Abstract
In addition to biochemical gradients and transcriptional networks, cell behavior is regulated by endogenous bioelectrical cues originating in the activity of ion channels and pumps, operating in a wide variety of cell types. Instructive signals mediated by changes in resting potential control proliferation, differentiation, cell shape, and apoptosis of stem, progenitor, and somatic cells. Of importance, however, cells are regulated not only by their own Vmem but also by the Vmem of their neighbors, forming networks via electrical synapses known as gap junctions. Spatiotemporal changes in Vmem distribution among nonneural somatic tissues regulate pattern formation and serve as signals that trigger limb regeneration, induce eye formation, set polarity of whole-body anatomical axes, and orchestrate craniofacial patterning. New tools for tracking and functionally altering Vmem gradients in vivo have identified novel roles for bioelectrical signaling and revealed the molecular pathways by which Vmem changes are transduced into cascades of downstream gene expression. Because channels and gap junctions are gated posttranslationally, bioelectrical networks have their own characteristic dynamics that do not reduce to molecular profiling of channel expression (although they couple functionally to transcriptional networks). The recent data provide an exciting opportunity to crack the bioelectric code, and learn to program cellular activity at the level of organs, not only cell types. The understanding of how patterning information is encoded in bioelectrical networks, which may require concepts from computational neuroscience, will have transformative implications for embryogenesis, regeneration, cancer, and synthetic bioengineering.
All these facts, sufficiently numerous, ... will open a very wide field of reflection, and of view, not only curious, but particularly interesting to medicine. There will be a great deal to occupy the anatomist, the physiologist, and the practitioner.
Allesandro Volta (1800), communicating to the Royal Society his invention of the electric battery
INTRODUCTION
Cell behavior is regulated by numerous distinct cues that impinge on them in vivo. Alongside chemical gradients (Huang et al., 2005; Geard and Willadsen, 2009; Niehrs, 2010; Ben-Zvi et al., 2011; Gershenson, 2012) and physical forces (Beloussov and Grabovsky, 2006; Beloussov, 2008; Nelson, 2009; von Dassow and Davidson, 2011; Davidson, 2012), cell activity is orchestrated toward the creation and repair of high-order anatomical structures by a set of bioelectrical cues (Levin, 2012a, b; Levin and Stevenson, 2012). Here bioelectricity refers to endogenous electrical signaling via ion channels and pumps at the plasma membrane; specifically excluded due to length constraints is the rich literature on external electromagnetic fields (Funk et al., 2009; Cifra et al., 2011; Hronik-Tupaj and Kaplan, 2012), ultraweak photon emission (Farhadi et al., 2007; Fels, 2009; Sun et al., 2010; Beloussov, 2011), and subcellular organelle potentials (Bustamante et al., 1995; Mazzanti et al., 2001; Yamashita, 2011).
The importance of bioelectricity for cells beyond excitable nerve and muscle was realized long ago, and solid functional data implicate steady ion currents in embryogenesis and wound healing (Burr and Northrop, 1935; Lund, 1947; Jaffe and Nuccitelli, 1977; Nuccitelli et al., 1986; Borgens et al., 1989; Hotary and Robinson, 1992). By tracking developmental currents and applying physiological-strength electric fields, it was shown that transepithelial electric fields regulate cell migration, orientation, and nerve growth (Jaffe and Poo, 1979; Patel and Poo, 1982; Borgens et al., 1987; McCaig et al., 2005; Nishiyama et al., 2008; Cao et al., 2011, 2013; Ozkucur et al., 2011; Pullar, 2011; Reid et al., 2011b; Vieira et al., 2011; Pan and Borgens, 2012; Zhao et al., 2012; Yamashita, 2013). However, recent advances and development of molecular-level techniques (Adams, 2008; Adams and Levin, 2013; Levin, 2013; Tseng and Levin, 2013) have identified a new aspect of bioelectricity that regulates individual cell function and helps coordinate the embryogenesis and regenerative repair of complex structures. This review focuses on the instructive cues mediated by spatiotemporal patterns of voltage potentials across the membranes (Vmem; Figure 1A) of nonneural cells and the roles these play in coordinating cell behavior during regeneration, development, and cancer.
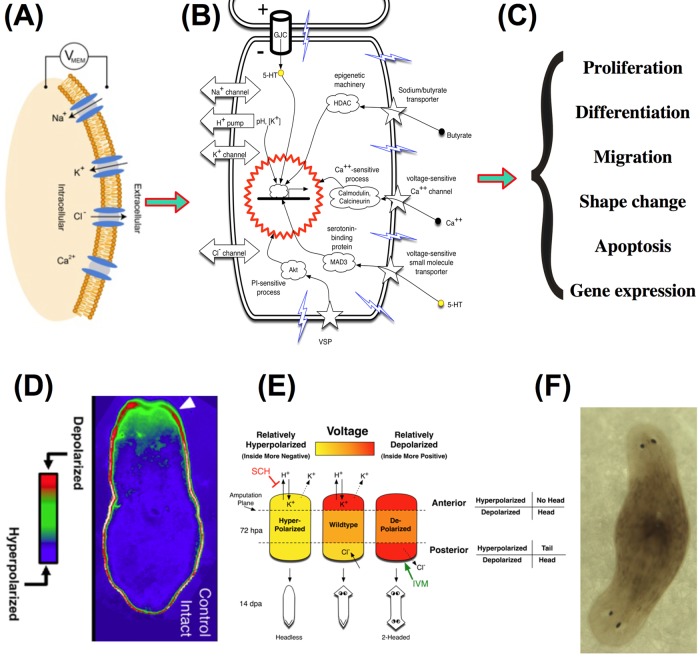
FIGURE 1:
Bioelectrical signaling at the cell and organism levels, At the level of single cells, bioelectrical signals are produced by ion channel proteins, transduced into second-messenger responses, and alter key aspects of cell behavior. (A) The voltage potential (Vmem) at the cell membrane is produced by the movement of ions through across a cell membrane. Ions move via many different ion channels and pumps, under the control of concentration and electric gradients. (B) Change of Vmem is transduced into cellular effector cascades by a range of mechanisms, including voltage-sensitive phosphatases, voltage-gated calcium channels, and voltage-sensitive transporters of signaling molecules such as serotonin and butyrate. (Diagram modified, with permission, from Figure 1B of Levin, 2007.) (C) Bioelectrical signals feed into epigenetic and transcriptional cascades and thus trigger changes in cell properties such as proliferation, differentiation, migration, shape change, and programmed cell death. (D) Voltage reporter dye reveals gradients of Vmem across the anterior-posterior axis of planarian flatworms. (Taken, with permission, from Figure 2B of Beane et al., 2013.) (E) In amputated worms, a circuit composed of proton and potassium conductances sets the voltage states at each blastema, which in turn determines the anatomical identity of each end of a regenerating fragment. (Diagram taken, with permission, from Figure 7C of Beane et al., 2011.) (F) Manipulating this circuit in amputated planaria using pharmacological or genetic techniques that target ion flux allows the programming of stem cell–mediated morphogenesis to specific anatomical outcomes, such as the creation of two-head animals shown here.
NEW CONTROL KNOBS: RESTING POTENTIAL DETERMINES SINGLE-CELL STATE
In general, terminally differentiated, quiescent cells tend to be strongly polarized (bearing a more-negative resting potential), whereas embryonic, stem, and tumor cells tend to be depolarized (closer to zero; Binggeli and Weinstein, 1986). The picture is complicated by two still poorly understood factors: the relationship of overall Vmem state to the cell cycle–dependent (sinusoidally varying) changes in voltage potential (Arcangeli et al., 1995; Higashimori and Sontheimer, 2007; Aprea and Calegari, 2012) and the fact that many cells in fact do not have a single Vmem but bear a set of distinct voltage domains over their surface (O'Connell and Tamkun, 2005; O'Connell et al., 2006; Levin, 2012a).
Crucially, Vmem is not simply a readout but is also a functional determinant of cell behavior, such as proliferative state and plasticity (Table 1), due to a number of mechanisms that functionally couple voltage potential changes to downstream cascades (Figure 1, B and C). These data derive from genetic experiments, as well as pharmacological screens designed to identify compounds that regulate stem cell differentiation or cancer progression (Alves et al., 2011; Sun et al., 2013). Differentiation and proliferation are controlled by changes in Vmem, as shown in human mesenchymal stem cells (Sundelacruz et al., 2008, 2013; You et al., 2012), cardiomyocytes (Lan et al., 2014), inhibitory postsynaptic currents (Jiang et al., 2009), vascular muscle (Jia et al., 2013), embryonic stem cells (Ng et al., 2010; Du et al., 2013), myoblasts (in which hyperpolarization driven by the Kir2.1 channel plays a key role; Hinard et al., 2008; Li et al., 2010), the specification of neurotransmitter types (Root et al., 2008), and the control of precursor differentiation (van Vliet et al., 2010; Yasuda and Adams, 2010; Lange et al., 2011; Liebau et al., 2011; Ring et al., 2012; Podda et al., 2013) in the developing nervous system and heart. Given the known roles of Vmem in regulating normal migration, differentiation, and proliferation (Aprea and Calegari, 2012; Ding et al., 2012; Inaba et al., 2012; Zhang et al., 2012; Cao et al., 2013; Yamashita, 2013), it is not surprising that control of ion flux (Park et al., 2008; House et al., 2010) and membrane voltage (Morokuma et al., 2008a; Blackiston et al., 2011; Chernet and Levin, 2013a, 2013b; Yang and Brackenbury, 2013) are also increasingly implicated in the cell dysregulation of cancer (Table 2).
TABLE 1:
Cell-level properties/behaviors controlled by bioelectric events.
TABLE 2:
Ion translocators implicated in cancer.
| Ion translocator protein | Species | References | Function |
|---|---|---|---|
| NaV1.5 sodium channel | Human | Onkal and Djamgoz (2009), House et al. (2010) | Oncogene |
| KCNK9 potassium channel | Mouse | Pei et al. (2003) | Oncogene |
| Ductin (proton V-ATPase component) | Mouse | Saito et al. (1998) | Oncogene |
| SLC5A8 sodium/butyrate transporter | Human | Gupta et al. (2006) | Oncogene |
| KCNE2 potassium channel | Mouse | Roepke et al. (2010) | Oncogene |
| KCNQ1 potassium channel | Human, mouse | Lee et al. (1997), Weksberg et al. (2001), Than et al. (2013) | Oncogene |
| SCN5A voltage-gated sodium channel | Human | House et al. (2010) | Oncogene |
| Metabotropic glutamate receptor | Mouse, human | Song et al. (2012), Speyer et al. (2012), Martino et al. (2013) | Oncogene |
| CFTR chloride channel | Human | Xie et al. (2013), Zhang et al. (2013) | Tumor suppressor |
| Connexin43 | Human | Sirnes et al. (2012) | Tumor suppressor |
| Acetylcholine receptor | Mouse | Felder et al. (1993) | Tumor suppressor |
Bioelectric cues also provide spatially patterned signals to cells. The differential activation of voltage-responsive transduction mechanisms on opposite sides of a cell allows bioelectric signals to regulate cell polarity. This was long ago shown in the symmetry breaking and control of outgrowth point in the algae Fucus (Jaffe, 1966, 1968) and has been recently shown using high-resolution imaging and genetic techniques in yeast (Minc and Chang, 2010) and pollen tubes (Certal et al., 2008; Michard et al., 2009). The cytoskeleton is one target of such signaling (Chifflet et al., 2003; Priel et al., 2006; Sekulic et al., 2011; Campetelli et al., 2012). Positional information can likewise be dictated by voltage properties of cells (Baglioni et al., 2012) and their neighbors (Shi and Borgens, 1995). Studies of embryonic left–right patterning of the Xenopus embryo have revealed how bioelectrical processes link individual cell dynamics to axial patterning of the entire body plan (Levin and Palmer, 2007; Aw and Levin, 2009): cytoskeletal chirality within the fertilized egg drives asymmetric distribution of ion transporter proteins in the early blastomeres, and the resulting gradient drives unidirectional (preneural) serotonin flow through cell fields, eventually triggering differential gene expression on the left versus right sides of the body (Levin, 2006; Levin et al., 2006; Aw et al., 2008; Lobikin et al., 2012b; Vandenberg et al., 2012, 2013). The dissection and synthesis of such systems at the genetic and physiological levels is beginning to reveal the properties of biophysical pathways by which individual cell polarity is integrated into large-scale patterning outcomes (Marshall, 2011).
MEASURING VMEM IN VIVO
The first step in analyzing a bioelectric signal is the characterization of the spatiotemporal distributions of ionic parameters and a determination of how they correlate with patterning events. Vmem in cells can be quantified using several approaches; unlike mRNA and protein levels revealed by sequencing or immunohistochemistry, bioelectric properties are only ascertainable in vivo and cannot be analyzed in fixed tissue. Voltage gradients can now be visualized continuously in situ using fluorescent reporters of transmembrane potential (Adams and Levin, 2012a, b; Figure 1D) and more exotic nanoscale materials (Tyner et al., 2007) suitable for use in any optically accessible tissue (Steinberg et al., 2007; Yun et al., 2007). These are a significant improvement on physiological impalement of single cells: far less invasive, and able to report multiple Vmem values across tissues and even within cell membrane subdomains (Lechleiter et al., 1991; Adams and Levin, 2013). Reagents include cell-permeant dyes such as CC2-DMPE and DiSBAC2(3) (Adams et al., 2006; Adams and Levin, 2012b; Oviedo et al., 2008; Ozkucur et al., 2010) and genetically encoded protein reporters (Tsutsui et al., 2008; Mutoh et al., 2011; Shen et al., 2011; Akemann et al., 2012).
Additional tools for the characterization of bioelectrical events include highly sensitive ion-selective extracellular electrode probes (Reid et al., 2007; Smith et al., 2007) that reveal ion flux, microelectrode arrays (Aryasomayajula et al., 2010; Schonecker et al., 2014), and reporters of individual ion species such as protons (Tantama et al., 2011) and sodium (Tseng et al., 2010; Dubach et al., 2011a, b). Significant opportunities exist for the development of specific, bright, ratiometric dyes that localize exclusively to the desired subcellular locale (e.g., plasma membrane or nucleus). Especially exciting will be the use of multiple physiological dyes in fluorescence-activated cell sorting experiments to identify subpopulations of “pure” stem and other cell types that differ in key bioelectric properties (Mello de Queiroz et al., 2008), as has been observed for human endothelial cells (Yu et al., 2002). Of importance, such experiments on dissociated cells will clearly highlight properties that are cell autonomous versus those physiological conditions that can only be maintained within a group context.
BIOELECTRIC SIGNALS INTERFACE WITH MOLECULAR GENETICS
The mechanistic investigation of bioelectric cues and their interactions with canonical biochemical pathways has been enriched by several new functional techniques (Adams and Levin, 2006b, 2013; Reid et al., 2007; Song et al., 2007). The comprehensive workflow for probing developmental bioelectricity can be illustrated by two examples. In the first, a tiered pharmacological screen (Adams and Levin, 2006a) implicated a proton pump and two channels as specifically required for tail regeneration but not for wound healing or development of the primary tail (Adams et al., 2007). These loss-of-function data were confirmed using reagents with molecular specificity by misexpression of a dominant-negative form of a V-ATPase subunit protein. Marker analysis was used to show why tails failed to regenerate in V-ATPase–inhibited tails (loss of regeneration-specific gene up-regulation, lack of the obligate increase of mitosis near the wound, and abrogation of innervation into the regenerate). Fluorescent dye imaging provided physiomic profiling of the changes of Vmem during the stages of regeneration and confirmed that the unique voltage changes characteristic of the regenerating state were blocked by V-ATPase inhibition and were absent during stages at which tadpoles normally are not competent to regenerate their tails. On the basis of these findings, to develop a gain-of-function application, a yeast P-type proton pump was misexpressed in regeneration-incompetent animals, leading to restoration of mitosis, gene expression (MSX-1, Notch), innervation, and morphological regeneration of a complete tail. Additional rescue experiments using net-electroneutral proton exchangers allowed the independent testing of pH versus voltage signaling.
One key result was that the anatomical outcome (regeneration rescue) can be induced by a completely heterologous hyperpolarizing pump, which has no sequence or structural homology to the native Xenopus protein endogenously driving regeneration. This demonstrated that the necessary and sufficient trigger for regeneration is not a specific gene product (V-ATPase), but a bioelectrical state, which can be implemented using a variety of different reagents. This finding facilitated development of a purely pharmacological method of modulating ion flows in the wound to induce tail (Tseng et al., 2010) and leg (Tseng and Levin, 2013) regeneration without the need for gene therapy.
The available tools enable a multistep strategy that combines pharmacological screening, physiological imaging, and molecular-genetic tools to generate loss- and gain-of-function data showing how a bioelectric pathway normally works and how it can be exploited to trigger pattern formation. A similar approach was taken with an initial gain-of-function screen, misexpressing ion channels in frog embryogenesis. One of the outcomes was the finding that a specific Vmem range was necessary and sufficient to trigger ectopic eye development (Pai et al., 2012). Dye imaging data showed that the location of the endogenous eyes is demarcated by a prepattern of Vmem states in the anterior neurectoderm and that experimental alteration of this prepattern results in abnormal craniofacial gene expression and eye and facial malformations (Vandenberg, 2011; Pai et al., 2012). To complement the data showing that bioelectric states are an endogenous component of eye development, it was then shown that driving eye-specific Vmem states in other body regions (by misexpression of ion channels) was sufficient to induce anatomically complete (well-formed) ectopic eyes (Figure 2A). Marker analysis revealed that this occurs via establishment of a positive feedback loop between hyperpolarization and Rx1/Pax6 expression, whereas a suppression screen of transduction mechanisms implicated voltage-gated calcium signaling as the transduction mechanism. However, note that, by themselves, “master” eye genes such as Pax6 do not produce eyes outside the head in vertebrates (Chow et al., 1999). Moreover, as with the tail, individual cell types appropriate to the eye did not have to be specified. Together these data revealed the unique properties of bioelectric triggers to reprogram body regions at the level of organ identity and overcome lineage specification limits observed with biochemical inducers.
FIGURE 2:
Bioelectric properties specify instructive, non–cell-autonomous patterning cues. (A) Targeted Vmem change, via misexpression of ion channels in the frog embryo, induces the formation of ectopic structures such as complete eyes, even in regions normally not competent to form eyes (such as on the gut). (Used, with permission, from Figure 3G of Pai et al., 2012.) (B) Tracking the ion channel expression using a lineage marker reveals that the effect is not cell-autonomous: in a lens created in the tail of a tadpole by ion channel expression, only about half of the ectopic cells express the heterologous ion channel (revealed by blue lacZ staining); the other half of the induced structure consists of host cells recruited to participate in making the appropriate shape but not themselves targeted by the Vmem-altering reagent. (C) Melanocytes seen in a cross section of a Xenopus tadpole are normally few in number, round, and confined to their normal locations. (D) Depolarization induced by ion channel modulation induces these cells to overproliferate, acquire an elongated shape, and invade many organs (red arrow). Of importance, this effect is also not cell autonomous, as seen in the melanocyte phenotype, which results when cells (marked by ion channel expression construct lineage label in blue) are depolarized at a considerable distance from the melanocytes. (Taken, with permission, from Figure 6A of Chernet and Levin, 2013b.) (E) A normal planarian has a head and tail and regenerates each at the appropriate end of an amputated fragment. When it is cut into thirds and the middle fragment is briefly exposed to octanol, which temporarily blocks long-range bioelectrical signaling between the wound and mature tissues, a two-headed worm results (F). Remarkably, upon further rounds of cutting in plain water (long after the octanol has left the tissues, as confirmed by HPLC), the two-headed form results (H, I; images of two-headed worms provided by Fallon Durant, Tufts University, Medford, MA). This change in the animal's target morphology (the shape to which it regenerates upon damage) appears to be permanent and persists across the animal's normal reproductive mode (fissioning), despite the fact that the genomic sequence has not been altered. Chromatin modifications alone do not explain this, because the posterior wound cells, which could have been epigenetically reprogrammed to a head fate, are discarded at each cut: the information encoding a bipolar two-head animal is present even in the normal gut fragment—it is distributed throughout the body. We propose that this information is a kind of memory, encoded in electrical networks of somatic cells coupled by gap junctions, and is stored at the level of bioelectrical dynamics. (E–I taken, with permission, from Figure 2 of Levin, 2014; photographs of planaria taken by Taisaku Nogi, Children's Health Research Institute, Canada, and Fallon Durant.)
Of interest, many forward genetic approaches have identified ion channel genes responsible for patterning phenotypes, as have unbiased transcriptional network analyses in development (Langlois and Martyniuk, 2013) and cancer (House et al., 2010). These include patterning of the face, limb, brain, and viscera in a range of model systems and a number of channelopathies that form an important class of human birth defects (Table 3). Thus upstream of endogenous bioelectrical signaling lie a set of ion channel and pump proteins that establish resting potential and alter it in response to physiological, transcriptional, and mechanical signals. Such data often come from studies that, unlike the previously discussed two examples, did not set out to investigate bioelectricity, and the overall structure of developmental bioelectric signaling is starting to emerge from the synthesis of bioelectric projects investigating molecular mechanisms and molecular biology efforts that implicate ion channel activity in instructive roles.
TABLE 3:
Ion translocators implicated in patterning by genetic approaches.
| Protein | Morphogenetic role or loss-of-function phenotype | Species | References |
|---|---|---|---|
| TMEM16A chloride channel | Tracheal morphogenesis | Mouse | Rock et al. (2008) |
| Kir7.1 potassium channel | Melanosome development | Zebrafish | Iwashita et al. (2006) |
| Cx41.8 gap junction | Pigmentation pattern | Zebrafish | Watanabe et al. (2006) |
| Cx45 gap junction | Cardiac defects (cushion patterning) | Mouse | Kumai et al. (2000), Nishii et al. (2001) |
| Cx43 gap junction | Oculodentodigital dysplasia, heart defects (outflow tract and conotruncal), left–right asymmetry defects, eye defect, osteoblast differentiation in bone patterning, syndactyly, microphthalmia | Human, mouse | Britz-Cunningham et al. (1995), Reaume et al. (1995), Ewart et al. (1997), Pizzuti et al. (2004), Debeer et al. (2005), Civitelli (2008), Zoidl and Dermietzel (2010), Gabriel et al. (2011) |
| Kir2.1 potassium channel | Wing patterning | Drosophila | Dahal et al. (2012) |
| Cx43 gap junction | Fin size and pattern regulation; craniofrontonasal syndrome | Zebrafish, mouse | Iovine et al. (2005), Davy et al. (2006), Hoptak-Solga et al. (2008), Sims et al. (2009) |
| Kir2.1 potassium channel | Andersen–Tawil syndrome, craniofacial and limb defects | Mouse, human | Bendahhou et al. (2003), Dahal et al. (2012) |
| CFTR chloride channel | Bilateral absence of vas deferens | Human | Uzun et al. (2005), Wilschanski et al. (2006) |
| KCNK9, TASK3 potassium channels | Birk–Barel dysmorphism syndrome, craniofacial defects | Human | Barel et al. (2008), Veale et al. (2014) |
| Girk2 potassium channel | Cerebellar development, retina patterning | Mouse | Rakic and Sidman (1973a, b), Hatten et al. (1986), Patil et al. (1995), Tong et al. (1996), Savy et al. (1999), Liesi et al. (2000) |
| GABA-A receptor (chloride channel) | Angelman syndrome, craniofacial patterning (e.g., cleft palate) and hand defects | Mouse, human | Wee and Zimmerman (1985), Culiat et al. (1995), Homanics et al. (1997) |
| KCNH2 K+ channel | Cardiac patterning | Mouse | Teng et al. (2008) |
| NHE2 Na+/H+ exchanger | Epithelial patterning | Drosophila | Simons et al. (2009) |
| V-ATPase proton pump | Wing-hair patterning, pigmentation and brain patterning, left–right asymmetry, eye development, tail regeneration, craniofacial patterning | Drosophila, medaka, human, chick, Xenopus, zebrafish | Hermle et al. (2010), Muller et al. (2013), Borthwick et al. (2003), Adams et al. (2006), Nuckels et al. (2009), Vandenberg et al. (2011), Monteiro et al. (2014) |
| Kv channel | Fin-size regulation | Zebrafish | Perathoner et al. (2014) |
| KCNQ1 potassium channel | Abnormalities of rectum, pancreas, and stomach, left–right patterning, Jervell and Lange-Nielsen syndrome, inner ear and limb defects | Mouse, Xenopus | Chouabe et al. (1997), Casimiro et al. (2004), Rivas and Francis (2005), Morokuma et al. (2008b), Than et al. (2013) |
| Kir6.2 potassium channel | Craniofacial defects, left–right patterning | Human, Xenopus | Gloyn et al. (2004), Aw et al. (2010) |
| NaV 1.5, Na+/K+-ATPase | Cardiac morphogenesis | Zebrafish | Shu et al. (2003), Chopra et al. (2010) |
| H+,K+-ATPase | Left–right patterning, polarity during regeneration | Xenopus, chick, sea urchin, zebrafish, planaria | Levin et al. (2002), Kawakami et al. (2005), Aw et al. (2008), Beane et al. (2011) |
| Innexin gap junctions | Foregut, cuticle (epithelial) patterning defects | Drosophila | Bauer et al. (2002), Bauer et al. (2004) |
| TRH1 K+ transporter | Root-hair patterning | Arabidopsis | Rigas et al. (2001) |
Downstream of voltage change lie two types of endpoints—at the mRNA and chromatin modification levels. Transcriptional responses to depolarization include genes such as Notch, BMP, Sox10, Nurr1, Slug, Fos, Jun, NPY, and Wnt (Bartel et al., 1989; Higuchi et al., 1990; Raya et al., 2004; Morokuma et al., 2008a; He et al., 2011; Lange et al., 2011; Tseng et al., 2011; Dahal et al., 2012; Swapna and Borodinsky, 2012; Adams et al., 2013). Epigenetic responses are triggered by movement of butyrate through an ion-dependent transporter, SLC5A8; butyrate is an HDAC1 inhibitor, and this allows voltage change to regulate chromatin acetylation (Davie, 2003; Tong et al., 2004; Gupta et al., 2006). This is believed to mediate control of tumorigenesis by depolarization and is also implicated in bioelectrical signaling during tail regeneration in Xenopus (Tseng et al., 2011; Chernet and Levin, 2013a, 2014).
A set of transduction mechanisms has been identified by which changes of resting potential affect events at the nucleus (Figure 1, B and C, and Table 4). One involves voltage-gated calcium channels, which convert voltage change into signaling via this versatile second-messenger molecule (Nilius et al., 1993; Dolmetsch et al., 1998; Nakanishi and Okazawa, 2006; Greer and Greenberg, 2008). This mode has been implicated in control of growth-cone turning (Nishiyama et al., 2008), eye patterning (Pai et al., 2012), and flatworm regeneration (Nogi et al., 2009; Beane et al., 2011; Zhang et al., 2011). Another uses the voltage gradients among cells to move small signaling molecules such as serotonin through gap junction–coupled cell fields, as occurs in left–right patterning (Fukumoto et al., 2005b; Adams et al., 2006) and control of neuronal pathfinding (Blackiston et al., 2015). Finally, voltage-sensitive phosphatases couple Vmem change to the plethora of events regulated by PTEN phosphatases (Murata et al., 2005; Okamura and Dixon, 2011).
TABLE 4:
Known transduction mechanisms by which ion flows affects cell behavior.
| Developmental role | Key biophysical event | Transduction mechanism | References |
|---|---|---|---|
| Tail regeneration in Xenopus: first step | Voltage change (repolarization) | Guidance of neural growth | Adams et al. (2007) |
| Tail regeneration in Xenopus: second step | Intracellular sodium content | SIK2 (salt-inducible kinase) | Tseng et al. (2010) |
| Neoplastic conversion of melanocytes in Xenopus tadpoles | Voltage change (depolarization) | Serotonin movement | Morokuma et al. (2008a), Blackiston et al. (2011) |
| Polarity determination in planarian regeneration, length control of zebrafish fin | Voltage change | Ca2+ flux through voltage-gated calcium channel | Beane et al. (2011), Zhang et al. (2011), Chan et al. (2014), Kujawski et al. (2014) |
| Left–right patterning in Xenopus embryos, melanocyte transformation toward metastatic behavior | Voltage change | Serotonin movement | Levin et al. (2002), Fukumoto et al. (2005a, b), Adams et al. (2006), Blackiston et al. (2011), Lobikin et al. (2012a) |
| Trachea size control in Drosophila | Ion-independent function | Planar polarity, septate junction structure | Paul et al. (2007) |
Of interest, when they conflict, bioelectrical cues tend to trump chemical signals. One example is the guidance of cell motility: if a chemical gradient and an electric field are set up in opposite directions, the bioelectric vector trumps the chemical cue in directing cell movement (Zhao, 2009; Cao et al., 2011). Another example is the differentiation of human mesenchymal stem cells (hMSCs), which normally hyperpolarize as they differentiate; despite the presence of potent chemical inducers, hMSCs will not differentiate if kept artificially depolarized (Sundelacruz et al., 2008). Indeed, the voltage state can even partially reverse the differentiation state, inducing plasticity in differentiated hMSCs (Sundelacruz et al., 2013).
By identifying the specific ion channel genes that set Vmem states, the transduction mechanisms that sense Vmem change, and the downstream transcriptional or epigenetic targets (which include ion channels themselves), recent work has established the causal chain integrating bioelectrical cues with chemical pathways (Table 5). Neither signaling mode is entirely “upstream” of the other—cellular processes are regulated by the continuous cyclical interplay between transcriptional control of ion channel profiles within cells and the regulation of transcription by voltage dynamics. Future work will identify new ion channel genes important for specific functions, additional transduction mechanisms by which cells sense their depolarization and hyperpolarization, and genome-wide (next-generation sequencing [NGS] or microarray) profiles of transcriptional programs triggered by specific Vmem change.
TABLE 5:
Data on endogenous bioelectric signal roles in morphogenesis.
| Role | Species/system | References |
|---|---|---|
| Cellular polarization (anatomical asymmetry of cell or epithelium) | Alga Fucus, yeast | Jaffe (1982), Minc and Chang (2010) |
| Migration of neurons and positional information | Chick, amphibia | Shi and Borgens (1995), Pan and Borgens (2010) |
| Patterning in gastrulation, neurulation, and organogenesis | Chick, axolotl, frog | Stern (1982), Hotary and Robinson (1992), Borgens and Shi (1995), Shi and Borgens (1995), Levin et al. (2002), Adams et al. (2006) |
| Directional transport of maternal components into the oocyte | Moth, Drosophila | Woodruff (2005) |
| Growth control and size determination | Segmented worms | Kurtz and Schrank (1955) |
| Neural differentiation | Xenopus embryo | Uzman et al. (1998), Lange et al. (2011) |
| Polarity during regeneration | Planaria, plants, and annelids | Marsh and Beams (1947, 1949, 1950, 1952), Marsh and Beams (1957), Bentrup et al. (1967), Novák and Bentrup (1972), Novak and Sirnoval (1975), Beane et al. (2011) |
| Induction of limb and spinal cord regeneration | Amphibia | Borgens (1986), Borgens et al. (1986, 1990) |
| Control of gene expression and anatomy in craniofacial patterning | Xenopus embryo | Vandenberg et al. (2011) |
| Induction of eye development | Xenopus embryo | Pai et al. (2012) |
Of importance, however, Vmem regulation extends beyond the state of single cells. Cells can sense the voltage states of their neighbors through gap junctions (GJs)—versatile (and themselves voltage-sensitive) channels allowing the direct sharing of current and other small molecules between cells (Palacios-Prado and Bukauskas, 2009; Pereda et al., 2013). The importance of GJ-mediated cues for cellular decision making has been shown, for example, in the development of the neocortex (Sutor and Hagerty, 2005) and more broadly in setting up the patterns of chemical synapses (Anava et al., 2013). Cells can also read the bioelectrical state of distant regions via the chemical molecules redistributed (and transported or diffused) across long distances by bioelectric state change. This was long ago suggested by Burr, who used voltage readings at remote locations of the body to detect transplanted or induced tumors (Burr et al., 1940; Burr, 1941). Recent data in the frog model implicate long-range signaling via bioelectrical control of butyrate (Chernet and Levin, 2014) and serotonin (Blackiston et al., 2011; Lobikin et al., 2012a) in tumorigenesis and metastatic induction. Additional modes for nonlocal bioelectrical signaling include tunneling nanotubes (Chinnery et al., 2008; Wittig et al., 2012) and exosomes, which contain numerous ion channels (Lotvall and Valadi, 2007; Valadi et al., 2007; Wahlgren et al., 2012) and could regulate bioelectric states of cells that incorporate them. Because bioelectrical gradients mediate signaling beyond the single-cell level, they form a versatile medium for carrying information.
BIOELECTRIC STATES CAN ACT AS NECESSARY, SUFFICIENT, AND INSTRUCTIVE PATTERNING SIGNALS
Spatiotemporal gradients of Vmem among cells in vivo are now known to regulate organ identity, positional information, size control, and polarity of anatomical axes. One mode of Vmem signaling is as a prepattern. Much like Hox genes, whose combinatorial patterns of gene expression encode specific body regions during development, it has recently been shown that bioelectric prepatterns in the developing face of the frog and planarian models regulate the gene expression, size, and shape of craniofacial components (Vandenberg et al., 2011; Beane et al., 2013). In the frog, for example, patterns of hyperpolarization in the nascent face reveal the prospective locations of the eyes and other structures; experimental perturbation of these distributions alters the boundaries of expression of face patterning genes such as Frizzled, with the expected effects on craniofacial anatomy. Bioelectric gradients also specify orientation of the left–right axis in frog and chick embryos (Levin et al., 2002; Adams et al., 2006) and set the size of regenerating structures in segmented worms and regenerating zebrafish tails (Kurtz and Schrank, 1955; Beane et al., 2013; Perathoner et al., 2014). Ion transporters, such as the V-ATPase, are required for normal left–right patterning in several vertebrate models (Adams et al., 2006), zebrafish fin regeneration (Monteiro et al., 2014), and zebrafish eye development (Nuckels et al., 2009). These examples illustrate that bioelectric patterns can be necessary aspects of development because, when they are specifically disrupted, predictable and coherent changes in morphogenesis occur. Of importance, many of these data sets used distinct ion species (potassium, sodium, chloride, or protons) to show that the necessary parameter is indeed the voltage potential, not any one channel gene (which could have had scaffold or binding roles) or even any one ion type (which could have had chemical, not electrical, roles). As with the gain-of-function examples discussed later, the voltage is what matters for the outcome, not which ion or channel was used to set it.
In addition to specifying directly the pattern of subsequent anatomy, some bioelectric signals seem to trigger whole developmental modules. In the case of tail regeneration in Xenopus, genetic, optogenetic, and pharmacological experiments have been used to recapitulate a regeneration-specific bioelectric state in nonregenerative animals and induce complete regrowth of this complex neuromuscular appendage (Adams et al., 2007; Tseng et al., 2010). Not only could appropriate Vmem state overcome physiological, chemical, and age-dependent blockade of regenerative capacity, but it was seen that a very simple (low information content) stimulus, such as “pump protons,” could be sufficient to trigger a complete and self-limiting cascade of events that rebuilt the appendage (Tseng and Levin, 2013), in essence providing a “build whatever normally goes here” signal. These examples reveal that bioelectric state can function as a sufficient signal or master regulator; this bodes well for the use of this approach in regenerative medicine, as we may not need to micromanage the morphogenesis of complex structures but instead rely on patterning subroutines already present in the host.
Bioelectric signals can also set the identity of whole embryonic regions to different organs. The morphogenesis of new regeneration blastemas in planaria (Figure 1, D–F) can be directed to make heads or tails by appropriate modulation of resting potential (Beane et al., 2011, 2013). In vertebrates, whole-eye formation can be induced ectopically, far outside the head, even in mesoderm or endoderm (Figure 2A) by misexpression of specific ion channels in vivo (Pai et al., 2012); this process is mediated by a feedback loop between hyperpolarization and expression of eye-specific genes such as Rx1 and Pax6, which in its absence cannot initiate eye formation outside of the head. It is also interesting that this signaling is not cell autonomous: cells with unique voltage characteristics serve as organizers, recruiting wild-type host tissues to participate in the ectopic morphogenesis (Figure 2B).
These examples illustrate the fact that bioelectric state provides instructive information to patterning processes and reveal that cell groups can be programmed at the level of complex organs, not only at the level of specifying individual cell types. Understanding in detail the mapping between bioelectric states and the anatomical outcomes—quantitatively cracking the bioelectric code—is a major open direction in this field. Possibilities for the parameters that functionally determine distinct organ types include spatial distribution of absolute Vmem values within a cell group, relative differences in Vmem across cell borders, and/or time-dependent changes of Vmem within cells. One technology that is likely to be instrumental in testing hypotheses about the bioelectric code is optogenetics (Knopfel et al., 2010; Liu and Tonegawa, 2010), which will facilitate the reading and writing of bioelectric patterning information in vivo. The first steps have been taken, showing regulation of stem cells via optogenetic signaling (Stroh et al., 2010; Wang et al., 2014), and a recent report showed the induction of tail regeneration by optical modulation of bioelectric state after amputation (Adams et al., 2013).
BIOELECTRICITY DOES NOT REDUCE TO MOLECULAR GENETICS
The information-bearing signal (the necessary and sufficient trigger) for events such as eye induction, head determination, and tail regeneration via Vmem change is a physiological state, not a gene product (Levin, 2013; Tseng and Levin, 2013). Studies reveal that the exact identity of the channel or pump used to trigger such morphological changes is often irrelevant—many sodium, potassium, chloride, or proton conductances can be used, as long as the appropriate Vmem state is reached. This means that the actual cause of the given morphological change can be a bioelectrical property not necessarily in 1:1 correspondence with any genetic locus.
Because channels and pumps can open and close posttranslationally, two cells expressing precisely the same mRNA and protein can be in very different bioelectrical states. Thus rich patterns of bioelectrical gradients can exist in a transcriptionally homogeneous tissue and be completely invisible to protein and mRNA profiling until they trigger distinct downstream transcriptional targets. Conversely, cells with very different channel and pump complements may have the same Vmem, since resting potential is an ensemble state that is a function of many different ion flows. The implication is that mRNA and protein profiling approaches are insufficient to detect and characterize important biophysical determinants of morphogenesis, and knockout screens may completely miss bioelectric pathways, since knockouts of single ion channels will be subject to compensation and redundancy by other channels contributing to Vmem.
One context in which bioelectric and genetic state information can diverge is cancer (Yang and Brackenbury, 2013; Chernet and Levin, 2013b). A metastatic phenotype (overproliferation, matrix metalloprotease–dependent invasion of body tissues, and drastic arborization) can be induced in genetically normal melanocytes by depolarization of somatic cells (Blackiston et al., 2011; Lobikin et al., 2012a). This effect is not cell autonomous (Figure 2, C and D), showing that the bioelectric state of cells at considerable distance can trigger metastatic behavior. Conversely, the formation of tumors by human oncogenes such as p53 and KRAS mutations can be suppressed, despite the strong presence of oncogene protein within the cells, by artificially preventing the depolarization that occurs during oncogenic transformation (Chernet and Levin, 2013a). These examples reveal the potential dissociation between genetic state and disease outcome; an implication of these data is that the neoplastic state cannot always be predicted from examination of the genome, transcriptomes, or proteome, although in some cases, ion channel expression is altered (Onkal and Djamgoz, 2009; Becchetti, 2011; Lang and Stournaras, 2014). On the other hand, the functionally determinative voltage states cannot be seen in fixed tissue, stressing the importance of gathering real-time in vivo bioelectric information over and above analysis of mutations, mRNA profiles, and protein levels. Another implication for cancer biology is that although expression of some ion channel might be a useful marker (Wang, 2004; Fraser et al., 2005; Stuhmer et al., 2006), there will also be many cases in which the transcriptional profile reveals nothing (because of signaling via posttranslational gating of channel state), and drugs targeting one specific channel type (Arcangeli et al., 2009, 2012) may have no effect (due to compensation and redundancy of channel types). If indeed cancer is augmented or induced by a depolarized bioelectric state (Binggeli and Weinstein, 1986; Olivotto et al., 1996; Yang and Brackenbury, 2013), we will have to think less about individual ion channels as oncogenes (Pillozzi et al., 2002; Bennett et al., 2004; Lallet-Daher et al., 2013; Than et al., 2013) and focus instead on the way in which many channels contribute to a bioelectrical oncostate, to develop strategies for dominating the resting potential irrespective of native channel identity (Sharmeen et al., 2010; Chernet and Levin, 2013a).
BIOELECTRIC GRADIENTS HAVE DISTINCT, AUTONOMOUS DYNAMICS
Bioelectric patterns are clearly important drivers of cell behavior and pattern formation, but how do these patterns originate? Diverse resting potentials across a tissue can arise from preexisting differences in ion channel transcription, but that is not the only way (Justet et al., 2013). Such regionalized patterns of Vmem can also form de novo in transcriptionally and proteomically identical cells because cells coupled by gap junctions (electrical synapses) form a (slow) electrically excitable medium; this is a particularly interesting aspect because such media are known to have powerful computational capabilities (Fenton et al., 1999; Gorgcki and Gorgcka, 2007; Adamatzky et al., 2011). Positive feedback loops implemented by elements such as voltage-gated ion channels, which both set and respond to Vmem changes, can drive spontaneous symmetry breaking and amplification of physiological noise. Considerable self-organization dynamics can take place without a need for preexisting chemical prepattern (Toko et al., 1987; Schiffmann, 1991, 1997; Palacios-Prado and Bukauskas, 2009) or transcriptional activity; for example, human red blood cells have a physiological, not genetic, circadian clock rhythm driven by a slow ionic oscillation (Chakravarty and Rizvi, 2011; O'Neill and Reddy, 2011). Such dynamics has been studied in nerve and muscle (Zykov, 1990; Chen et al., 1997; Boettiger et al., 2009; Boettiger and Oster, 2009), and Turing-type self-organization has long been appreciated in chemical signaling (Takagi and Kaneko, 2005; Muller et al., 2012; Sheth et al., 2012). However, capabilities and properties of self-organization of voltage patterns in groups of nonneural cells remain to be formally analyzed. Quantitative analysis of in silico models of bioelectric dynamics will need to be integrated with deep new data sets from appropriate physiomic technologies to fully understand and control developmental patterning in vivo.
One unexpected recent finding illustrates the storage of patterning information in physiological networks and has significant implications for evolution. Planarian flatworms have the remarkable ability to regenerate completely from partial body fragments (Reddien and Sanchez Alvarado, 2004; Salo et al., 2009; Lobo et al., 2012). After a surgical bisection, the cells at one edge make a tail, whereas those at the other edge make a head, revealing that the adult stem cells that implement regeneration are not locally controlled (since the cells were direct neighbors until the scalpel separated them) but must communicate with the remaining tissue to decide what anatomical structures must be formed. It was shown that this long-range communication occurs via gap junction–mediated electrical synapses (Scemes et al., 2007; Marder, 2009; Pereda et al., 2013), and works together with a bioelectric circuit that determines head versus tail identity in each end's blastema (Beane et al., 2011, 2013). Brief inhibition of this gap junction–mediated communication results in worms developing heads at both ends (Nogi and Levin, 2005; Oviedo et al., 2010).
What is remarkable (Figure 2, E–I) is that weeks later, when these two-headed animals have their heads and tails amputated again (in just water, with no further perturbation), the same two-headed phenotype results, and this is repeated upon subsequent amputations. Thus a transient perturbation of physiological cell:cell communication stably changes the pattern to which the animal regenerates upon damage, despite normal genomic sequence. This again illustrates the potential divergence of genetic versus physiological information, especially since the phenotype is stable across fission (this animal's most frequent reproductive mode), and thus could have significant implications for evolution. Although epigenetic processes may be involved, chromatin modification mechanisms alone are not a sufficient explanation, since the ectopic heads (tissue that might be suggested to have been epigenetically reprogrammed into a head state from its original tail identity) are thrown away at each generation of cutting. What remains is a gut fragment, which somehow knows that it is to form two heads, not one, upon further cutting; the information about basic anatomical polarity and body organization must be stored in a distributed form throughout the animal. Quantitative, field-like models of this circuit remain to be developed to understand precisely how information guiding specific shape outcomes is encoded in (represented by) bioelectric states among cells.
CONCLUSION: NEXT STEPS AND BEYOND
Major open questions for future progress include the mechanisms by which cells compare bioelectric state across distances, additional molecular details of the interactions of bioelectrical signals with chemical gradients and physical forces, and the development of quantitative models of bioelectric circuits that store stable patterning information during morphogenesis. Expansions of the toolkit of synthetic biology will soon allow the rational top-down programming of bioelectric circuits, which will have important implications for regenerative medicine, cancer biology, and bioengineering (Reid et al., 2011a; Levin, 2013). Optogenetics, once expanded to facilitate the control of stable Vmem in large, nonexcitable cell groups, will play a large part, and there is significant room for advances in better voltage reporters and techniques for in vivo modulation of bioelectric state. One hypothesis for the development of deep, quantitative theory in this field is that pattering information may be stored within nonneural bioelectric cell networks using the same molecular mechanisms and information-processing algorithms that underlie behavioral memory in the nervous system. This is being tested in our lab. It is thus possible that the techniques such as those now used to extract mental imagery from electrical measurements of living human brains (Nishimoto et al., 2011) may shed crucial light on the encoding of anatomical pattern in the electrical circuits of somatic cells; conversely, the cracking of the bioelectric code in development and regeneration may have important benefits for the understanding of the semantics of electric states in the brain.
In practical terms, the molecular biologist needs to consider not only transcriptional and protein profiles when working to understand regulation of single-cell behavior and pattern formation. Significant instructive information is generated at the level of bioelectricity; ion channels and gap junctions are the molecular elements of such circuits, but bioelectrical signaling has its own unique dynamics that will become increasingly tractable with development of new technology specifically targeting stable Vmem states. The existence of bioelectric signaling among most cell types, not only neurons, suggests that the field of applicability of electroceuticals (Famm et al., 2013; Sinha, 2013; Birmingham et al., 2014) is much wider than anticipated by current plans to target neural function. More broadly, to the extent that the data of developmental bioelectricity are erasing artificial distinctions between neural and nonneural cell types, the insights of computational neuroscience and cognitive science will become relevant to cell and developmental biology. It is possible that the most effective ways to understand high-order (anatomical-level) outcomes will involve not only bottom-up models of molecular pathways but also top-down models in which information and control theory concepts play central roles. In this way, molecular bioelectricity may be revealing a mechanistic path toward understanding the intelligence exhibited by cell behavior and harnessing it toward transformative advances in biomedicine and the information sciences (Albrecht-Buehler, 1985; Rubenstein et al., 2009; Marshall, 2011; Aur, 2012).
Acknowledgments
This Perspective is dedicated to G. Marsh and H. W. Beams, who were among the first to demonstrate bioelectrical reprogramming of whole body regions. I thank the members of the Levin lab and the bioelectricity community for many helpful discussions on these issues and Gary McDowell, Jean-Francois Pare, and Juanita Mathews for their comments on an early draft of the manuscript. I gratefully acknowledge support of the National Science Foundation (DBI-1152279 and Emergent Behaviors of Integrated Cellular Systems Subaward CBET-0939511), the National Institutes of Health (AR055993), the W. M. Keck Foundation, and the G. Harold and Leila Y. Mathers Charitable Foundation.
Abbreviations used:
- dpa
days postamputation
- hMSC
human mesenchymal stem cells
- hpa
hours postamputation
- HPLC
high-performance liquid chromatography
- 5-HT
serotonin
- Vmem
transmembrane voltage potential
- VSP
voltage-sensitive phosphatase.
Footnotes
REFERENCES
- Adamatzky A, Costello B, Bull L, Holley J. Towards arithmetic circuits in sub-excitable chemical media. Israel J Chem. 2011;51:56–66. [Google Scholar]
- Adams DS. A new tool for tissue engineers: ions as regulators of morphogenesis during development and regeneration. Tissue Eng Part A. 2008;14:1461–1468. doi: 10.1089/ten.tea.2008.0080. [DOI] [PubMed] [Google Scholar]
- Adams DS, Levin M. Inverse drug screens: a rapid and inexpensive method for implicating molecular targets. Genesis. 2006a;44:530–540. doi: 10.1002/dvg.20246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Levin M. Strategies and techniques for investigation of biophysical signals in patterning. In: Whitman M, Sater AK, editors. Analysis of Growth Factor Signaling in Embryos. Oxford, UK: Taylor and Francis; 2006b. pp. 177–262. [Google Scholar]
- Adams DS, Levin M. General principles for measuring resting membrane potential and ion concentration using fluorescent bioelectricity reporters. Cold Spring Harb Protoc. 2012a;2012:385–397. doi: 10.1101/pdb.top067710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Levin M. Measuring resting membrane potential using the fluorescent voltage reporters DiBAC4(3) and CC2-DMPE. Cold Spring Harb Protoc. 2012b;2012:459–464. doi: 10.1101/pdb.prot067702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Levin M. Endogenous voltage gradients as mediators of cell-cell communication: strategies for investigating bioelectrical signals during pattern formation. Cell Tissue Res. 2013;352:95–122. doi: 10.1007/s00441-012-1329-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Masi A, Levin M. H+ pump-dependent changes in membrane voltage are an early mechanism necessary and sufficient to induce Xenopus tail regeneration. Development. 2007;134:1323–1335. doi: 10.1242/dev.02812. [DOI] [PubMed] [Google Scholar]
- Adams DS, Robinson KR, Fukumoto T, Yuan S, Albertson RC, Yelick P, Kuo L, McSweeney M, Levin M. Early, H+-V-ATPase-dependent proton flux is necessary for consistent left-right patterning of non-mammalian vertebrates. Development. 2006;133:1657–1671. doi: 10.1242/dev.02341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams DS, Tseng AS, Levin M. Light-activation of the Archaerhodopsin H(+)-pump reverses age-dependent loss of vertebrate regeneration: sparking system-level controls in vivo. Biol Open. 2013;2:306–313. doi: 10.1242/bio.20133665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akemann W, Mutoh H, Perron A, Kyung Park Y, Iwamoto Y, Knopfel T. Imaging neural circuit dynamics with a voltage-sensitive fluorescent protein. J Neurophys. 2012;108:2323–2337. doi: 10.1152/jn.00452.2012. [DOI] [PubMed] [Google Scholar]
- Albrecht-Buehler G. Is cytoplasm intelligent too. Cell Muscle Motil. 1985;6:1–21. doi: 10.1007/978-1-4757-4723-2_1. [DOI] [PubMed] [Google Scholar]
- Alves H, Dechering K, Van Blitterswijk C, De Boer J. High-throughput assay for the identification of compounds regulating osteogenic differentiation of human mesenchymal stromal cells. PLoS One. 2011;6:e26678. doi: 10.1371/journal.pone.0026678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anava S, Saad Y, Ayali A. The role of gap junction proteins in the development of neural network functional topology. Insect Mol Biol. 2013;2:457–472. doi: 10.1111/imb.12036. [DOI] [PubMed] [Google Scholar]
- Anderson JD. Galvanotaxis of slime mold. J Gen Physiol. 1951;35:1–16. doi: 10.1085/jgp.35.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aprea J, Calegari F. Bioelectric state and cell cycle control of mammalian neural stem cells. Stem Cells Int 2012. 2012:816049. doi: 10.1155/2012/816049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli A, Bianchi L, Becchetti A, Faravelli L, Coronnello M, Mini E, Olivotto M, Wanke E. A novel inward-rectifying K+ current with a cell-cycle dependence governs the resting potential of mammalian neuroblastoma cells. J Physiol. 1995;489:455–471. doi: 10.1113/jphysiol.1995.sp021065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli A, Carla M, Bene M, Becchetti A, Wanke E, Olivotto M. Polar/apolar compounds induce leukemia cell differentiation by modulating cell-surface potential. Proc Natl Acad Sci USA. 1993;90:5858–5862. doi: 10.1073/pnas.90.12.5858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arcangeli A, Crociani O, Lastraioli E, Masi A, Pillozzi S, Becchetti A. Targeting ion channels in cancer: a novel frontier in antineoplastic therapy. Curr Med Chem. 2009;16:66–93. doi: 10.2174/092986709787002835. [DOI] [PubMed] [Google Scholar]
- Arcangeli A, Pillozzi S, Becchetti A. Targeting ion channels in leukemias: a new challenge for treatment. Curr Med Chem. 2012;19:683–696. doi: 10.2174/092986712798992093. [DOI] [PubMed] [Google Scholar]
- Aryasomayajula A, Derix J, Perike S, Gerlach G, Funk RH. DC microelectrode array for investigating the intracellular ion changes. Biosens Bioelectron. 2010;26:1268–1272. doi: 10.1016/j.bios.2010.06.068. [DOI] [PubMed] [Google Scholar]
- Aur D. From neuroelectrodynamics to thinking machines. Cogn Comput. 2012;4:4–12. [Google Scholar]
- Aw S, Adams DS, Qiu D, Levin M. H,K-ATPase protein localization and Kir4.1 function reveal concordance of three axes during early determination of left-right asymmetry. Mech Dev. 2008;125:353–372. doi: 10.1016/j.mod.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Koster J, Pearson W, Nichols C, Shi NQ, Carneiro K, Levin M. The ATP-sensitive K(+)-channel (K(ATP)) controls early left-right patterning in Xenopus and chick embryos. Dev Biol. 2010;346:39–53. doi: 10.1016/j.ydbio.2010.07.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aw S, Levin M. Is left-right asymmetry a form of planar cell polarity. Development. 2009;136:355–366. doi: 10.1242/dev.015974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baglioni S, Cantini G, Poli G, Francalanci M, Squecco R, Di Franco A, Borgogni E, Frontera S, Nesi G, Liotta F, et al. Functional differences in visceral and subcutaneous fat pads originate from differences in the adipose stem cell. PLoS One. 2012;7:e36569. doi: 10.1371/journal.pone.0036569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barel O, Shalev SA, Ofir R, Cohen A, Zlotogora J, Shorer Z, Mazor G, Finer G, Khateeb S, Zilberberg N, et al. Maternally inherited Birk Barel mental retardation dysmorphism syndrome caused by a mutation in the genomically imprinted potassium channel KCNK9. Am J Hum Genet. 2008;83:193–199. doi: 10.1016/j.ajhg.2008.07.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartel DP, Sheng M, Lau LF, Greenberg ME. Growth factors and membrane depolarization activate distinct programs of early response gene expression: dissociation of fos and jun induction. Genes Dev. 1989;3:304–313. doi: 10.1101/gad.3.3.304. [DOI] [PubMed] [Google Scholar]
- Barth LG, Barth LJ. Ionic regulation of embryonic induction and cell differentiation in Rana pipiens. Dev Biol. 1974a;39:1–22. doi: 10.1016/s0012-1606(74)80004-7. [DOI] [PubMed] [Google Scholar]
- Barth LJ, Barth LG. Effect of the potassium ion on induction of notochord from gastrula ectoderm of Rana pipiens. Biol Bull. 1974b;146:313–325. doi: 10.2307/1540407. [DOI] [PubMed] [Google Scholar]
- Bauer R, Lehmann C, Fuss B, Eckardt F, Hoch M. The Drosophila gap junction channel gene innexin 2 controls foregut development in response to Wingless signalling. J Cell Sci. 2002;115:1859–1867. doi: 10.1242/jcs.115.9.1859. [DOI] [PubMed] [Google Scholar]
- Bauer R, Lehmann C, Martini J, Eckardt F, Hoch M. Gap junction channel protein innexin 2 is essential for epithelial morphogenesis in the Drosophila embryo. Mol Biol Cell. 2004;15:2992–3004. doi: 10.1091/mbc.E04-01-0056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beane WS, Morokuma J, Adams DS, Levin M. A chemical genetics approach reveals H,K-ATPase-mediated membrane voltage is required for planarian head regeneration. Chem Biol. 2011;18:77–89. doi: 10.1016/j.chembiol.2010.11.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beane WS, Morokuma J, Lemire JM, Levin M. Bioelectric signaling regulates head and organ size during planarian regeneration. Development. 2013;140:313–322. doi: 10.1242/dev.086900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Becchetti A. Ion channels and transporters in cancer. 1. Ion channels and cell proliferation in cancer. Am J Physiol. Cell Physiol. 2011;301:C255–265. doi: 10.1152/ajpcell.00047.2011. [DOI] [PubMed] [Google Scholar]
- Beloussov LV. Mechanically based generative laws of morphogenesis. Phys Biol. 2008;5:015009. doi: 10.1088/1478-3975/5/1/015009. [DOI] [PubMed] [Google Scholar]
- Beloussov L. Mechanoelectrical and photon-generating devices in cells and organisms: from molecular machines to macroscopic fields. J Phys Conf Ser. 2011 329, 012008. [Google Scholar]
- Beloussov LV, Grabovsky VI. Morphomechanics: goals, basic experiments and models. Int J Dev Biol. 2006;50:81–92. doi: 10.1387/ijdb.052056lb. [DOI] [PubMed] [Google Scholar]
- Bendahhou S, Donaldson MR, Plaster NM, Tristani-Firouzi M, Fu YH, Ptacek LJ. Defective potassium channel Kir2.1 trafficking underlies Andersen-Tawil syndrome. J Biol Chem. 2003;278:51779–51785. doi: 10.1074/jbc.M310278200. [DOI] [PubMed] [Google Scholar]
- Bennett ES, Smith BA, Harper JM. Voltage-gated Na+ channels confer invasive properties on human prostate cancer cells. Pflugers Arch. 2004;447:908–914. doi: 10.1007/s00424-003-1205-x. [DOI] [PubMed] [Google Scholar]
- Bentrup F, Sandan T, Jaffe L. Induction of polarity in Fucus eggs by potassium ion gradients. Protoplasma. 1967;64 254. [Google Scholar]
- Ben-Zvi D, Shilo BZ, Barkai N. Scaling of morphogen gradients. Curr Opin Genet Dev. 2011;21:704–710. doi: 10.1016/j.gde.2011.07.011. [DOI] [PubMed] [Google Scholar]
- Binggeli R, Weinstein R. Membrane potentials and sodium channels: hypotheses for growth regulation and cancer formation based on changes in sodium channels and gap junctions. J Theor Biol. 1986;123:377–401. doi: 10.1016/s0022-5193(86)80209-0. [DOI] [PubMed] [Google Scholar]
- Birmingham K, Gradinaru V, Anikeeva P, Grill WM, Pikov V, McLaughlin B, Pasricha P, Weber D, Ludwig K, Famm K. Bioelectronic medicines: a research roadmap. Nat Rev Drug Discov. 2014;13:399–400. doi: 10.1038/nrd4351. [DOI] [PubMed] [Google Scholar]
- Blackiston D, Adams DS, Lemire JM, Lobikin M, Levin M. Transmembrane potential of GlyCl-expressing instructor cells induces a neoplastic-like conversion of melanocytes via a serotonergic pathway. Dis Models Mech. 2011;4:67–85. doi: 10.1242/dmm.005561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackiston D, Anderson GM, Rahman N, Bieck C, Levin M. A novel method for inducing nerve growth via modulation of host resting potential. Neurotherapeutics. 2015 doi: 10.1007/s13311-014-0317-7. (in press) [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger A, Ermentrout B, Oster G. The neural origins of shell structure and pattern in aquatic mollusks. Proc Natl Acad Sci USA. 2009;106:6837–6842. doi: 10.1073/pnas.0810311106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boettiger AN, Oster G. Emergent complexity in simple neural systems. Commun Integr Biol. 2009;2:467–470. doi: 10.4161/cib.2.6.9260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgens RB. The role of natural and applied electric fields in neuronal regeneration and development. Prog Clin Biol Res. 1986;210:239–250. [PubMed] [Google Scholar]
- Borgens RB, Blight AR, McGinnis ME. Behavioral recovery induced by applied electric fields after spinal cord hemisection in guinea pig. Science. 1987;238:366–369. doi: 10.1126/science.3659920. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Blight AR, McGinnis ME. Functional recovery after spinal cord hemisection in guinea pigs: the effects of applied electric fields. J Comp Neurol. 1990;296:634–653. doi: 10.1002/cne.902960409. [DOI] [PubMed] [Google Scholar]
- Borgens RB, Blight AR, Murphy DJ. Axonal regeneration in spinal cord injury: a perspective and new technique. J Comp Neurol. 1986;250:157–167. doi: 10.1002/cne.902500203. [DOI] [PubMed] [Google Scholar]
- Borgens R, Robinson K, Vanable J, McGinnis M. Electric Fields in Vertebrate Repair. New York: Alan R. Liss; 1989. [Google Scholar]
- Borgens RB, Shi R. Uncoupling histogenesis from morphogenesis in the vertebrate embryo by collapse of the transneural tube potential. Dev Dyn. 1995;203:456–467. doi: 10.1002/aja.1002030408. [DOI] [PubMed] [Google Scholar]
- Borthwick KJ, Kandemir N, Topaloglu R, Kornak U, Bakkaloglu A, Yordam N, Ozen S, Mocan H, Shah GN, Sly WS, et al. A phenocopy of CAII deficiency: a novel genetic explanation for inherited infantile osteopetrosis with distal renal tubular acidosis. J Med Genet. 2003;40:115–121. doi: 10.1136/jmg.40.2.115. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Britz-Cunningham S, Shah M, Zuppan C, Fletcher W. Mutations of the connexin-43 gap-junction gene in patients with heart malformations and defects of laterality. N Engl J Med. 1995;332:1323–1329. doi: 10.1056/NEJM199505183322002. [DOI] [PubMed] [Google Scholar]
- Burr HS. Changes in the field properties of mice with transplanted tumors. Yale J Biol Med. 1941;13:783–788. [PMC free article] [PubMed] [Google Scholar]
- Burr HS, Northrop FSC. The electro-dynamic theory of life. Q Rev Biol. 1935;10:322–333. [Google Scholar]
- Burr HS, Smith GM, Strong LC. Electrometric studies of tumors in mice induced by the external application of benzpyrene. Yale J Biol Med. 1940;12:711–717. [PMC free article] [PubMed] [Google Scholar]
- Bustamante JO, Hanover JA, Liepins A. The ion channel behavior of the nuclear pore complex. J Membr Biol. 1995;146:239–251. doi: 10.1007/BF00233944. [DOI] [PubMed] [Google Scholar]
- Campetelli A, Bonazzi D, Minc N. Electrochemical regulation of cell polarity and the cytoskeleton. Cytoskeleton (Hoboken) 2012;69:601–612. doi: 10.1002/cm.21047. [DOI] [PubMed] [Google Scholar]
- Cao L, Pu J, Zhao M. GSK-3beta is essential for physiological electric field-directed Golgi polarization and optimal electrotaxis. Cell Mol Life Sci. 2011;68:3081–3093. doi: 10.1007/s00018-010-0608-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cao L, Wei D, Reid B, Zhao S, Pu J, Pan T, Yamoah E, Zhao M. Endogenous electric currents might guide rostral migration of neuroblasts. EMBO Rep 14, 184–190. 2013 doi: 10.1038/embor.2012.215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casimiro MC, Knollmann BC, Yamoah EN, Nie L, Vary JC, Jr, Sirenko SG, Greene AE, Grinberg A, Huang SP, Ebert SN, Pfeifer K. Targeted point mutagenesis of mouse Kcnq1: phenotypic analysis of mice with point mutations that cause Romano-Ward syndrome in humans. Genomics. 2004;84:555–564. doi: 10.1016/j.ygeno.2004.06.007. [DOI] [PubMed] [Google Scholar]
- Certal AC, Almeida RB, Carvalho LM, Wong E, Moreno N, Michard E, Carneiro J, Rodriguez-Leon J, Wu HM, Cheung AY, et al. Exclusion of a proton ATPase from the apical membrane is associated with cell polarity and tip growth in Nicotiana tabacum pollen tubes. Plant Cell. 2008;20:614–634. doi: 10.1105/tpc.106.047423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chakravarty S, Rizvi SI. Circadian modulation of sodium-potassium ATPase and sodium - proton exchanger in human erythrocytes: in vitro effect of melatonin. Cell Mol Biol. 2011;57:80–86. [PubMed] [Google Scholar]
- Chan JD, Agbedanu PN, Zamanian M, Gruba SM, Haynes CL, Day TA, Marchant JS. “Death and axes”: unexpected ca(2+) entry phenologs predict new anti-schistosomal agents. PLoS Pathog. 2014;10:e1003942. doi: 10.1371/journal.ppat.1003942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen PS, Garfinkel A, Weiss JN, Karagueuzian HS. Spirals, chaos, and new mechanisms of wave propagation. Pacing Clin Electrophysiol. 1997;20:414–421. doi: 10.1111/j.1540-8159.1997.tb06200.x. [DOI] [PubMed] [Google Scholar]
- Chernet BT, Levin M. Transmembrane voltage potential is an essential cellular parameter for the detection and control of tumor development in a Xenopus model. Dis Models Mech. 2013a;6:595–607. doi: 10.1242/dmm.010835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernet B, Levin M. Endogenous voltage potentials and the microenvironment: bioelectric signals that reveal, induce and normalize cancer. J Exp Clin Oncol. 2013b doi: 10.4172/2324-9110.S1-002. doi:10.4172/2324-9110.S1-002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chernet BT, Levin M. Transmembrane voltage potential of somatic cells controls oncogene-mediated tumorigenesis at long-range. Oncotarget. 2014;5:3287–3306. doi: 10.18632/oncotarget.1935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chifflet S, Hernandez JA, Grasso S, Cirillo A. Nonspecific depolarization of the plasma membrane potential induces cytoskeletal modifications of bovine corneal endothelial cells in culture. Exp Cell Res. 2003;282:1–13. doi: 10.1006/excr.2002.5664. [DOI] [PubMed] [Google Scholar]
- Chinnery HR, Pearlman E, McMenamin PG. Cutting edge: membrane nanotubes in vivo: a feature of MHC class II +cells in the mouse cornea. J Immunol. 2008;180:5779–5783. doi: 10.4049/jimmunol.180.9.5779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chopra SS, Stroud DM, Watanabe H, Bennett JS, Burns CG, Wells KS, Yang T, Zhong TP, Roden DM. Voltage-gated sodium channels are required for heart development in zebrafish. Circ Res. 2010;106:1342–1350. doi: 10.1161/CIRCRESAHA.109.213132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chouabe C, Neyroud N, Guicheney P, Lazdunski M, Romey G, Barhanin J. Properties of KvLQT1 K +channel mutations in Romano-Ward and Jervell and Lange-Nielsen inherited cardiac arrhythmias. EMBO J. 1997;16:5472–5479. doi: 10.1093/emboj/16.17.5472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chow RL, Altmann CR, Lang RA, Hemmati-Brivanlou A. Pax6 induces ectopic eyes in a vertebrate. Dev Suppl. 1999;126:4213–4222. doi: 10.1242/dev.126.19.4213. [DOI] [PubMed] [Google Scholar]
- Cifra M, Fields JZ, Farhadi A. Electromagnetic cellular interactions. Prog Biophys Mol Biol. 2011;105:223–246. doi: 10.1016/j.pbiomolbio.2010.07.003. [DOI] [PubMed] [Google Scholar]
- Civitelli R. Cell-cell communication in the osteoblast/osteocyte lineage. Arch Biochem Biophys. 2008;473:188–192. doi: 10.1016/j.abb.2008.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cone CD., Jr Variation of the transmembrane potential level as a basic mechanism of mitosis control. Oncology. 1970;24:438–470. doi: 10.1159/000224545. [DOI] [PubMed] [Google Scholar]
- Cone CD. Unified theory on the basic mechanism of normal mitotic control and oncogenesis. J Theor Biol. 1971;30:151–181. doi: 10.1016/0022-5193(71)90042-7. [DOI] [PubMed] [Google Scholar]
- Cone CD. The role of the surface electrical transmembrane potential in normal and malignant mitogenesis. Ann NY Acad Sci. 1974;238:420–435. doi: 10.1111/j.1749-6632.1974.tb26808.x. [DOI] [PubMed] [Google Scholar]
- Cone CD, Cone CM. Induction of mitosis in mature neurons in central nervous system by sustained depolarization. Science. 1976;192:155–158. doi: 10.1126/science.56781. [DOI] [PubMed] [Google Scholar]
- Cone CD, Tongier M. Control of somatic cell mitosis by simulated changes in the transmembrane potential level. Oncology. 1971;25:168–182. doi: 10.1159/000224567. [DOI] [PubMed] [Google Scholar]
- Cone CD, Tongier M. Contact inhibition of division: involvement of the electrical transmembrane potential. J Cell Physiol. 1973;82:373–386. doi: 10.1002/jcp.1040820307. [DOI] [PubMed] [Google Scholar]
- Culiat CT, Stubbs LJ, Woychik RP, Russell LB, Johnson DK, Rinchik EM. Deficiency of the beta 3 subunit of the type A gamma-aminobutyric acid receptor causes cleft palate in mice. Nat Genet. 1995;11:344–346. doi: 10.1038/ng1195-344. [DOI] [PubMed] [Google Scholar]
- Dahal GR, Rawson J, Gassaway B, Kwok B, Tong Y, Ptacek LJ, Bates E. An inwardly rectifying K+ channel is required for patterning. Development. 2012;139:3653–3664. doi: 10.1242/dev.078592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson LA. Epithelial machines that shape the embryo. Trends Cell Biol. 2012;22:82–87. doi: 10.1016/j.tcb.2011.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davie JR. Inhibition of histone deacetylase activity by butyrate. J Nutr. 2003;133:2485S-2493S. doi: 10.1093/jn/133.7.2485S. [DOI] [PubMed] [Google Scholar]
- Davy A, Bush JO, Soriano P. Inhibition of gap junction communication at ectopic Eph/ephrin boundaries underlies craniofrontonasal syndrome. PLoS Biol. 2006;4:e315. doi: 10.1371/journal.pbio.0040315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Debeer P, Van Esch H, Huysmans C, Pijkels E, De Smet L, Van de Ven W, Devriendt K, Fryns JP. Novel GJA1 mutations in patients with oculo-dento-digital dysplasia (ODDD) Eur J Med Genet. 2005;48:377–387. doi: 10.1016/j.ejmg.2005.05.003. [DOI] [PubMed] [Google Scholar]
- Ding F, Zhang G, Liu L, Jiang L, Wang R, Zheng Y, Wang G, Xie M, Duan Y. Involvement of cationic channels in proliferation and migration of human mesenchymal stem cells. Tissue Cell. 2012;44:358–364. doi: 10.1016/j.tice.2012.06.001. [DOI] [PubMed] [Google Scholar]
- Dolmetsch RE, Xu K, Lewis RS. Calcium oscillations increase the efficiency and specificity of gene expression. Nature. 1998;392:933–936. doi: 10.1038/31960. [DOI] [PubMed] [Google Scholar]
- Du Y, Du Z, Zheng H, Wang D, Li S, Yan Y, Li Y. GABA exists as a negative regulator of cell proliferation in spermaogonial stem cells. Cell Mol Biol Lett. 2013;18:149–162. doi: 10.2478/s11658-013-0081-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubach JM, Balaconis MK, Clark HA. Fluorescent nanoparticles for the measurement of ion concentration in biological systems. J Vis Exp. 2011a doi: 10.3791/2896. DOI:10.3791/2896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dubach JM, Lim E, Zhang N, Francis KP, Clark H. In vivo sodium concentration continuously monitored with fluorescent sensors. Integr Biol (Camb) 2011b;3:142–148. doi: 10.1039/c0ib00020e. [DOI] [PubMed] [Google Scholar]
- Ewart JL, Cohen MF, Meyer RA, Huang GY, Wessels A, Gourdie RG, Chin AJ, Park SM, Lazatin BO, Villabon S, et al. Heart and neural tube defects in transgenic mice overexpressing the Cx43 gap junction gene. Development. 1997;124:1281–1292. doi: 10.1242/dev.124.7.1281. [DOI] [PubMed] [Google Scholar]
- Famm K, Litt B, Tracey KJ, Boyden ES, Slaoui M. Drug discovery: a jump-start for electroceuticals. Nature. 2013;496:159–161. doi: 10.1038/496159a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farhadi A, Forsyth C, Banan A, Shaikh M, Engen P, Fields JZ, Keshavarzian A. Evidence for non-chemical, non-electrical intercellular signaling in intestinal epithelial cells. Bioelectrochemistry. 2007;71:142–148. doi: 10.1016/j.bioelechem.2007.03.001. [DOI] [PubMed] [Google Scholar]
- Felder CC, MacArthur L, Ma AL, Gusovsky F, Kohn EC. Tumor-suppressor function of muscarinic acetylcholine receptors is associated with activation of receptor-operated calcium influx. Proc Natl Acad Sci USA. 1993;90:1706–1710. doi: 10.1073/pnas.90.5.1706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fels D. Cellular communication through light. PLoS One. 2009;4:e5086. doi: 10.1371/journal.pone.0005086. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fenton FH, Evans SJ, Hastings HM. Memory in an excitable medium: a mechanism for spiral wave breakup in the low-excitability limit. Phys Rev Lett. 1999;83:3964–3967. [Google Scholar]
- Fraser SP, Diss JK, Chioni AM, Mycielska ME, Pan H, Yamaci RF, Pani F, Siwy Z, Krasowska M, Grzywna Z, et al. Voltage-gated sodium channel expression and potentiation of human breast cancer metastasis. Clin Cancer Res. 2005;11:5381–5389. doi: 10.1158/1078-0432.CCR-05-0327. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Blakely R, Levin M. Serotonin transporter function is an early step in left-right patterning in chick and frog embryos. Dev Neurosci. 2005a;27:349–363. doi: 10.1159/000088451. [DOI] [PubMed] [Google Scholar]
- Fukumoto T, Kema IP, Levin M. Serotonin signaling is a very early step in patterning of the left-right axis in chick and frog embryos. Curr Biol. 2005b;15:794–803. doi: 10.1016/j.cub.2005.03.044. [DOI] [PubMed] [Google Scholar]
- Funk RH, Monsees T, Ozkucur N. Electromagnetic effects—from cell biology to medicine. Prog Histochem Cytochem. 2009;43:177–264. doi: 10.1016/j.proghi.2008.07.001. [DOI] [PubMed] [Google Scholar]
- Gabriel LA, Sachdeva R, Marcotty A, Rockwood EJ, Traboulsi EI. Oculodentodigital dysplasia: new ocular findings and a novel connexin 43 mutation. Arch Ophthalmol. 2011;129:781–784. doi: 10.1001/archophthalmol.2011.113. [DOI] [PubMed] [Google Scholar]
- Geard N, Willadsen K. Dynamical approaches to modeling developmental gene regulatory networks. Birth Defects Res C Embryo Today. 2009;87:131–142. doi: 10.1002/bdrc.20150. [DOI] [PubMed] [Google Scholar]
- Gershenson C. Guiding the self-organization of random Boolean networks. Theory Biosci. 2012;131:181–191. doi: 10.1007/s12064-011-0144-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gloyn AL, Pearson ER, Antcliff JF, Proks P, Bruining GJ, Slingerland AS, Howard N, Srinivasan S, Silva JM, Molnes J, et al. Activating mutations in the gene encoding the ATP-sensitive potassium-channel subunit Kir6.2 and permanent neonatal diabetes. N Engl J Med. 2004;350:1838–1849. doi: 10.1056/NEJMoa032922. [DOI] [PubMed] [Google Scholar]
- Gorgcki J, Gorgcka FN. Chemical wave based programming in reaction-diffusion systems. Int J Unconv Comput. 2007;3:259–270. [Google Scholar]
- Greer PL, Greenberg ME. From synapse to nucleus: calcium-dependent gene transcription in the control of synapse development and function. Neuron. 2008;59:846–860. doi: 10.1016/j.neuron.2008.09.002. [DOI] [PubMed] [Google Scholar]
- Gupta N, Martin PM, Prasad PD, Ganapathy V. SLC5A8 (SMCT1)-mediated transport of butyrate forms the basis for the tumor suppressive function of the transporter. Life Sci. 2006;78:2419–2425. doi: 10.1016/j.lfs.2005.10.028. [DOI] [PubMed] [Google Scholar]
- Harrington DB, Becker RO. Electrical stimulation of RNA and protein synthesis in the frog erythrocyte. Exp Cell Res. 1973;76:95–98. doi: 10.1016/0014-4827(73)90423-0. [DOI] [PubMed] [Google Scholar]
- Hatten ME, Liem RK, Mason CA. Weaver mouse cerebellar granule neurons fail to migrate on wild-type astroglial processes in vitro. J Neurosci. 1986;6:2676–2683. doi: 10.1523/JNEUROSCI.06-09-02676.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He XB, Yi SH, Rhee YH, Kim H, Han YM, Lee SH, Lee H, Park CH, Lee YS, Richardson E, et al. Prolonged membrane depolarization enhances midbrain dopamine neuron differentiation via epigenetic histone modifications. Stem Cells. 2011;29:1861–1873. doi: 10.1002/stem.739. [DOI] [PubMed] [Google Scholar]
- Hermle T, Saltukoglu D, Grunewald J, Walz G, Simons M. Regulation of Frizzled-dependent planar polarity signaling by a V-ATPase subunit. Curr Biol. 2010;20:1269–1276. doi: 10.1016/j.cub.2010.05.057. [DOI] [PubMed] [Google Scholar]
- Higashimori H, Sontheimer H. Role of Kir4.1 channels in growth control of glia. Glia. 2007;55:1668–1679. doi: 10.1002/glia.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Higuchi H, Iwasa A, Yoshida H, Miki N. Long lasting increase in neuropeptide Y gene expression in rat adrenal gland with reserpine treatment: positive regulation of transsynaptic activation and membrane depolarization. Mol Pharmacol. 1990;38:614–623. [PubMed] [Google Scholar]
- Hinard V, Belin D, Konig S, Bader CR, Bernheim L. Initiation of human myoblast differentiation via dephosphorylation of Kir2.1 K+ channels at tyrosine 242. Development. 2008;135:859–867. doi: 10.1242/dev.011387. [DOI] [PubMed] [Google Scholar]
- Homanics GE, DeLorey TM, Firestone LL, Quinlan JJ, Handforth A, Harrison NL, Krasowski MD, Rick CE, Korpi ER, Makela R, et al. Mice devoid of gamma-aminobutyrate type A receptor beta3 subunit have epilepsy, cleft palate, and hypersensitive behavior. Proc Natl Acad Sci USA. 1997;94:4143–4148. doi: 10.1073/pnas.94.8.4143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoptak-Solga AD, Nielsen S, Jain I, Thummel R, Hyde DR, Iovine MK. Connexin43 (GJA1) is required in the population of dividing cells during fin regeneration. Dev Biol. 2008;317:541–548. doi: 10.1016/j.ydbio.2008.02.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotary KB, Robinson KR. Evidence of a role for endogenous electrical fields in chick embryo development. Development. 1992;114:985–996. doi: 10.1242/dev.114.4.985. [DOI] [PubMed] [Google Scholar]
- House CD, Vaske CJ, Schwartz AM, Obias V, Frank B, Luu T, Sarvazyan N, Irby R, Strausberg RL, Hales TG, et al. Voltage-gated Na+ channel SCN5A is a key regulator of a gene transcriptional network that controls colon cancer invasion. Cancer Res. 2010;70:6957–6967. doi: 10.1158/0008-5472.CAN-10-1169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hronik-Tupaj M, Kaplan DL. A review of the responses of two- and three-dimensional engineered tissues to electric fields. Tissue Eng Part B Rev. 2012;18:167–180. doi: 10.1089/ten.teb.2011.0244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang S, Eichler G, Bar-Yam Y, Ingber DE. Cell fates as high-dimensional attractor states of a complex gene regulatory network. Phys Rev Lett. 2005;94:128701. doi: 10.1103/PhysRevLett.94.128701. [DOI] [PubMed] [Google Scholar]
- Hyman L, Bellamy A. Studies on the correlation between metabolic gradients, electrical gradients, and galvanotaxis I. Biol Bull. 1922;43:313–347. [Google Scholar]
- Inaba M, Yamanaka H, Kondo S. Pigment pattern formation by contact-dependent depolarization. Science. 2012;335:677. doi: 10.1126/science.1212821. [DOI] [PubMed] [Google Scholar]
- Iovine MK, Higgins EP, Hindes A, Coblitz B, Johnson SL. Mutations in connexin43 (GJA1) perturb bone growth in zebrafish fins. Dev Biol. 2005;278:208–219. doi: 10.1016/j.ydbio.2004.11.005. [DOI] [PubMed] [Google Scholar]
- Iwashita M, Watanabe M, Ishii M, Chen T, Johnson SL, Kurachi Y, Okada N, Kondo S. Pigment pattern in jaguar/obelix zebrafish is caused by a Kir7.1 mutation: implications for the regulation of melanosome movement. PLoS Genet. 2006;2:e197. doi: 10.1371/journal.pgen.0020197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe LF. Electrical currents through the developing fucus egg. Proc Natl Acad Sci USA. 1966;56:1102–1109. doi: 10.1073/pnas.56.4.1102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaffe LF. Localization in the developing Fucus egg and the general role of localizing currents. Adv Morphog. 1968;7:295–328. doi: 10.1016/b978-1-4831-9954-2.50012-4. [DOI] [PubMed] [Google Scholar]
- Jaffe L. Developmental currents, voltages, and gradients. In: Subtelny S, editor. Developmental Order: its origin and regulation. New York: Alan R. Liss; 1982. pp. 183–215. [Google Scholar]
- Jaffe LF, Nuccitelli R. Electrical controls of development. Annu Rev Biophys Bioeng. 1977;6:445–476. doi: 10.1146/annurev.bb.06.060177.002305. [DOI] [PubMed] [Google Scholar]
- Jaffe LF, Poo MM. Neurites grow faster towards the cathode than the anode in a steady field. J Exp Zool. 1979;209:115–128. doi: 10.1002/jez.1402090114. [DOI] [PubMed] [Google Scholar]
- Jia X, Yang J, Song W, Li P, Wang X, Guan C, Yang L, Huang Y, Gong X, Liu M, et al. Involvement of large conductance Ca(2+)-activated K (+) channel in laminar shear stress-induced inhibition of vascular smooth muscle cell proliferation. Pflugers Arch. 2013;465:221–232. doi: 10.1007/s00424-012-1182-z. [DOI] [PubMed] [Google Scholar]
- Jiang P, Rushing S, Kong CW, Fu J, Lieu DK, Chan C, Deng W, Li R. Electrophysiological properties of human induced pluripotent stem cells. Am J Physiol Cell Physiol. 2009;298:C486–C495. doi: 10.1152/ajpcell.00251.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Justet C, Evans F, Vasilskis E, Hernandez JA, Chifflet S. ENaC contribution to epithelial wound healing is independent of the healing mode and of any increased expression in the channel. Cell Tissue Res. 2013;353:53–64. doi: 10.1007/s00441-013-1635-5. [DOI] [PubMed] [Google Scholar]
- Kawakami Y, Raya A, Raya RM, Rodriguez-Esteban C, Belmonte JC. Retinoic acid signalling links left-right asymmetric patterning and bilaterally symmetric somitogenesis in the zebrafish embryo. Nature. 2005;435:165–171. doi: 10.1038/nature03512. [DOI] [PubMed] [Google Scholar]
- Knopfel T, Lin MZ, Levskaya A, Tian L, Lin JY, Boyden ES. Toward the second generation of optogenetic tools. J Neurosci. 2010;30:14998–15004. doi: 10.1523/JNEUROSCI.4190-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Konig S, Beguet A, Bader CR, Bernheim L. The calcineurin pathway links hyperpolarization (Kir2.1)-induced Ca2+ signals to human myoblast differentiation and fusion. Development. 2006;133:3107–3114. doi: 10.1242/dev.02479. [DOI] [PubMed] [Google Scholar]
- Kujawski S, Lin W, Kitte F, Bormel M, Fuchs S, Arulmozhivarman G, Vogt S, Theil D, Zhang Y, Antos CL. Calcineurin regulates coordinated outgrowth of zebrafish regenerating fins. Dev Cell. 2014;28:573–587. doi: 10.1016/j.devcel.2014.01.019. [DOI] [PubMed] [Google Scholar]
- Kumai M, Nishii K, Nakamura K, Takeda N, Suzuki M, Shibata Y. Loss of connexin45 causes a cushion defect in early cardiogenesis. Development. 2000;127:3501–3512. doi: 10.1242/dev.127.16.3501. [DOI] [PubMed] [Google Scholar]
- Kurtz I, Schrank AR. Bioelectrical properties of intact and regenerating earthworms Eisenia foetida. Physiol Zool. 1955;28:322–330. [Google Scholar]
- Lallet-Daher H, Wiel C, Gitenay D, Navaratnam N, Augert A, Le Calve B, Verbeke S, Carling D, Aubert S, Vindrieux D, et al. Potassium channel KCNA1 modulates oncogene-induced senescence and transformation. Cancer Res. 2013;73:5253–5265. doi: 10.1158/0008-5472.CAN-12-3690. [DOI] [PubMed] [Google Scholar]
- Lan J-Y, Williams C, Levin M, Black LD., III Depolarization of cellular resting membrane potential promotes neonatal cardiomyocyte proliferation in vitro. Cell Mol Bioeng. 2014;7:1–14. doi: 10.1007/s12195-014-0346-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lang F, Foller M, Lang KS, Lang PA, Ritter M, Gulbins E, Vereninov A, Huber SM. Ion channels in cell proliferation and apoptotic cell death. J Membr Biol. 2005;205:147–157. doi: 10.1007/s00232-005-0780-5. [DOI] [PubMed] [Google Scholar]
- Lang F, Stournaras C. Ion channels in cancer: future perspectives and clinical potential. Philos Trans R Soc Lond B Biol Sci. 2014;369:20130108. doi: 10.1098/rstb.2013.0108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lange C, Prenninger S, Knuckles P, Taylor V, Levin M, Calegari F. The H(+) vacuolar ATPase maintains neural stem cells in the developing mouse cortex. Stem Cells Dev. 2011;20:843–850. doi: 10.1089/scd.2010.0484. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Langlois VS, Martyniuk CJ. Genome wide analysis of Silurana (Xenopus) tropicalis development reveals dynamic expression using network enrichment analysis. Mech Dev. 2013;130:304–322. doi: 10.1016/j.mod.2012.12.002. [DOI] [PubMed] [Google Scholar]
- Lauritzen I, Zanzouri M, Honore E, Duprat F, Ehrengruber MU, Lazdunski M, Patel AJ. K+-dependent cerebellar granule neuron apoptosis. Role of task leak K +channels. J Biol Chem. 2003;278:32068–32076. doi: 10.1074/jbc.M302631200. [DOI] [PubMed] [Google Scholar]
- Lechleiter J, Girard S, Peralta E, Clapham D. Spiral calcium wave propagation and annihilation in Xenopus laevis oocytes. Science. 1991;252:123–126. doi: 10.1126/science.2011747. [DOI] [PubMed] [Google Scholar]
- Lee MP, Hu RJ, Johnson LA, Feinberg AP. Human KVLQT1 gene shows tissue-specific imprinting and encompasses Beckwith-Wiedemann syndrome chromosomal rearrangements. Nat Genet. 1997;15:181–185. doi: 10.1038/ng0297-181. [DOI] [PubMed] [Google Scholar]
- Levin M. Is the early left-right axis like a plant, a kidney, or a neuron? The integration of physiological signals in embryonic asymmetry. Birth Defects Res C Embryo Today. 2006;78:191–223. doi: 10.1002/bdrc.20078. [DOI] [PubMed] [Google Scholar]
- Levin M. Large-scale biophysics: ion flows and regeneration. Trends Cell Biol. 2007;17:262–271. doi: 10.1016/j.tcb.2007.04.007. [DOI] [PubMed] [Google Scholar]
- Levin M. Molecular bioelectricity in developmental biology: new tools and recent discoveries: control of cell behavior and pattern formation by transmembrane potential gradients. BioEssays. 2012a;34:205–217. doi: 10.1002/bies.201100136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Morphogenetic fields in embryogenesis, regeneration, and cancer: non-local control of complex patterning. Bio Systems. 2012b;109:243–261. doi: 10.1016/j.biosystems.2012.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Reprogramming cells and tissue patterning via bioelectrical pathways: molecular mechanisms and biomedical opportunities. Wiley Interdiscip Rev Syst Biol Med. 2013;5:657–676. doi: 10.1002/wsbm.1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M. Endogenous bioelectrical networks store non-genetic patterning information during development and regeneration. J Physiol. 2014;592:2295–2305. doi: 10.1113/jphysiol.2014.271940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Buznikov GA, Lauder JM. Of minds and embryos: left-right asymmetry and the serotonergic controls of pre-neural morphogenesis. Dev Neurosci. 2006;28:171–185. doi: 10.1159/000091915. [DOI] [PubMed] [Google Scholar]
- Levin M, Palmer AR. Left-right patterning from the inside out: widespread evidence for intracellular control. Bioessays. 2007;29:271–287. doi: 10.1002/bies.20545. [DOI] [PubMed] [Google Scholar]
- Levin M, Stevenson CG. Regulation of cell behavior and tissue patterning by bioelectrical signals: challenges and opportunities for biomedical engineering. Annu Rev Biomed Eng. 2012;14:295–323. doi: 10.1146/annurev-bioeng-071811-150114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Thorlin T, Robinson KR, Nogi T, Mercola M. Asymmetries in H+/K+-ATPase and cell membrane potentials comprise a very early step in left-right patterning. Cell. 2002;111:77–89. doi: 10.1016/s0092-8674(02)00939-x. [DOI] [PubMed] [Google Scholar]
- Li F, Yin J, Yue T, Liu L, Zhang H. The chloride intracellular channel 5 (CLIC5) involved in C2C12 myoblasts proliferation and differentiation. Cell Biol Int. 2010;34:379–384. doi: 10.1042/CBI20090334. [DOI] [PubMed] [Google Scholar]
- Liebau S, Propper C, Bockers T, Lehmann-Horn F, Storch A, Grissmer S, Wittekindt OH. Selective blockage of Kv1.3 and Kv3.1 channels increases neural progenitor cell proliferation. J Neurochem. 2006;99:426–437. doi: 10.1111/j.1471-4159.2006.03967.x. [DOI] [PubMed] [Google Scholar]
- Liebau S, Tischendorf M, Ansorge D, Linta L, Stockmann M, Weidgang C, Iacovino M, Boeckers T, von Wichert G, Kyba M, et al. An inducible expression system of the calcium-activated potassium channel 4 to study the differential impact on embryonic stem cells. Stem Cells Int 2011. 2011:456815. doi: 10.4061/2011/456815. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liesi P, Stewart RR, Wright JM. Involvement of GIRK2 in postnatal development of the weaver cerebellum. J Neurosci Res. 2000;60:164–173. doi: 10.1002/(SICI)1097-4547(20000415)60:2<164::AID-JNR5>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- Liu X, Tonegawa S. Optogenetics 3.0. Cell. 2010;141:22–24. doi: 10.1016/j.cell.2010.03.019. [DOI] [PubMed] [Google Scholar]
- Lobikin M, Chernet B, Lobo D, Levin M. Resting potential, oncogene-induced tumorigenesis, and metastasis: the bioelectric basis of cancer in vivo. Phys Biol. 2012a;9:065002. doi: 10.1088/1478-3975/9/6/065002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobikin M, Wang G, Xu J, Hsieh YW, Chuang CF, Lemire JM, Levin M. Early, nonciliary role for microtubule proteins in left-right patterning is conserved across kingdoms. Proc Natl Acad Sci USA. 2012b;109:12586–12591. doi: 10.1073/pnas.1202659109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lobo D, Beane WS, Levin M. Modeling planarian regeneration: a primer for reverse-engineering the worm. PLoS Comput Biol. 2012;8:e1002481. doi: 10.1371/journal.pcbi.1002481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh Migr. 2007;1:156–158. doi: 10.4161/cam.1.3.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund E. Bioelectric Fields and Growth. Austin: University of Texas Press; 1947. [Google Scholar]
- MacFarlane SN, Sontheimer H. Changes in ion channel expression accompany cell cycle progression of spinal cord astrocytes. Glia. 2000;30:39–48. doi: 10.1002/(sici)1098-1136(200003)30:1<39::aid-glia5>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Marder E. Electrical synapses: rectification demystified. Curr Biol. 2009;19:R34–35. doi: 10.1016/j.cub.2008.11.008. [DOI] [PubMed] [Google Scholar]
- Marsh G, Beams HW. Electrical control of growth polarity in regenerating Dugesia-Tigrina. Fed Proc. 1947;6:163–164. [PubMed] [Google Scholar]
- Marsh G, Beams HW. Electrical control of axial polarity in a regenerating annelid. Anat Rec. 1949;105:513–514. [Google Scholar]
- Marsh G, Beams HW. Electrical control of growth axis in a regenerating annelid. Anat Rec. 1950;108:512. [Google Scholar]
- Marsh G, Beams HW. Electrical control of morphogenesis in regenerating Dugesia Tigrina.1. Relation of axial polarity to field strength. J Cell Comp Physiol. 1952;39 doi: 10.1002/jcp.1030390203. 191. [DOI] [PubMed] [Google Scholar]
- Marsh G, Beams H. Electrical control of morphogenesis in regenerating Dugesia tigrina. J Cell Comp Physiol. 1957;39:191–211. doi: 10.1002/jcp.1030390203. [DOI] [PubMed] [Google Scholar]
- Marshall WF. Origins of cellular geometry. BMC Biol. 2011;9:57. doi: 10.1186/1741-7007-9-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martino JJ, Wall BA, Mastrantoni E, Wilimczyk BJ, La Cava SN, Degenhardt K, White E, Chen S. Metabotropic glutamate receptor 1 (Grm1) is an oncogene in epithelial cells. Oncogene. 2013;32:4366–4376. doi: 10.1038/onc.2012.471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mazzanti M, Bustamante JO, Oberleithner H. Electrical dimension of the nuclear envelope. Physiol Rev. 2001;81:1–19. doi: 10.1152/physrev.2001.81.1.1. [DOI] [PubMed] [Google Scholar]
- McCaig CD, Rajnicek AM, Song B, Zhao M. Controlling cell behavior electrically: current views and future potential. Physiol Rev. 2005;85:943–978. doi: 10.1152/physrev.00020.2004. [DOI] [PubMed] [Google Scholar]
- Mello de Queiroz F, Ponte CG, Bonomo A, Vianna-Jorge R, Suarez-Kurtz G. Study of membrane potential in T lymphocytes subpopulations using flow cytometry. BMC Immunol. 2008;9:63. doi: 10.1186/1471-2172-9-63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michard E, Alves F, Feijo JA. The role of ion fluxes in polarized cell growth and morphogenesis: the pollen tube as an experimental paradigm. Int J Dev Biol. 2009;53:1609–1622. doi: 10.1387/ijdb.072296em. [DOI] [PubMed] [Google Scholar]
- Miki T, Iwanaga T, Nagashima K, Ihara Y, Seino S. Roles of ATP-sensitive K +channels in cell survival and differentiation in the endocrine pancreas. Diabetes. 2001;50(Suppl 1):S48–S51. doi: 10.2337/diabetes.50.2007.s48. [DOI] [PubMed] [Google Scholar]
- Minc N, Chang F. Electrical control of cell polarization in the fission yeast Schizosaccharomyces pombe. Curr Biol. 2010;20:710–716. doi: 10.1016/j.cub.2010.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteiro J, Aires R, Becker JD, Jacinto A, Certal AC, Rodriguez-Leon J. V-ATPase proton pumping activity is required for adult zebrafish appendage regeneration. PLoS One. 2014;9:e92594. doi: 10.1371/journal.pone.0092594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morokuma J, Blackiston D, Adams DS, Seebohm G, Trimmer B, Levin M. Modulation of potassium channel function confers a hyperproliferative invasive phenotype on embryonic stem cells. Proc Natl Acad Sci USA. 2008a;105:16608–16613. doi: 10.1073/pnas.0808328105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morokuma J, Blackiston D, Levin M. KCNQ1 and KCNE1 K+ channel components are involved in early left-right patterning in Xenopus laevis embryos. Cell Physiol Biochem. 2008b;21:357–372. doi: 10.1159/000129628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller C, Maeso I, Wittbrodt J, Martinez-Morales JR. The medaka mutation tintachina sheds light on the evolution of V-ATPase B subunits in vertebrates. Sci Rep. 2013;3:3217. doi: 10.1038/srep03217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muller P, Rogers KW, Jordan BM, Lee JS, Robson D, Ramanathan S, Schier AF. Differential diffusivity of Nodal and Lefty underlies a reaction-diffusion patterning system. Science. 2012;336:721–724. doi: 10.1126/science.1221920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata Y, Iwasaki H, Sasaki M, Inaba K, Okamura Y. Phosphoinositide phosphatase activity coupled to an intrinsic voltage sensor. Nature. 2005;435:1239–1243. doi: 10.1038/nature03650. [DOI] [PubMed] [Google Scholar]
- Mutoh H, Perron A, Akemann W, Iwamoto Y, Knopfel T. Optogenetic monitoring of membrane potentials. Exp Physiol. 2011;96:13–18. doi: 10.1113/expphysiol.2010.053942. [DOI] [PubMed] [Google Scholar]
- Nakanishi S, Okazawa M. Membrane potential-regulated Ca2+ signalling in development and maturation of mammalian cerebellar granule cells. J Physiol. 2006;575:389–395. doi: 10.1113/jphysiol.2006.113340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson CM. Geometric control of tissue morphogenesis. Biochim Biophys Acta. 2009;1793:903–910. doi: 10.1016/j.bbamcr.2008.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ng SY, Chin CH, Lau YT, Luo J, Wong CK, Bian ZX, Tsang SY. Role of voltage-gated potassium channels in the fate determination of embryonic stem cells. J Cell Physiol. 2010;224:165–177. doi: 10.1002/jcp.22113. [DOI] [PubMed] [Google Scholar]
- Niehrs C. On growth and form: a Cartesian coordinate system of Wnt and BMP signaling specifies bilaterian body axes. Development. 2010;137:845–857. doi: 10.1242/dev.039651. [DOI] [PubMed] [Google Scholar]
- Nilius B, Schwarz G, Droogmans G. Control of intracellular calcium by membrane potential in human melanoma cells. Am J Physiol. 1993;265:C1501–1510. doi: 10.1152/ajpcell.1993.265.6.C1501. [DOI] [PubMed] [Google Scholar]
- Nishii K, Kumai M, Shibata Y. Regulation of the epithelial-mesenchymal transformation through gap junction channels in heart development. Trends Cardiovasc Med. 2001;11:213–218. doi: 10.1016/s1050-1738(01)00103-7. [DOI] [PubMed] [Google Scholar]
- Nishimoto S, Vu AT, Naselaris T, Benjamini Y, Yu B, Gallant JL. Reconstructing visual experiences from brain activity evoked by natural movies. Curr Biol. 2011;21:1641–1646. doi: 10.1016/j.cub.2011.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishiyama M, von Schimmelmann MJ, Togashi K, Findley WM, Hong K. Membrane potential shifts caused by diffusible guidance signals direct growth-cone turning. Nat Neurosci. 2008;11:762–771. doi: 10.1038/nn.2130. [DOI] [PubMed] [Google Scholar]
- Nogi T, Levin M. Characterization of innexin gene expression and functional roles of gap-junctional communication in planarian regeneration. Dev Biol. 2005;287:314–335. doi: 10.1016/j.ydbio.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Nogi T, Zhang D, Chan JD, Marchant JS. A novel biological activity of praziquantel requiring voltage-operated ca channel Beta subunits: subversion of flatworm regenerative polarity. PLoS Negl Trop Dis. 2009;3:e464. doi: 10.1371/journal.pntd.0000464. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novák B, Bentrup FW. An electrophysiological study of regeneration in Acetabularia mediterranea. Planta. 1972;108:227–244. doi: 10.1007/BF00384111. [DOI] [PubMed] [Google Scholar]
- Novak B, Sirnoval C. Inhibition of regeneration of Acetabularia mediterranea enucleated posterior stalk segments by electrical isolation. Plant Sci Lett. 1975;5:183–188. [Google Scholar]
- Nuccitelli R, Robinson K, Jaffe L. On electrical currents in development. Bioessays. 1986;5:292–294. doi: 10.1002/bies.950050616. [DOI] [PubMed] [Google Scholar]
- Nuckels RJ, Ng A, Darland T, Gross JM. The vacuolar-ATPase complex regulates retinoblast proliferation and survival, photoreceptor morphogenesis, and pigmentation in the zebrafish eye. Invest Ophthalmol Vis Sci. 2009;50:893–905. doi: 10.1167/iovs.08-2743. [DOI] [PubMed] [Google Scholar]
- O'Connell KM, Rolig AS, Whitesell JD, Tamkun MM. Kv2.1 potassium channels are retained within dynamic cell surface microdomains that are defined by a perimeter fence. J Neurosci. 2006;26:9609–9618. doi: 10.1523/JNEUROSCI.1825-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connell KM, Tamkun MM. Targeting of voltage-gated potassium channel isoforms to distinct cell surface microdomains. J Cell Sci. 2005;118:2155–2166. doi: 10.1242/jcs.02348. [DOI] [PubMed] [Google Scholar]
- Okamura Y, Dixon JE. Voltage-sensing phosphatase: its molecular relationship with PTEN. Physiology (Bethesda) 2011;26:6–13. doi: 10.1152/physiol.00035.2010. [DOI] [PubMed] [Google Scholar]
- Olivotto M, Arcangeli A, Carla M, Wanke E. Electric fields at the plasma membrane level: a neglected element in the mechanisms of cell signalling. Bioessays. 1996;18:495–504. doi: 10.1002/bies.950180612. [DOI] [PubMed] [Google Scholar]
- O'Neill JS, Reddy AB. Circadian clocks in human red blood cells. Nature. 2011;469:498–503. doi: 10.1038/nature09702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onkal R, Djamgoz MB. Molecular pharmacology of voltage-gated sodium channel expression in metastatic disease: clinical potential of neonatal Nav1.5 in breast cancer. Eur J Pharmacol. 2009;625:206–219. doi: 10.1016/j.ejphar.2009.08.040. [DOI] [PubMed] [Google Scholar]
- Oviedo NJ, Morokuma J, Walentek P, Kema IP, Gu MB, Ahn JM, Hwang JS, Gojobori T, Levin M. Long-range neural and gap junction protein-mediated cues control polarity during planarian regeneration. Dev Biol. 2010;339:188–199. doi: 10.1016/j.ydbio.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oviedo NJ, Nicolas CL, Adams DS, Levin M. Live imaging of planarian membrane potential using DiBAC4(3) CSH Protoc 2008. 2008 doi: 10.1101/pdb.prot5055. pdb prot5055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozkucur N, Epperlein HH, Funk RH. Ion imaging during axolotl tail regeneration in vivo. Dev Dyn. 2010;239:2048–2057. doi: 10.1002/dvdy.22323. [DOI] [PubMed] [Google Scholar]
- Ozkucur N, Perike S, Sharma P, Funk RH. Persistent directional cell migration requires ion transport proteins as direction sensors and membrane potential differences in order to maintain directedness. BMC Cell Biol. 2011;12:4. doi: 10.1186/1471-2121-12-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai VP, Aw S, Shomrat T, Lemire JM, Levin M. Transmembrane voltage potential controls embryonic eye patterning in Xenopus laevis. Development. 2012;139:313–323. doi: 10.1242/dev.073759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palacios-Prado N, Bukauskas FF. Heterotypic gap junction channels as voltage-sensitive valves for intercellular signaling. Proc Natl Acad Sci USA. 2009;106:14855–14860. doi: 10.1073/pnas.0901923106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pan L, Borgens RB. Perpendicular organization of sympathetic neurons within a required physiological voltage. Exp Neurol. 2010;222:161–164. doi: 10.1016/j.expneurol.2009.12.002. [DOI] [PubMed] [Google Scholar]
- Pan L, Borgens RB. Strict perpendicular orientation of neural crest-derived neurons in vitro is dependent on an extracellular gradient of voltage. J Neurosci Res. 2012;90:1335–1346. doi: 10.1002/jnr.22809. [DOI] [PubMed] [Google Scholar]
- Park JY, Helm JF, Zheng W, Ly QP, Hodul PJ, Centeno BA, Malafa MP. Silencing of the candidate tumor suppressor gene solute carrier family 5 member 8 (SLC5A8) in human pancreatic cancer. Pancreas. 2008;36:e32–39. doi: 10.1097/MPA.0b013e3181630ffe. [DOI] [PubMed] [Google Scholar]
- Patel N, Poo MM. Orientation of neurite growth by extracellular electric fields. J Neurosci. 1982;2:483–496. doi: 10.1523/JNEUROSCI.02-04-00483.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patil N, Cox DR, Bhat D, Faham M, Myers RM, Peterson AS. A potassium channel mutation in weaver mice implicates membrane excitability in granule cell differentiation. Nat Genet. 1995;11:126–129. doi: 10.1038/ng1095-126. [DOI] [PubMed] [Google Scholar]
- Paul SM, Palladino MJ, Beitel GJ. A pump-independent function of the Na,K-ATPase is required for epithelial junction function and tracheal tube-size control. Development. 2007;134:147–155. doi: 10.1242/dev.02710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pei L, Wiser O, Slavin A, Mu D, Powers S, Jan LY, Hoey T. Oncogenic potential of TASK3 (Kcnk9) depends on K+ channel function. Proc Natl Acad Sci USA. 2003;100:7803–7807. doi: 10.1073/pnas.1232448100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perathoner S, Daane JM, Henrion U, Seebohm G, Higdon CW, Johnson SL, Nusslein-Volhard C, Harris MP. Bioelectric signaling regulates size in zebrafish fins. PLoS Genet. 2014;10:e1004080. doi: 10.1371/journal.pgen.1004080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereda AE, Curti S, Hoge G, Cachope R, Flores CE, Rash JE. Gap junction-mediated electrical transmission: regulatory mechanisms and plasticity. Biochim Biophys Acta. 2013;1828:134–146. doi: 10.1016/j.bbamem.2012.05.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillozzi S, Brizzi MF, Balzi M, Crociani O, Cherubini A, Guasti L, Bartolozzi B, Becchetti A, Wanke E, et al. HERG potassium channels are constitutively expressed in primary human acute myeloid leukemias and regulate cell proliferation of normal and leukemic hemopoietic progenitors. Leukemia. 2002;16:1791–1798. doi: 10.1038/sj.leu.2402572. [DOI] [PubMed] [Google Scholar]
- Pizzuti A, Flex E, Mingarelli R, Salpietro C, Zelante L, Dallapiccola B. A homozygous GJA1 gene mutation causes a Hallermann-Streiff/ODDD spectrum phenotype. Hum Mutat. 2004;23:286. doi: 10.1002/humu.9220. [DOI] [PubMed] [Google Scholar]
- Podda MV, Piacentini R, Barbati SA, Mastrodonato A, Puzzo D, D'Ascenzo M, Leone L, Grassi C. Role of cyclic nucleotide-gated channels in the modulation of mouse hippocampal neurogenesis. PLoS One. 2013;8:e73246. doi: 10.1371/journal.pone.0073246. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Priel A, Ramos AJ, Tuszynski JA, Cantiello HF. A biopolymer transistor: electrical amplification by microtubules. Biophys J. 2006;90:4639–4643. doi: 10.1529/biophysj.105.078915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pullar CE. The Physiology of Bioelectricity in Development, Tissue Regeneration, and Cancer. Boca Raton, FL: CRC Press; 2011. [Google Scholar]
- Pullar CE, Isseroff RR. Cyclic AMP mediates keratinocyte directional migration in an electric field. J Cell Sci. 2005;118:2023–2034. doi: 10.1242/jcs.02330. [DOI] [PubMed] [Google Scholar]
- Rakic P, Sidman RL. Sequence of developmental abnormalities leading to granule cell deficit in cerebellar cortex of weaver mutant mice. J Comp Neurol. 1973a;152:103–132. doi: 10.1002/cne.901520202. [DOI] [PubMed] [Google Scholar]
- Rakic P, Sidman RL. Weaver mutant mouse cerebellum: defective neuronal migration secondary to abnormality of Bergmann glia. Proc Natl Acad Sci USA. 1973b;70:240–244. doi: 10.1073/pnas.70.1.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raya A, Kawakami Y, Rodriguez-Esteban C, Ibanes M, Rasskin-Gutman D, Rodriguez-Leon J, Buscher D, Feijo JA, Izpisua Belmonte JC. Notch activity acts as a sensor for extracellular calcium during vertebrate left-right determination. Nature. 2004;427:121–128. doi: 10.1038/nature02190. [DOI] [PubMed] [Google Scholar]
- Reaume AG, De Sousa PA, Kilkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J. Cardiac malformation in neonatal mice lacking connexin43. Science. 1995;267:1831–1834. doi: 10.1126/science.7892609. [DOI] [PubMed] [Google Scholar]
- Reddien PW, Sanchez Alvarado A. Fundamentals of planarian regeneration. Annu Rev Cell Dev Biol. 2004;20:725–757. doi: 10.1146/annurev.cellbio.20.010403.095114. [DOI] [PubMed] [Google Scholar]
- Reid B, Graue-Hernandez EO, Mannis MJ, Zhao M. Modulating endogenous electric currents in human corneal wounds–a novel approach of bioelectric stimulation without electrodes. Cornea. 2011a;30:338–343. doi: 10.1097/ICO.0b013e3181f7f2de. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reid B, Nuccitelli R, Zhao M. Non-invasive measurement of bioelectric currents with a vibrating probe. Nat Protoc. 2007;2:661–669. doi: 10.1038/nprot.2007.91. [DOI] [PubMed] [Google Scholar]
- Reid B, Vieira AC, Cao L, Mannis MJ, Schwab IR, Zhao M. Specific ion fluxes generate cornea wound electric currents. Commun Integr Biol. 2011b;4:462–465. doi: 10.4161/cib.4.4.15545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rigas S, Debrosses G, Haralampidis K, Vicente-Agullo F, Feldmann KA, Grabov A, Dolan L, Hatzopoulos P. TRH1 encodes a potassium transporter required for tip growth in Arabidopsis root hairs. Plant Cell. 2001;13:139–151. doi: 10.1105/tpc.13.1.139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ring H, Mendu SK, Shirazi-Fard S, Birnir B, Hallbook F. GABA maintains the proliferation of progenitors in the developing chick ciliary marginal zone and non-pigmented ciliary epithelium. PLoS One. 2012;7:e36874. doi: 10.1371/journal.pone.0036874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rivas A, Francis HW. Inner ear abnormalities in a Kcnq1 (Kvlqt1) knockout mouse: a model of Jervell and Lange-Nielsen syndrome. Otol Neurotol. 2005;26:415–424. doi: 10.1097/01.mao.0000169764.00798.84. [DOI] [PubMed] [Google Scholar]
- Rock JR, Futtner CR, Harfe BD. The transmembrane protein TMEM16A is required for normal development of the murine trachea. Dev Biol. 2008;321:141–149. doi: 10.1016/j.ydbio.2008.06.009. [DOI] [PubMed] [Google Scholar]
- Roepke TK, Purtell K, King EC, La Perle KM, Lerner DJ, Abbott GW. Targeted deletion of Kcne2 causes gastritis cystica profunda and gastric neoplasia. PLoS One. 2010;5:e11451. doi: 10.1371/journal.pone.0011451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root CM, Velazquez-Ulloa NA, Monsalve GC, Minakova E, Spitzer NC. Embryonically expressed GABA and glutamate drive electrical activity regulating neurotransmitter specification. J Neurosci. 2008;28:4777–4784. doi: 10.1523/JNEUROSCI.4873-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouzaire-Dubois B, Gerard V, Dubois JM. Involvement of K+ channels in the quercetin-induced inhibition of neuroblastoma cell growth. Pflugers Arch. 1993;423:202–205. doi: 10.1007/BF00374395. [DOI] [PubMed] [Google Scholar]
- Rubenstein M, Sai Y, Chuong CM, Shen WM. Regenerative patterning in Swarm Robots: mutual benefits of research in robotics and stem cell biology. Int J Dev Biol. 2009;53:869–881. doi: 10.1387/ijdb.092937mr. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saito T, Schlegel R, Andresson T, Yuge L, Yamamoto M, Yamasaki H. Induction of cell transformation by mutated 16K vacuolar H+-atpase (ductin) is accompanied by down-regulation of gap junctional intercellular communication and translocation of connexin 43 in NIH3T3 cells. Oncogene. 1998;17:1673–1680. doi: 10.1038/sj.onc.1202092. [DOI] [PubMed] [Google Scholar]
- Salo E, Abril JF, Adell T, Cebria F, Eckelt K, Fernandez-Taboada E, Handberg-Thorsager M, Iglesias M, Molina MD, Rodriguez-Esteban G. Planarian regeneration: achievements and future directions after 20 years of research. Int J Dev Biol. 2009;53:1317–1327. doi: 10.1387/ijdb.072414es. [DOI] [PubMed] [Google Scholar]
- Savy C, Martin-Martinelli E, Simon A, Duyckaerts C, Verney C, Adelbrecht C, Raisman-Vozari R, Nguyen-Legros J. Altered development of dopaminergic cells in the retina of weaver mice. J Comp Neurol. 1999;412:656–668. [PubMed] [Google Scholar]
- Scemes E, Suadicani SO, Dahl G, Spray DC. Connexin and pannexin mediated cell-cell communication. Neuron Glia Biol. 2007;3:199–208. doi: 10.1017/S1740925X08000069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiffmann Y. An hypothesis: phosphorylation fields as the source of positional information and cell differentiation—(cAMP, ATP) as the universal morphogenetic Turing couple. Prog Biophys Mol Biol. 1991;56:79–105. doi: 10.1016/0079-6107(91)90015-k. [DOI] [PubMed] [Google Scholar]
- Schiffmann Y. Self-organization in biology and development. Prog Biophys Mol Biol. 1997;68:145–205. doi: 10.1016/s0079-6107(97)00023-0. [DOI] [PubMed] [Google Scholar]
- Schonecker S, Kraushaar U, Dufer M, Sahr A, Hardtner C, Guenther E, Walther R, Lendeckel U, Barthlen W, Krippeit-Drews P, et al. Long-term culture and functionality of pancreatic islets monitored using microelectrode arrays. Integr Biol (Camb) 2014;6:540–544. doi: 10.1039/c3ib40261d. [DOI] [PubMed] [Google Scholar]
- Schwab A. Function and spatial distribution of ion channels and transporters in cell migration. Am J Physiol Renal Physiol. 2001;280:F739–747. doi: 10.1152/ajprenal.2001.280.5.F739. [DOI] [PubMed] [Google Scholar]
- Schwab A, Gabriel K, Finsterwalder F, Folprecht G, Greger R, Kramer A, Oberleithner H. Polarized ion transport during migration of transformed Madin-Darby canine kidney cells. Pflugers Arch. 1995;430:802–807. doi: 10.1007/BF00386179. [DOI] [PubMed] [Google Scholar]
- Sekulic DL, Sataric BM, Tuszynski JA, Sataric MV. Nonlinear ionic pulses along microtubules. Eur Phys J E Soft Matter. 2011;34:1–11. doi: 10.1140/epje/i2011-11049-0. [DOI] [PubMed] [Google Scholar]
- Sharmeen S, Skrtic M, Sukhai MA, Hurren R, Gronda M, Wang X, Fonseca SB, Sun H, Wood TE, Ward R, et al. The antiparasitic agent ivermectin induces chloride-dependent membrane hyperpolarization and cell death in leukemia cells. Blood. 2010;116:3593–3603. doi: 10.1182/blood-2010-01-262675. [DOI] [PubMed] [Google Scholar]
- Shen JX, Qin D, Wang H, Wu C, Shi FD, Wu J. Roles of nicotinic acetylcholine receptors in stem cell survival/apoptosis, proliferation and differentiation. Curr Mol Med. 2013;13:1455–1464. doi: 10.2174/15665240113139990074. [DOI] [PubMed] [Google Scholar]
- Shen B, Xiang Z, Miller B, Louie G, Wang W, Noel JP, Gage FH, Wang L. Genetically encoding unnatural amino acids in neural stem cells and optically reporting voltage-sensitive domain changes in differentiated neurons. Stem Cells. 2011;9:1231–1240. doi: 10.1002/stem.679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sheth R, Marcon L, Bastida MF, Junco M, Quintana L, Dahn R, Kmita M, Sharpe J, Ros MA. Hox genes regulate digit patterning by controlling the wavelength of a Turing-type mechanism. Science. 2012;338:1476–1480. doi: 10.1126/science.1226804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi R, Borgens RB. Three-dimensional gradients of voltage during development of the nervous system as invisible coordinates for the establishment of embryonic pattern. Dev Dyn. 1995;202:101–114. doi: 10.1002/aja.1002020202. [DOI] [PubMed] [Google Scholar]
- Shu X, Cheng K, Patel N, Chen F, Joseph E, Tsai HJ, Chen JN. Na,K-ATPase is essential for embryonic heart development in the zebrafish. Development. 2003;130:6165–6173. doi: 10.1242/dev.00844. [DOI] [PubMed] [Google Scholar]
- Simons M, Gault WJ, Gotthardt D, Rohatgi R, Klein TJ, Shao Y, Lee HJ, Wu AL, Fang Y, Satlin LM, et al. Electrochemical cues regulate assembly of the Frizzled/Dishevelled complex at the plasma membrane during planar epithelial polarization. Nat Cell Biol. 2009;11:286–294. doi: 10.1038/ncb1836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sims K, Jr, Eble DM, Iovine MK. Connexin43 regulates joint location in zebrafish fins. Dev Biol. 2009;327:410–418. doi: 10.1016/j.ydbio.2008.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha G. Charged by GSK investment, battery of electroceuticals advance. Nat Med. 2013;19:654. doi: 10.1038/nm0613-654. [DOI] [PubMed] [Google Scholar]
- Sirnes S, Bruun J, Kolberg M, Kjenseth A, Lind GE, Svindland A, Brech A, Nesbakken A, Lothe RA, Leithe E, et al. Connexin43 acts as a colorectal cancer tumor suppressor and predicts disease outcome. Int J Cancer. 2012;131:570–581. doi: 10.1002/ijc.26392. [DOI] [PubMed] [Google Scholar]
- Smith PJS, Sanger RS, Messerli MA. Principles, development and applications of self-referencing electrochemical microelectrodes to the determination of fluxes at cell membranes. In: Michael AC, editor. In: Methods and New Frontiers in Neuroscience. Boca Raton, FL: CRC Press; 2007. pp. 373–405. [PubMed] [Google Scholar]
- Song B, Gu Y, Pu J, Reid B, Zhao Z, Zhao M. Application of direct current electric fields to cells and tissues in vitro and modulation of wound electric field in vivo. Nat Protoc. 2007;2:1479–1489. doi: 10.1038/nprot.2007.205. [DOI] [PubMed] [Google Scholar]
- Song Z, He CD, Liu J, Sun C, Lu P, Li L, Gao L, Zhang Y, Xu Y, Shan L, et al. Blocking glutamate-mediated signalling inhibits human melanoma growth and migration. Exp Dermatol. 2012;21:926–931. doi: 10.1111/exd.12048. [DOI] [PubMed] [Google Scholar]
- Speyer CL, Smith JS, Banda M, DeVries JA, Mekani T, Gorski DH. Metabotropic glutamate receptor-1: a potential therapeutic target for the treatment of breast cancer. Breast Cancer Res Treat. 2012;132:565–573. doi: 10.1007/s10549-011-1624-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg BE, Touret N, Vargas-Caballero M, Grinstein S. In situ measurement of the electrical potential across the phagosomal membrane using FRET and its contribution to the proton-motive force. Proc Natl Acad Sci USA. 2007;104:9523–9528. doi: 10.1073/pnas.0700783104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stern C. Experimental reversal of polarity in chick embryo epiblast sheets in vitro. Exp Cell Res. 1982;140:468–471. doi: 10.1016/0014-4827(82)90143-4. [DOI] [PubMed] [Google Scholar]
- Stillwell EF, Cone CM, Cone CD. Stimulation of DNA synthesis in CNS neurones by sustained depolarisation. Nat New Biol. 1973;246:110–111. doi: 10.1038/newbio246110a0. [DOI] [PubMed] [Google Scholar]
- Stroh A, Tsai HC, Ping Wang L, Zhang F, Kressel J, Aravanis A, Santhanam N, Deisseroth K, Konnerth A, Schneider MB. Tracking stem cell differentiation in the setting of automated optogenetic stimulation. Stem Cells. 2010;29:78–88. doi: 10.1002/stem.558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stuhmer W, Alves F, Hartung F, Zientkowska M, Pardo LA. Potassium channels as tumour markers. FEBS Lett. 2006;580:2850–2852. doi: 10.1016/j.febslet.2006.03.062. [DOI] [PubMed] [Google Scholar]
- Stump RF, Robinson KR. Xenopus neural crest cell migration in an applied electrical field. J Cell Biol. 1983;97:1226–1233. doi: 10.1083/jcb.97.4.1226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Dong Z, Jin T, Ang KH, Huang M, Haston KM, Peng J, Zhong TP, Finkbeiner S, Weiss WA, et al. Imaging-based chemical screening reveals activity-dependent neural differentiation of pluripotent stem cells. Elife. 2013;2:e00508. doi: 10.7554/eLife.00508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun Y, Wang C, Dai J. Biophotons as neural communication signals demonstrated by in situ biophoton autography. Photochem Photobiol Sci. 2010;9:315–322. doi: 10.1039/b9pp00125e. [DOI] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. Membrane potential controls adipogenic and osteogenic differentiation of mesenchymal stem cells. PLoS One. 2008;3:e3737. doi: 10.1371/journal.pone.0003737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sundelacruz S, Levin M, Kaplan DL. Depolarization alters phenotype, maintains plasticity of predifferentiated mesenchymal stem cells. Tiss Eng. Part A. 2013;19:1889–1908. doi: 10.1089/ten.tea.2012.0425.rev. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutor B, Hagerty T. Involvement of gap junctions in the development of the neocortex. Biochim Biophys Acta. 2005;1719:59–68. doi: 10.1016/j.bbamem.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Swapna I, Borodinsky LN. Interplay between electrical activity and bone morphogenetic protein signaling regulates spinal neuron differentiation. Proc Natl Acad Sci USA. 2012;109(40):16336–16341. doi: 10.1073/pnas.1202818109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takagi H, Kaneko K. Dynamical systems basis of metamorphosis: diversity and plasticity of cellular states in reaction diffusion network. J Theor Biol. 2005;234:173–186. doi: 10.1016/j.jtbi.2004.11.030. [DOI] [PubMed] [Google Scholar]
- Tantama M, Hung YP, Yellen G. Imaging intracellular pH in live cells with a genetically encoded red fluorescent protein sensor. J Am Chem Soc. 2011;133:10034–10037. doi: 10.1021/ja202902d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teng GQ, Zhao X, Lees-Miller JP, Quinn FR, Li P, Rancourt DE, London B, Cross JC, Duff HJ. Homozygous missense N629D hERG (KCNH2) potassium channel mutation causes developmental defects in the right ventricle and its outflow tract and embryonic lethality. Circ Res. 2008;103:1483–1491. doi: 10.1161/CIRCRESAHA.108.177055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Than BL, Goos JA, Sarver AL, O'Sullivan MG, Rod A, Starr TK, Fijneman RJ, Meijer GA, Zhao L, Zhang Y, et al. The role of KCNQ1 in mouse and human gastrointestinal cancers. Oncogene. 2013;33:3861–3868. doi: 10.1038/onc.2013.350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toko K, Iiyama S, Tanaka C, Hayashi K, Yamafuji K. Relation of growth process to spatial patterns of electric potential and enzyme activity in bean roots. Biophys Chem. 1987;27:39–58. doi: 10.1016/0301-4622(87)80045-5. [DOI] [PubMed] [Google Scholar]
- Tong Y, Wei J, Zhang S, Strong JA, Dlouhy SR, Hodes ME, Ghetti B, Yu L. The weaver mutation changes the ion selectivity of the affected inwardly rectifying potassium channel GIRK2. FEBS Lett. 1996;390:63–68. doi: 10.1016/0014-5793(96)00632-1. [DOI] [PubMed] [Google Scholar]
- Tong X, Yin L, Giardina C. Butyrate suppresses Cox-2 activation in colon cancer cells through HDAC inhibition. Biochem Biophys Res Commun. 2004;317:463–471. doi: 10.1016/j.bbrc.2004.03.066. [DOI] [PubMed] [Google Scholar]
- Tseng AS, Beane WS, Lemire JM, Masi A, Levin M. Induction of vertebrate regeneration by a transient sodium current. J Neurosci. 2010;30:13192–13200. doi: 10.1523/JNEUROSCI.3315-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng AS, Carneiro K, Lemire JM, Levin M. HDAC activity is required during Xenopus tail regeneration. PLoS One. 2011;6:e26382. doi: 10.1371/journal.pone.0026382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tseng A, Levin M. Cracking the bioelectric code: Probing endogenous ionic controls of pattern formation. Commun Integr Biol. 2013;6:1–8. doi: 10.4161/cib.22595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsutsui H, Karasawa S, Okamura Y, Miyawaki A. Improving membrane voltage measurements using FRET with new fluorescent proteins. Nat Methods. 2008;5:683–685. doi: 10.1038/nmeth.1235. [DOI] [PubMed] [Google Scholar]
- Tyner KM, Kopelman R, Philbert MA. “Nanosized voltmeter” enables cellular-wide electric field mapping. Biophys J. 2007;93:1163–1174. doi: 10.1529/biophysj.106.092452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzman JA, Patil S, Uzgare AR, Sater AK. The role of intracellular alkalinization in the establishment of anterior neural fate in Xenopus. Dev Biol. 1998;193:10–20. doi: 10.1006/dbio.1997.8782. [DOI] [PubMed] [Google Scholar]
- Uzun S, Gokce S, Wagner K. Cystic fibrosis transmembrane conductance regulator gene mutations in infertile males with congenital bilateral absence of the vas deferens. Tohoku J Exp Med. 2005;207:279–285. doi: 10.1620/tjem.207.279. [DOI] [PubMed] [Google Scholar]
- Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- Vandenberg LN, Lemire JM, Levin M. Serotonin has early, cilia-independent roles in Xenopus left-right patterning. Dis Models Mech. 2012;6:261–268. doi: 10.1242/dmm.010256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Morrie RD, Adams DS. V-ATPase-dependent ectodermal voltage and pH regionalization are required for craniofacial morphogenesis. Dev Dyn. 2011;240:1889–1904. doi: 10.1002/dvdy.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandenberg LN, Morrie RD, Seebohm G, Lemire JM, Levin M. Rab GTPases are required for early orientation of the left-right axis in Xenopus. Mech Dev. 2013;130:254–271. doi: 10.1016/j.mod.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Vliet P, de Boer TP, van der Heyden MA, El Tamer MK, Sluijter JP, Doevendans PA, Goumans MJ. Hyperpolarization induces differentiation in human cardiomyocyte progenitor cells. Stem Cell Rev. 2010;6:178–185. doi: 10.1007/s12015-010-9142-5. [DOI] [PubMed] [Google Scholar]
- Veale EL, Hassan M, Walsh Y, Al-Moubarak E, Mathie A. Recovery of current through mutated TASK3 potassium channels underlying Birk Barel syndrome. Mol Pharmacol. 2014;85:397–407. doi: 10.1124/mol.113.090530. [DOI] [PubMed] [Google Scholar]
- Vieira AC, Reid B, Cao L, Mannis MJ, Schwab IR, Zhao M. Ionic components of electric current at rat corneal wounds. PLoS One. 2011;6:e17411. doi: 10.1371/journal.pone.0017411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volta A. On the electricity excited by the mere contact of conducting substances of different kinds. Abstracts Philos Trans R Soc Lond. 1800;1:27–29. [Google Scholar]
- von Dassow M, Davidson LA. Physics and the canalization of morphogenesis: a grand challenge in organismal biology. Phys Biol. 2011;8:045002. doi: 10.1088/1478-3975/8/4/045002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wahlgren J, De LKT, Brisslert M, Vaziri Sani F, Telemo E, Sunnerhagen P, Valadi H. Plasma exosomes can deliver exogenous short interfering RNA to monocytes and lymphocytes. Nucleic Acids Res. 2012;40:e130. doi: 10.1093/nar/gks463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L, Zhou P, Craig RW, Lu L. Protection from cell death by mcl-1 is mediated by membrane hyperpolarization induced by K(+) channel activation. J Membr Biol. 1999;172:113–120. doi: 10.1007/s002329900589. [DOI] [PubMed] [Google Scholar]
- Wang SJ, Weng CH, Xu HW, Zhao CJ, Yin ZQ. Effect of optogenetic stimulus on the proliferation and cell cycle progression of neural stem cells. J Membr Biol. 2014;247:493–500. doi: 10.1007/s00232-014-9659-7. [DOI] [PubMed] [Google Scholar]
- Wang Z. Roles of K +channels in regulating tumour cell proliferation and apoptosis. Pflugers Arch. 2004;448:274–286. doi: 10.1007/s00424-004-1258-5. [DOI] [PubMed] [Google Scholar]
- Watanabe M, Iwashita M, Ishii M, Kurachi Y, Kawakami A, Kondo S, Okada N. Spot pattern of leopard Danio is caused by mutation in the zebrafish connexin41.8 gene. EMBO Rep. 2006;7:893–897. doi: 10.1038/sj.embor.7400757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wee EL, Zimmerman EF. GABA uptake in embryonic palate mesenchymal cells of two mouse strains. Neurochem Res. 1985;10:1673–1688. doi: 10.1007/BF00988609. [DOI] [PubMed] [Google Scholar]
- Weksberg R, Nishikawa J, Caluseriu O, Fei YL, Shuman C, Wei C, Steele L, Cameron J, Smith A, Ambus I, et al. Tumor development in the Beckwith-Wiedemann syndrome is associated with a variety of constitutional molecular 11p15 alterations including imprinting defects of KCNQ1OT1. Hum Mol Genet. 2001;10:2989–3000. doi: 10.1093/hmg/10.26.2989. [DOI] [PubMed] [Google Scholar]
- Wilschanski M, Dupuis A, Ellis L, Jarvi K, Zielenski J, Tullis E, Martin S, Corey M, Tsui LC, Durie P. Mutations in the cystic fibrosis transmembrane regulator gene and in vivo transepithelial potentials. Am J Respir Crit Care Med. 2006;174:787–794. doi: 10.1164/rccm.200509-1377OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wittig D, Wang X, Walter C, Gerdes HH, Funk RH, Roehlecke C. Multi-level communication of human retinal pigment epithelial cells via tunneling nanotubes. PLoS One. 2012;7:e33195. doi: 10.1371/journal.pone.0033195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wonderlin WF, Strobl JS. Potassium channels, proliferation and G1 progression. J Membr Biol. 1996;154:91–107. doi: 10.1007/s002329900135. [DOI] [PubMed] [Google Scholar]
- Woodruff RI. Calmodulin transit via gap junctions is reduced in the absence of an electric field. J Insect Physiol. 2005;51:843–852. doi: 10.1016/j.jinsphys.2005.03.013. [DOI] [PubMed] [Google Scholar]
- Xie C, Jiang XH, Zhang JT, Sun TT, Dong JD, Sanders AJ, Diao RY, Wang Y, Fok KL, Tsang LL, et al. CFTR suppresses tumor progression through miR-193b targeting urokinase plasminogen activator (uPA) in prostate cancer. Oncogene. 2013;32:e1–7. doi: 10.1038/onc.2012.251. 2282–2291, 2291. [DOI] [PubMed] [Google Scholar]
- Yamashita M. Fluctuations in nuclear envelope's potential mediate synchronization of early neural activity. Biochem Biophys Res Commun. 2011;406:107–111. doi: 10.1016/j.bbrc.2011.02.004. [DOI] [PubMed] [Google Scholar]
- Yamashita M. Electric axon guidance in embryonic retina: galvanotropism revisited. Biochem Biophys Res Commun. 2013;431:280–283. doi: 10.1016/j.bbrc.2012.12.115. [DOI] [PubMed] [Google Scholar]
- Yan X, Han J, Zhang Z, Wang J, Cheng Q, Gao K, Ni Y, Wang Y. Lung cancer A549 cells migrate directionally in DC electric fields with polarized and activated EGFRs. Bioelectromagnetics. 2009;30:29–35. doi: 10.1002/bem.20436. [DOI] [PubMed] [Google Scholar]
- Yang M, Brackenbury WJ. Membrane potential and cancer progression. Front Physiol. 2013;4:185. doi: 10.3389/fphys.2013.00185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda T, Adams DJ. Physiological roles of ion channels in adult neural stem cells and their progeny. J Neurochem. 2010;114:946–959. doi: 10.1111/j.1471-4159.2010.06822.x. [DOI] [PubMed] [Google Scholar]
- You MH, Song MS, Lee SK, Ryu PD, Lee SY, Kim DY. Voltage-gated K(+) channels in adipogenic differentiation of bone marrow-derived human mesenchymal stem cells. Acta Pharmacol Sin. 2012;34:129–136. doi: 10.1038/aps.2012.142. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu K, Ruan DY, Ge SY. Three electrophysiological phenotypes of cultured human umbilical vein endothelial cells. Gen Physiol Biophys. 2002;21:315–326. [PubMed] [Google Scholar]
- Yun Z, Zhengtao D, Jiachang Y, Fangqiong T, Qun W. Using cadmium telluride quantum dots as a proton flux sensor and applying to detect H9 avian influenza virus. Anal Biochem. 2007;364:122–127. doi: 10.1016/j.ab.2007.02.031. [DOI] [PubMed] [Google Scholar]
- Zhang D, Chan JD, Nogi T, Marchant JS. Opposing roles of voltage-gated Ca2+ channels in neuronal control of regenerative patterning. J Neurosci. 2011;31:15983–15995. doi: 10.1523/JNEUROSCI.3029-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J, Chan YC, Ho JC, Siu CW, Lian Q, Tse HF. Regulation of cell proliferation of human induced pluripotent stem cell-derived mesenchymal stem cells via Ether a go-go 1 (hEAG1) potassium channel. Am J Physiol Cell Physiol. 2012;303:C115–C125. doi: 10.1152/ajpcell.00326.2011. [DOI] [PubMed] [Google Scholar]
- Zhang JT, Jiang XH, Xie C, Cheng H, Da Dong J, Wang Y, Fok KL, Zhang XH, Sun TT, Tsang LL, et al. Downregulation of CFTR promotes epithelial-to-mesenchymal transition and is associated with poor prognosis of breast cancer. Biochim Biophys Acta. 2013;1833:2961–2969. doi: 10.1016/j.bbamcr.2013.07.021. [DOI] [PubMed] [Google Scholar]
- Zhao M. Electrical fields in wound healing—an overriding signal that directs cell migration. Semin Cell Dev Biol. 2009;20:674–682. doi: 10.1016/j.semcdb.2008.12.009. [DOI] [PubMed] [Google Scholar]
- Zhao M, Chalmers L, Cao L, Vieira AC, Mannis M, Reid B. Electrical signaling in control of ocular cell behaviors. Progr Retin Eye Res. 2012;31:65–88. doi: 10.1016/j.preteyeres.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao M, McCaig CD, Agius-Fernandez A, Forrester JV, Araki-Sasaki K. Human corneal epithelial cells reorient and migrate cathodally in a small applied electric field. Curr Eye Res. 1997;16:973–984. doi: 10.1076/ceyr.16.10.973.9014. [DOI] [PubMed] [Google Scholar]
- Zoidl G, Dermietzel R. Gap junctions in inherited human disease. Pflugers Arch. 2010;460:451–466. doi: 10.1007/s00424-010-0789-1. [DOI] [PubMed] [Google Scholar]
- Zykov VS. Spiral waves in two-dimensional excitable media. Ann NY Acad Sci. 1990;591:75–85. doi: 10.1111/j.1749-6632.1990.tb15082.x. [DOI] [PubMed] [Google Scholar]