Abstract
Low/medium-bleeding-risk populations undergoing percutaneous coronary intervention (PCI) show significantly less bleeding with bivalirudin (BIV) than with unfractionated heparin (UFH), but this has not been established for high-risk patients. We performed a randomized double-blind prospective trial comparing efficacy and safety of BIV versus UFH combined with dual antiplatelet therapy during PCI among 100 high-risk patients with non-ST elevation myocardial infarction (NSTEMI) or angina pectoris. The baseline characteristics were similar in both treatment arms. A radial approach was used in 84% of patients with a higher rate in the BIV group (90 vs. 78%, p < 0.05). Study end points were: major and minor bleeding, port-of-entry complications, major adverse cardiac events (MACE) in-hospital, and at long-term follow-up. There was one case of major gastrointestinal bleeding in the BIV group and 7% minor bleeding complications in both categories. Rate of periprocedural myocardial infarction (PPMI) in the BIV group was twice that in the UFH group (20 vs. 10%, p < 0.16). In-hospital MACE rate was higher in BIV patients as well (12 vs. 2%, p = 0.1). By univariate analysis, the femoral approach was the predictor of PPMI and in-hospital MACE. In a multivariate model, the independent predictor of PPMI was previous MI (odds ratio, 7.7; p < 0.0158). PPMI was 49.7 times more likely with the femoral approach plus BIV than the nonfemoral approach plus UFH (p < 0.0021). At 41.5 ± 14 months' follow-up, end points did not significantly differ between the groups. In patients at high risk for bleeding undergoing PCI, BIV was not superior to UFH for bleeding complications, and early and late clinical outcomes.
Keywords: bivalirudin, unfractionated heparin, PCI, bleeding, high-risk patient
The overall rate of bleeding complications during interventional therapeutic procedures for coronary heart disease by the femoral approach is 1.5 to 9%.1 Although bleeding is considered a minor complication related to percutaneous coronary intervention (PCI),2 on rare occasions, it might lead to life-threatening situations that must be treated by intensive medical or surgical approaches.3 These events are multifactorial, involving patient-, physician-, and nursing staff–related prognosis-worsening factors. Patient-related factors include: female gender, age > 70 years, and comorbidities such as diabetes mellitus, obesity, hypertension, and diseases with poor clot formation, such as uremia and thrombocytopenia.4 Physician-related factors include: port of access, mode of arterial puncture (single vs. multiple trials) and anticoagulation regimen.5 Early data using abciximab during high-risk coronary angioplasty (EPIC trial) showed that bleeding complications occur more often in the IIb-/IIIa-treated groups,6 a trend that persisted under low-dose (EPILOG trial), weight-adjusted heparin infusion.7
One of the alternatives to unfractionated heparin (UFH) is bivalirudin (BIV), a direct and reversible thrombin-inhibition oligopeptide with a relatively short half-life. In previous studies of patients at low-to-moderate risk for bleeding, BIV, with or without added IIb/IIIa antagonists, was found to be superior to UFH, with a lower rate of bleeding complications and noninferior clinical outcomes.8 9 10 11 12 However, patients with a high-risk profile for bleeding complications were excluded from these studies.
We performed a randomized double-blind prospective trial to compare efficacy and safety of the BIV treatment with the UFH regimen combined with dual antiplatelet therapy during PCI among patients with angina pectoris or non-ST elevation myocardial infarction (NSTEMI) who are at high risk for bleeding.
Patients and Methods
Study Population
We enrolled patients > 18 years of age with stable or unstable angina pectoris or NSTEMI, undergoing elective or urgent PCI, who met the following criteria indicating a high tendency for bleeding: age above 75 years, creatinin clearance rate less than 60 mL/min, anemia with plasma hemoglobin level between 9 and 11 mg%, systemic blood pressure measured between 180/95 and 210/110 mm Hg, diabetes mellitus, current treatment with steroids, recent (within 6 weeks) nonmajor surgery, and/or systemic hematological disorders. The exclusion criteria were acute ST elevation myocardial infarction, active bleeding, possible use of IIb/IIIa antagonists during PCI, treatment by subcutaneous low-molecular-weight heparin within 8 hours or intravenous UFH within 4 hours of PCI, international normalized ratio level > 1.5 on the day of the procedure, concurrent pregnancy or women of reproductive age not using contraceptives, and known allergy to UFH, BIV, or its components.
Protocol
The study complied with the Declaration of Helsinki, and the Institutional Ethics Committee approved the study protocol. After preliminary screening and explanation, all eligible patients gave their written informed consent and were pretreated with aspirin (75–325 mg daily) and clopidogrel (300 mg loading dose at least 6 hours before starting PCI, followed by 75 mg daily).
An arterial approach for coronary angiography was undertaken by the transfemoral or transradial approach, using a 5F/6F introducer sheath. After the decision had been made to perform coronary intervention, 100 consecutive patients underwent randomization with 1:1 distribution between the groups in a double-blind manner via the use of opaque, sealed envelopes that contained the assignment. Non-blinded staff (nurses working in the catheterization laboratory) prepared the study drug and the placebo in weight-adjusted doses according to the study arm written in the patient's envelope and gave them to the patient. Before the guide wire crossed the lesion, study participants in the UFH group received a bolus of 60 U/kg. Patients in the BIV group received a loading dose of 0.75 mg/kg, followed by infusion of 1.75 mg/kg per hour for the duration of the procedure. Doses were adjusted for patients with chronic renal failure and creatinin clearance rate < 30 mL/min in accordance with acceptable BIV protocol. Placebos in volumes similar to active unused drugs were added respective to the group to ensure the blindness of the study. When activated clotting time (ACT) was checked during the PCI and found to be < 250 second a nonblinded cardiologist ordered an additional dose of heparin or placebo from the nurse. All operators were unaware of the ACT values. Balloon angioplasty, or coronary bare-metal or drug-eluting stenting was used according to the caregiver's choice. Femoral sheaths were removed 3 hour after guide-wire withdrawal. Sheaths from atransradial approaches were removed at the end of the procedure. A closure device was not used in this trial. Postprocedural therapy included aspirin (75–325 mg/day) for life, as well as clopidogrel (75 mg/day) for at least 9 months, in patients who underwent a simple balloon procedure or a bare-metal stent, or for 12 months, in patients with a drug-eluting stent. According to protocol, electrocardiography and blood sampling for measurements of cardiac enzymes, renal functions, hemoglobin levels, and platelet counts were performed before and 12 hour after the indexed PCI. Patients were interviewed by telephone at a 30-day and a long-term follow-up, and all patients with cardiac symptoms underwent a complete medical evaluation (Fig. 1).
Fig. 1.
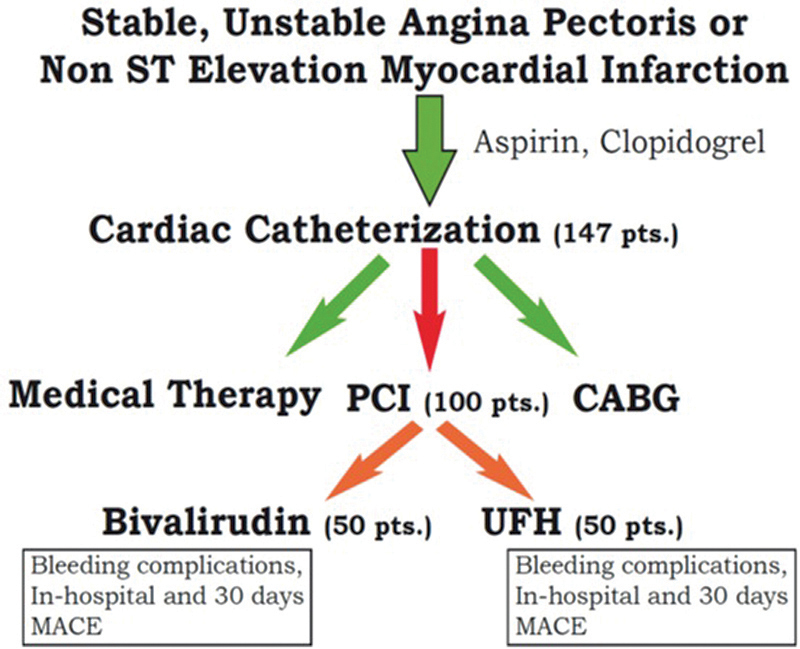
ACRIPAB trial flow chart. CABG, coronary artery bypass graft; MACE, major adverse cardiac event; PCI, percutaneous coronary intervention; UFH, unfractionated heparin.
Study End Points and Definitions
The primary study end points were: major and minor bleeding and port-of-entry complications; secondary end points were: periprocedural myocardial infarction (PPMI) and major adverse cardiac events (MACE) in-hospital and after long-term follow-up.
Major bleeding was defined as intracerebral, intraocular, or retroperitoneal hemorrhage, overt hemoglobin loss of more than 3 gr%, need for a blood transfusion or for surgical or percutaneous intervention to stop blood loss, or groin hematoma with a circumference of more than 6 cm. Minor bleeding was determined as overt bleeding that did not meet the criteria of major bleeding. Port-of-entry–related complications included pseudoaneurysm, arteriovenous fistula, or huge hematoma with pressure on nearby important structures (veins, nerves, etc.). PPMI was defined as procedure-related ischemic symptoms and/or electrocardiogram changes with an over threefold increase in ischemic serum markers relative to the reference pre-PCI level. MACE was defined as cardiac death, target-vessel revascularization, stent thrombosis, or any post-PCI ischemic event.
Statistical Analysis
All analyses were performed in a blinded manner on the basis of the intention-to-treat principle. Categorical variables were analyzed by χ 2 test or Fisher exact test (where 20% or more of the cells in a χ 2 table had an expected count of < 5). For continuous data, differences between the two treatment groups (patients receiving UFH vs. patients treated with BIV) were assessed by a Student t-test, or alternatively, by a Mann–Whitney U test when the data were not normally distributed. Binary logistic regression analysis was performed to derive univariate and multivariate predictors of adverse procedural results. A two-tailed value of p < 0.05 was considered significant.
Results
Baseline Characteristics
In total, 147 patients were screened and catheterized in this trial. For 47 of them, the decision was to recommend medical therapy or a coronary artery bypass graft operation. The other 100 patients were candidates for PCI and were randomized into UFH or BIV treatment arms (50 patients in each group). Of these, 87% had diabetes mellitus, 98% hypertension, 22% chronic renal failure, 30% were older than 75 years, 21% had anemia, and 58% systolic blood pressure ≥ 180 mm Hg; 32% of participants were catheterized due to NSTEMI. Two or more vessels were treated in the same session in 24% of the patients. A radial approach was used in 84% of the patients, with a higher rate in the BIV group (90 vs. 78% in the UFH group, p < 0.05). There were no significant differences in baseline characteristics between the groups except for a higher rate of males in the BIV group (Table 1). There were no differences in angiographic and procedural variables between the groups (Table 2).
Table 1. Baseline characteristics of the patients.
| Characteristic | Total (n = 100) | Unfractionated heparin (n = 50) | Bivalirudin (n = 50) |
|---|---|---|---|
| Age (y), mean ± SD | 66.6 ± 12.3 | 64.9 ± 13.4 | 68.3 ± 10.9 |
| Male gender, n (%) | 69 (69) | 30 (60)a | 39 (78)a |
| Diabetes mellitus, n (%) | 87 (87) | 45 (90) | 42 (84) |
| Hypertension, n (%) | 98 (98) | 49 (98) | 49 (98) |
| Old myocardial infarction, n (%) | 37 (37) | 17 (34) | 20 (40) |
| Left ventricle ejection fraction (%), mean ± SD | 56.7 ± 12.6 | 56.7 ± 13.5 | 56.7 ± 11.8 |
| Old ischemic CVA, n (%) | 7 (7) | 5 (10) | 2 (4) |
| Old hemorrhagic CVA, n (%) | 1 (1) | 1 (2) | 0 (0) |
| Old GI bleeding, n (%) | 4 (4) | 4 (8) | 0 (0) |
| Chronic renal failure, n (%) | 22 (22) | 9 (18) | 13 (26) |
| Age >75 y, n (%) | 30 (30) | 16 (32) | 14 (28) |
| Cr clearance < 60 mL/min, n (%) | 28 (28) | 14 (28) | 14 (28) |
| 9 mg% < Hb < 11 mg%, n (%) | 21 (21) | 11 (22) | 10 (20) |
| Systemic BP > 180/95 mm Hg, n (%) | 58 (58) | 30 (60) | 28 (56) |
| Steroid treatment, n (%) | 3 (3) | 1 (2) | 2 (4) |
| Hematological problems, n (%) | 7 (7) | 2 (4) | 5 (10) |
| NSTEMI on admission, n (%) | 32 (32) | 19 (38) | 13 (26) |
Abbreviations: BP, blood pressure; Cr, creatinin; CVA, cerebrovascular accident; GI, gastrointestinal; Hb, hemoglobin; NSTEMI, non-ST elevation myocardial infarction; SD, standard deviation.
p = 0.052.
Table 2. Angiographic and procedural characteristics.
| Characteristic | Total (n = 100) | Unfractionated heparin (n = 50) | Bivalirudin (n = 50) |
|---|---|---|---|
| Sheath size (French) | 5.6 ± 0.5 | 5.5 ± 0.5 | 5.6 ± 0.5 |
| Radial approach, n (%) | 84 (84) | 39 (78)a | 45 (90)a |
| Single vessel disease, n (%) | 14 (14) | 7 (14) | 7 (14) |
| Double vessel disease, n (%) | 42 (42) | 22 (44) | 20 (40) |
| Triple vessel disease, n (%) | 34 (44.7) | 17 (44.7) | 17 (44.7) |
| Left main disease, n (%) | 7 (7) | 1 (2) | 6 (12) |
| Multivessel PCI, n (%) | 23 (23) | 13 (26) | 10 (20) |
| Number of treated lesions (n) | 123 | 63 | 60 |
| Calcified-treated lesions, n (%) | 52 (42.3) | 22 (34.9)a | 30 (50)a |
| Bifurcation-treated lesions, n (%) | 24 (19.5) | 12 (19) | 12 (20) |
| Ostial-treated lesions, n (%) | 12 (9.8) | 5 (7.9) | 7 (11.6) |
| Intervention on SVG, n (%) | 6 (4.8) | 2 (3.2) | 4(6.7) |
| Intervention on LM, n (%) | 1 (0.8) | 1 (1.6) | − |
| Intervention on LAD, n (%) | 54 (43.9) | 31 (49.2) | 23 (38.3) |
| Intervention on CX, n (%) | 35 (28.4) | 19 (30.1) | 16 (26.7) |
| Intervention on RCA, n (%) | 27 (21.9) | 10 (15.9) | 17 (28.3) |
| Stent usage, n (%) | 99 (80.5) | 52 (82.5) | 47 (78.3) |
| Bare metal stent usage, n (%) | 79 (79.8) | 40 (76.9) | 39 (82.9) |
| Drug eluting stent usage, n (%) | 20 (20.2) | 12 (23.1) | 8 (17) |
| Stent length (mm), mean ± SD | 14 ± 4.9 | 13.7 ± 4.8 | 14.4 ± 4.9 |
| Stent width (mm), mean ± SD | 3.1 ± 0.4 | 3 ± 0.4 | 3.3 ± 0.5 |
Abbreviations: CX, circumflex artery; LAD, left anterior descending; LM, left main; PCI, percutaneous coronary intervention; RCA, right coronary artery; SD, standard deviation; SVG, saphenous vein graft.
p < 0.05.
In-Hospital and Long-Term Follow-Up Results
There was one case of major gastrointestinal bleeding in the BIV group and 7% of minor bleeding complications in both categories (Fig. 2). PPMI in the BIV group was twice that in the UFH group (20 vs. 10%, p < 0.16). In-hospital MACE rate was higher in BIV patients as well (12 vs. 2%, p = 0.1). In the UFH group, there was one case of cardiogenic shock and death 2 days after PCI in a 73-year-old woman with anemia and severe multivessel disease (Fig. 3).
Fig. 2.
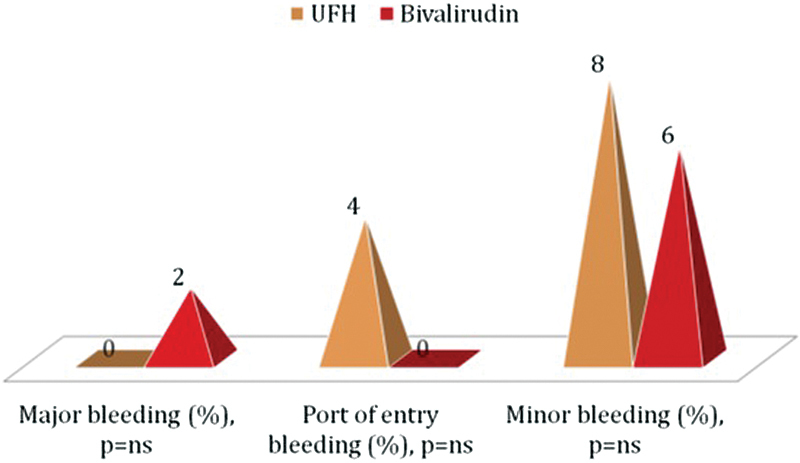
Bleeding complications. UFH, unfractionated heparin.
Fig. 3.
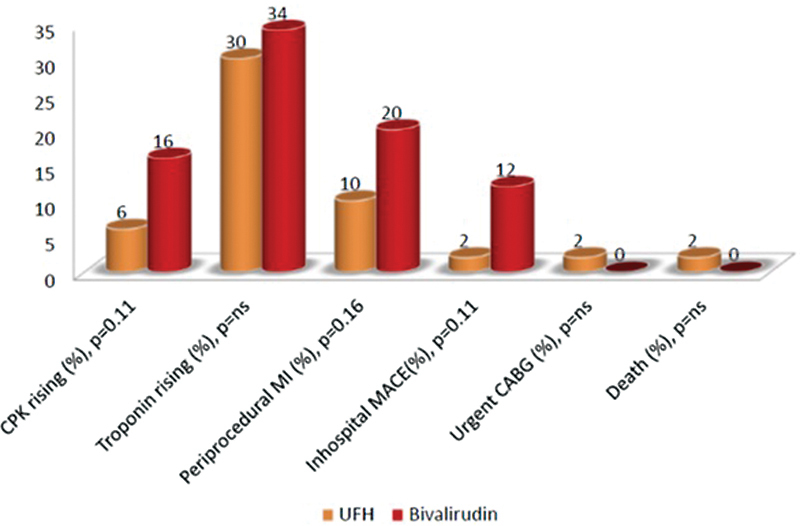
Periprocedural results. CABG, coronary artery bypass graft; CPK, creatin-phospho-kinase; MACE, major adverse cardiac event; MI, myocardial infarction; UFH, unfractionated heparin.
By univariate analysis, for patients treated via the femoral approach, the odds of having PPMI and in-hospital MACE were 7.39 and 9.0 times higher than for patients treated via the radial approach (Table 3).
Table 3. Influence of the approach on immediate clinical outcomes.
| Clinical immediate outcomes | Radial approach | Femoral approach | p Value | Odds ratio (95% CI) |
|---|---|---|---|---|
| Periprocedural MI, n (%) | 8 (9.52) | 7 (43.75) | 0.0022 | 7.39 (2.16–25.21) |
| In-hospital MACE, n (%) | 3 (3.57) | 4 (25) | 0.0118 | 9.00 (1.78–45.25) |
| Major bleeding, n (%) | 1 (1.19) | 0 (0) | 1.00 | – |
| Port of entry bleeding, n (%) | 2 (2.38) | 0 (0) | 1.00 | – |
| CPK rising, n (%) | 10 (11.9) | 1 (6.25) | 1.00 | 0.49 (0.057–4.14) |
| Troponin rising, n (%) | 29 (34.52) | 3 (18.75) | 0.2151 | 0.43 (0.11–1.66) |
Abbreviations: CI, confidence interval; CPK, creatin-phospho-kinase; MACE, major adverse cardiac event; MI, myocardial infarction.
By multivariate analysis, previous myocardial infarction was found to be a strong predictor for PPMI with a hazard ratio of 7.7. For subjects that were treated with the femoral approach and BIV, the odds of having PPMI were 49.69 times higher than for study participants who were treated with the radial approach and UFH (Table 4).
Table 4. Multivariate analysis of predictors for periprocedural MI.
| Variable | p Value | OR (95% CI) |
|---|---|---|
| Previous myocardial infarction | 0.0158 | 7.714 (1.467–40.562) |
| Femoral approach and Bivalirudina | 0.0021 | 49.694 (4.134–597.292) |
| Femoral approach and UFHa | 0.1271 | 5.46 (0.617–48.348) |
| Radial approach and Bivalirudin | 0.7568 | 1.35 (0.202–9.014) |
Abbreviations: CI, confidence interval; OR, odds ratio; UFH, unfractionated heparin.
Note: Model significance was 0.0025, C-statistic 0.871.
Compared with patients treated with radial approach and UFH.
There were no significant differences between the groups in 41.5 ± 14 months' outcomes (Table 5, Fig. 4).
Table 5. Data from 41.5 ± 14 months' follow-up.
| Characteristic | Total (n = 100) | Unfractionated heparin (n = 50) | Bivalirudin (n = 50) |
|---|---|---|---|
| MACE, n (%) | 22 (22) | 11 (22) | 11 (22) |
| Reinfarction, n (%) | 16 (16) | 7 (14) | 9 (18) |
| Death from any reason, n (%) | 7 (7) | 5 (10) | 2 (4) |
| Target vessel revascularization, n (%) | 15 (15) | 8 (16) | 7 (14) |
| Cardiac rehospitalization, n (%) | 52 (52) | 25 (50) | 27 (54) |
| Noncardiac rehospitalization, n (%) | 54 (54) | 22 (44)a | 32 (64)* |
Abbreviation: MACE, major adverse cardiac event.
p = 0.056.
Fig. 4.
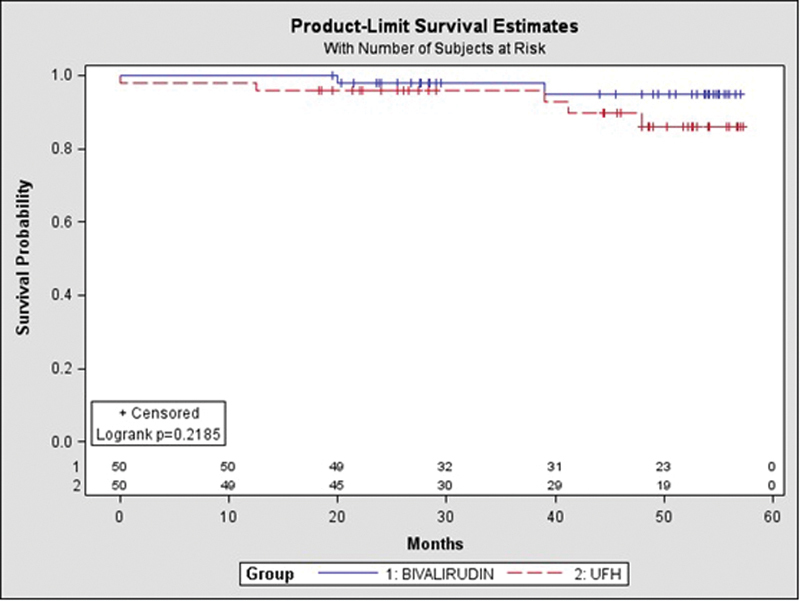
Long-term survival. Survival curves in patients treated by Bivalirudin or UFH. The mortality assessed by Kaplan–Meier analysis was similar between the groups (log-rank p = 0.358). UFH, unfractionated heparin.
Discussion
Randomized studies and real-world cohorts of patients undergoing PCI8 9 10 11 12 showed that the BIV-based anticoagulation strategy is associated with a decreased risk of bleeding complications without an increase of MACE, compared with a UFH-based regimen.13 Nonaccess-site bleeding after PCI, which represents approximately two-thirds of all thrombolysis in myocardial infarction bleeding effects and is associated with a fourfold increase in 1-year mortality, is also decreased by approximately 40% with the use of BIV rather than heparin plus GP IIb/IIIa inhibitors.14 Patients with renal failure,15 anemia, high blood pressure, diabetes mellitus, steroid treatment, and recent surgery are at even higher risk for bleeding during PCI. However, generally these patients have been excluded from previous trials studying BIV safety and efficacy.
In this double-blind study, we compared BIV and UFH in patients with angina pectoris and NSTEMI who were at high risk for bleeding and underwent PCI combined with dual antiplatelet therapy. The main findings unexpectedly showed that BIV treatment among high-risk patients was not superior, especially in patients treated with the femoral approach. A low rate of all types of bleeding complications in both groups could be explained by the high percentage of use of transradial or transulnar approaches in our population. However, patients with increased risk for bleeding have a high-risk profile for ischemic complications as well. Thus, presumably for these reasons, BIV lost its protective effect on bleeding in our trial and the scale of composite outcome of bleeding and ischemic events turned against BIV. It should be noted that in the REPLACE-2 study9 16 where most of the procedures were performed via the femoral approach, debatable results were found, with a trend toward higher PPMI in BIV-treated patients compared with the controls (6.6 vs. 5.8%) and, at the same time, a reduction in bleeding complications in a renal failure group. It is also interesting to note that Saltzman et al17 found no benefit of BIV treatment compared with UFH and GP IIb/IIIa inhibitors in clinical outcomes, including bleeding complications in patients with chronic renal failure undergoing primary PCI for acute ST elevation myocardial infarction.
According to our data, in the specific category of high-risk patients, the best treatment approach for PCI combined with dual antiplatelet therapy includes a transradial site of entry and a UFH-based anticoagulation regimen, that provide the lowest profile of bleeding and ischemic complications.
Study Limitations
This is a single-center study with a relatively small number of patients and outcomes. The low percentage of patients treated through a femoral approach could be another limiting factor. However, the design allowed us to control for established clinical and angiographic data.
Summary and Conclusion
In our study of a unique group of patients at high risk for bleeding, who underwent PCI for stable or unstable angina pectoris or NSTEMI along with dual antiplatelet therapy, treatment with BIV was found inferior to UFH for all types of bleeding complications, as well as early and late clinical outcomes. These findings can be partially explained by a high percentage of transradial approach interventions and the high-risk characteristics of our patients.
Footnotes
Conflict of Interest The authors report no financial relationships or conflicts of interest regarding the content herein.
References
- 1.Nasser T K, Mohler E R III, Wilensky R L, Hathaway D R. Peripheral vascular complications following coronary interventional procedures. Clin Cardiol. 1995;18(11):609–614. doi: 10.1002/clc.4960181105. [DOI] [PubMed] [Google Scholar]
- 2.Ellis S G. Philadelphia, PA: W.B. Saunders; 1993. Elective coronary angioplasty: technique and complications; pp. 186–207. [Google Scholar]
- 3.Bredlau C E, Roubin G S, Leimgruber P P, Douglas J S Jr, King S B III, Gruentzig A R. In-hospital morbidity and mortality in patients undergoing elective coronary angioplasty. Circulation. 1985;72(5):1044–1052. doi: 10.1161/01.cir.72.5.1044. [DOI] [PubMed] [Google Scholar]
- 4.Green G S, McKinnon C M, Rösch J, Judkins M P. Complications of selective percutaneous transfemoral coronary arteriography and their prevention. A review of 445 consecutive examinations. Circulation. 1972;45(3):552–557. doi: 10.1161/01.cir.45.3.552. [DOI] [PubMed] [Google Scholar]
- 5.Kelsey S F, Mullin S M, Detre K M. et al. Effect of investigator experience on percutaneous transluminal coronary angioplasty. Am J Cardiol. 1984;53(12):56C–64C. doi: 10.1016/0002-9149(84)90747-1. [DOI] [PubMed] [Google Scholar]
- 6.Topol E J, Califf R M, Weisman H F. et al. Randomised trial of coronary intervention with antibody against platelet IIb/IIIa integrin for reduction of clinical restenosis: results at six months. Lancet. 1994;343(8902):881–886. doi: 10.1016/s0140-6736(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 7.Lincoff A M, Tcheng J E, Califf R M. et al. Sustained suppression of ischemic complications of coronary intervention by platelet GP IIb/IIIa blockade with abciximab: one-year outcome in the EPILOG trial. Evaluation in PTCA to Improve Long-term Outcome with abciximab GP IIb/IIIa blockade. Circulation. 1999;99(15):1951–1958. doi: 10.1161/01.cir.99.15.1951. [DOI] [PubMed] [Google Scholar]
- 8.Kastrati A, Mehilli J, Schühlen H. et al. A clinical trial of abciximab in elective percutaneous coronary intervention after pretreatment with clopidogrel. N Engl J Med. 2004;350(3):232–238. doi: 10.1056/NEJMoa031859. [DOI] [PubMed] [Google Scholar]
- 9.Lincoff A M, Bittl J A, Harrington R A. et al. Bivalirudin and provisional glycoprotein IIb/IIIa blockade compared with heparin and planned glycoprotein IIb/IIIa blockade during percutaneous coronary intervention: REPLACE-2 randomized trial. JAMA. 2003;289(7):853–863. doi: 10.1001/jama.289.7.853. [DOI] [PubMed] [Google Scholar]
- 10.Stone G W, McLaurin B T, Cox D A. et al. Bivalirudin for patients with acute coronary syndromes. N Engl J Med. 2006;355(21):2203–2216. doi: 10.1056/NEJMoa062437. [DOI] [PubMed] [Google Scholar]
- 11.Kastrati A, Neumann F J, Mehilli J. et al. Bivalirudin versus unfractionated heparin during percutaneous coronary intervention. N Engl J Med. 2008;359(7):688–696. doi: 10.1056/NEJMoa0802944. [DOI] [PubMed] [Google Scholar]
- 12.Kastrati A, Neumann F J, Schulz S. et al. Abciximab and heparin versus bivalirudin for non-ST-elevation myocardial infarction. N Engl J Med. 2011;365(21):1980–1989. doi: 10.1056/NEJMoa1109596. [DOI] [PubMed] [Google Scholar]
- 13.Vidi V D, Matheny M E, Agarwal V. et al. Validation of long-term benefits of bivalirudin versus unfractionated heparin in routine clinical practice after percutaneous coronary intervention. Am J Cardiol. 2010;106(9):1234–1240. doi: 10.1016/j.amjcard.2010.06.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Verheugt F W, Steinhubl S R, Hamon M. et al. Incidence, prognostic impact, and influence of antithrombotic therapy on access and nonaccess site bleeding in percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4(2):191–197. doi: 10.1016/j.jcin.2010.10.011. [DOI] [PubMed] [Google Scholar]
- 15.Hanna E B, Chen A Y, Roe M T, Wiviott S D, Fox C S, Saucedo J F. Characteristics and in-hospital outcomes of patients with non-ST-segment elevation myocardial infarction and chronic kidney disease undergoing percutaneous coronary intervention. JACC Cardiovasc Interv. 2011;4(9):1002–1008. doi: 10.1016/j.jcin.2011.05.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chew D P, Lincoff A M, Gurm H. et al. Bivalirudin versus heparin and glycoprotein IIb/IIIa inhibition among patients with renal impairment undergoing percutaneous coronary intervention (a subanalysis of the REPLACE-2 trial) Am J Cardiol. 2005;95(5):581–585. doi: 10.1016/j.amjcard.2004.11.003. [DOI] [PubMed] [Google Scholar]
- 17.Saltzman A J, Stone G W, Claessen B E. et al. Long-term impact of chronic kidney disease in patients with ST-segment elevation myocardial infarction treated with primary percutaneous coronary intervention: the HORIZONS-AMI (Harmonizing Outcomes With Revascularization and Stents in Acute Myocardial Infarction) trial. JACC Cardiovasc Interv. 2011;4(9):1011–1019. doi: 10.1016/j.jcin.2011.06.012. [DOI] [PubMed] [Google Scholar]


