Abstract
Infection of normal individuals with human parvovirus (B19) results in a mild disease (erythema infectiosum) but gives rise to aplastic crises in patients with chronic hemolytic anemias. The effects of this disease on hemopoiesis were investigated following intranasal inoculation of the virus into three volunteers. A typical disease ensued with a viremia peaking at 9 d. Marrow morphology 6 d after inoculation appeared normal but at 10 d there was a severe loss of erythroid precursors followed by a 1-2-g drop in hemoglobin, and an increase in serum immunoreactive erythropoietin. Erythroid burst-forming units (BFU-E) from the peripheral blood were considerably reduced, starting at the time of viremia and persisting for 4-8 d depending on the individual. Granulocyte-macrophage colony-forming units (CFU-GM) were also affected but the loss started 2 d later. Both CFU-GM and BFU-E showed a sharp overshoot at recovery. In the marrow, BFU-E and CFU-E were reduced at 6 and 10 d in the individual having the longest period of peripheral progenitor loss. In contrast, there was an increase in BFU-E and CFU-E in the subject with least change in peripheral progenitors. In the third subject, with an intermediate picture, there was a loss at 6 d but an increase at 10 d of erythroid progenitors. It is suggested that the architecture of the marrow might partially isolate progenitors from high titers of virus in the serum and individual variation in this respect might give the results observed.
Full text
PDF

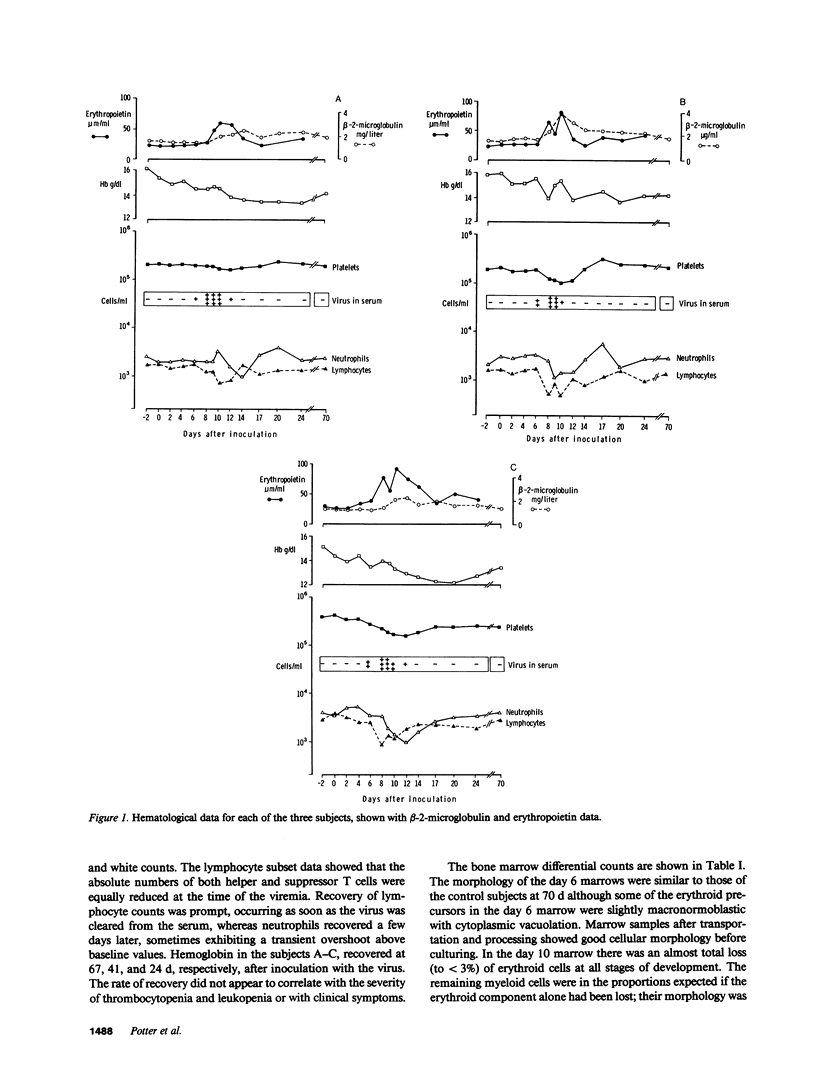
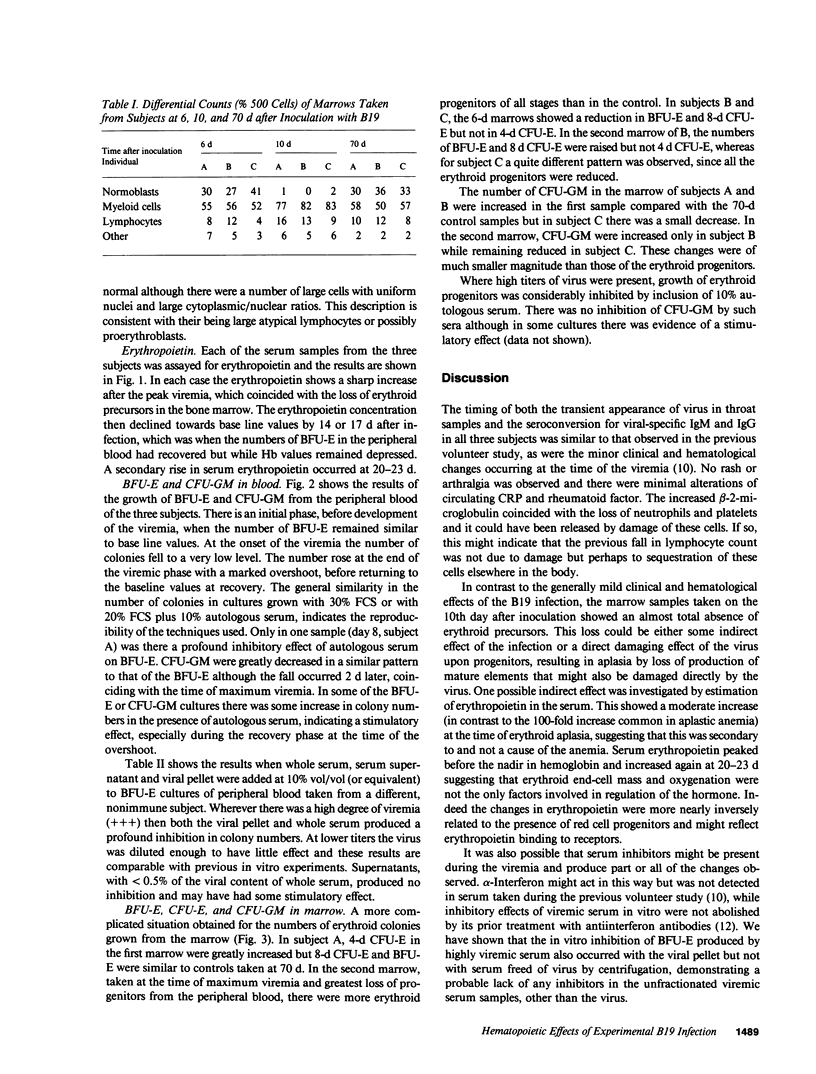
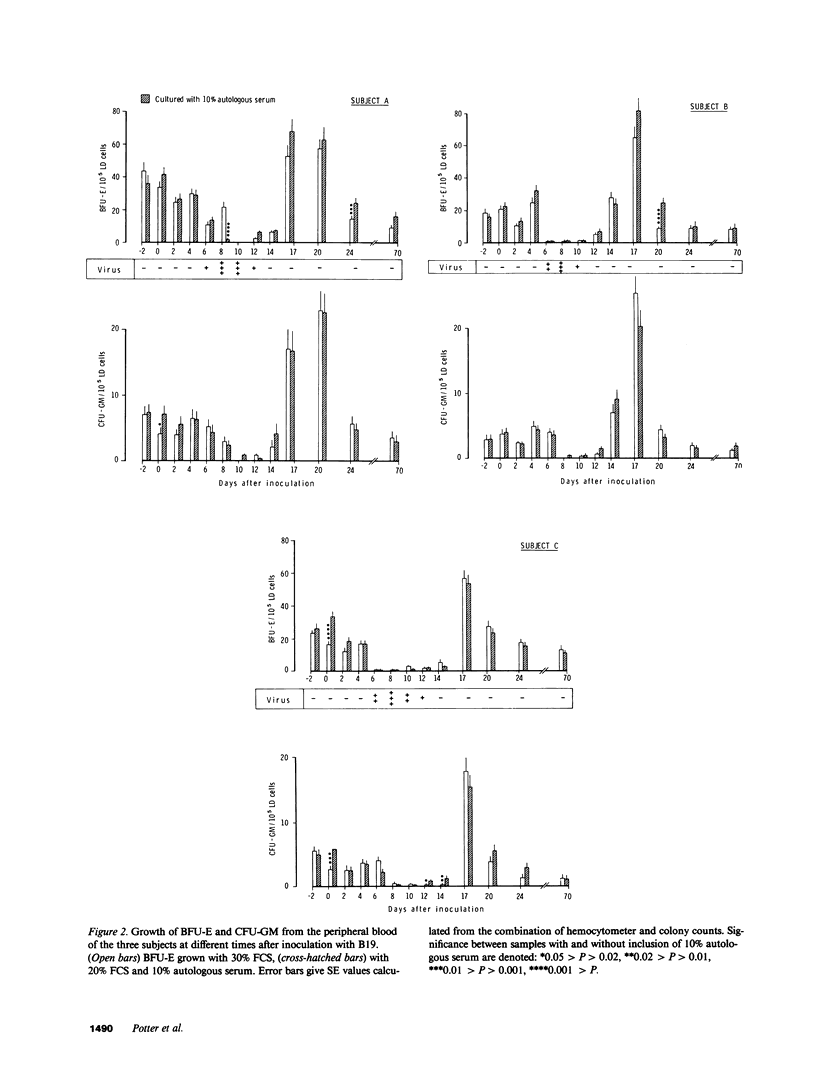


Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson M. J., Davis L. R., Jones S. E., Pattison J. R., Serjeant G. R. The development and use of an antibody capture radioimmunoassay for specific IgM to a human parvovirus-like agent. J Hyg (Lond) 1982 Apr;88(2):309–324. doi: 10.1017/s0022172400070169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson M. J., Higgins P. G., Davis L. R., Willman J. S., Jones S. E., Kidd I. M., Pattison J. R., Tyrrell D. A. Experimental parvoviral infection in humans. J Infect Dis. 1985 Aug;152(2):257–265. doi: 10.1093/infdis/152.2.257. [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Jones S. E., Fisher-Hoch S. P., Lewis E., Hall S. M., Bartlett C. L., Cohen B. J., Mortimer P. P., Pereira M. S. Human parvovirus, the cause of erythema infectiosum (fifth disease)? Lancet. 1983 Jun 18;1(8338):1378–1378. doi: 10.1016/s0140-6736(83)92152-9. [DOI] [PubMed] [Google Scholar]
- Anderson M. J., Jones S. E., Minson A. C. Diagnosis of human parvovirus infection by dot-blot hybridization using cloned viral DNA. J Med Virol. 1985 Feb;15(2):163–172. doi: 10.1002/jmv.1890150209. [DOI] [PubMed] [Google Scholar]
- Bloom M. E., Race R. E., Aasted B., Wolfinbarger J. B. Analysis of Aleutian disease virus infection in vitro and in vivo: demonstration of Aleutian disease virus DNA in tissues of infected mink. J Virol. 1985 Sep;55(3):696–703. doi: 10.1128/jvi.55.3.696-703.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossart Y. E., Field A. M., Cant B., Widdows D. Parvovirus-like particles in human sera. Lancet. 1975 Jan 11;1(7898):72–73. doi: 10.1016/s0140-6736(75)91074-0. [DOI] [PubMed] [Google Scholar]
- Cotes P. M. Immunoreactive erythropoietin in serum. I. Evidence for the validity of the assay method and the physiological relevance of estimates. Br J Haematol. 1982 Mar;50(3):427–438. doi: 10.1111/j.1365-2141.1982.tb01938.x. [DOI] [PubMed] [Google Scholar]
- Cronkite E. P., Feinendegen L. E. Notions about human stem cells. Nouv Rev Fr Hematol Blood Cells. 1976;17(1-2):269–284. doi: 10.1007/978-3-642-66312-3_18. [DOI] [PubMed] [Google Scholar]
- Duncan J. R., Potter C. B., Cappellini M. D., Kurtz J. B., Anderson M. J., Weatherall D. J. Aplastic crisis due to parvovirus infection in pyruvate kinase deficiency. Lancet. 1983 Jul 2;2(8340):14–16. doi: 10.1016/s0140-6736(83)90005-3. [DOI] [PubMed] [Google Scholar]
- Kelleher J. F., Luban N. L., Mortimer P. P., Kamimura T. Human serum "parvovirus": a specific cause of aplastic crisis in children with hereditary spherocytosis. J Pediatr. 1983 May;102(5):720–722. doi: 10.1016/s0022-3476(83)80243-1. [DOI] [PubMed] [Google Scholar]
- Mortimer P. P., Humphries R. K., Moore J. G., Purcell R. H., Young N. S. A human parvovirus-like virus inhibits haematopoietic colony formation in vitro. 1983 Mar 31-Apr 6Nature. 302(5907):426–429. doi: 10.1038/302426a0. [DOI] [PubMed] [Google Scholar]
- Ogawa M., MacEachern M. D., Avila L. Human marrow erythropoiesis in culture: II. Heterogeneity in the morphology, time course of colony formation, and sedimentation velocities of the colony-forming cells. Am J Hematol. 1977;3:29–36. doi: 10.1002/ajh.2830030104. [DOI] [PubMed] [Google Scholar]
- Ozawa K., Kurtzman G., Young N. Replication of the B19 parvovirus in human bone marrow cell cultures. Science. 1986 Aug 22;233(4766):883–886. doi: 10.1126/science.3738514. [DOI] [PubMed] [Google Scholar]
- Pattison J. R., Jones S. E., Hodgson J., Davis L. R., White J. M., Stroud C. E., Murtaza L. Parvovirus infections and hypoplastic crisis in sickle-cell anaemia. Lancet. 1981 Mar 21;1(8221):664–665. doi: 10.1016/s0140-6736(81)91579-8. [DOI] [PubMed] [Google Scholar]
- Plummer F. A., Hammond G. W., Forward K., Sekla L., Thompson L. M., Jones S. E., Kidd I. M., Anderson M. J. An erythema infectiosum-like illness caused by human parvovirus infection. N Engl J Med. 1985 Jul 11;313(2):74–79. doi: 10.1056/NEJM198507113130203. [DOI] [PubMed] [Google Scholar]
- Potter C. G., Cappellini M. D. Improved culture of BFU-E and CFC-GM by the use of an oil seal. Br J Haematol. 1983 May;54(1):153–154. doi: 10.1111/j.1365-2141.1983.tb02077.x. [DOI] [PubMed] [Google Scholar]
- Rao K. R., Patel A. R., Anderson M. J., Hodgson J., Jones S. E., Pattison J. R. Infection with parvovirus-like virus and aplastic crisis in chronic hemolytic anemia. Ann Intern Med. 1983 Jun;98(6):930–932. doi: 10.7326/0003-4819-98-6-930. [DOI] [PubMed] [Google Scholar]
- Reid D. M., Reid T. M., Brown T., Rennie J. A., Eastmond C. J. Human parvovirus-associated arthritis: a clinical and laboratory description. Lancet. 1985 Feb 23;1(8426):422–425. doi: 10.1016/s0140-6736(85)91146-8. [DOI] [PubMed] [Google Scholar]
- Schlunk T., Schleyer M. The influence of culture conditions on the production of colony-stimulating activity by human placenta. Exp Hematol. 1980 Feb;8(2):179–184. [PubMed] [Google Scholar]
- Serjeant G. R., Topley J. M., Mason K., Serjeant B. E., Pattison J. R., Jones S. E., Mohamed R. Outbreak of aplastic crises in sickle cell anaemia associated with parvovirus-like agent. Lancet. 1981 Sep 19;2(8247):595–597. doi: 10.1016/s0140-6736(81)92739-2. [DOI] [PubMed] [Google Scholar]
- Young N. S., Mortimer P. P., Moore J. G., Humphries R. K. Characterization of a virus that causes transient aplastic crisis. J Clin Invest. 1984 Jan;73(1):224–230. doi: 10.1172/JCI111195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Young N., Harrison M., Moore J., Mortimer P., Humphries R. K. Direct demonstration of the human parvovirus in erythroid progenitor cells infected in vitro. J Clin Invest. 1984 Dec;74(6):2024–2032. doi: 10.1172/JCI111625. [DOI] [PMC free article] [PubMed] [Google Scholar]



