Abstract
This study aims to retrospectively evaluate the outcomes following nitinol stent placement for malignant superior vena cava syndrome. A total of 25 patients with thoracic malignancies were treated with self-expanding nitinol stents for superior vena cava syndrome (E*Luminexx [Bard GmbH/Angiomed, Karlsruhe, Germany], Sinus-XL [OptiMed Medizinische Instrumente GmbH, Ettlingen, Germany], and Zilver Vena [Cook Medical Inc., Bloomington, IN]). It was seen that the procedural success rate was 76% with all stents deployed as intended and no procedure-related complications but in five patients with 50% residual stenosis and one patient with stent occlusion within 48 hours after stent deployment. Stent occlusion occurred in further two patients during follow-up: one patient developed infection, thrombosis, and occlusion in the stent seen at 2-month follow-up, and one patient had stent occlusion at 4-month follow-up. The clinical success rate was 96%. Stent compression leading to a greater than 50% reduction in stent diameter was observed in three patients at follow-up. Overall 22 patients died at a mean follow-up of 3.5 months for reasons related to their underlying malignancy. It was concluded that the stent treatment for superior vena cava syndrome is a safe treatment with good clinical effect in patients with superior vena cava syndrome in the terminal phase of malignant disease. In this small patient population, no trends were observed which would suggest that outcomes vary by stent type, though additional, large-scale studies are needed.
Keywords: superior vena cava syndrome, stent, treatment, palliative, interventional radiology
Superior vena cava (SVC) syndrome is a clinical diagnosis, which is confirmed by radiological methods. It is caused by compromised venous return from the head and upper extremities due to a compressed or obstructed SVC. Orthopnea, stridor, dysphagia, swelling, and venous dilatation of the head and upper body are among the main symptoms, but neurologic symptoms due to cerebral edema may also evolve.1 2 3 SVC syndrome may be a complication associated with indwelling catheters and other implantable central venous access devices such as pacemakers4 5 or due to fibrosing mediastinitis.6 Intrathoracic malignancy, however, is the most common cause of SVC syndrome in which the obstruction is caused by direct invasion or compression of the neoplasm or by lymph node invasion of the mediastinum. Extensive complicating thrombus formation may accompany the compromised SVC lumen and flow. Palliative treatment of SVC syndrome with stents has been described for more than 25 years and is now a well-accepted treatment in the terminal phase of malignant thoracic diseases. Results have demonstrated that endovascular treatment of malignant SVC syndrome is safe and yields rapid relief of symptoms, and therefore it has become the first-line treatment in recent years.7 8 9 10 11 Nitinol stents are recommended, as opposed to stainless steel stents, as recurrence of SVC syndrome was previously found to significantly increase with use of stainless steel stents compared with nitinol stents.9 However, it remains unknown if the different nitinol stent types are associated with different outcomes.
Therefore, the aim of the present study was to retrospectively evaluate the procedural and clinical outcomes following the placement of self-expanding nitinol stents in terminal cancer patients with SVC syndrome. Further, the aim was to investigate if any trends or differences between compression and patency outcomes could be identified among the three commercially available nitinol stents utilized in this study.
Material and Methods
Patients
This was a retrospective analysis of 25 consecutive cancer patients with clinical SVC syndrome, verified by contrast-enhanced computed tomography (CT), who underwent placement of 46 self-expanding nitinol stents (Table 1) between January 2012 and July 2013 at Odense University Hospital. The mean patient age was 65 years (range: 49–86 years), and nine patients were male. All patients presented with clinical SVC syndrome, and the compressed or obstructed SVC was verified by contrast-enhanced CT. All patients had advanced cancer in the right upper lung lobe, metastases to mediastinal lymph nodes, and/or invasive disease into the mediastinum. No patient had malignant lymphoma. All patients had terminal cancer without further possibility of curative treatment and had received maximal adjunct therapy with chemotherapy and radiotherapy. Overall 18 patients had nonsmall cell lung cancer and 7 had small cell lung cancer. Some data of 10 of the patients have also been included in another study.12
Table 1. Patients listed with implanted stent types, preprocedural stenosis, and outcome after 1, 1–30, 30–60, 60–90, 90–120, and more than 120 days' interval.
| Patient no. | Implanted stent type | Stenosis percentage | ||||||
|---|---|---|---|---|---|---|---|---|
| Preprocedure | Day 1 (procedure) | Day 1–30 | Day 30–60 | Day 60–90 | Day 90–120 | Day> 120 | ||
| CT and vena cavagrams | Postprocedure vena cavagrams | CT | ||||||
| 1 | E*Luminexx | 90 | 10 | 10 | 10a | |||
| 2 | Sinux-XL, Sinux-XL, Sinux-XL | 100 | 100b | 100 | 100a | |||
| 3 | Zilver Vena, Zilver Vena, Zilver Vena | 99 | 25 | 100 | 100a | |||
| 4 | Sinux-XL, Sinux-XL | 99 | 10 | 75a | ||||
| 5 | Zilver Vena, Zilver Vena | 95 | 25 | 50 | 50 | 100 | a | |
| 6 | Zilver Vena, Zilver Vena, Sinux-XL | 75 | 0 | 0a | ||||
| 7 | E*Luminexx, Sinux-XL | 90 | 0 | 0 | 0 | a | ||
| 8 | Sinux-XL | 99 | 25 | 25a | ||||
| 9 | E*Luminexx | 99 | 20 | 20 | 20 | 20 | ||
| 10 | Zilver Vena | 90 | 10 | 10a | ||||
| 11 | E*Luminexx, Zilver Vena, Sinux-XL | 99 | 20 | 20 | 20 | a | ||
| 12 | E*Luminexx | 95 | 10 | 50 | 50 | 50a | ||
| 13 | E*Luminexx, Sinux-XL | 100 | 25 | 75 | 75a | |||
| 14 | E*Luminexx | 75 | 0 | 0 | 0a | |||
| 15 | Sinux-XL | 80 | 0 | 0 | 0a | |||
| 16 | Zilver Vena, Zilver Vena, E*Luminexx | 95 | 0 | 0a | ||||
| 17 | Zilver Vena, Zilver Vena | 100 | 50 | 50 | 50a | |||
| 18 | Sinux-XL, Sinux-XL | 99 | 0 | 0 | 0 | 0 | 75 | |
| 19 | Zilver Vena | 95 | 50 | 50a | ||||
| 20 | Sinux-XL | 99 | 50b | 50 | 50 | 66a | ||
| 21 | Sinux-XL, Sinux-XL, Sinux-XL | 95 | 10 | 50 | a | |||
| 22 | Sinux-XL, Sinux-XL, E*Luminexx | 100 | 50 | 50 | 50 | 50 | ||
| 23 | Sinux-XL | 80 | 25 | 25a | ||||
| 24 | Sinux-XL | 90 | 0 | 0 | 75a b | |||
| 25 | Zilver Vena, Zilver Vena | 90 | 50 | 50 | ||||
Note: E*Luminexx (Bard GmbH/Angiomed, Karlsruhe, Germany), Sinus-XL (OptiMed Medizinische Instrumente GmbH, Ettlingen, Germany), and Zilver Vena (Cook Medical Inc., Bloomington, IN).
Indicates patient death occurred before the next follow-up.
Indicates percentage stenosis is associated with external stent compression.
Endovascular Technique
The technique was performed according to the quality assurance guidelines for superior vena cava stenting in malignant disease.1 All patients underwent a contrast-enhanced CT within 2 days of the interventional treatment. In addition, vena cavagrams were performed before and immediately after stent placement. Venous access was gained under local analgesia, typically through the right femoral vein, and alternatively through the internal jugular vein. Stenotic lesions were crossed with 5F catheters over a hydrophilic guide wire, and subsequently a superior vena cavagram was performed to define the landing zone for the stents (Fig. 1, patient no. 7). If the obstruction extended to the venous confluence or to a brachiocephalic vein, stents were placed from the ipsilateral brachiocephalic vein into the SVC, and if the obstruction involved both brachiocephalic veins, stents were placed in one or both brachiocephalic veins and in the SVC (Fig. 2A ,B patient no. 18). If possible, stents were placed such that the occlusion/stenosis was covered and there was at least 1 cm of disease-free vessel at both ends.1 All patients had a bolus of 5,000 units of unfractionated heparin during the procedure, and all were on an antiplatelet aggregation regimen with aspirin after the procedure. Further, some were on anticoagulation as a consequence of decision made by the referring department. Procedural success was defined as stent deployment in the intended location with < 50% residual stenosis and no adverse events during the procedure or within the first 48 hours after the procedure. Clinical success was defined as very good or good clinical effect with relief of SVC symptoms within the first 48 hours according to the clinical findings and the patients' satisfaction.
Fig. 1.
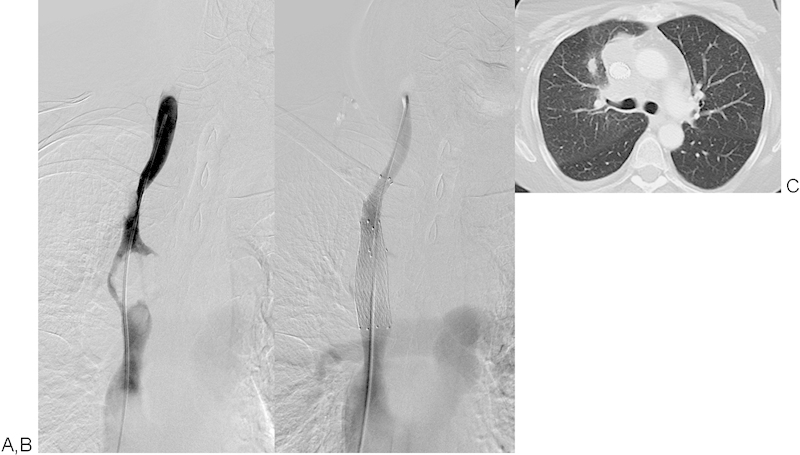
Patient No. 7: A 62-year-old woman with SCLC (T4N3M1b). (A) 90% SVC stenosis prestenting. One E*Luminexx 14 × 40 mm stent and one Sinus-XL 18 × 60 mm stent were implanted and postdilated with a 14 mm balloon. (B) No residual stenosis poststenting. (C) Very good clinical results and imaging results at > 120 days follow-up. SCLC, small cell lung cancer; SVC, superior vena cava. (E*Luminexx [Bard GmbH/Angiomed, Karlsruhe, Germany] and Sinus-XL [OptiMed Medizinische Instrumente GmbH, Ettlingen, Germany].)
Fig. 2.
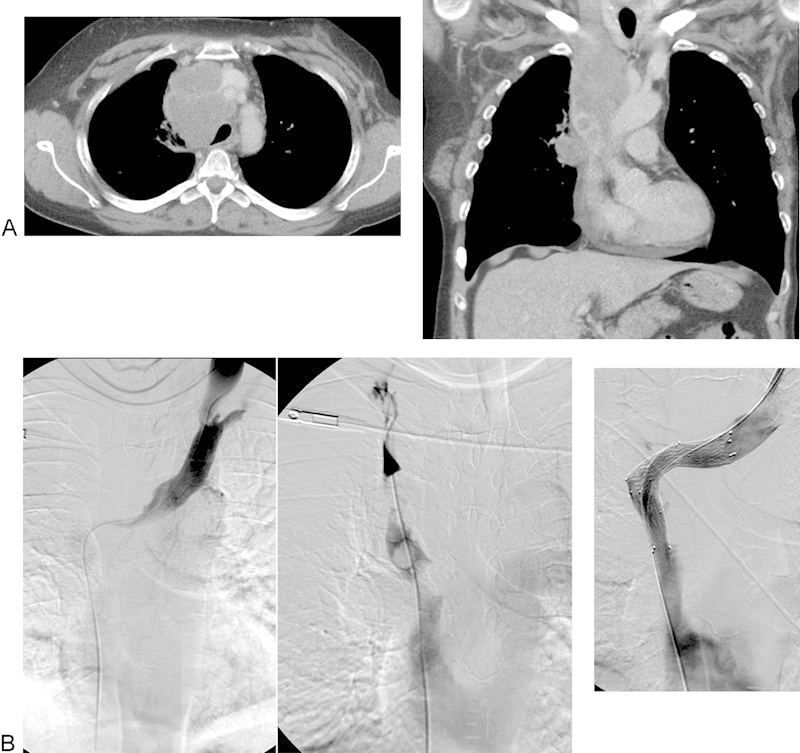
(A) Patient No. 18: A 50-year-old woman with disseminated SCLC (T4N2M1a). Contrast-enhanced CT shows occlusion of SVC and thrombi in cava and right brachiocephalic trunk. (B) Same patient as in (A). Venografia showing occluded SVC and thrombi in both brachiocephalic trunks. Two Sinus-XL stents of 16 × 60 mm were implanted, one into SVC from the right femoral vein, and the other one into left brachiocephalic trunk from the left jugular vein. Postdilatation with 14 mm balloon with good flow. Very good clinical effect. Reintervention 4 months later because of recurrent SVC syndrome (Patient No. 14) and stenosis of 75% with one E*Luminexx 14 × 60 mm stent in cava with very good clinical effect lasting until death 8 months later. CT, computed tomography; SCLC, small cell lung cancer; SVC, superior vena cava. (E*Luminexx [Bard GmbH/Angiomed, Karlsruhe, Germany].)
Stents
Patients received one of three types of self-expanding nitinol stents: E*Luminexx Vascular stent (Bard GmbH/Angiomed, Karlsruhe, Germany), Sinus-XL stent (OptiMed Medizinische Instrumente GmbH, Ettlingen, Germany), or Zilver Vena stent (Cook Medical, Inc., Bloomington, IN). The E*Luminexx stent is available in diameters up to 14 mm and lengths up to 120 mm and requires a 6F (in small stent sizes) or 7F introducer sheath. The Sinus-XL stent is the first stent registered for use in the SVC and is available in diameters up to 34 mm and lengths up to 100 mm (only in the 28 mm diameter size) and requires a 10F introducer sheath. The Zilver Vena stent is designed for iliofemoral venous stenting, and is available in diameters up to 16 mm and lengths up to 140 mm and requires a 7F introducer sheath. All three stent types are flexible, self-expanding nitinol stents, and are preloaded on over-the-wire delivery systems that are compatible with 0.035 in. guide wires. They all come on long shafts so they can also be deployed from the groin.
Clinical Follow-Up
All patients were followed up by their referring clinicians and all patients had repeated contrast-enhanced CT 1 to 3 months after stent implantation and in case of recurrence of SVC syndrome symptoms. Stent stenosis, stent compression or thrombi and recurrence of SVC syndrome were recorded. The primary endpoint of the study was clinical outcome 48 hours after stent placement, and secondary endpoints were SVC syndrome recurrence-free survival.
Results
Procedural Outcome
Based on prestenting CT, 4 venae cava were occluded, 17 had stenoses of 90 to 99%, and 4 had stenosis of 75 to 89%. The stenoses estimated on prestenting vena cavagram (mean 89%, median 90%) were significantly less than those estimated on the contrast-enhanced CT (mean 95%, median 98%), p = 0.003. Unlike atherosclerotic arterial stenoses, the SVC stenoses were always eccentric and flattened out or sickle-shaped because of the external compression, and thus difficult to estimate on single-plane cavography (Fig. 3, patient no. 10).
Fig. 3.
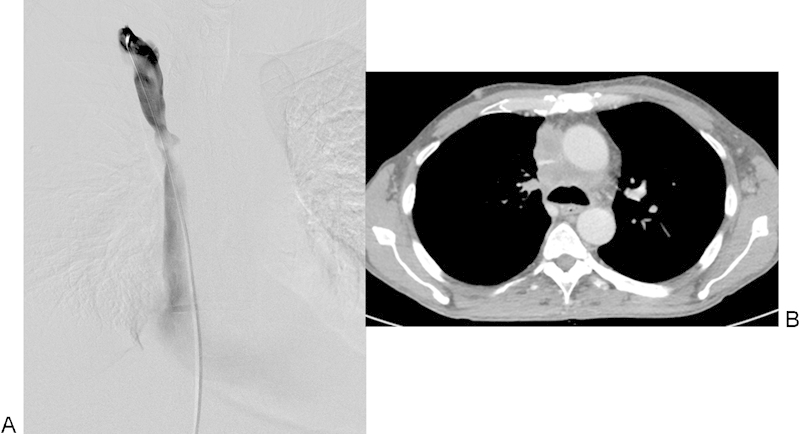
Patient No. 19: A 58-year-old man with SCLC. (A) Approximately 66% stenosis on pretreatment venography (arrow) and (B) a slit-shaped stenosis of about 90% on pretreatment contrast-enhanced CT (arrow). CT, computed tomography; SCLC, small cell lung cancer.
In three patients with 99 to 100% stenosis, prestenting dilatation was performed with 4 to 5 mm diameter balloons. Overall 46 stents were placed in the 25 patients: 42 stents were delivered via the right femoral vein and 4 stents were delivered via the left jugular vein. Left jugular vein access was required in one patient (three stents; patient no. 16) because of an inability to access through the femoral veins as a consequence of earlier femoral/femoral artery cross-over bypass surgery in the groins and an occluded right jugular vein, and was required in the other patient (one stent; patient no. 18) because it was impossible to cross the left brachiocephalic lesion from the cava site (this patient had one stent deployed from the femoral vein and one from the jugular vein).
A total of 21 Sinus-XL stents, 16 Zilver Vena stents, and 9 E*Luminexx stents were implanted (Table 1). Eleven patients received one stent, seven patients received two stents, and seven patients received three stents (Table 1); a mean of 1.8 stents (range 1–3) were inserted per patient. In six patients more than one stent type was implanted; this was done based solely on the stent sizes available at the time of the procedure. Stents with diameters between 12 and 20 mm and lengths between 40 and 100 mm were implanted; the most commonly used stent size was the 16 mm diameter by 60 mm length (n = 25). Stent diameters were oversized approximately 10 to 20% according to the normal diameter of the SVC. Poststenting balloon dilatation was performed in 23 patients (92%).
All stents were deployed in the intended position and without associated complications or adverse effects. In one patient (patient no. 2) with an extensive occlusion of the SVC involving the venous confluence, and with thrombus in both the brachiocephalic branches and prestenting thrombus aspiration, reocclusion occurred within 48 hours (three Sinus-XL stents). In five patients there was a 50% residual stenosis after stent deployment (Table 1) but all these patients had a very good clinical effect as well. Thus, the procedural success rate was 76% (19/25 patients).
Poststenting, the mean SVC stenosis had been significantly reduced from 93%, based on contrast-enhanced CT pretreatment (median 95%), to 22% based on vena cavagram poststent placement (median 15%).
Follow-Up
Overall 22 patients were followed until their death (mean 3.5 months, range 1–12 months after stent implantation); follow-up for the remaining 3 patients was for a mean of 8 months (range 1–16 months). In follow-up, two additional patients (no. 3 and no. 5) had reocclusions: one patient (three Zilver Vena stents) had a reocclusion after less than 1 month, and one patient (two Zilver Vena stents) had a reocclusion at 4 months. All other stents were patent in follow-up. External stent compression because of tumor growth with > 50% diameter reduction during follow-up was seen in three patients with Sinus-XL stents (patient nos. 2, 20, and 24; Table 1). One patient (patient no. 18) had a reintervention; an additional stent was placed 4 months after the primary procedure (during which two stents were implanted) because of 75% in-stent restenosis and recurrence of symptoms. In this small patient population, no trends were observed which suggests one stent type is associated with worse patency outcomes (either due to internal obstruction or external compression).
Clinical Outcomes
All patients with residual stenosis of 50% at procedure end had very good clinical success with relief of SVC symptoms. There were no clinical signs of pulmonary embolism in relation to stent placement in any patient. Specifically, the rate of clinical success (i.e., relief of SVC symptoms within the first 48 hours) was 96% (24 out of 25 patients). In the unsuccessful case (patient no. 2) the stent reoccluded within 48 hours. Very good clinical success with complete resolution of SVC symptoms within 48 hours was achieved in 21 patients (84%), good clinical success with partial resolution of SVC symptoms within 48 hours was achieved in 3 patients (12%), and no change in SVC symptoms was observed in 1 patient (4%). In one patient, air inside and below the stents was observed at 2 month follow-up (Fig. 4, patient no. 3), but this patient did not have sepsis or severe signs of infection. Finally, none of the mortalities (n = 22) could be ascribed to a complication of the interventional procedure or because of SVC syndrome.
Fig. 4.
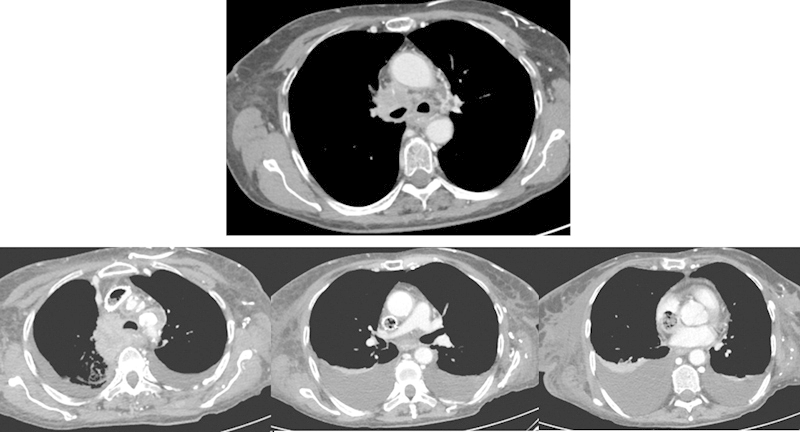
Patient No. 3: A 68-year-old woman with disseminated NSCLC. Approximately 99% stenosis of the SVC on pretreatment CT. After deployment of three Zilver Vena stents the stenosis was reduced to 25%. Good primary clinical effect, but at 2 months follow-up air and thrombus were seen inside and distal to the stents. CT, computed tomography; NSCLC, nonsmall cell lung cancer; SVC, superior vena cava. (Zilver Vena [Cook Medical Inc., Bloomington, IN])
Discussion
SVC stenting is a well-established palliative treatment for malignant SVC syndrome. It is a safe effective treatment1; peri- and postprocedural complication rates are about 6%, and the mortality rate is approximately 3%.1 8 10 13 Severe and potentially fatal complications after percutaneous stenting of SVC are few. Venous rupture, cardiac tamponade, and stent migration (into the pulmonary artery and the right atrium) have been described,14 15 16 17 18 and pulmonary embolism may be a potential hazard.
This study was a retrospective review of our clinical experience with nitinol stent placement in 25 patients with malignant SVC syndrome. We placed the Sinux-XL, the Zilver Vena, and the E*Luminexx based on available device size at our institution. Stainless steel stents were not utilized in the present study, as they have been shown to be inferior to nitinol stents with regard to recurrence of SVC syndrome.9 All stents were deployed as intended and without procedural complications and the rate of clinical success was 96%. Stent occlusion occurred in three patients in follow-up and stent compression greater than 50% occurred in three patients in follow-up (one patient with compression also had an occlusion).
As described previously, in relation to iliofemoral venous stenting, the desired attributes of a venous stent are different than for an arterial stent: venous stents must be characterized by larger diameters, longer lengths, and higher radial force to prevent compression (Fig. 5, patient no. 24), and high flexibility and adaptability to the curves of the vessels (Fig. 2B).19 The same stent attributes are important when stenting the SVC. In this regard, the limitations of E*Luminexx stent is that it is only available in diameters up to 14 mm and therefore only nine stents could be implanted in this study; it is also not approved for use in any vein. The limitations of the Sinus-XL stent is that a 10F introducer is required, which may occlude the access vein during the interventional procedure and potentially cause thrombus of the vein. The Zilver Vena stent is newest on the market and is approved for the iliofemoral veins19, though it may also be of value in the SVC because of its design features (including its radial force and radial strength, which are higher than the arterial stents from the same company).
Fig. 5.
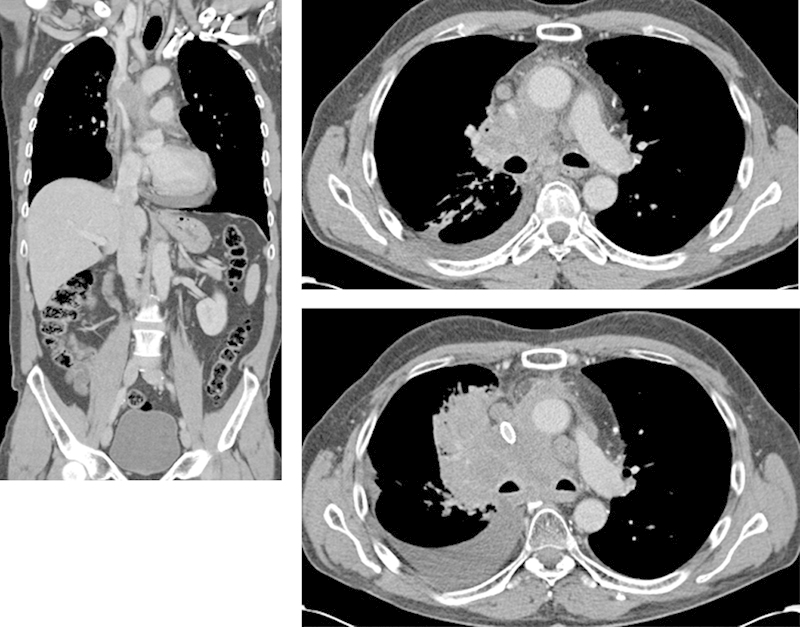
Patient No. 24: A 56-year-old man with neuroendocrine NSCLC. Approximately 90% stenosis of the SVC on pretreatment CT. One Sinus-XL 16 × 60 mm stent was implanted and postdilated with a 12 mm balloon. There was no residual stenosis and very good clinical result. At follow-up 2 months later the stent was patent, but compressed with a reduced diameter of 75%. The patient died a few weeks later without SVC syndrome. CT, computed tomography; NSCLC, nonsmall cell lung cancer; SVC, superior vena cava. (Sinus-XL [OptiMed Medizinische Instrumente GmbH, Ettlingen, Germany].)
Radial force and radial strength are important factors when stenting near a tumor and when stenting for SVC syndrome, as a stent's ability to resist compression is important for patency outcome (Fig. 5). In this study, patency failures were attributed to stent occlusions and external compression, consistent with expectations in this patient population and previous reports. Only the Sinus-XL stents were associated with compression > 50% due to external masses, and occlusions were observed in both the Zilver Vena and Sinus-XL stents. However, in this small patient population, no further analyses can be performed.
We did not perform reintervention in patients with stent compression or in-stent thrombosis/stenosis if they did not have recurrent SVC symptoms; this decision was based on the fact that the patients' residual lives were limited because of the malignant disease itself. The study is limited by the relatively small number of patients and retrospective design. A prospective designed study including higher number of patients and patient randomization to the different types of nitinol stents is warranted to determine whether outcome differences exist among stent type.
Conclusion
Stenting of SVC has become widely accepted as a palliative treatment in malignant diseases for SVC syndrome. Three types of nitinol stents have been used and all shown to be safe and efficacious.
Acknowledgment
The authors thank Jennifer A. McCann-Brown, PhD (MED Institute, Inc., West Lafayette, IN a Cook group company) for assistance in article preparation.
Footnotes
Conflict of Interest None.
References
- 1.Uberoi R. Quality assurance guidelines for superior vena cava stenting in malignant disease. Cardiovasc Intervent Radiol. 2006;29(3):319–322. doi: 10.1007/s00270-005-0284-9. [DOI] [PubMed] [Google Scholar]
- 2.Kee S T, Kinoshita L, Razavi M K, Nyman U R, Semba C P, Dake M D. Superior vena cava syndrome: treatment with catheter-directed thrombolysis and endovascular stent placement. Radiology. 1998;206(1):187–193. doi: 10.1148/radiology.206.1.9423671. [DOI] [PubMed] [Google Scholar]
- 3.Wan J F, Bezjak A. Superior vena cava syndrome. Hematol Oncol Clin North Am. 2010;24(3):501–513. doi: 10.1016/j.hoc.2010.03.003. [DOI] [PubMed] [Google Scholar]
- 4.Warren P, Burke C. Endovascular management of chronic upper extremity deep vein thrombosis and superior vena cava syndrome. Semin Intervent Radiol. 2011;28(1):32–38. doi: 10.1055/s-0031-1273938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Klop B, Scheffer M G, McFadden E, Bracke F, van Gelder B. Treatment of pacemaker-induced superior vena cava syndrome by balloon angioplasty and stenting. Neth Heart J. 2011;19(1):41–46. doi: 10.1007/s12471-010-0052-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Albers E L, Pugh M E, Hill K D, Wang L, Loyd J E, Doyle T P. Percutaneous vascular stent implantation as treatment for central vascular obstruction due to fibrosing mediastinitis. Circulation. 2011;123(13):1391–1399. doi: 10.1161/CIRCULATIONAHA.110.949180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Duvnjak S, Andersen P. Endovascular treatment of superior vena cava syndrome. Int Angiol. 2011;30(5):458–461. [PubMed] [Google Scholar]
- 8.Lanciego C, Pangua C, Chacón J I. et al. Endovascular stenting as the first step in the overall management of malignant superior vena cava syndrome. AJR Am J Roentgenol. 2009;193(2):549–558. doi: 10.2214/AJR.08.1904. [DOI] [PubMed] [Google Scholar]
- 9.Fagedet D, Thony F, Timsit J-F. et al. Endovascular treatment of malignant superior vena cava syndrome: results and predictive factors of clinical efficacy. Cardiovasc Intervent Radiol. 2013;36(1):140–149. doi: 10.1007/s00270-011-0310-z. [DOI] [PubMed] [Google Scholar]
- 10.Urruticoechea A, Mesía R, Domínguez J. et al. Treatment of malignant superior vena cava syndrome by endovascular stent insertion. Experience on 52 patients with lung cancer. Lung Cancer. 2004;43(2):209–214. doi: 10.1016/s0169-5002(03)00361-1. [DOI] [PubMed] [Google Scholar]
- 11.Watkinson A F, Yeow T N, Fraser C. Endovascular stenting to treat obstruction of the superior vena cava. BMJ. 2008;336(7658):1434–1437. doi: 10.1136/bmj.39562.512789.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andersen P E Midtgaard A Brenoe A S Elle B Duvnjak S A new Nitinol stent for use in superior vena cava syndrome Initial clinical experience. J Cardiovasc Surg, in press [PubMed] [Google Scholar]
- 13.Smayra T, Otal P, Chabbert V. et al. Long-term results of endovascular stent placement in the superior caval venous system. Cardiovasc Intervent Radiol. 2001;24(6):388–394. doi: 10.1007/s00270-001-0055-1. [DOI] [PubMed] [Google Scholar]
- 14.Maleux G, Gillardin P, Fieuws S, Heye S, Vaninbroukx J, Nackaerts K. Large-bore nitinol stents for malignant superior vena cava syndrome: factors influencing outcome. AJR Am J Roentgenol. 2013;201(3):667–674. doi: 10.2214/AJR.12.9582. [DOI] [PubMed] [Google Scholar]
- 15.Da Ines D, Chabrot P, Motreff P. et al. Cardiac tamponade after malignant superior vena cava stenting: Two case reports and brief review of the literature. Acta Radiol. 2010;51(3):256–259. doi: 10.3109/02841850903578807. [DOI] [PubMed] [Google Scholar]
- 16.Khalid I, Omari M, Khalid T J, Castillo E, Khandelwal A, Kattoo R. Pericardial tamponade after superior vena cava stent: are nitinol stents safe? Asian Cardiovasc Thorac Ann. 2010;18(3):294–296. doi: 10.1177/0218492310368730. [DOI] [PubMed] [Google Scholar]
- 17.Anand G Lewanski C R Cowman S A Jackson J E Superior vena cava stent migration into the pulmonary artery causing fatal pulmonary infarction Cardiovasc Intervent Radiol 2011342, Suppl 02S198–S201. [DOI] [PubMed] [Google Scholar]
- 18.Brown K T, Getrajdman G I. Balloon dilation of the superior vena cava (SVC) resulting in SVC rupture and pericardial tamponade: a case report and brief review. Cardiovasc Intervent Radiol. 2005;28(3):372–376. doi: 10.1007/s00270-004-0001-0. [DOI] [PubMed] [Google Scholar]
- 19.O'Sullivan G J, Sheehan J, Lohan D, McCann-Brown J A. Iliofemoral venous stenting extending into the femoral region: initial clinical experience with the purpose-designed Zilver Vena stent. J Cardiovasc Surg (Torino) 2013;54(2):255–261. [PubMed] [Google Scholar]


