Supplemental Digital Content is available in the text.
Keywords: cardiac resynchronization therapy; comparative effectiveness research; defibrillators, implantable; heart failure
Abstract
Background—
Cardiac resynchronization therapy with defibrillator (CRT-D) reduces morbidity and mortality among selected patients with heart failure in clinical trials. The effectiveness of this therapy in clinical practice has not been well studied.
Methods and Results—
We compared a cohort of 4471 patients from the National Cardiovascular Data Registry’s Implantable Cardioverter-Defibrillator (ICD) Registry hospitalized primarily for heart failure and who received CRT-D between April 1, 2006, and December 31, 2009, to a historical control cohort of 4888 patients with heart failure without CRT-D from the Acute Decompensated Heart Failure National Registry (ADHERE) hospitalized between January 1, 2002, and March 31, 2006. Both registries were linked with Medicare claims to evaluate longitudinal outcomes. We included patients from the ICD Registry with left ventricular ejection fraction ≤35% and QRS duration ≥120 ms who were admitted for heart failure. We used Cox proportional hazards models to compare outcomes with and without CRT-D after adjustment for important covariates. After multivariable adjustment, CRT-D was associated with lower 3-year risks of death (hazard ratio, 0.52; 95% confidence interval, 0.48–0.56; P<0.001), all-cause readmission (hazard ratio, 0.69; 95% confidence interval, 0.65–0.73; P<0.001), and cardiovascular readmission (hazard ratio, 0.60; 95% confidence interval, 0.56–0.64; P<0.001). The association of CRT-D with mortality did not vary significantly among subgroups defined by age, sex, race, QRS duration, and optimal medical therapy.
Conclusions—
CRT-D was associated with lower risks of mortality, all-cause readmission, and cardiovascular readmission than medical therapy alone among patients with heart failure in community practice.
Cardiac resynchronization therapy with defibrillator (CRT-D) can improve functional status, morbidity, and survival in select populations of patients with heart failure, reduced left ventricular ejection fraction (EF), and QRS prolongation.1–12 Accordingly, clinical guidelines recommend CRT-D in patients with symptomatic heart failure, left ventricular EF ≤35%, and a wide QRS complex.13 However, the extent to which the efficacy of CRT-D in randomized clinical trials translates into effectiveness in clinical practice is unknown.14,15
Clinical Perspective on p 934
Clinical trials in heart failure enroll selected participants with idealized care compared with what is seen in real-world practice.16 This is particularly true for CRT-D, for which the landmark randomized trials included relatively young, predominantly male populations with few comorbid conditions. In the Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION),6 the first randomized trial to demonstrate the efficacy of CRT-D, median age was 66 years and 67% of participants were men.
Given the differences in demographic characteristics and coexisting illnesses often seen in patients with heart failure in clinical practice, it is unclear whether the benefits and safety of CRT-D demonstrated in clinical trials reflect outcomes in a broader population of older patients, women, patients in minority racial or ethnic groups, and patients with moderate prolongation of QRS duration. We examined associations between CRT-D and mortality and readmission among patients with heart failure who received CRT-D in clinical practice compared with potentially eligible patients who received medical therapy alone.
Methods
Data Sources
We obtained clinical data from 2 registries, the Acute Decompensated Heart Failure National Registry (ADHERE) and the National Cardiovascular Data Registry’s Implantable Cardioverter-Defibrillator (ICD) Registry. ADHERE was established to study the characteristics, treatments, and inpatient outcomes of patients hospitalized with acute decompensated heart failure. More than 185 000 patients from ≥300 community and academic centers were enrolled between January 2001 and March 2006.17 The ICD Registry, an initiative of the American College of Cardiology Foundation and the Heart Rhythm Society, became the official repository of ICD implantation data for Medicare beneficiaries in April 2006 and is used in >1400 hospitals in the United States.18 Both registries include demographic characteristics, medical history, and discharge medications, as well as clinical and procedural information.
To analyze long-term follow-up data, we obtained research-identifiable files for all fee-for-service Medicare inpatient claims and the corresponding denominator files for 2000 through 2010 from the US Centers for Medicare & Medicaid Services. These files contain encrypted identifiers that allow for longitudinal follow-up of Medicare beneficiaries. The inpatient files contain hospital claims covered under Medicare Part A and include service dates and diagnosis and procedure codes. The denominator files include patient demographic characteristics, information about Medicare eligibility and enrollment, and death dates if applicable. We linked records from both registries to the Medicare inpatient files using methods described previously.19 After linking the data, we used Medicare beneficiary identifiers to obtain subsequent events for beneficiaries with eligible hospitalizations.
Study Cohort
We used the ICD Registry to identify patients who received CRT-D, and we used ADHERE to identify a comparison group of patients who did not receive CRT-D during the hospital stay. We included patients from the ICD Registry who were admitted between April 1, 2006, and December 31, 2009, and underwent CRT-D implantation during the hospital stay. To ensure comparability with the ADHERE population, we only included patients from the ICD Registry who were admitted primarily for heart failure and not specifically for the device implantation. We excluded patients who received CRT-D for secondary prevention or had an epicardial lead placement. We included patients from ADHERE who were admitted between January 1, 2002, and March 31, 2006, and did not receive an ICD during the hospital stay. We excluded patients from ADHERE who underwent defibrillation during the admission.
From both registries, we included patients who were ≥65 years, were enrolled in fee-for-service Medicare for ≥12 months before the index admission, were discharged alive but not to a skilled nursing facility or hospice, and did not leave against medical advice. We also required that the patients have QRS duration ≥120 ms and EF ≤35%, consistent with guideline recommendations.13,20–22 We also required that patients not have a previous implantation of a cardiac device, previous cardiac arrest, myocardial infarction in the previous 40 days, or revascularization during the admission. For patients with multiple eligible hospitalizations, we selected the earliest as the index hospitalization.
Treatment
The treatment of interest was CRT-D as recorded in the ICD Registry.
Outcomes
The outcomes of interest were death, all-cause readmission, and cardiovascular readmission after the index hospitalization. We analyzed noncardiovascular readmission in a post hoc analysis. We determined all-cause mortality on the basis of death dates in the Medicare denominator files. We determined all-cause readmission on the basis of new nonelective Medicare inpatient claims, excluding the index hospitalization claim, transfers to or from another hospital, and admissions for rehabilitation (International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code V57.xx). Cardiovascular readmissions were the subset of these all-cause readmissions having one of the following diagnosis-related group codes: 104 to 112, 115 to 118, 121 to 145, 479, 514 to 518, 525 to 527, 535, 536, or 547 to 558 before October 1, 2007, and 215 to 238, 242 to 254, 258 to 262, or 280 to 316 on or after October 1, 2007. We defined the remaining readmissions as noncardiovascular. The follow-up period for all outcomes was 3 years after discharge from the index hospitalization.
Covariates
We used the registries to identify patient demographic characteristics (age, sex, and race), medical history (atrial fibrillation/flutter, cerebrovascular disease, chronic lung disease, diabetes mellitus, hypertension, ischemic heart disease, previous myocardial infarction, and renal failure dialysis), laboratory test results and vital signs (blood urea nitrogen, EF, left bundle-branch block, QRS duration, serum creatinine, serum sodium, and systolic blood pressure), and discharge medications (angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker, β-blocker, digoxin, and diuretic). We used inpatient claims to identify the urgency of the index admission and hospital admissions for a primary diagnosis of heart failure (International Classification of Diseases, Ninth Revision, Clinical Modification diagnosis code 428.x, 402.x1, 404.x1, or 404.x3) in the 6 months before the index hospitalization. We used Hierarchical Condition Category (HCC) codes derived from inpatient claims in the 12 months before the index hospitalization to identify protein-calorie malnutrition (HCC 21), dementia (HCC 49, 50), disability (HCC 100–102, 68, 69, 177, and 178), major psychiatric disorders (HCC 54–56), and chronic liver disease (HCC 25–27). These variables have independent prognostic value for modeling mortality and readmission after a heart failure hospitalization.23,24
Statistical Analysis
We describe the baseline characteristics of the study population using proportions for categorical variables and means with SDs for continuous variables. We tested for differences between treatment groups using χ2 tests for categorical variables and Wilcoxon rank-sum tests for continuous variables. We report observed event rates by treatment group. For mortality, we used Kaplan–Meier methods to calculate event rates and log-rank tests to assess differences between groups. For readmission end points, we used the cumulative incidence function to calculate event rates and Gray tests to assess differences between groups. The cumulative incidence function accounts for the competing risk of mortality, which is high in this population.25 We censored data for patients who did not experience an event at the earliest of 3 years after discharge, the end of the period for which data were available (December 31, 2010), or the date on which the patient’s data were no longer available because the patient enrolled in a Medicare managed care plan. We treated death as a competing risk for readmission.
We used Cox proportional hazards models to estimate the risk-adjusted association between CRT-D and each outcome. In addition to an indicator for receipt of CRT-D, the model included all of the covariates described above, except left bundle-branch block, which was only available for the CRT-D group. We report hazard ratios (HRs) and 95% confidence intervals (CIs) based on robust SEs to account for clustering of patients by hospital. For the main analysis, we used α≤0.05 to determine statistical significance.
We also estimated the risk-adjusted association between CRT-D and each outcome in prespecified subgroups based on age, sex, race, QRS duration, presence of atrial fibrillation, and receipt of optimal medical therapy. We defined optimal medical therapy as receipt of a β-blocker and either an angiotensin-converting enzyme inhibitor or an angiotensin II receptor blocker in the absence of contraindications, as defined by guidelines for ICD and CRT during the study period.21,26,27 We estimated risk-adjusted associations for each subgroup by adding a subgroup variable and an interaction term between this variable and the CRT-D indicator to the models. We assessed differences in the strength of the treatment–outcome association between subgroups by testing the significance of the interaction term. We calculated estimates of the association within each subgroup using model contrasts. Because of the multiple comparisons in the subgroup analysis, we used α≤0.01 to establish statistical significance and we report 99% CIs.
In a secondary analysis, we applied the methods used in the primary analysis to a propensity-matched cohort.28 The propensity score treatment model was fit as a logistic regression model with receipt of CRT-D as the dependent variable and demographic (age, sex, and race) and clinical characteristics (atrial fibrillation/flutter, cerebrovascular disease, chronic lung disease, diabetes mellitus, hypertension, ischemic heart disease, previous myocardial infarction, renal failure dialysis, protein-calorie malnutrition, dementia, disability, major psychiatric disorders, chronic liver disease, blood urea nitrogen, EF, QRS duration, serum creatinine, serum sodium, systolic blood pressure, and admission urgency) as covariates. Using greedy matching techniques, we matched comparison patients 1:1 to treated patients on the linear predictor from the treatment model with calipers equal to 0.2 SD of the linear predictor distribution. We used standardized differences to check the distribution of patient characteristics for balance. To estimate the association of CRT-D with each outcome, we used Cox proportional hazards models with an indicator for receipt of CRT-D as the only covariate.
We used SAS version 9.2 (SAS Institute Inc, Cary, NC) for all analyses. The Institutional Review Board of the Duke University Health System approved the study.
Results
The final study population included 4471 patients from the ICD Registry and 4888 patients from ADHERE (Figure 1). When compared with historical controls, the CRT-D group was slightly younger and had more men and white patients (Table 1). CRT-D recipients were more likely to have atrial fibrillation, hypertension, previous hospitalization for heart failure, or previous myocardial infarction, and they had a longer mean QRS duration and a lower mean EF. Discharge medications also differed between groups: patients who received CRT-D were more likely to receive β-blockers, less likely to receive digoxin, and more likely to be on an angiotensin-converting enzyme inhibitor or angiotensin II receptor blocker and a β-blocker.
Figure 1.

Derivation of the study population. *Patients may have more than one of these. †Includes implantable cardioverter-defibrillator (ICD), CRT-D, CRT-P, or other pacemaker. ADHERE indicates Acute Decompensated Heart Failure Registry; CRT-D, cardiac resynchronization therapy with defibrillator; and NCDR, National Cardiovascular Data Registry.
Table 1.
Characteristics of the Study Population
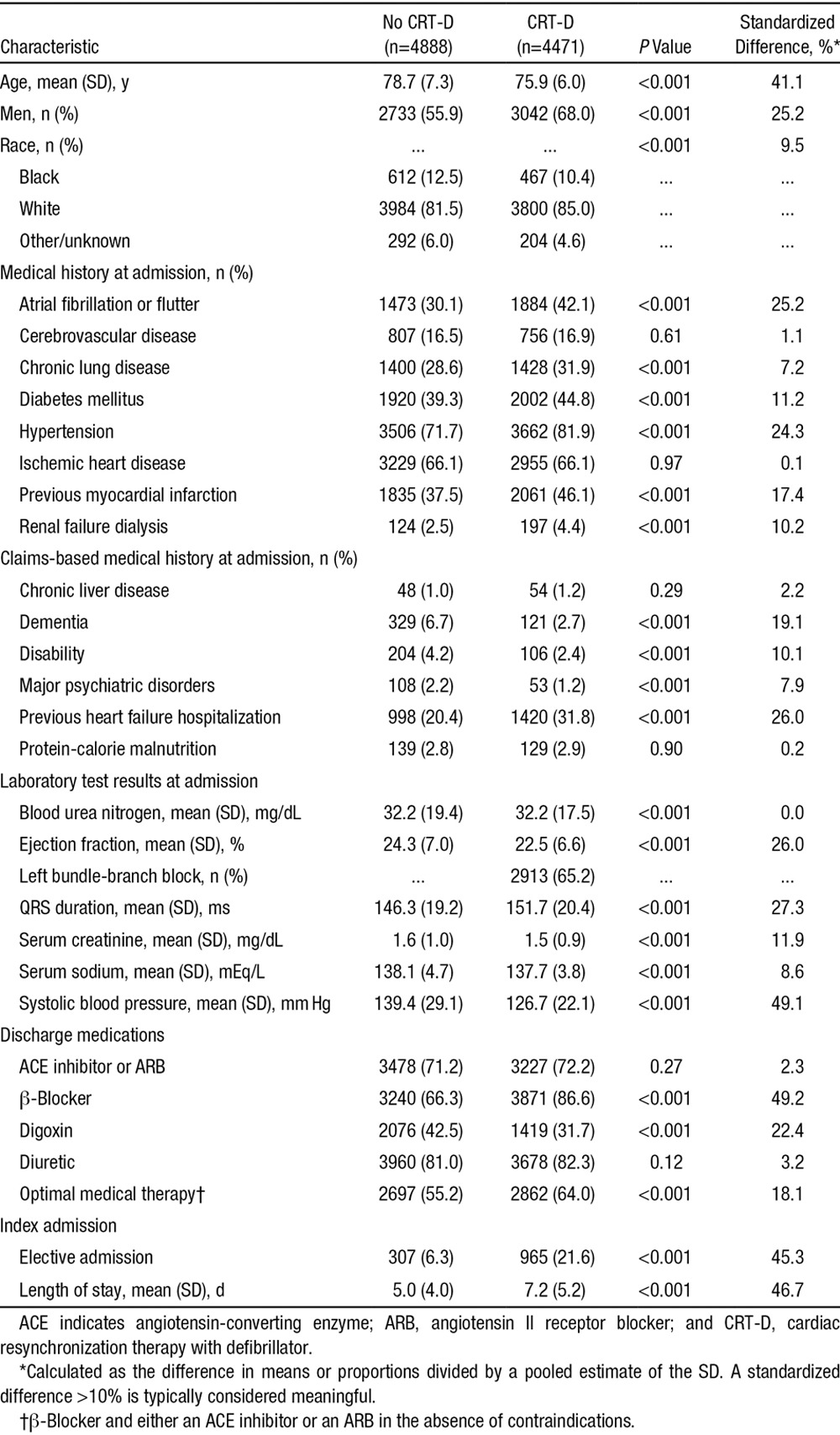
Table 2 shows the unadjusted outcomes within 1, 2, and 3 years after the index hospitalization. Patients who received CRT-D had a lower cumulative incidence of mortality, all-cause readmission, and cardiovascular readmission than historical controls. Slightly <40% of patients who received CRT-D died within 3 years of the index hospitalization when compared with 59% of those who did not (P<0.001). Differences were similar but less pronounced for all-cause and cardiovascular readmission. Within 3 years, 81% of patients who received CRT-D had a readmission from any cause when compared with 84% of those who did not (P<0.001). Similarly, 59% of patients who received CRT-D had a cardiovascular readmission when compared with 67% of those who did not (P<0.001). There were no significant differences in noncardiovascular readmission rates. After adjustment for demographic and clinical characteristics, the risks of 3-year mortality, all-cause readmission, and cardiovascular readmission were significantly lower among patients who received CRT-D (Figure 2). Patients who received CRT-D were at lower risk for mortality (HR, 0.52; 95% CI, 0.48–0.56; P<0.001), all-cause readmission (HR, 0.69; 95% CI, 0.65–0.73; P<0.001), cardiovascular readmission (HR, 0.60; 95% CI, 0.56–0.64; P<0.001), and noncardiovascular readmission (HR, 0.87; 95% CI, 0.81–0.93).
Table 2.
Cumulative Incidence of Mortality, All-Cause Readmission, and Cardiovascular Readmission
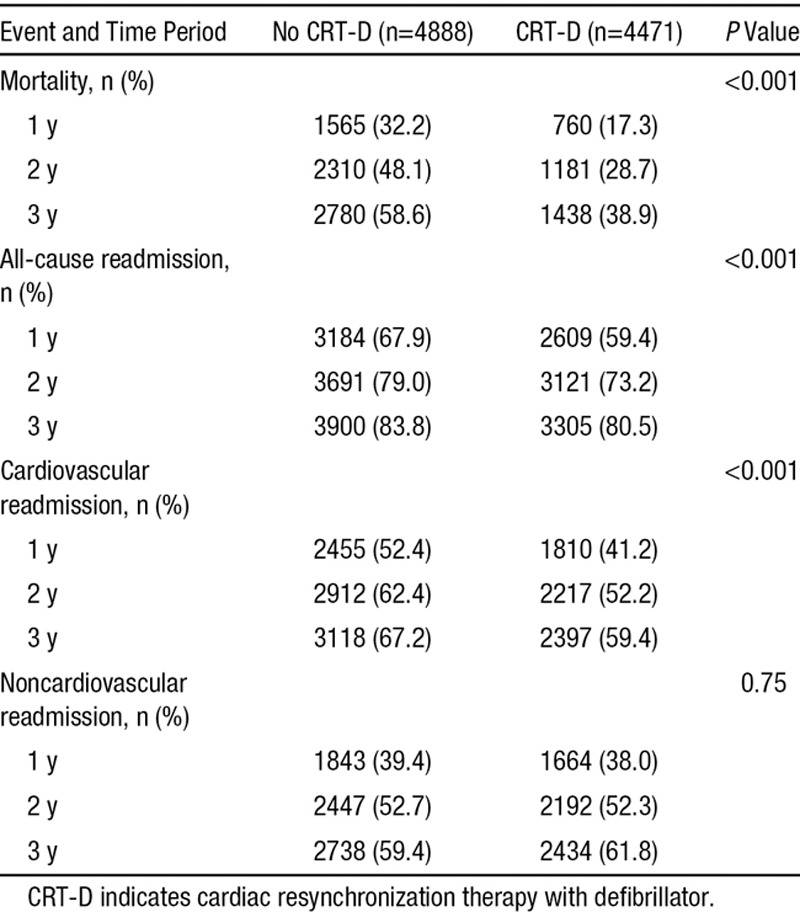
Figure 2.
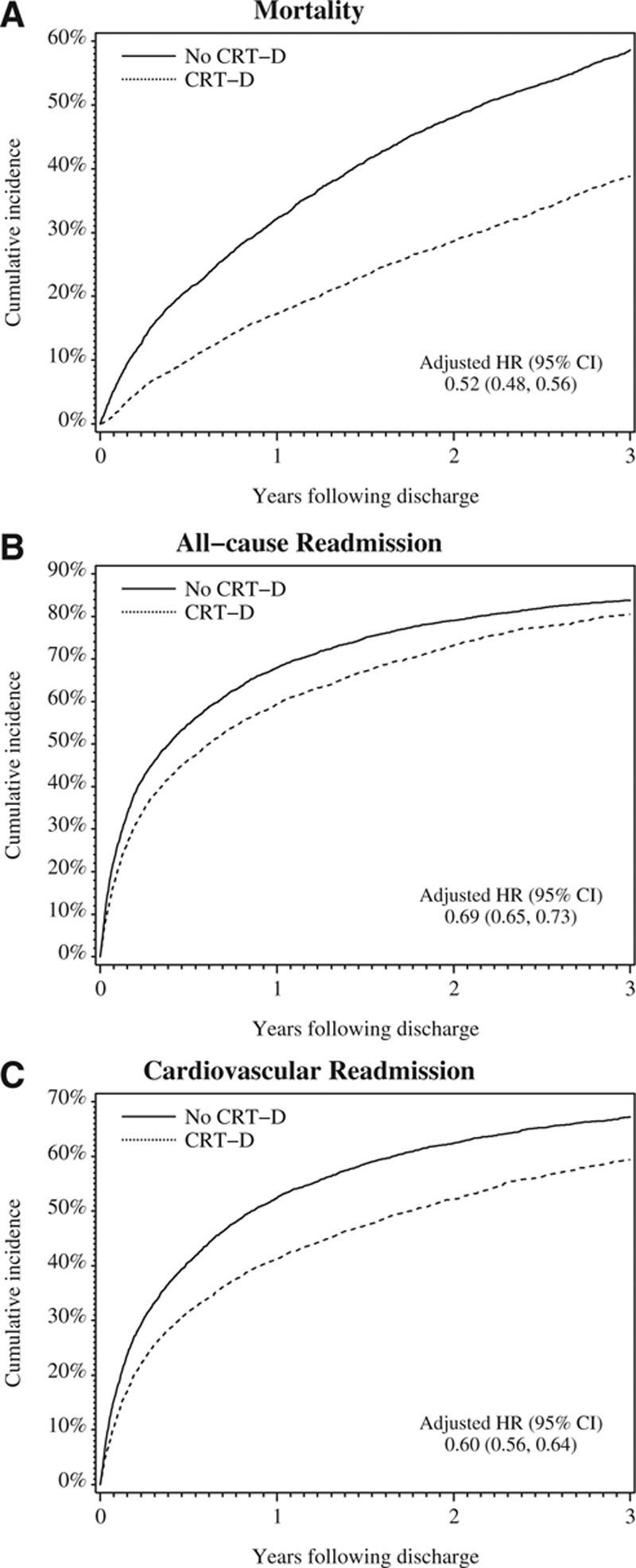
Cumulative incidence of (A) mortality, (B) all-cause readmission, and (C) cardiovascular readmission. Hazard ratios (HRs) reflect adjusted associations between cardiac resynchronization therapy with defibrillator (CRT-D) and mortality and cardiovascular readmission compared with medical therapy alone. Regression models controlled for demographic characteristics, medical history, test results, discharge medications, and admission urgency. CI indicates confidence interval.
Figure 3 shows adjusted associations between CRT-D and 3-year clinical outcomes by subgroup. Apart from the subgroup defined by QRS duration for all-cause and cardiovascular readmission, the strength of the CRT-D association with mortality, all-cause readmission, or cardiovascular readmission was similar between subgroups after adjustment for demographic and clinical characteristics. The hazard of all-cause readmission associated with CRT-D, compared with medical therapy alone, was higher among patients with QRS duration of 120 to 149 ms (HR, 0.77; 99% CI, 0.69–0.85) than among those with QRS duration ≥150 ms (HR, 0.61; 99% CI, 0.56–0.67; P<0.001 for the interaction). Similarly, the hazard of cardiovascular readmission associated with CRT-D, compared with medical therapy alone, was higher among patients with QRS duration of 120 to 149 ms (HR, 0.70; 99% CI, 0.63–0.78) than among those with QRS duration ≥150 ms (HR, 0.50; 99% CI, 0.45–0.56; P<0.001 for the interaction). In contrast, CRT-D was associated with similar mortality benefit in patients with QRS duration of 120 to 149 ms and those with QRS duration ≥150 ms. Results in a propensity-matched sample of treated and comparison patients were similar to the results of the main analysis (Table I in the Data Supplement).
Figure 3.
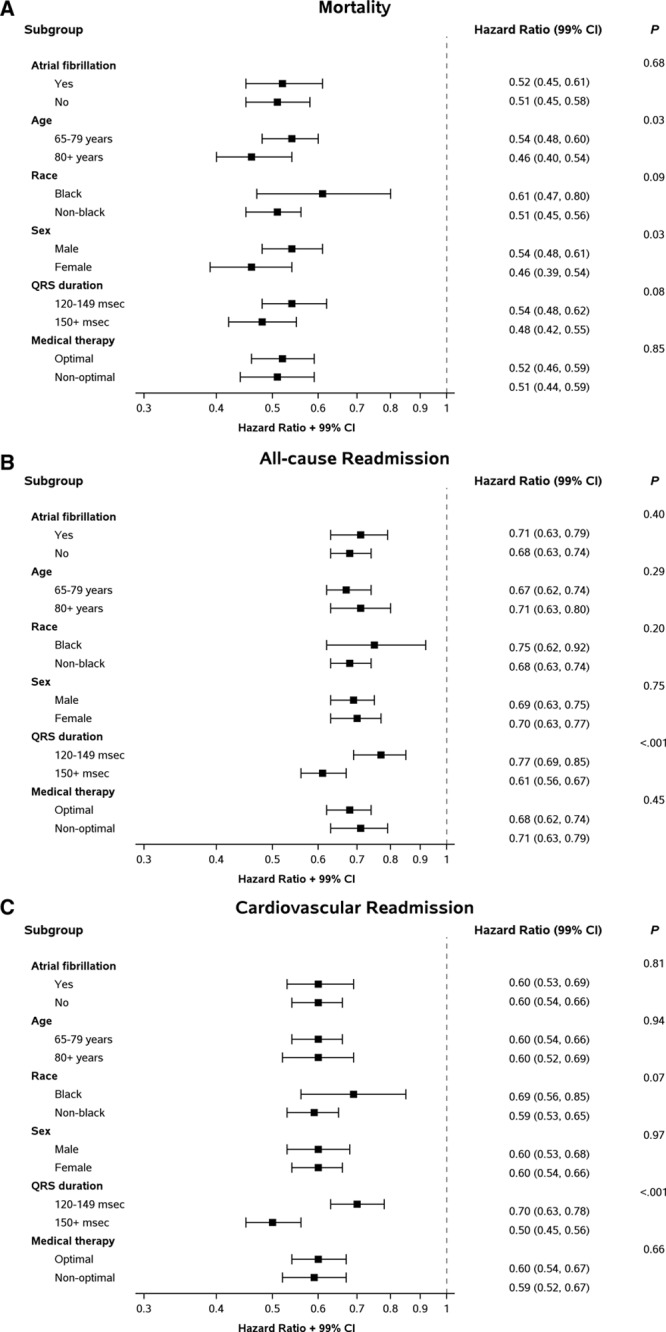
Associations between cardiac resynchronization therapy with defibrillator and 3-year outcomes by subgroup. CI indicates confidence interval.
Discussion
In the largest registry of patients with heart failure in the United States who received CRT-D and a control group of patients who were potentially eligible for CRT-D but did not receive it, CRT-D was associated with lower rates of mortality, all-cause readmission, and cardiovascular readmission. Although the study population was older and had greater comorbidity than participants in the landmark clinical trials, the magnitude of risk reduction associated with CRT-D was similar. CRT-D was also associated with lower mortality in prespecified clinical subgroups.
There is little evidence of the effectiveness of CRT-D in clinical practice in the literature. To our knowledge, ours is the first study to estimate the clinical effectiveness of CRT-D compared with medical therapy among Medicare beneficiaries. Real-world evidence is important for CRT-D because almost one third of patients in the landmark trials were randomized only after successful device implantation, potentially inflating the benefits and underestimating the risks of the therapy.29
Real-world evidence can be difficult to generate for therapies, such as CRT-D, for which eligibility criteria include information not routinely collected in relevant clinical registries. QRS duration is critical for identifying patients eligible for CRT, and ADHERE was the only heart failure registry we found with high-quality data on QRS duration on all patients, not just those who received a device. However, ADHERE completed data collection months before the ICD Registry was initiated, and the use of a historical control group can be problematic. General improvements in health and outcomes over time tend to favor contemporary patients over historical controls and inflate effectiveness estimates for a contemporary therapy. However, 1-year mortality among Medicare beneficiaries hospitalized for heart failure has been stable,30 and available therapeutic options for chronic heart failure did not change during the study period. Similar to other studies, which have demonstrated improvements over time in the use of and adherence to medical therapy, such as β-blockers,31–33 patients in ADHERE had lower rates of β-blocker use and optimal medical therapy than patients in the ICD Registry. Similarly, differential use of aldosterone antagonist therapy might be expected, but data for this class of medications were unavailable in the ICD Registry.
COMPANION, the first major trial to compare CRT alone and CRT-D to medical therapy, found that both CRT and CRT-D reduced the primary outcome of death or cardiovascular hospitalization but only CRT-D provided a survival advantage (ie, 36% relative risk reduction in death).6 After COMPANION, the US Food and Drug Administration approved the use of CRT-D. Subsequent studies reported relative risk reductions in death from 22% to 36%.7–12 The consistency of findings across trials and in our analysis supports current guideline recommendations for CRT-D in patients with symptomatic heart failure, left ventricular EF ≤35%, and prolonged QRS duration.13
We also found that CRT-D was associated with lower risks of all-cause and cardiovascular readmission, consistent with previous research.6,11 Because the risk reductions was dramatic, we performed a post hoc analysis of noncardiovascular readmission to account for factors that were different in the 2 populations. The adjusted risk of noncardiovascular readmission for CRT-D versus no CRT-D was 0.87. Although this reduction is modest and less than the cardiovascular readmission risk, it was not surprising. Heart failure may contribute to noncardiovascular readmission but may not be the main factor, so effectiveness is less. For example, a patient with chronic lower-extremity edema may be admitted for noncardiovascular causes, such as cellulitis. Also, CRT would not be expected to modify some causes of readmission, such as cancer.
In post hoc analyses of trial data, subgroup results by QRS duration have led to mixed conclusions. In the Cardiac Resynchronization in Heart Failure (CARE-HF) trial, which compared CRT plus pacemaker with medical therapy, there was no heterogeneity in the effect of CRT by QRS duration (120–159 versus ≥160 ms) on outcomes.7–9 In contrast, newer trials that broadened the indication of CRT-D to patients with mild heart failure suggested a significantly more beneficial effect of CRT for death or heart failure events among patients with QRS duration ≥150 ms when compared with patients with QRS duration of 120 to 149 ms.10–12,34 In our study, the effect of CRT versus medical therapy differed significantly by QRS duration for all-cause and cardiovascular readmission but not for mortality. For readmissions, patients with QRS duration ≥150 ms exhibited greater benefit of CRT when compared with patients with QRS duration of 120 to 149 ms.
Our findings suggest that black patients benefit as much as patients of other races, further emphasizing the importance of reducing racial disparities in the use of CRT-D.35,36 Moreover, we observed similar benefits between men and women. In contrast, recent clinical trial findings suggest that women may benefit more than men37 although differences in patient characteristics may have contributed to outcome differences by sex. Finally, patients aged 65 to 80 years and those aged ≥80 years seemed to benefit from CRT-D compared with medical therapy alone.
Our study has limitations. We used historical comparators from ADHERE, selecting patients who met eligibility requirements for CRT-D. We maximized the comparability of the groups through careful sample restriction and adjustment for measured confounders, but residual confounding is possible. The use of 2 registries with slightly different data definitions may have further increased the likelihood of confounding. Finally, the data were derived from clinical registries linked with fee-for-service Medicare claims. Registry data were collected by medical chart review and depended on the accuracy of documentation and abstraction, and the results may not be generalizable to Medicare beneficiaries in managed care or younger patients. Generalizability is further limited by our exclusion of 90% of patients in the ICD Registry and 50% of patients ADHERE to create comparable groups and improve internal validity.
In conclusion, CRT-D was associated with lower rates of mortality, all-cause readmission, and cardiovascular readmission among patients with heart failure in clinical practice, including patients with QRS duration ≥120 ms and in subgroups defined by race and sex.
Acknowledgments
Damon M. Seils, MA, Duke University, provided editorial assistance and prepared the article. D.M. Seils did not receive compensation for his assistance apart from his employment at the institution where the study was conducted.
Sources of Funding
This study was funded under contract HHSA29020050032I (Duke University Developing Evidence to Inform Decisions About Effectiveness [DEcIDE] Center) from the Agency for Healthcare Research and Quality as part of the DEcIDE program. The project was also supported, in part, by grant number U19HS021092 from the Agency for Healthcare Research and Quality. Dr Khazanie was supported, in part, by grant T32HL069749 from the National Heart, Lung, and Blood Institute.
Disclosures
Dr Fonarow reported serving as a consultant for Amgen, Gambro, GlaxoSmithKline, Johnson & Johnson, Medtronic, Merck & Co, Novartis, Pfizer, Relypsa, Scios, St. Jude, The Medicines Company, and Takeda; being used as the Eliot Corday Chair of Cardiovascular Medicine and Science and with the Ahmanson Foundation; receiving grants from the Ahmanson Foundation and GlaxoSmithKline; and receiving payment for lectures from Boston Scientific/Guidant, GlaxoSmithKline, Medtronic, Merck & Co, Novartis, Pfizer, and St. Jude Medical. Dr Heidenreich reported receiving research support from Medtronic. Dr Masoudi has contracts with the Oklahoma Foundation for Medical Quality and the American College of Cardiology Foundation. Dr Peterson reported serving as a consultant for Merck & Co. Dr Piccini reported serving as a consultant for Bristol-Myers Squibb, Johnson & Johnson, Medtronic, and Spectranetics; receiving fees from Forest Laboratories; and receiving grants from GE Healthcare and Johnson & Johnson. Dr Curtis reported that his institution received funding as the data center for the American College of Cardiology’s National Cardiovascular Data Registry; providing expert testimony for Piedmont Liability Trust; receiving research grants from the American College of Cardiology Foundation; and having stock in Medtronic. Dr Hernandez reported serving as a consultant for AstraZeneca, Bristol-Myers Squibb, Corthera, Cytokinetics, and Johnson & Johnson; and receiving grants from Amylin, Bristol-Myers Squibb, Johnson & Johnson, and Portola. Dr Curtis reported receiving research grants from GlaxoSmithKline and Johnson & Johnson. The other authors report no conflicts.
Footnotes
The authors of this report are responsible for its content. Statements in the report should not be construed as endorsement by the Agency for Healthcare Research and Quality, the US Department of Health and Human Services, or the National Cardiovascular Data Registry or its associated professional societies identified at www.ncdr.com, or to represent the official views of the National Heart, Lung, and Blood Institute or the National Institutes of Health.
The Data Supplement is available at http://circheartfailure.ahajournals.org/lookup/suppl/doi:10.1161/CIRCHEARTFAILURE.113.000838/-/DC1.
CLINICAL PERSPECTIVE
Although clinical guidelines recommend cardiac resynchronization therapy with defibrillator (CRT-D) to reduce morbidity and mortality among eligible patients with reduced ejection fraction and QRS prolongation, the comparative effectiveness of CRT-D compared with medical therapy is unknown. Patients receiving CRT-D in clinical practice are typically older and have greater comorbidity than patients in the landmark clinical trials. We examined associations between CRT-D and mortality and readmission rates among patients with heart failure who received CRT-D compared with potentially eligible patients who received medical therapy alone using clinical data from 2 registries linked with Medicare claims. We used Cox proportional hazards models to compare outcomes with and without CRT-D after adjustment for important covariates. CRT-D was associated with lower rates of mortality, all-cause readmission, and cardiovascular readmission among patients with heart failure in clinical practice, including patients with QRS duration ≥120 ms and in subgroups defined by race and sex. The magnitudes of risk reduction associated with CRT-D were similar to clinical trials. Data such as these are helpful to the clinician because they demonstrate that, despite an older and sicker population, the efficacy of CRT-D as shown in clinical trials translates into effectiveness in clinical practice when compared with medical therapy alone.
References
- 1.Cazeau S, Leclercq C, Lavergne T, Walker S, Varma C, Linde C, Garrigue S, Kappenberger L, Haywood GA, Santini M, Bailleul C, Daubert JC Multisite Stimulation in Cardiomyopathies (MUSTIC) Study Investigators. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 2.Abraham WT, Fisher WG, Smith AL, Delurgio DB, Leon AR, Loh E, Kocovic DZ, Packer M, Clavell AL, Hayes DL, Ellestad M, Trupp RJ, Underwood J, Pickering F, Truex C, McAtee P, Messenger J MIRACLE Study Group. Multicenter InSync Randomized Clinical Evaluation. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 3.Young JB, Abraham WT, Smith AL, Leon AR, Lieberman R, Wilkoff B, Canby RC, Schroeder JS, Liem LB, Hall S, Wheelan K Multicenter InSync ICD Randomized Clinical Evaluation (MIRACLE ICD) Trial Investigators. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD Trial. JAMA. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 4.Linde C, Leclercq C, Rex S, Garrigue S, Lavergne T, Cazeau S, McKenna W, Fitzgerald M, Deharo JC, Alonso C, Walker S, Braunschweig F, Bailleul C, Daubert JC. Long-term benefits of biventricular pacing in congestive heart failure: results from the MUltisite STimulation in cardiomyopathy (MUSTIC) study. J Am Coll Cardiol. 2002;40:111–118. doi: 10.1016/s0735-1097(02)01932-0. [DOI] [PubMed] [Google Scholar]
- 5.Higgins SL, Hummel JD, Niazi IK, Giudici MC, Worley SJ, Saxon LA, Boehmer JP, Higginbotham MB, De Marco T, Foster E, Yong PG. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454–1459. doi: 10.1016/s0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 6.Bristow MR, Saxon LA, Boehmer J, Krueger S, Kass DA, De Marco T, Carson P, DiCarlo L, DeMets D, White BG, DeVries DW, Feldman AM Comparison of Medical Therapy, Pacing, and Defibrillation in Heart Failure (COMPANION) Investigators. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 7.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L Cardiac Resynchronization-Heart Failure (CARE-HF) Study Investigators. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 8.Cleland JG, Daubert JC, Erdmann E, Freemantle N, Gras D, Kappenberger L, Tavazzi L. Longer-term effects of cardiac resynchronization therapy on mortality in heart failure [the CArdiac REsynchronization-Heart Failure (CARE-HF) trial extension phase]. Eur Heart J. 2006;27:1928–1932. doi: 10.1093/eurheartj/ehl099. [DOI] [PubMed] [Google Scholar]
- 9.Cleland JG, Freemantle N, Erdmann E, Gras D, Kappenberger L, Tavazzi L, Daubert JC. Long-term mortality with cardiac resynchronization therapy in the Cardiac Resynchronization-Heart Failure (CARE-HF) trial. Eur J Heart Fail. 2012;14:628–634. doi: 10.1093/eurjhf/hfs055. [DOI] [PubMed] [Google Scholar]
- 10.Linde C, Abraham WT, Gold MR, St John Sutton M, Ghio S, Daubert C REVERSE (REsynchronization reVErses Remodeling in Systolic left vEntricular dysfunction) Study Group. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–1843. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 11.Moss AJ, Hall WJ, Cannom DS, Klein H, Brown MW, Daubert JP, Estes NA, 3rd, Foster E, Greenberg H, Higgins SL, Pfeffer MA, Solomon SD, Wilber D, Zareba W MADIT-CRT Trial Investigators. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 12.Tang AS, Wells GA, Talajic M, Arnold MO, Sheldon R, Connolly S, Hohnloser SH, Nichol G, Birnie DH, Sapp JL, Yee R, Healey JS, Rouleau JL Resynchronization-Defibrillation for Ambulatory Heart Failure Trial Investigators. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 13.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO American College of Cardiology Foundation; American Heart Association Task Force on Practice Guidelines; Heart Rhythm Society. 2012 ACCF/AHA/HRS focused update incorporated into the ACCF/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. Circulation. 2013;127:e283–e352. doi: 10.1161/CIR.0b013e318276ce9b. [DOI] [PubMed] [Google Scholar]
- 14.Fonarow GC, Albert NM, Curtis AB, Gheorghiade M, Heywood JT, Liu Y, Mehra MR, O’Connor CM, Reynolds D, Walsh MN, Yancy CW. Associations between outpatient heart failure process-of-care measures and mortality. Circulation. 2011;123:1601–1610. doi: 10.1161/CIRCULATIONAHA.110.989632. [DOI] [PubMed] [Google Scholar]
- 15.Fonarow GC, Albert NM, Curtis AB, Gheorghiade M, Liu Y, Mehra MR, O’Connor CM, Reynolds D, Walsh MN, Yancy CW. Incremental Reduction in Risk of Death Associated With Use of Guideline-Recommended Therapies in Patients With Heart Failure: A Nested Case-Control Analysis of IMPROVE HF. J Am Heart Assoc. 2012;1:16–26. doi: 10.1161/JAHA.111.000018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Masoudi FA, Havranek EP, Wolfe P, Gross CP, Rathore SS, Steiner JF, Ordin DL, Krumholz HM. Most hospitalized older persons do not meet the enrollment criteria for clinical trials in heart failure. Am Heart J. 2003;146:250–257. doi: 10.1016/S0002-8703(03)00189-3. [DOI] [PubMed] [Google Scholar]
- 17.Adams KF, Jr, Fonarow GC, Emerman CL, LeJemtel TH, Costanzo MR, Abraham WT, Berkowitz RL, Galvao M, Horton DP ADHERE Scientific Advisory Committee and Investigators. Characteristics and outcomes of patients hospitalized for heart failure in the United States: rationale, design, and preliminary observations from the first 100,000 cases in the Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2005;149:209–216. doi: 10.1016/j.ahj.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 18.Hammill SC, Stevenson LW, Kadish AH, Kremers MS, Heidenreich P, Lindsay BD, Mirro MJ, Radford MJ, Wang Y, Lang CM, Harder JC, Brindis RG. Review of the registry’s first year, data collected, and future plans. Heart Rhythm. 2007;4:1260–1263. doi: 10.1016/j.hrthm.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 19.Hammill BG, Hernandez AF, Peterson ED, Fonarow GC, Schulman KA, Curtis LH. Linking inpatient clinical registry data to Medicare claims data using indirect identifiers. Am Heart J. 2009;157:995–1000. doi: 10.1016/j.ahj.2009.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gregoratos G, Abrams J, Epstein AE, Freedman RA, Hayes DL, Hlatky MA, Kerber RE, Naccarelli GV, Schoenfeld MH, Silka MJ, Winters SL, Gibbons RJ, Antman EM, Alpert JS, Gregoratos G, Hiratzka LF, Faxon DP, Jacobs AK, Fuster V, Smith SC, Jr American College of Cardiology/American Heart Association Task Force on Practice Guidelines/North American Society for Pacing and Electrophysiology Committee to Update the 1998 Pacemaker Guidelines. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines). Circulation. 2002;106:2145–2161. doi: 10.1161/01.cir.0000035996.46455.09. [DOI] [PubMed] [Google Scholar]
- 21.Epstein AE, DiMarco JP, Ellenbogen KA, Estes NA, III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO, Smith SC, Jr, Jacobs AK, Adams CD, Anderson JL, Buller CE, Creager MA, Ettinger SM, Faxon DP, Halperin JL, Hiratzka LF, Hunt SA, Krumholz HM, Kushner FG, Lytle BW, Nishimura RA, Ornato JP, Page RL, Riegel B, Tarkington LG, Yancy CW American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices); American Association for Thoracic Surgery; Society of Thoracic Surgeons. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–e408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 22.Stevenson WG, Hernandez AF, Carson PE, Fang JC, Katz SD, Spertus JA, Sweitzer NK, Tang WH, Albert NM, Butler J, Westlake Canary CA, Collins SP, Colvin-Adams M, Ezekowitz JA, Givertz MM, Hershberger RE, Rogers JG, Teerlink JR, Walsh MN, Stough WG, Starling RC Heart Failure Society of America Guideline Committee. Indications for cardiac resynchronization therapy: 2011 update from the Heart Failure Society of America Guideline Committee. J Card Fail. 2012;18:94–106. doi: 10.1016/j.cardfail.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 23.Keenan PS, Normand SL, Lin Z, Drye EE, Bhat KR, Ross JS, Schuur JD, Stauffer BD, Bernheim SM, Epstein AJ, Wang Y, Herrin J, Chen J, Federer JJ, Mattera JA, Wang Y, Krumholz HM. An administrative claims measure suitable for profiling hospital performance on the basis of 30-day all-cause readmission rates among patients with heart failure. Circ Cardiovasc Qual Outcomes. 2008;1:29–37. doi: 10.1161/CIRCOUTCOMES.108.802686. [DOI] [PubMed] [Google Scholar]
- 24.Krumholz HM, Wang Y, Mattera JA, Wang Y, Han LF, Ingber MJ, Roman S, Normand SL. An administrative claims model suitable for profiling hospital performance based on 30-day mortality rates among patients with heart failure. Circulation. 2006;113:1693–1701. doi: 10.1161/CIRCULATIONAHA.105.611194. [DOI] [PubMed] [Google Scholar]
- 25.Gray RJ. A class of k-sample tests for comparing the cumulative incidence of a competing risk. Ann Stat. 1988;16:1141–1154. [Google Scholar]
- 26.Hunt SA, Abraham WT, Chin MH, Feldman AM, Francis GS, Ganiats TG, Jessup M, Konstam MA, Mancini DM, Michl K, Oates JA, Rahko PS, Silver MA, Stevenson LW, Yancy CW, Antman EM, Smith SC, Jr, Adams CD, Anderson JL, Faxon DP, Fuster V, Halperin JL, Hiratzka LF, Jacobs AK, Nishimura R, Ornato JP, Page RL, Riegel B American College of Cardiology; American Heart Association Task Force on Practice Guidelines; American College of Chest Physicians; International Society for Heart and Lung Transplantation; Heart Rhythm Society. ACC/AHA 2005 Guideline Update for the Diagnosis and Management of Chronic Heart Failure in the Adult: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Update the 2001 Guidelines for the Evaluation and Management of Heart Failure): developed in collaboration with the American College of Chest Physicians and the International Society for Heart and Lung Transplantation: endorsed by the Heart Rhythm Society. Circulation. 2005;112:e154–e235. doi: 10.1161/CIRCULATIONAHA.105.167586. [DOI] [PubMed] [Google Scholar]
- 27.Jessup M, Abraham WT, Casey DE, Feldman AM, Francis GS, Ganiats TG, Konstam MA, Mancini DM, Rahko PS, Silver MA, Stevenson LW, Yancy CW. 2009 focused update: ACCF/AHA Guidelines for the Diagnosis and Management of Heart Failure in Adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:1977–2016. doi: 10.1161/CIRCULATIONAHA.109.192064. [DOI] [PubMed] [Google Scholar]
- 28.Rosenbaum PR, Rubin DB. The central role of the propensity score in observational studies for causal effects. Biometrika. 1983;70:41–55. [Google Scholar]
- 29.Linde C, Ellenbogen K, McAlister FA. Cardiac resynchronization therapy (CRT): clinical trials, guidelines, and target populations. Heart Rhythm. 2012;9(8 suppl):S3–S13. doi: 10.1016/j.hrthm.2012.04.026. [DOI] [PubMed] [Google Scholar]
- 30.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998-2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fonarow GC, Heywood JT, Heidenreich PA, Lopatin M, Yancy CW ADHERE Scientific Advisory Committee and Investigators. Temporal trends in clinical characteristics, treatments, and outcomes for heart failure hospitalizations, 2002 to 2004: findings from Acute Decompensated Heart Failure National Registry (ADHERE). Am Heart J. 2007;153:1021–1028. doi: 10.1016/j.ahj.2007.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Fonarow GC, Abraham WT, Albert NM, Gattis Stough W, Gheorghiade M, Greenberg BH, O’Connor CM, Pieper K, Sun JL, Yancy CW, Young JB OPTIMIZE-HF Investigators and Hospitals. Influence of a performance-improvement initiative on quality of care for patients hospitalized with heart failure: results of the Organized Program to Initiate Lifesaving Treatment in Hospitalized Patients With Heart Failure (OPTIMIZE-HF). Arch Intern Med. 2007;167:1493–1502. doi: 10.1001/archinte.167.14.1493. [DOI] [PubMed] [Google Scholar]
- 33.Fonarow GC, Albert NM, Curtis AB, Stough WG, Gheorghiade M, Heywood JT, McBride ML, Inge PJ, Mehra MR, O’Connor CM, Reynolds D, Walsh MN, Yancy CW. Improving evidence-based care for heart failure in outpatient cardiology practices: primary results of the Registry to Improve the Use of Evidence-Based Heart Failure Therapies in the Outpatient Setting (IMPROVE HF). Circulation. 2010;122:585–596. doi: 10.1161/CIRCULATIONAHA.109.934471. [DOI] [PubMed] [Google Scholar]
- 34.Gold MR, Thébault C, Linde C, Abraham WT, Gerritse B, Ghio S, St John Sutton M, Daubert JC. Effect of QRS duration and morphology on cardiac resynchronization therapy outcomes in mild heart failure: results from the Resynchronization Reverses Remodeling in Systolic Left Ventricular Dysfunction (REVERSE) study. Circulation. 2012;126:822–829. doi: 10.1161/CIRCULATIONAHA.112.097709. [DOI] [PubMed] [Google Scholar]
- 35.Hernandez AF, Fonarow GC, Liang L, Al-Khatib SM, Curtis LH, LaBresh KA, Yancy CW, Albert NM, Peterson ED. Sex and racial differences in the use of implantable cardioverter-defibrillators among patients hospitalized with heart failure. JAMA. 2007;298:1525–1532. doi: 10.1001/jama.298.13.1525. [DOI] [PubMed] [Google Scholar]
- 36.Eapen ZJ, Al-Khatib S, Lopes RD, Wang Y, Bao H, Curtis J, Heidenreich PA, Hernandez AF, Peterson ED, Hammill SC. Are racial/ethnic gaps in the use of cardiac resynchronization therapy narrowing?: an analysis of 107,096 patients from the National Cardiovascular Data Registry’s ICD Registry. J Am Coll Cardiol. 2012;60:1577–1578. doi: 10.1016/j.jacc.2012.06.024. [DOI] [PubMed] [Google Scholar]
- 37.Arshad A, Moss AJ, Foster E, Padeletti L, Barsheshet A, Goldenberg I, Greenberg H, Hall WJ, McNitt S, Zareba W, Solomon S, Steinberg JS MADIT-CRT Executive Committee. Cardiac resynchronization therapy is more effective in women than in men: the MADIT-CRT (Multicenter Automatic Defibrillator Implantation Trial with Cardiac Resynchronization Therapy) trial. J Am Coll Cardiol. 2011;57:813–820. doi: 10.1016/j.jacc.2010.06.061. [DOI] [PubMed] [Google Scholar]


