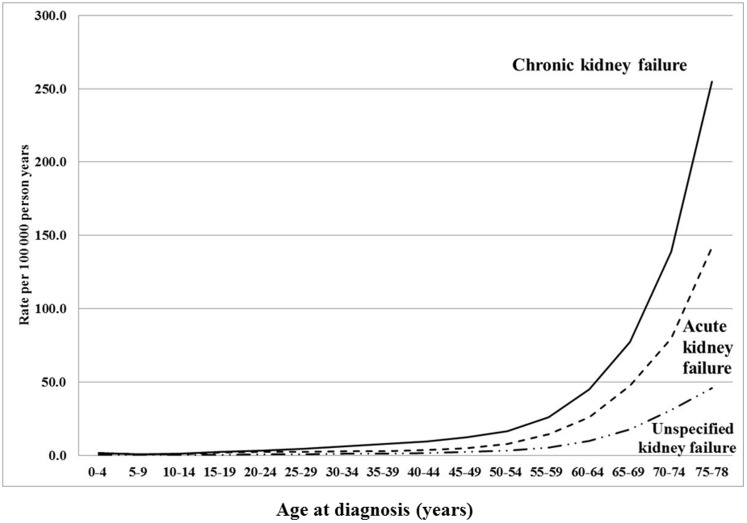
We analyzed familial risks of kidney failure in the siblings/offspring (aged 0–78 years) of individuals with kidney failure between 1987 and 2010 in Sweden. The population and number of diagnosis for kidney failure are presented in Table 1. A total of 8054071 individuals were included in this cohort. A total of 32462 individuals were diagnosed with kidney failure, 64% (20688) were males and 36% (11774) females (Table 1). Of these patients, 31.0% (10063) were diagnosed with acute kidney failure, 57.5% (18668) with chronic kidney failure, and 11.5% (3731) with unspecified kidney failure. Comorbidities were more common in patients with kidney failure than in the general population (Table 1). The lowest incidence rates for kidney failure were observed for children (Figure 1). The incidence rate for kidney failure increased with age in both sexes (Figure 1). At older ages, the incidence rate for kidney failure was higher for males than females (Figure 1). The incidence rate was highest for chronic kidney failure, and lowest for unspecified kidney failure (Figure 2).
Table 1. Study population and number of kidney failure events in individuals aged 0 to 78 years (born 1932 and later and alive in 1987).
| Males | Females | All | ||||||||||
| Population | Kidney failure events | Population | Kidney failure events | Population | Kidney failure events | |||||||
| No | % | No | % | No | % | No | % | No | % | No | % | |
| Age at diagnosis (years) | ||||||||||||
| 0–9 | 286 | 1.4 | 263 | 2.2 | 549 | 1.7 | ||||||
| 10–19 | 424 | 2.0 | 419 | 3.6 | 843 | 2.6 | ||||||
| 20–29 | 998 | 4.8 | 706 | 6.0 | 1704 | 5.2 | ||||||
| 30–39 | 1695 | 8.2 | 1100 | 9.3 | 2795 | 8.6 | ||||||
| 40–49 | 2698 | 13.0 | 1585 | 13.5 | 4283 | 13.2 | ||||||
| 50–59 | 4712 | 22.8 | 2557 | 21.7 | 7269 | 22.4 | ||||||
| 60–69 | 6700 | 32.4 | 3477 | 29.5 | 10177 | 31.4 | ||||||
| 70–78 | 3175 | 15.3 | 1667 | 14.2 | 4842 | 14.9 | ||||||
| Subtype of kidney failure | ||||||||||||
| Acute kidney failure | 6385 | 30.9 | 3678 | 31.2 | 10063 | 31.0 | ||||||
| Chronic kidney failure | 11872 | 57.4 | 6796 | 57.7 | 18668 | 57.5 | ||||||
| Unspecified kidney failure | 2431 | 11.7 | 1300 | 11.1 | 3731 | 11.5 | ||||||
| Socioeconomic status | ||||||||||||
| Farmer | 69645 | 1.7 | 609 | 2.9 | 50935 | 1.3 | 263 | 2.2 | 120580 | 1.5 | 872 | 2.7 |
| Self-employed | 161705 | 3.9 | 1591 | 7.7 | 106967 | 2.7 | 476 | 4.0 | 268672 | 3.3 | 2067 | 6.4 |
| Professional | 359536 | 8.7 | 2435 | 11.8 | 251700 | 6.4 | 720 | 6.1 | 611236 | 7.6 | 3155 | 9.7 |
| White collar worker | 1192177 | 29.0 | 5898 | 28.5 | 1390397 | 35.3 | 4421 | 37.5 | 2582574 | 32.1 | 10319 | 31.8 |
| Blue-collar worker | 1848695 | 45.0 | 9952 | 48.1 | 1689070 | 42.8 | 5750 | 48.8 | 3537765 | 43.9 | 15702 | 48.4 |
| Other | 480443 | 11.7 | 203 | 1.0 | 452801 | 11.5 | 144 | 1.2 | 933244 | 11.6 | 347 | 1.1 |
| Region of residence | ||||||||||||
| Northern Sweden | 427832 | 10.4 | 2120 | 10.2 | 402535 | 10.2 | 1290 | 11.0 | 830367 | 10.3 | 3410 | 10.5 |
| Large city | 1632588 | 39.7 | 8828 | 42.7 | 1575746 | 40.0 | 4861 | 41.3 | 3208334 | 39.8 | 13689 | 42.2 |
| Southern Sweden | 2051781 | 49.9 | 9740 | 47.1 | 1963589 | 49.8 | 5623 | 47.8 | 4015370 | 49.9 | 15363 | 47.3 |
| Chronic obstructive pulmonary disease | ||||||||||||
| No | 3910183 | 95.1 | 18979 | 91.7 | 3763810 | 95.5 | 10467 | 88.9 | 7673993 | 95.3 | 29446 | 90.7 |
| Yes | 202018 | 4.9 | 1709 | 8.3 | 178060 | 4.5 | 1307 | 11.1 | 380078 | 4.7 | 3016 | 9.3 |
| Obesity | ||||||||||||
| No | 4080976 | 99.2 | 20058 | 97.0 | 3885685 | 98.6 | 11217 | 95.3 | 7966661 | 98.9 | 31275 | 96.3 |
| Yes | 31225 | 0.8 | 630 | 3.0 | 56185 | 1.4 | 557 | 4.7 | 87410 | 1.1 | 1187 | 3.7 |
| Alcoholism and related liver disease | ||||||||||||
| No | 3994406 | 97.1 | 18453 | 89.2 | 3883021 | 98.5 | 11184 | 95.0 | 7877427 | 97.8 | 29637 | 91.3 |
| Yes | 117795 | 2.9 | 2235 | 10.8 | 58849 | 1.5 | 590 | 5.0 | 176644 | 2.2 | 2825 | 8.7 |
| Diabetes Mellitus | ||||||||||||
| No | 3998103 | 97.2 | 13930 | 67.3 | 3868649 | 98.1 | 8345 | 70.9 | 7866752 | 97.7 | 22275 | 68.6 |
| Yes | 114098 | 2.8 | 6758 | 32.7 | 73221 | 1.9 | 3429 | 29.1 | 187319 | 2.3 | 10187 | 31.4 |
| Hyptertension | ||||||||||||
| No | 3928928 | 95.5 | 10841 | 52.4 | 3791314 | 96.2 | 6965 | 59.2 | 7720242 | 95.9 | 17806 | 54.9 |
| Yes | 183273 | 4.5 | 9847 | 47.6 | 150556 | 3.8 | 4809 | 40.8 | 333829 | 4.1 | 14656 | 45.1 |
| Coronary heart disease | ||||||||||||
| No | 3975828 | 96.7 | 15160 | 73.3 | 3879307 | 98.4 | 9561 | 81.2 | 7855135 | 97.5 | 24721 | 76.2 |
| Yes | 136373 | 3.3 | 5528 | 26.7 | 62563 | 1.6 | 2213 | 18.8 | 198936 | 2.5 | 7741 | 23.8 |
| Stroke | ||||||||||||
| No | 4038432 | 98.2 | 17537 | 84.8 | 3892316 | 98.7 | 10276 | 87.3 | 7930748 | 98.5 | 27813 | 85.7 |
| Yes | 73769 | 1.8 | 3151 | 15.2 | 49554 | 1.3 | 1498 | 12.7 | 123323 | 1.5 | 4649 | 14.3 |
| Hyperlipidemia | ||||||||||||
| No | 4067712 | 98.9 | 19437 | 94.0 | 3917158 | 99.4 | 11233 | 95.4 | 7984870 | 99.1 | 30670 | 94.5 |
| Yes | 44489 | 1.1 | 1251 | 6.0 | 24712 | 0.6 | 541 | 4.6 | 69201 | 0.9 | 1792 | 5.5 |
| Heart failure | ||||||||||||
| No | 4068915 | 98.9 | 16408 | 79.3 | 3920737 | 99.5 | 9833 | 83.5 | 7989652 | 99.2 | 26241 | 80.8 |
| Yes | 43286 | 1.1 | 4280 | 20.7 | 21133 | 0.5 | 1941 | 16.5 | 64419 | 0.8 | 6221 | 19.2 |
| All | 4112201 | 100.0 | 20688 | 100.0 | 3941870 | 100.0 | 11774 | 100.0 | 8054071 | 100.0 | 32462 | 100.0 |
Figure 1. Age-specific incidence rates (per 100000 person years) of kidney failure for males and females in offspring/siblings born in 1932 and later.
Figure 2. Age-specific incidence rates (per 100000 person years) of chronic kidney failure, acute kidney failure, and unspecified kidney failure ( = others) in offspring/siblings born in 1932 and later.
Familial risk of kidney failure
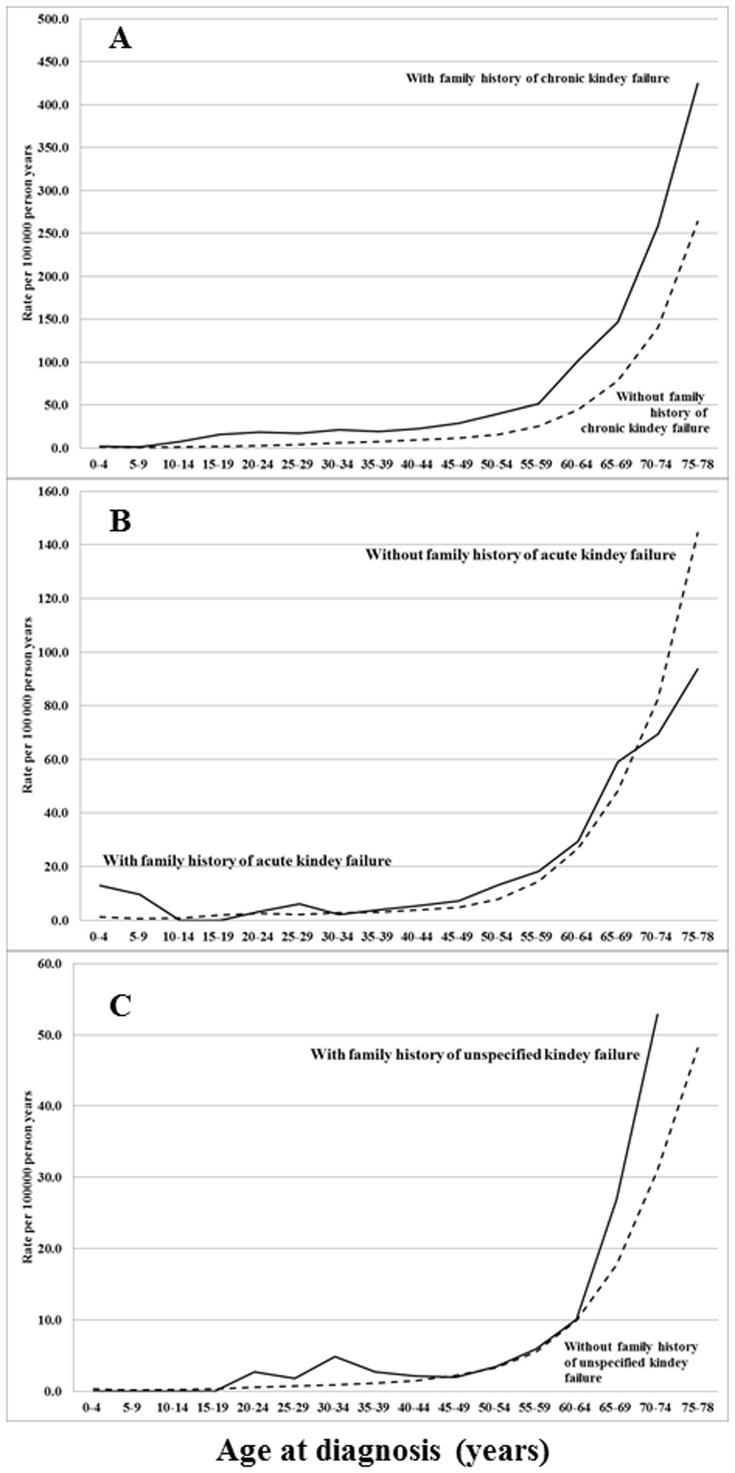
Familial risks of kidney failure according to disease subtypes are presented in Table 2. Familial risks were adjusted for age, sex, time period, region of residence, socioeconomic status, and comorbidities. The incidence rates for familial and non-familial kidney failure are presented Figure 3. Concordant (same disease in proband and exposed relative) and discordant (different disease in proband and exposed relative) risks were determined. The familial risks were highest for chronic kidney failure: the concordant familial SIR for chronic kidney failure was 2.02. The concordant familial risk was not significantly increased for acute kidney failure (SIR = 1.08) and for unspecified kidney failure (SIR = 1.25) (Table 2). However, discordant risks show that family history (sibling/parent) of chronic kidney failure is a risk factor for both acute kidney failure (SIR = 1.19) and unspecific kidney failure (SIR = 1.63) (Table 2). Moreover, discordant risks show that family history (sibling/parent) of acute kidney failure is a risk factor for both chronic kidney failure (SIR = 1.10) and unspecific kidney failure (SIR = 1.30) (Table 2). Family history of unspecified kidney failure (sibling/parent) was a risk factor for chronic kidney failure (SIR = 1.31) (Table 2). Family history of all kidney failure was a risk factor for all types of kidney failure (Table 2). Familial risks of kidney failure were determined in both males and females. There were no major sex differences (Table 2).
Table 2. Familial concordant and discordant risk (sibling/parent history) of kidney failure in males and females.
| Males | Females | All | |||||||||||
| Type of kidney failure in proband | Subtype of kidney failure in offspring/sibling | O | SIR | 95% CI | O | SIR | 95% CI | O | SIR | 95% CI | |||
| Acute kidney failure | Acute kidney failure | 153 | 1.09 | 0.92 | 1.27 | 84 | 1.05 | 0.84 | 1.30 | 237 | 1.08 | 0.94 | 1.22 |
| Chronic kidney failure | 282 | 1.07 | 0.95 | 1.20 | 153 | 1.15 | 0.98 | 1.35 | 435 | 1.10 | 1.00 | 1.21 | |
| Unspecified kidney failure | 64 | 1.29 | 1.00 | 1.65 | 36 | 1.31 | 0.92 | 1.81 | 100 | 1.30 | 1.06 | 1.58 | |
| All kidney failure | 499 | 1.10 | 1.01 | 1.20 | 273 | 1.14 | 1.01 | 1.28 | 772 | 1.11 | 1.04 | 1.19 | |
| Chronic kidney failure | Acute kidney failure | 201 | 1.15 | 0.99 | 1.32 | 129 | 1.26 | 1.05 | 1.50 | 330 | 1.19 | 1.06 | 1.32 |
| Chronic kidney failure | 717 | 2.04 | 1.90 | 2.20 | 395 | 1.97 | 1.78 | 2.17 | 1112 | 2.02 | 1.90 | 2.14 | |
| Unspecified kidney failure | 104 | 1.56 | 1.28 | 1.89 | 65 | 1.76 | 1.36 | 2.24 | 169 | 1.63 | 1.40 | 1.90 | |
| All kidney failure | 1022 | 1.72 | 1.62 | 1.83 | 589 | 1.73 | 1.59 | 1.88 | 1611 | 1.73 | 1.64 | 1.81 | |
| Unspecified kidney failure | Acute kidney failure | 72 | 1.07 | 0.84 | 1.35 | 42 | 1.04 | 0.75 | 1.41 | 114 | 1.06 | 0.88 | 1.28 |
| Chronic kidney failure | 176 | 1.31 | 1.12 | 1.52 | 101 | 1.31 | 1.07 | 1.60 | 277 | 1.31 | 1.16 | 1.47 | |
| Unspecified kidney failure | 33 | 1.18 | 0.81 | 1.65 | 21 | 1.38 | 0.85 | 2.11 | 54 | 1.25 | 0.94 | 1.63 | |
| All kidney failure | 281 | 1.22 | 1.09 | 1.38 | 164 | 1.24 | 1.06 | 1.44 | 445 | 1.23 | 1.12 | 1.35 | |
| All kidney failure | Acute kidney failure | 426 | 1.11 | 1.01 | 1.22 | 255 | 1.15 | 1.01 | 1.30 | 681 | 1.12 | 1.04 | 1.21 |
| Chronic kidney failure | 1175 | 1.57 | 1.48 | 1.66 | 649 | 1.58 | 1.46 | 1.71 | 1824 | 1.57 | 1.50 | 1.65 | |
| Unspecified kidney failure | 201 | 1.39 | 1.21 | 1.60 | 122 | 1.53 | 1.27 | 1.83 | 323 | 1.44 | 1.29 | 1.61 | |
| All kidney failure | 1802 | 1.41 | 1.35 | 1.48 | 1026 | 1.44 | 1.35 | 1.53 | 2828 | 1.42 | 1.37 | 1.48 | |
Familial risks were adjusted for age, sex, time period, region of residence, socioeconomic status, and comorbidities.
Bold type: 95% CI does not include 1.00.
O = observed number of cases with family history of kidney failure; SIR = standardized incidence ratio; CI = confidence interval.
Figure 3. Age-specific incidence rate (per 100000 person years) of kidney failure by concordant family history of kidney failure in individuals born in 1932 and later.
A Chronic kidney failure. B Acute kidney failure. C Unspecified kidney failure.
In Table 3, familial concordant risks are presented according to the affected relative. Familial risks were adjusted for age, sex, time period, region of residence, socioeconomic status, and comorbidities. Sibling history of chronic kidney failure showed the highest familial risk, with a concordant SIR of 2.52. The familial concordant risk for individuals with a parental history of chronic kidney failure was 1.67. There were no major sex differences. The familial concordant risks for acute and unspecified kidney failure were not significant (Table 3).
Table 3. Familial risk of concordant kidney failure in males and females.
| Males | Females | All | |||||||||||
| Probands with any type of kidney failure | Subtype of kidney failure in offspring/siblings | O | SIR | 95% CI | O | SIR | 95% CI | O | SIR | 95% CI | |||
| Family history (parent/sibling) | Acute kidney failure | 153 | 1.09 | 0.92 | 1.27 | 84 | 1.05 | 0.84 | 1.30 | 237 | 1.08 | 0.94 | 1.22 |
| Chronic kidney failure | 717 | 2.04 | 1.90 | 2.20 | 395 | 1.97 | 1.78 | 2.17 | 1112 | 2.02 | 1.90 | 2.14 | |
| Unspecified kidney failure | 33 | 1.18 | 0.81 | 1.65 | 21 | 1.38 | 0.85 | 2.11 | 54 | 1.25 | 0.94 | 1.63 | |
| All kidney failure | 1802 | 1.41 | 1.35 | 1.48 | 1026 | 1.44 | 1.35 | 1.53 | 2828 | 1.42 | 1.37 | 1.48 | |
| Parents history | Acute kidney failure | 103 | 1.06 | 0.87 | 1.29 | 58 | 1.10 | 0.83 | 1.42 | 161 | 1.07 | 0.91 | 1.25 |
| Chronic kidney failure | 378 | 1.71 | 1.54 | 1.89 | 204 | 1.62 | 1.40 | 1.85 | 582 | 1.67 | 1.54 | 1.82 | |
| Unspecified kidney failure | 22 | 1.00 | 0.63 | 1.52 | 16 | 1.50 | 0.86 | 2.45 | 38 | 1.16 | 0.82 | 1.60 | |
| All kidney failure | 1124 | 1.28 | 1.21 | 1.36 | 637 | 1.32 | 1.22 | 1.43 | 1761 | 1.29 | 1.23 | 1.35 | |
| Paternal history | Acute kidney failure | 62 | 1.20 | 0.92 | 1.54 | 26 | 1.03 | 0.67 | 1.51 | 88 | 1.14 | 0.92 | 1.41 |
| Chronic kidney failure | 212 | 1.73 | 1.50 | 1.98 | 105 | 1.45 | 1.19 | 1.76 | 317 | 1.62 | 1.45 | 1.81 | |
| Unspecified kidney failure | 9 | 0.74 | 0.33 | 1.40 | 11 | 1.87 | 0.93 | 3.36 | 20 | 1.10 | 0.67 | 1.71 | |
| All kidney failure | 637 | 1.35 | 1.24 | 1.45 | 326 | 1.21 | 1.08 | 1.35 | 963 | 1.29 | 1.21 | 1.38 | |
| Maternal history | Acute kidney failure | 42 | 0.92 | 0.66 | 1.25 | 33 | 1.19 | 0.82 | 1.67 | 75 | 1.02 | 0.80 | 1.28 |
| Chronic kidney failure | 178 | 1.69 | 1.45 | 1.96 | 108 | 1.88 | 1.54 | 2.27 | 286 | 1.76 | 1.56 | 1.97 | |
| Unspecified kidney failure | 13 | 1.26 | 0.67 | 2.16 | 7 | 1.39 | 0.55 | 2.89 | 20 | 1.31 | 0.80 | 2.02 | |
| All kidney failure | 513 | 1.20 | 1.10 | 1.31 | 326 | 1.47 | 1.31 | 1.63 | 839 | 1.29 | 1.21 | 1.38 | |
| Sibling history | Acute kidney failure | 52 | 1.15 | 0.86 | 1.50 | 27 | 0.98 | 0.64 | 1.43 | 79 | 1.08 | 0.86 | 1.35 |
| Chronic kidney failure | 366 | 2.52 | 2.27 | 2.80 | 213 | 2.52 | 2.19 | 2.88 | 579 | 2.52 | 2.32 | 2.73 | |
| Unspecified kidney failure | 12 | 1.65 | 0.85 | 2.89 | 6 | 1.08 | 0.39 | 2.37 | 18 | 1.40 | 0.83 | 2.22 | |
| All kidney failure | 738 | 1.68 | 1.56 | 1.80 | 430 | 1.70 | 1.54 | 1.87 | 1168 | 1.69 | 1.59 | 1.78 | |
Familial risks were adjusted for age, sex, time period, region of residence, socioeconomic status, and comorbidities.
Bold type: 95% CI does not include 1.00.
O = observed number of cases with family history of kidney failure; SIR = standardized incidence ratio; CI = confidence interval.
The familial concordant risks (parent/sibling history) were stratified according to age at diagnosis (Table 4). The familial risks for chronic kidney failure were highly age dependent and were highest risks at younger ages (SIR = 6.33 between the age of 10 and 19 years). Increased concordant familial risk of 1.81 was noted also for chronic kidney failure for those aged 60 years or more (Table 4). The familial concordant risks for chronic kidney failure were increased in all age groups except those younger than 10 years. For acute kidney failure, the familial concordant risks were only significantly increased only in two age groups (Table 4). The familial risk for acute kidney failure before age of 10 years was high (SIR = 14.21). The age of these six children with familial acute kidney failure were 0, 1, 1, 5, 5, and 7 years. For three children, the diagnosis was unknown (two had ICD diagnosis = Z038 and one had no additional diagnosis). One child was prematurely born (<28 weeks) and/or had a very low birth weight (<1000 g) (ICD-9 = 765A), one had unspecified infectious gastroenteritis (ICD-9 = 009B), and one had gastroenteritis with Escherichia coli (ICD-9 = 008A). No significant increased risk for unspecified kidney failure was observed for any other age groups. However, the familial risk for all kidney failure was increased in all age groups (Table 4).
Table 4. Familial risk (sibling/parent history) of concordant kidney failure in males and females by age diagnosis.
| Males | Females | All | ||||||||||
| Age at diagnosis (years) | O | SIR | 95% CI | O | SIR | 95% CI | O | SIR | 95% CI | |||
| Acute kidney failure | ||||||||||||
| <10 | 4 | 16.78 | 4.36 | 43.39 | 2 | 10.88 | 1.03 | 40.02 | 6 | 14.21 | 5.11 | 31.14 |
| 10–19 | 0 | 0 | 0 | |||||||||
| 20–29 | 6 | 2.00 | 0.72 | 4.37 | 5 | 3.70 | 1.17 | 8.70 | 11 | 2.52 | 1.25 | 4.53 |
| 30–39 | 11 | 1.43 | 0.71 | 2.57 | 2 | 0.61 | 0.06 | 2.24 | 13 | 1.18 | 0.63 | 2.03 |
| 40–49 | 22 | 1.23 | 0.77 | 1.87 | 14 | 1.57 | 0.86 | 2.64 | 36 | 1.34 | 0.94 | 1.86 |
| 50–59 | 52 | 1.35 | 1.01 | 1.78 | 21 | 0.84 | 0.52 | 1.28 | 73 | 1.15 | 0.90 | 1.45 |
| > = 60 | 58 | 0.80 | 0.61 | 1.03 | 40 | 0.99 | 0.71 | 1.35 | 98 | 0.87 | 0.70 | 1.06 |
| All | 153 | 1.09 | 0.92 | 1.27 | 84 | 1.05 | 0.84 | 1.30 | 237 | 1.08 | 0.94 | 1.22 |
| Chronic kidney failure | ||||||||||||
| <10 | 2 | 3.92 | 0.37 | 14.41 | 0 | 2 | 2.09 | 0.20 | 7.70 | |||
| 10–19 | 16 | 6.94 | 3.96 | 11.30 | 11 | 5.60 | 2.78 | 10.06 | 27 | 6.33 | 4.16 | 9.22 |
| 20–29 | 41 | 4.87 | 3.49 | 6.61 | 33 | 4.35 | 2.99 | 6.12 | 74 | 4.62 | 3.63 | 5.81 |
| 30–39 | 91 | 2.36 | 1.90 | 2.90 | 43 | 1.76 | 1.27 | 2.37 | 134 | 2.13 | 1.78 | 2.52 |
| 40–49 | 135 | 2.08 | 1.75 | 2.47 | 75 | 2.11 | 1.66 | 2.65 | 210 | 2.09 | 1.82 | 2.40 |
| 50–59 | 187 | 1.86 | 1.60 | 2.14 | 92 | 1.74 | 1.40 | 2.14 | 279 | 1.82 | 1.61 | 2.04 |
| > = 60 | 245 | 1.81 | 1.59 | 2.05 | 141 | 1.81 | 1.52 | 2.14 | 386 | 1.81 | 1.63 | 2.00 |
| All | 717 | 2.04 | 1.90 | 2.20 | 395 | 1.97 | 1.78 | 2.17 | 1112 | 2.02 | 1.90 | 2.14 |
| Unspecified kidney failure | ||||||||||||
| <10 | 0 | 0 | 0 | |||||||||
| 10–19 | 0 | 0 | 0 | |||||||||
| 20–29 | 1 | 2.39 | 0.00 | 13.67 | 1 | 4.41 | 0.00 | 25.26 | 2 | 3.10 | 0.29 | 11.38 |
| 30–39 | 6 | 2.80 | 1.01 | 6.12 | 1 | 1.04 | 0.00 | 5.95 | 7 | 2.25 | 0.89 | 4.66 |
| 40–49 | 2 | 0.58 | 0.05 | 2.12 | 4 | 1.62 | 0.42 | 4.18 | 6 | 1.01 | 0.36 | 2.21 |
| 50–59 | 8 | 1.04 | 0.45 | 2.07 | 4 | 1.18 | 0.31 | 3.05 | 12 | 1.09 | 0.56 | 1.90 |
| > = 60 | 16 | 1.13 | 0.64 | 1.83 | 11 | 1.35 | 0.67 | 2.43 | 27 | 1.21 | 0.80 | 1.76 |
| All | 33 | 1.18 | 0.81 | 1.65 | 21 | 1.38 | 0.85 | 2.11 | 54 | 1.25 | 0.94 | 1.63 |
| All kidney failure | ||||||||||||
| <10 | 6 | 3.28 | 1.18 | 7.19 | 6 | 3.52 | 1.27 | 7.71 | 12 | 3.40 | 1.75 | 5.95 |
| 10–19 | 23 | 3.43 | 2.17 | 5.15 | 24 | 4.02 | 2.57 | 5.98 | 47 | 3.70 | 2.72 | 4.93 |
| 20–29 | 72 | 2.53 | 1.98 | 3.18 | 56 | 2.83 | 2.14 | 3.68 | 128 | 2.65 | 2.21 | 3.15 |
| 30–39 | 180 | 1.74 | 1.49 | 2.01 | 87 | 1.47 | 1.18 | 1.82 | 267 | 1.64 | 1.45 | 1.85 |
| 40–49 | 290 | 1.45 | 1.29 | 1.63 | 178 | 1.58 | 1.36 | 1.83 | 468 | 1.50 | 1.37 | 1.64 |
| 50–59 | 515 | 1.41 | 1.29 | 1.54 | 260 | 1.30 | 1.15 | 1.47 | 775 | 1.37 | 1.28 | 1.47 |
| > = 60 | 716 | 1.25 | 1.16 | 1.35 | 415 | 1.32 | 1.20 | 1.46 | 1131 | 1.28 | 1.20 | 1.35 |
| All | 1802 | 1.41 | 1.35 | 1.48 | 1026 | 1.44 | 1.35 | 1.53 | 2828 | 1.42 | 1.37 | 1.48 |
Familial risks were adjusted for age, sex, time period, region of residence, socioeconomic status, and comorbidities.
Bold type: 95% CI does not include 1.00. O = observed number of cases with family history of kidney failure; SIR = standardized incidence ratio; CI = confidence interval.
Test for the extent of the shared non-genetic familial contribution
In order to test for the extent of environmental sharing in the observed risks of kidney failure SIRs for siblings according to difference in age were calculated (Table S1). Familial risks were adjusted for age, sex, time period, region of residence, socioeconomic status, and comorbidities. Overall, the age difference had little effect. Siblings with an age difference of <5 years showed a SIR for all kidney failure of 1.64 (95% CI, 1.50 to 1.79) compared with 1.72 (95% CI, 1.59 to 1.86) for those with an age difference of ≥5 years. The concordant sibling risk for chronic kidney failure was 2.36 (95% CI 2.07–2.67) for siblings with an age difference of <5 years, compared with 2.65 (95% CI, 2.38 to 2.95) for those with an age difference of ≥5 years.
Additional analyses
In Table S2, familial concordant and discordant risks are presented according to the affected relative. Familial risks were adjusted for age, sex, time period, region of residence, socioeconomic status, and comorbidities. The results were basically similar to the familial concordant/discordant risk in Table 2. Thus, concordant and discordant risk was generally highest for chronic kidney failure, followed by unspecified kidney failure, and weakest for acute kidney failure independent of the type of affected relative (sibling/parent, parents, mother, father or sibling).
Table S3 shows age stratified concordant and discordant familial risks (parent/siblings) of kidney failure. Familial risks were adjusted for age, sex, time period, region of residence, socioeconomic status, and comorbidities. The results were basically similar to the familial age stratified concordant risks in Table 4. Thus, age stratified concordant and discordant risks were generally highest for chronic kidney failure, followed by unspecified kidney failure, and weakest for acute kidney failure independent of the type of affected relative (sibling/parent, parents, mother, father or sibling). However, for acute kidney failure, the familial concordant risks were highly increased in the two youngest age groups (Table S3).
Sensitivity analysis
Table S4 presents concordant and discordant familial risks (parent/siblings) after exclusion of patients with kidney cancer in parents/offspring. This did not change the results to any major degree.
Table S5 shows concordant and discordant familial risks (parent/siblings) for the follow up period 2001–2010. The familial risks were similar compared to using a follow-up period from 1987–2010. Thus, inclusion of outpatients with kidney failure diagnosis from 2001 until 2010 did not change the results to any major degree.
Discussion
The present study is the first nationwide follow-up study to evaluate the familial risks of chronic, acute, and unspecified kidney failure among offspring/siblings of affected individuals. The results confirm previous case-series and case-control studies, which showed that familial factors are important for chronic kidney failure [9]–[18]. The present study adds follow-up data for a whole country. Previously a follow-up study only showed moderately increased familial risk (HR = 1.40) [19]. The present results indicate that familial factors are important for chronic kidney failure in both males and females of all ages (except <10 years), although the familial risks were highest at ages 10–19 years (Table 4). This is in contrast to the findings that most risk alleles from genome wide association studies add little to the prediction of CKD [21]–[23]. It is possible that there are a large number of risk alleles that have yet to be discovered that may account for this discrepancy. Unique familial (environmental or genetic) factors may predispose individuals to chronic kidney failure. Support for a genetic contribution to chronic kidney failure comes from the observation that age difference between siblings had little influence (Table S1). If environmental factors were strong, one would expect higher risks for siblings with smaller age differences. For chronic kidney failure, the familial concordant risks were high (Table 2). The familial concordant risks for acute and unspecified kidney failure were not significant (Table 2). However, increased discordant familial risks show that familial factors also are involved in acute and unspecified kidney failure (Table 2), though to a lower degree than for chronic kidney failure. Acute kidney failure is instead more related to precipitating factors such as sepsis, complex surgery, diagnostic procedures requiring intravenous contrast continue, and drug-induced kidney injury [20]. Unspecified kidney failure is probably a mixture of patients with chronic and acute kidney failure. No previous study has reported familial risks for acute and unspecified kidney failure.
Of interest is the high familial risk for acute renal failure among children younger than ten years (SIR = 14.21) and also children and teenagers between 10 and 19 years of age (SIR = 2.52) (Table 4). Among the children younger than 10 years, 2 were related to infection and one to preterm birth and or/low birth weight, which argue against a genetic cause for these cases of acute renal failure. Three cases were unknown and we cannot exclude that in rare cases familial factors are important among children and teenagers. A study from Norway of acute renal failure identified 315 cases of acute renal failure among children under the age of 16 years [30]. The estimated incidence rate was 3.3 cases per 100 000 children. This is in range of out overall kidney failure incidence rate among children (Table 1). Most cases (43%) in the Norwegian study were children under the age of five years [30]. The authors identified 53 aetiologies and classified these into 30 aetiological groups: 25% were prerenal failure (n = 75), 74% were intrinsic/renal failure (n = 234), and 2% were postrenal failure (n = 5). Nephritic syndromes was the most common cause (44%) of acute kidney failure, followed by haemolytic-uraemic syndrome (HUS) (15%) [30].
The present design has potential advantages and disadvantages. Strengths of the study include complete nationwide coverage from 1987 in a country with high standards of diagnosis, with diagnoses often being made by specialists during extended examinations in clinics. The Swedish hospital discharge register contains no information about diagnostic procedures, which is a limitation. Moreover, the validity of ICD codes for kidney disease has not been reported. However, the Swedish hospital discharge register has been extensively validated and its overall diagnostic validity is close to 90% [31]–[32]. A limitation is the inclusion of asymptomatic early stages of renal failure. The Swedish ICD-9 code 585 has no sub codes for different stages of ESRD. Thus, a number of patients with unidentified early stages of renal failure are not included in the study, which most likely is a non-differential bias with regards to familial risks. Another likely non-differential bias regarding familial risks is that cases in probands and relatives before 1987 are unknown. Moreover, the number of comorbidities is rather low (Table 1), possible due to that diagnosis made in primary health are not included. No nationwide primary health care register exists in Sweden.
Another important strength of our study is that it was based on nationwide registers and was thus free of selection and recall bias. The Swedish multi-generation register and the Swedish hospital discharge register are validated data sources that have been proven to be reliable in the study of many diseases [26], [31]. Data in our dataset are almost 100% complete [26].
In summary, the present study found indications of strong aggregation of chronic kidney failure, while familial factors are less important in acute and unspecified kidney failure. Familial non-genetic factors contribute among husbands but not wives. Identification of the unique familial factors in chronic kidney failure will advance our knowledge about the pathogenesis of kidney failure.
Supporting Information
Familial risk of concordant kidney failure among siblings by age at difference in siblings.
(DOCX)
Familial risk of concordant and discordant kidney failure in men and women.
(DOCX)
Familial risk (sibling/parent history) of concordant and discordant kidney failure in males and females by age at diagnosis.
(DOCX)
Familial risk (sibling/offspring) of concordant and discordant kidney failure in males and females, after excluding kidney cancer in parents/offspring.
(DOCX)
Familial risk of concordant and discordant kidney failure in males and females, follow-up 2001–2010.
(DOCX)
Acknowledgments
The authors wish to thank the CPF's Science Editor Stephen Gilliver for his useful comments on the text. The registers used in the present study are maintained by Statistics Sweden and the National Board of Health and Welfare.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its supporting information files.
Funding Statement
This work was supported by grants awarded to Dr. Bengt Zöller by the Swedish Heart-Lung Foundation, ALF funding from RegionSkåne awarded to Dr. Bengt Zöller and Prof. Kristina Sundquist, grants awarded to Prof. Kristina Sundquist by the Swedish Research Council (K2009-70X-15428-05-3 and K2012-70X-15428-08-3), and grants awarded to Prof. Jan Sundquist by the Swedish Council for Working Life and Social Research (2007-1754) and King Gustaf V and Queen Victoria's Foundation of Freemasons. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Levey AS, Coresh J (2012) Chronic kidney disease. Lancet 379:165–180. [DOI] [PubMed] [Google Scholar]
- 2. Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, et al. (2007) Chronic kidney disease as a global public health problem: approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72:247–259. [DOI] [PubMed] [Google Scholar]
- 3. Weiner DE, Tighiouart H, Amin MG, Stark PC, Macleod B, et al. (2004) Chronic kidney disease as a risk factor for cardiovascular disease and all-cause mortality: a pooled analysis of community-based studies. J Am Soc Nephrol 15:1307–1315. [DOI] [PubMed] [Google Scholar]
- 4. Satko SG, Freedman BI (2004) The importance of family history on the development of renal disease. Curr Opin Nephrol Hypertens 13:337–341. [DOI] [PubMed] [Google Scholar]
- 5. Satko SG, Sedor JR, Iyengar SK, Freedman BI (2007) Familial clustering of chronic kidney disease. Semin Dial 20:229–236. [DOI] [PubMed] [Google Scholar]
- 6. Witasp A, Nordfors L, Carrero JJ, Luttropp K, Lindholm B, et al. (2012) Genetic studies in chronic kidney disease: interpretation and clinical applicability. J Nephrol 25:851–864. [DOI] [PubMed] [Google Scholar]
- 7. Drawz PE, Sedor JR (2011) The genetics of common kidney disease: a pathway toward clinical relevance. Nat Rev Nephrol 7:458–468. [DOI] [PubMed] [Google Scholar]
- 8. O'Seaghdha CM, Fox CS (2011) Genome-wide association studies of chronic kidney disease: what have we learned? Nat Rev Nephrol 8:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Ferguson R, Grim CE, Opgenorth TJ (1988) A familial risk of chronic renal failure among blacks on dialysis? J Clin Epidemiol 41:1189–1196. [DOI] [PubMed] [Google Scholar]
- 10. Freedman BI, Spray BJ, Tuttle AB, Buckalew VM Jr (1993) The familial risk of end-stage renal disease in African Americans. Am J Kidney Dis 21:387–393. [DOI] [PubMed] [Google Scholar]
- 11. Lei HH, Perneger TV, Klag MJ, Whelton PK, Coresh J (1998) Familial aggregation of renal disease in a population-based case-control study. J Am Soc Nephrol 9:1270–1276. [DOI] [PubMed] [Google Scholar]
- 12. O'Dea DF, Murphy SW, Hefferton D, Parfrey PS (1998) Higher risk for renal failure in first-degree relatives of white patients with end-stage renal disease: A population-based study. Am J Kidney Dis 32:794–801. [DOI] [PubMed] [Google Scholar]
- 13. Bergman S, Key BO, Kirk KA, Warnock DG, Rostant SG (1996) Kidney disease in the first-degree relatives of African-Americans with hypertensive end-stage renal disease. Am J Kidney Dis 27:341–346. [DOI] [PubMed] [Google Scholar]
- 14. Jurkovitz C, Franch H, Shoham D, Bellenger J, McClellan W (2002) Family members of patients treated for ESRD have high rates of undetected kidney disease. Am J Kidney Dis 40:1173–1178. [DOI] [PubMed] [Google Scholar]
- 15. McClellan W, Speckman R, McClure L, Howard V, Campbell RC, et al. (2007) Prevalence and characteristics of a family history of end-stage renal disease among adults in the United States population: Reasons for Geographic and Racial Differences in Stroke (REGARDS) renal cohort study. J Am Soc Nephrol 18:1344–1352. [DOI] [PubMed] [Google Scholar]
- 16. Jurkovitz C, Hylton TN, McClellan WM (2005) Prevalence of family history of kidney disease and perception of risk for kidney disease: A population-based study. Am J Kidney Dis 46:11–17. [DOI] [PubMed] [Google Scholar]
- 17. Freedman BI, Soucie JM, McClellan WM (1997) Family history of end-stage renal disease among incident dialysis patients. J Am Soc Nephrol 8:1942–1945. [DOI] [PubMed] [Google Scholar]
- 18. Freedman BI, Volkova NV, Satko SG, Krisher J, Jurkovitz C, et al. (2005) Population-based screening for family history of end-stage renal disease among incident dialysis patients. Am J Nephrol 25:529–535. [DOI] [PubMed] [Google Scholar]
- 19. Hsu CY, Iribarren C, McCulloch CE Darbinian J, Go AS (2009) Risk factors for end-stage renal disease: 25-year follow-up. Arch Intern Med 169:342–350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Li PK, Burdmann EA, Mehta RL, World Kidney Day Steering Committee 2013 (2013) Acute kidney injury: global health alert. Transplantation 95:653–657. [DOI] [PubMed] [Google Scholar]
- 21. O'Seaghdha CM, Fox CS (2011) Genome-wide association studies of chronic kidney disease: what have we learned? Nat Rev Nephrol 8:89–99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Köttgen A, Glazer NL, Dehghan A, Hwang SJ, Katz R, et al. (2009) Multiple loci associated with indices of renal function and chronic kidney disease. Nat Genet 41:712–717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Köttgen A, Pattaro C, Böger CA, Fuchsberger C, Olden M, et al. (2010) New loci associated with kidney function and chronic kidney disease. Nat Genet 42:376–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Burton PR, Tobin MD, Hopper JL (2005) Key concepts in genetic epidemiology. Lancet 366:941–951. [DOI] [PubMed] [Google Scholar]
- 25.Lawlor DA, Mishra GD (2009) Family matters. Deigning, analyzing and understanding family based studies in life course epidemiology. New York: Oxford University Press. [Google Scholar]
- 26.Rosen M, Hakulinen T (2005) Use of disease registers. In: Handbook of epidemiology. Berlin: Springer-Verlag. pp.231–251.
- 27. Zöller B, Li X, Sundquist J, Sundquist K (2011) Age- and gender-specific familial risks for venous thromboembolism: a nationwide epidemiological study based on hospitalizations in Sweden. Circulation 124:1012–1020. [DOI] [PubMed] [Google Scholar]
- 28. Hemminki K, Vaittinen P, Dong C, Easton D (2001) Sibling risks in cancer: clues to recessive or X-linked genes? Br J Cancer 84:388–391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Breslow NE, Day NE (1987) Statistical methods in cancer research. Volume II–The design and analysis of cohort studies. IARC Sci Publ 82:1–406. [PubMed] [Google Scholar]
- 30.Jenssen GR, Hovland E, Bangstad HJ, Nygård K, Vold L, et al. (2014) The incidence and aetiology of acute kidney injury in children in Norway between 1999 and 2008. Acta Paediatr Jul 10. Epub ahead of print doi: 10.1111/apa.12742 [DOI] [PMC free article] [PubMed]
- 31.The National Board of Health and Welfare (2000) [Validity of the diagnoses from the Swedish In-Care Register 1987 and 1995]. In Swedish. Stockholm: Epidemiologiskt Centrum, National Board of Health and Welfare. [Google Scholar]
- 32. Ludvigsson JF, Andersson E, Ekbom A, Feychting M, Kim JL, et al. (2011) External review and validation of the Swedish national inpatient register. BMC Public Health 11:450. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Familial risk of concordant kidney failure among siblings by age at difference in siblings.
(DOCX)
Familial risk of concordant and discordant kidney failure in men and women.
(DOCX)
Familial risk (sibling/parent history) of concordant and discordant kidney failure in males and females by age at diagnosis.
(DOCX)
Familial risk (sibling/offspring) of concordant and discordant kidney failure in males and females, after excluding kidney cancer in parents/offspring.
(DOCX)
Familial risk of concordant and discordant kidney failure in males and females, follow-up 2001–2010.
(DOCX)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its supporting information files.