Abstract
Mast cells (MCs) are important effector cells in asthma and pulmonary inflammation, and their proliferation and maturation is maintained by stem cell factor (SCF) via its receptor, c-Kit. Cysteinyl leukotrienes (cys-LTs) are potent inflammatory mediators that signal through CysLT1R and CysLT2R located on the MC surface, and they enhance MC inflammatory responses. However, it is not known if SCF and cys-LTs cross-talk and influence MC hyperplasia and activation in inflammation. Here, we report the concerted effort of the growth factor SCF and the inflammatory mediator LTD4 in MC activation. Stimulation of MCs by LTD4 in the presence of SCF enhances c-Kit-mediated proliferative responses. Similarly, SCF synergistically enhances LTD4-induced calcium, c-fos expression and phosphorylation, as well as MIP1β generation in MCs. These findings suggest that integration of SCF and LTD4 signals may contribute to MC hyperplasia and hyper-reactivity during airway hyper-response and inflammation.
Keywords: calcium; c-fos; c-Kit; cys-LTs; LTD4; mast cells; MIP1β, proliferation; calcium; stem cell factor
Introduction
Mast cells (MCs) are stem cell factor (SCF)-dependent hematopoietic cells that are ubiquitously distributed throughout the body (Gurish and Boyce, 2006; Wedemeyer et al., 2000) and they initiate inflammatory responses to allergens and infectious agents (Okayama and Kawakami, 2006). MCs play an important role in asthma through the secretion of several soluble inflammatory mediators. c-Kit is a member of the type III subclass of receptor tyrosine kinases, comprising of an N-terminal extracellular ligand binding domain, a transmembrane domain and a cytoplasmic kinase domain, which is activated by ligand-mediated receptor dimerization (Mani et al., 2009). C-Kit activation by SCF is crucial for the survival and proliferation of human MCs and and the deregulation of the c-kit/SCF axis can lead to uncontrolled proliferation as seen during systemic mastocytosis. Mastocytosis is the disturbance of the homeostatic mechanisms that control the accumulation, proliferation, survival, and turnover rates of MCs, contributing to inflammation, and remodeling. The mechanistic basis of MC hyperplasia in asthma (or in any allergic disease) is not completely understood. The importance of c-Kit signaling in MC proliferation makes it crucial to understand the basic mechanisms by which Kit regulates MC function. Although the role of c-Kit signaling is extensively studied in porcine aortic endothelial cells (Blume-Jensen et al., 1994; Blume-Jensen et al., 1993), its role in MC proliferation is still not well known.
Cysteinyl leukotrienes (cys-LTs), comprising of LTC4, LTD4 and LTE4 are potent bronchoconstrictors and they play an important role in asthma and airway inflammation (Davidson et al., 1987; Drazen and Austen, 1987). They are derivatives of arachidonic acid generated by MCs, eosinophils, basophils, macrophages, and myeloid dendritic cells (Kanaoka and Boyce, 2004), and act through two main receptors, CysLT1R and CysLT2R (Heise et al., 2000; Lynch et al., 1999). MCs not only generate cys-LTs, but also express CysLT1R and CysLT2R (Mellor et al., 2003; Mellor et al., 2001). We and others have previously shown that stimulation of human cord blood-derived MCs hMCs) with LTD4 potently induces calcium flux and cytokine generation through CysLT1R (Mellor et al., 2002; Paruchuri et al., 2008).
During inflammation, various mediators prime each other’s responses, resulting in amplified inflammatory milieu. For example, SCF-induced prolonged activation of mast cells has been shown to play critical role in the progression of allergen-induced airway hyper responsiveness (AHR) and chronic airway hypersensitivity (Hundley et al., 2004). Also, SCF has been implicated in the induction of airway hyper-reactivity during allergy-induced pulmonary responses in mouse models (Campbell et al., 1999). Interestingly, SCF-induced airway hyper-reactivity has been shown to depend on leukotriene production (Oliveira et al., 2001). Enhanced proliferation of MCs is commonly seen at the site of inflammation and this increase in MC number correlates with the severity of AHR. Given that both SCF and leukotrienes are produced at the site of inflammation and induce AHR together with the fact that MCs express receptors for both SCF and leukotrienes and enhanced MC proliferation is seen during inflammation, we asked if c-Kit and CysLT1R can cross-talk and influence MC proliferatory and inflammatory phenotypes. Therefore, in the current study, we tested the MC proliferatory and inflammatory responses in the presence of both SCF and LTD4. We hypothesized that the growth factor SCF can boost LTD4-mediated inflammatory signals and an inflammatory mediator, LTD4, can increase SCF-induced, c-Kit-mediated proliferative responses. Our results suggest that LTD4 and SCF synergistically enhance MC proliferative (c-Kit phosphorylation and proliferation) and inflammatory responses (c-fos phosphorylation, expression, and MIP1β production).
Materials and Methods
Reagents
LTC4, LTD4, LTE4, and MK571 were purchased from Cayman Chemicals, Fura-2 AM from Molecular Probes, All phospho-specific antibodies and corresponding controls from Cell Signaling Technology, GAPDH antibody from Fitzgerald (Acton, MA), XTT proliferation assay kit was from Trevigen (Gaithersburg, MD), BrdU proliferation assay kit was from Calbiochem, MIP1β ELISA kit was from R&D systems. All cytokines were purchased from R&D systems.
Cell culture
The LAD2 MC leukemia line (Kirshenbaum et al., 2003) was a generous gift from Dr. Arnold Kirshenbaum, NIH. LAD2 cells were cultured in stemPro-34 (Invitrogen) supplemented with 2mM L-Glutamine (Invitrogen), Pen-strep (100 IU/ml) (Invitrogen), and SCF (R&D systems) (100 ng/ml). Cell culture medium was hemi-depleted every week with fresh medium and 100 ng/ml SCF. Cells were SCF-starved overnight before stimulating with SCF in all the experiments. LAD2 cells are dependent on SCF for their proliferation and retain excellent responses to cys-LTs in inducing calcium flux, secretion of MIP1β and other chemokines, similar to isolated hMCs. (Paruchuri et al., 2008; Paruchuri et al., 2009). The expression pattern of cys-LT receptors in these cells are also similar to hMCs with high levels of CysLT1R compared to CysLT2R (Paruchuri et al., 2008). Bone marrow derived mast cells (BMMCs) were isolated from C57BL/6 mice. Mice were euthanized according to guidelines and approval of the Institutional Animal Care and Use Committee (IACUC) of the Northeast Ohio Medical University (NEOMED). BMMCs were cultured in 80% RPMI 1640 supplemented with 10% fetal bovine serum, 2mM L-Glutamine, Pen-Strep (100UI/ml), Sodium Pyruvate (1mM), Non-Essential Amino acids, HEPES buffer (25mM) and β-Mercaptoethanol (50uM) and 20% WEHI-3 cell conditioned medium for 4–6 weeks. Maturity of BMMCs was examined by Toluidine blue staining and >90% mature BMMCs were used for experiments.
Cord blood was obtained from Cleveland Cord Blood Center and MCs were isolated as described (Mellor et al., 2002). Briefly, heparin-treated cord blood was sedimented with 4.5% dextran solution and the buffy coat was layered onto Ficoll-Hypaque and mononuclear cells (MNC) were obtained after centrifugation at the interphase. Erythrocytes were further removed from MNC by hypotonic lysis and cultured in RPMI-1640 (Gibco), 10%FBS, 2 mM L-glutamine, 0.1 mM non- essential amino acids, Penicillin-Streptomycin, Gentamicin and 0.2µM 2-Mercaptoethanol in the presence of SCF (100ng/ml), IL-6 (50 ng/ml) and IL-10 (10 ng/ml). Non-adherent cells were transferred to fresh medium containing cytokines every week for 6–9 weeks. Maturity of hMCs was examined by Toluidine blue staining and >90% mature hMCs were used for experiments.
Calcium flux
Cells were cultured in SCF-free medium overnight. Thereafter, cells (0.5–1 × 106/sample) were washed and labeled with fura 2-AM for 30 minutes at 37°C. Cells were further washed and stimulated with 500 nM of LTD4 and/or 100 ng/ml of SCF, and the changes in intracellular calcium were measured using excitation at 340 and 380nm in a fluorescence spectrophotometer (Hitachi F-4500) as described earlier (Paruchuri et al., 2008). In some experiments, MK571 was added 10 minutes before the addition of indicated agonists. The relative ratios of fluorescence emitted at 510 nm were recorded and displayed as a reflection of intracellular calcium concentration.
Cell activation
Cells were either stimulated with 500 nM LTD4 and/or 100 ng/ml SCF (or with indicated concentrations) for indicated time points (phosphorylation of c-Kit for 15 min, phosphorylation and expression of c-fos for 1h, measurement of cytokines at transcript level for 2h, and protein level for 6h). The concentration of MIP1β was measured with ELISAs according to the manufacturer’s protocol (Paruchuri et al., 2008).
Cell lysates and western blotting
After stimulation with the respective agonists, MCs (0.5×106) were lysed with lysis buffer (BD Bioscience) supplemented with protease inhibitor cocktail (Roche) and phosphatase inhibitor cocktail (Pierce). Immunoblotting was performed as described previously (Paruchuri et al., 2002). Briefly, lysates were subjected to 4–12% SDS-PAGE and transferred to PVDF membrane. Membranes were incubated with respective primary phospho- and total antibodies diluted in 1× TBS, 5% dry milk, 0.1% Tween-20 (1:1000) overnight at 4°C on shaker, and then with secondary antibody (peroxidase-conjugated anti-rabbit or anti-mouse). Western blot was incubated with ECL, and the bands were visualized using imager (Protein Simple) and quantified using Alpha View software (Protein Simple).
Real-time Quantitative PCR
The expressions of COX-2, MIP1β and TNFα transcripts were determined with real-time PCR performed on Light cycler 480 (Roche). Cells were cultured and treated as described above, and total RNA was isolated with an RNAeasy minikit (Qiagen) according to manufacturer’s instructions. RNA concentration was determined using a Take 3 module of Epoch micro plate reader (Biotek) at 260/280 nm. 1µg of total RNA was used for reverse transcription using cDNA synthesis kit from Quanta Biosciences, containing MgCl2, dNTPs, recombinant RNAse inhibitor protein, qScript Reverse Transcriptase, random primers, oligo (dT) primers and stabilizers. Gene expression was assayed by quantitative real-time PCR on LightCycler® 480 II (Roche Applied Science) using LightCycler® 480 SYBR Green I Master mix, cDNA prepared as described above and COX-2, MIP1β, TNFα and GAPDH forward and reverse Primers. Following are the forward (F) and reverse (R) primers used
| MIP1β– | F- CCAGCCAGCTGTGGTATT |
| R-CAGTTCAGTTCCAGGTCATACA | |
| TNFα- | F- CCAGGGACCTCTCTCTAATCA |
| R-TCAGCTTGAGGGTTTGCTAC | |
| COX-2- | F-CAACTCTATATTGCTGGAACATGGA |
| R-TGGAAGCCTGTGATACTTTCTGTACT | |
| GAPDH- | F-TGCACCACCAACTGCTTAGC |
| R-GGCATGGACTGTGGTCATGAG |
The ΔΔCt values for COX-2, MIP1β, and TNFα were calculated relative to the GAPDH levels and values were expressed as fold change over the control (Duah et al., 2013).
Cell proliferation
Cells were plated at a density of 5000 cells/well of 96 well plate, cultured in SCF-free medium overnight, and treated with increasing concentration of SCF and/or 500 nM of LTD4. After 72 h, the proliferation was assayed either by XTT assay (Trevigen) or BrdU ELISA (Millipore) according to the manufacturer’s protocol.
Data Analysis
Data is expressed as mean ± SEM from at least three experiments except where otherwise indicated. Significance was determined using one way ANOVA and post-hoc analysis.
Results
SCF induces concentration-dependent phosphorylation of c-Kit in MCs
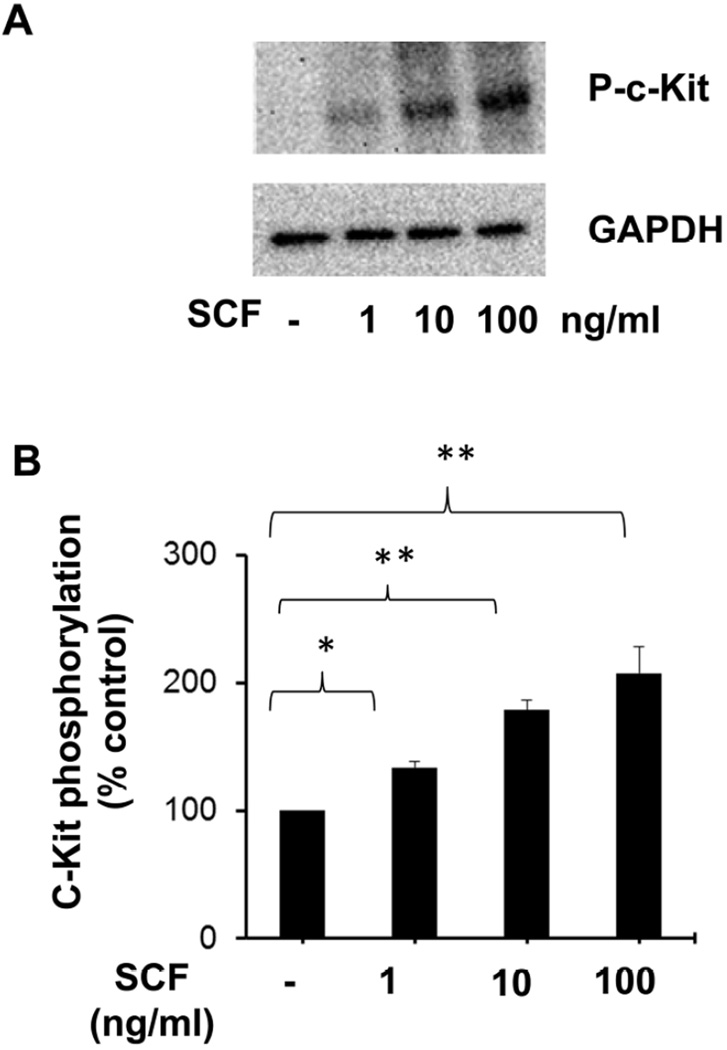
SCF is the major growth factor for the proliferation of MCs, and it relays its responses through the cell surface receptor, c-Kit (Galli et al., 1995; Iemura et al., 1994; Tsai et al., 1991). To confirm if c-Kit is activated in response to SCF stimulation in LAD2 cells, we first determined the effect of SCF on phosphorylation of c-Kit receptor, by treating the cells with different concentrations of SCF. Stimulation with SCF for 15 minutes led to dose-dependent phosphorylation (Fig. 1A, B) of c-Kit receptor. SCF induced phosphorylation of c-Kit receptor at doses as low as 1ng/ml with a maximum response at 100 ng/ml concentration.
Figure 1. Dose dependent phosphorylation of c-Kit receptor by SCF.
(A) LAD2 cells were stimulated with the indicated doses of SCF for 15 minutes and the c-Kit phosphorylation was assessed by western blotting using phospho-specific c-Kit antibodies. Blots were stripped and re-blotted for GAPDH to confirm equal loading. The data shown are representative of three separate experiments. (B) Densitometric analysis of data shown in A. The data represents mean ± SEM of three separate experiments. The significance was tested using one way ANOVA and post-hoc analysis *P<0.05, **P<0.001.
C-Kit phosphorylation is synergistically activated by SCF and LTD4
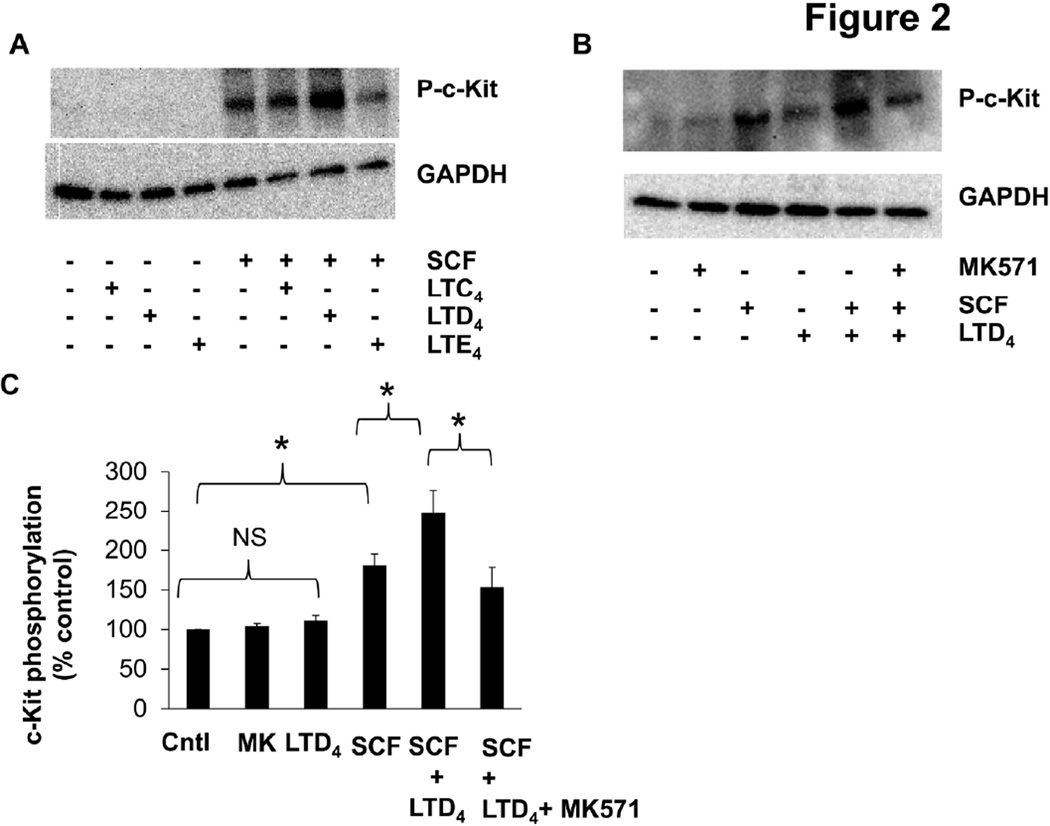
To determine if there is crosstalk between c-Kit and CysLTRs, we stimulated LAD2 cells with cys-LTs with or without SCF and analyzed c-Kit phosphorylation. We could not detect any significant phosphorylation of c-Kit by cys-LTs alone. However, LTD4 significantly strengthened SCF-induced c-Kit phosphorylation (Fig. 2A, B, C). We also found similar synergistic activation of c-Kit by SCF and LTD4 in BMMCs (Suppl. Fig. 1). This synergism with SCF was not observed with LTC4 or LTE4 treatment (Fig. 2A). Further, we found that treatment of cells with CysLT1R antagonist, MK571 prior to stimulation with SCF and LTD4, inhibited this synergistic effect suggesting that it is mediated through CysLT1R (Fig. 2B, C).
Figure 2. LTD4 and SCF synergistically phosphorylate c-Kit.
c-Kit phosphorylation was analyzed by western blotting in LAD2 cell lysates (A) stimulated with 500 nM of LTC4, LTD4 and LTE4 in presence or absence of 100ng/ml SCF for 15 minutes. (B) Phospho-c-Kit levels upon stimulation with 500 nM LTD4 and/or of 100 ng/ml SCF for 15 minutes with/without MK571 (1µM) pre-treatment (30 min). (C) Densitometric analysis of phospho-c-Kit levels upon SCF and /or LTD4 stimulation in the presence or absence of MK571 (1µM). The data represents mean ± SEM of three separate experiments. The significance was tested using one way ANOVA and post-hoc analysis *P<0.05.
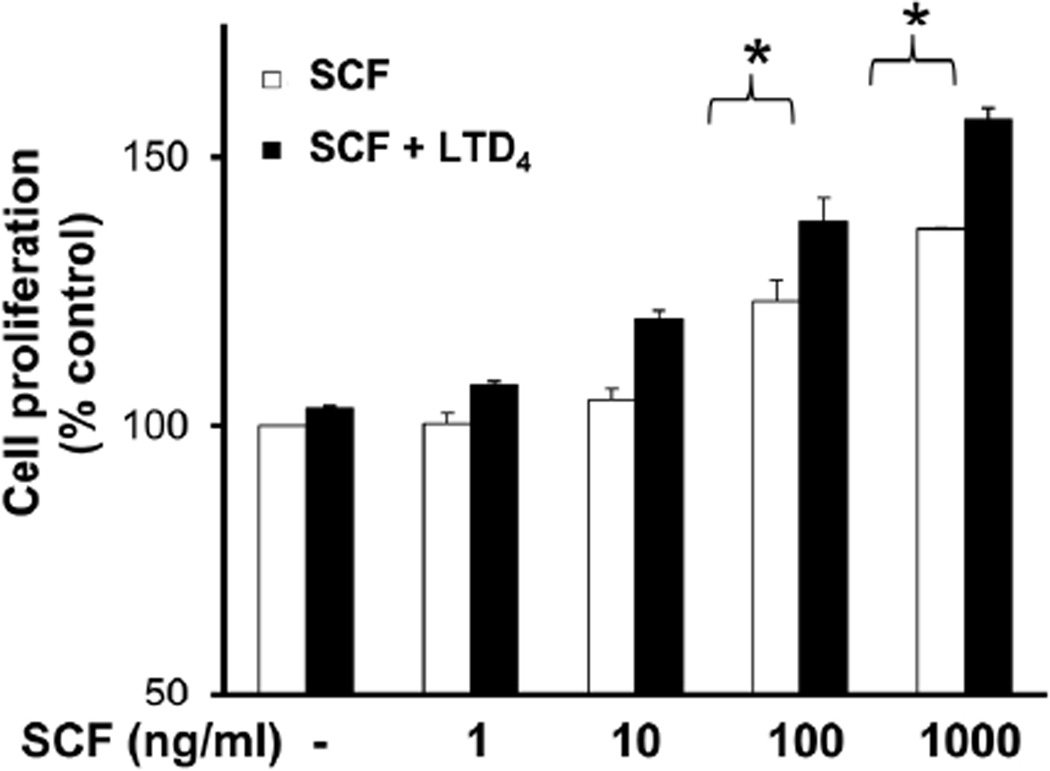
LTD4 potentiates SCF-induced MC proliferation
The fact that LTD4 augmented c-Kit phosphorylation by SCF prompted us further to understand the significance of this potentiation. We analyzed if LTD4 treatment together with SCF would amplify LAD2 cell proliferation by using XTT assay. Consistent with earlier results (Laidlaw et al., 2011), we found that SCF promoted proliferation of LAD2 cells in a dose-dependent manner (Fig. 3). Interestingly, a significant increment in cell proliferation was observed with combined stimulation of SCF and LTD4 at high doses of SCF. These findings indicate that LTD4 can enhance c-Kit responses in LAD2 cells and increase their proliferation. We also found that hMCs cultured in the presence of both SCF and LTD4 induced around 2 fold increase in proliferation compared to SCF treatment alone (Suppl. Fig. 2A). Taken together, these results suggest that LTD4 in concert with SCF can modulate primary mast cell (hMC) and MC cell line (LAD2) proliferation.
Figure 3. LTD4 enhances SCF-mediated cell proliferation.
Proliferation of LAD2 cells stimulated with 500 nM LTD4 and/or the indicated dose of SCF was measured by XTT assay. Changes in cell proliferation were expressed as percentage of control. The data represents mean ± SEM of three separate experiments. Data was analyzed with one way ANOVA and post-hoc analysis. *P<0.05.
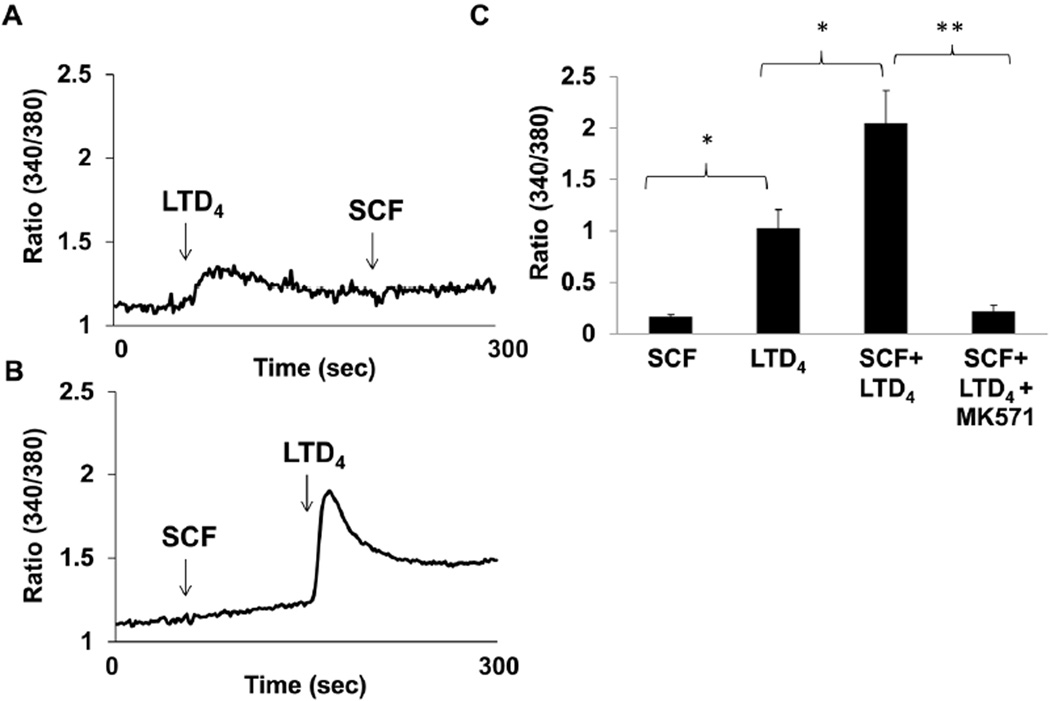
SCF pre-treatment amplifies CysLT1R function as measured by LTD4-induced calcium flux
Above results clearly depict that combined stimulation of SCF and LTD4 enhances c-Kit-mediated proliferative responses. Next, we asked if this cross-talk is bi-directional i.e. if combined stimulation with SCF can augment LTD4-mediated inflammatory responses, the same way as LTD4 can modulate SCF-induced proliferation. We have previously shown that stimulation of LAD2 cells with LTD4 induces calcium flux, c-fos phosphorylation and expression and MIP1β generation (Kondeti et al., 2013; Paruchuri et al., 2008). First, we asked if SCF pre-treatment influences LTD4-mediated calcium flux. We have previously shown that LTD4 was the most potent agonist among the cys-LTs for eliciting calcium flux and completely desensitized MC to the calcium fluxes induced by the two other cys-LTs (Paruchuri et al., 2008). We observed that SCF (100 ng/ml) induced minimal or no calcium flux by itself (Fig. 4B) or followed by priming with LTD4 (Fig. 4A). However, stimulating cells with SCF prior to LTD4 significantly amplified LTD4-induced calcium flux (Fig. 4B, C). Further, this calcium flux was inhibited by prior treatment with CysLT1R antagonist, MK571, confirming that this signal is mediated by CysLT1R (Fig. 4C).
Figure 4. SCF primes LTD4 -induced calcium flux.
(A, B) LAD2 cells were loaded with Fura-2AM and stimulated with 500 nM LTD4 and 100ng/ml SCF at indicated times arrows) and changes in intracellular calcium concentration were measured. (C) Quantitative analysis of the three experiments performed. The data represents mean ± SEM of three separate experiments. Data was analyzed with one way ANOVA and post-hoc analysis. *P<0.05, **P<0.001.
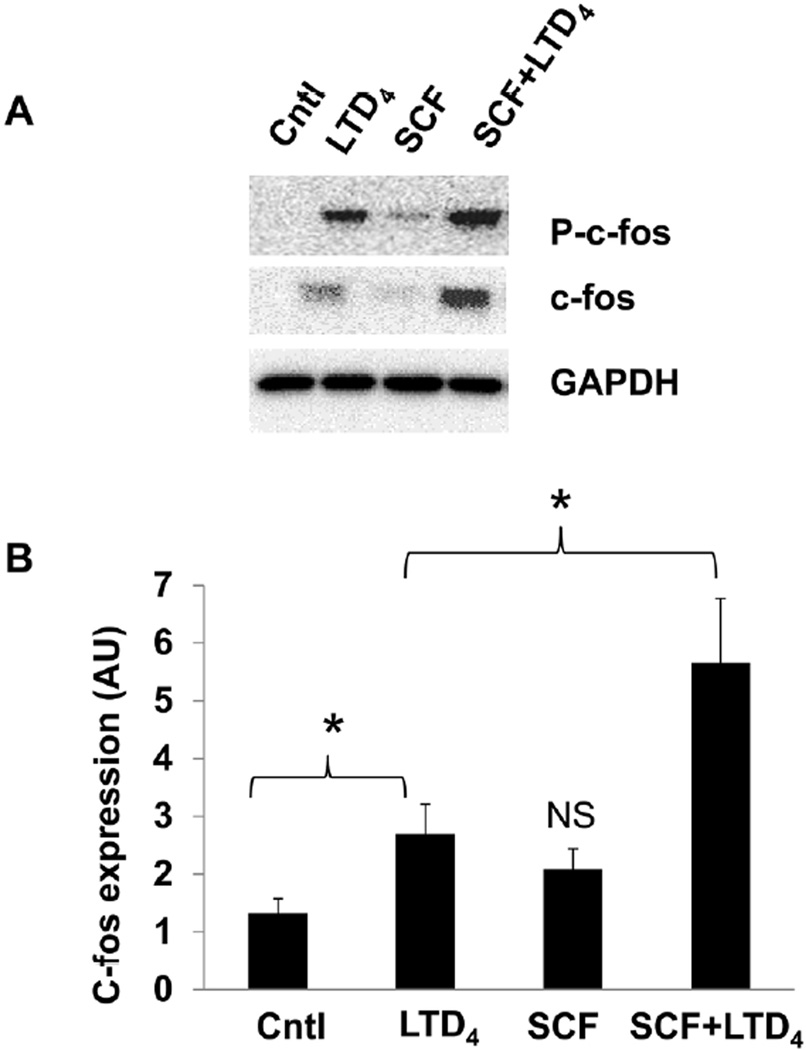
SCF and LTD4 treatment synergistically amplifies c-fos phosphorylation and expression
Next, we investigated if SCF has the potential to amplify other LTD4-induced MC responses such as c-fos phosphorylation and expression. We have reported earlier that stimulation of LAD2 cells with LTD4 led to phosphorylation and increased expression of c-fos (Kondeti et al., 2013). SCF treatment alone did not induce significant c-fos phosphorylation or expression, however, both LTD4-induced c-fos phosphorylation and expression were synergistically enhanced in the presence of SCF (Fig. 5A, B), further suggesting the ability of SCF/c-Kit to modulate LTD4-induced responses. We also found that treatment of hMCs with a combination of SCF and LTD4 caused potentiation of c-fos phosphorylation and expression compared to treatment with either of the agonists (Suppl. Fig.2B) further confirming the synergistic activation in primary MCs.
Figure 5. SCF potentiates LTD4-induced c-fos phosphorylation and expression.
LAD2 cells were treated with 500 nM LTD4 and/or 100 ng/ml of SCF for 1 h and the phospho- and total c-fos levels were evaluated by western blotting. (B) Densitometric analysis of c-fos expression shown in (A). The data represents mean ± SEM of three separate experiments. Data was analyzed with one way ANOVA and post-hoc analysis. P<0.05.
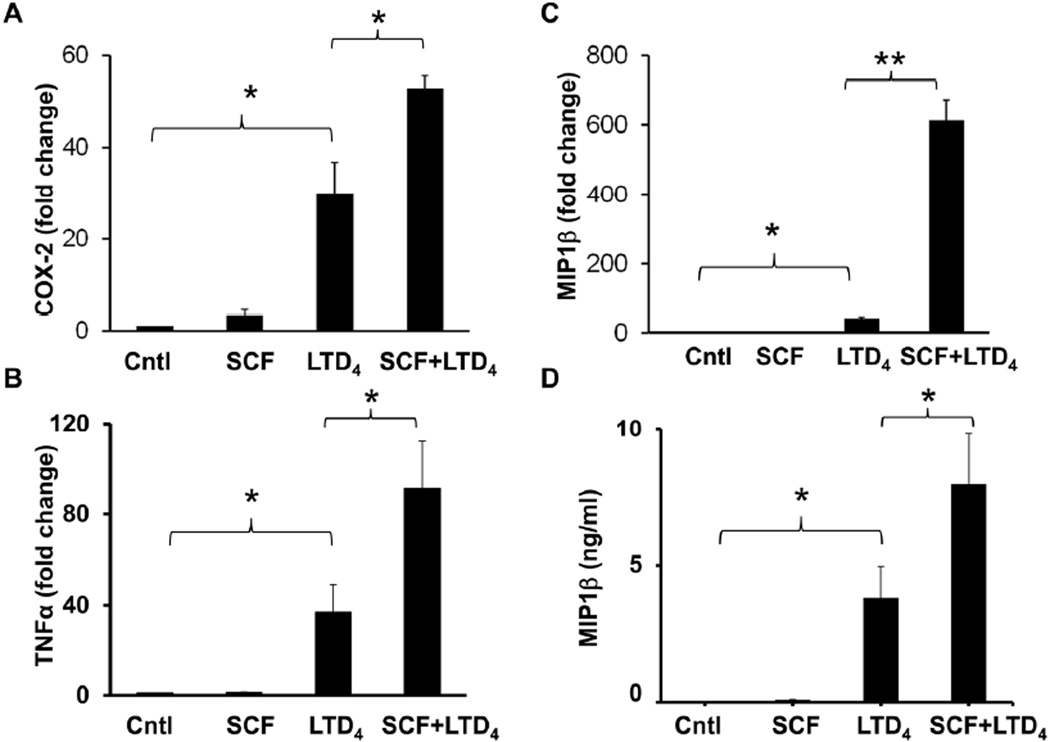
Amplification of inflammatory signals by LTD4 and SCF treatment
COX-2 activation, MIP1β secretion and up-regulation of other inflammatory cytokine expression are major MC responses downstream of CysLT1R signaling induced by LTD4 (Paruchuri et al., 2008; Paruchuri et al., 2009). These responses further enhance inflammation by recruiting other immune cells. To find out if SCF can increase LTD4-induced MC inflammatory signals, we measured LTD4-induced inflammatory signals in the presence or absence of SCF. LTD4 stimulation alone, but not SCF, induced the expression of COX-2, TNF-α, and MIP1β in LAD2 cells. Interestingly, we found that LTD4-induced expression of these inflammatory genes is significantly boosted by SCF (Fig. 6 A, B, C). Finally, consistent with our transcript data, we found that LTD4 and SCF synergistically increased the secretion of MIP1β protein in LAD2 cells (Fig.6 D). However, either LTD4 or SCF treatment, or the combination of both failed to induce any degranulation (data not shown) as determined by β-hexosaminidase assay using p-NAG (p-Nitrophenyl-N-acetyl-β-D-glucosaminidine) as substrate.
Figure 6. SCF augments LTD4-induced inflammatory gene repertoire.
LAD2 cells were treated with 500 nM LTD4 and/or 100ng/ml of SCF for 2 h, followed by mRNA extraction and cDNA synthesis. Transcript levels of COX-2 (A), TNFα (B), and MIP1β (C) were analyzed in these cDNAs using respective real time primers and were analyzed compared to GAPDH. The graph represents fold change in the level of transcripts compared to controls from three separate experiments. (D) LAD2 cells were treated with 500 nM LTD4 in the presence or absence of 100ng/ml SCF for 6h. Culture medium was collected and analyzed for secreted MIP1β protein by ELISA. The data shown represents mean ± SEM of three separate experiments. Data was analyzed with one way ANOVA and post-hoc analysis. *P<0.05, **P<0.001.
Discussion
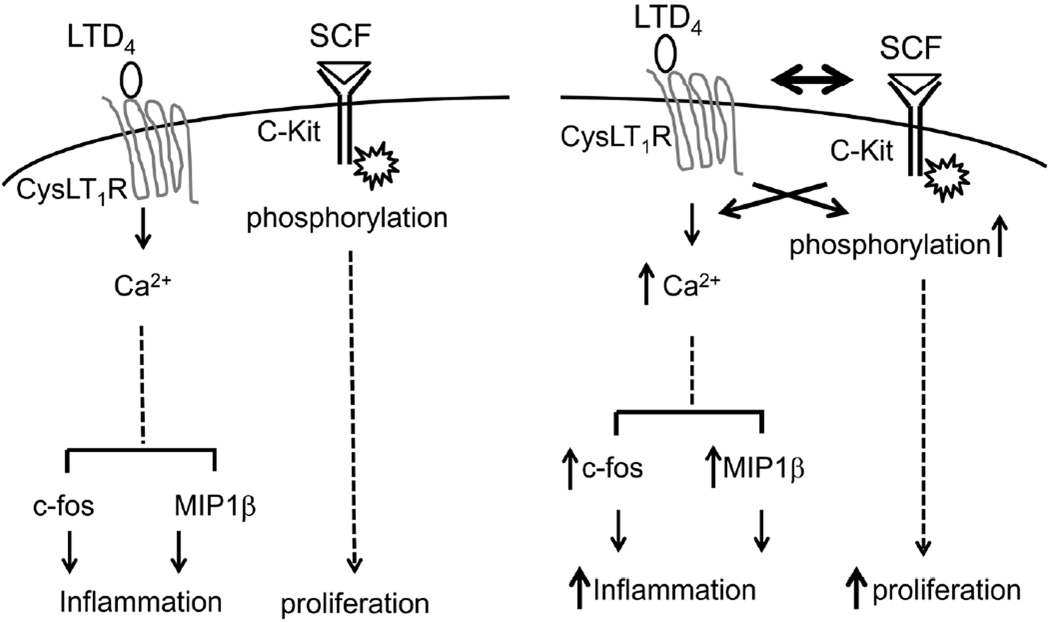
In the present study, we demonstrate that an inflammatory mediator, LTD4, and a growth factor, SCF synergistically regulate MC function. We clearly demonstrate that LTD4 can prime SCF-induced c-Kit phosphorylation and proliferation of MCs, and that SCF in turn can strengthen LTD4-induced calcium flux and inflammatory responses such as c-fos phosphorylation and expression, as well as MIP1β production, suggesting a potential cross-talk between the LTD4 and SCF receptors, CysLT1R and c-Kit (Fig. 7).
Figure 7. Schematic showing the possible cross-talk between LTD4 and SCF in mast cells.
LTD4 alone induces calcium influx via CysLT1R leading to activation of c-fos and generation of inflammatory chemokine, MIP1β in MCs, while SCF alone enhances their proliferation through the activation of C-kit. However, simultaneous stimulation of MCs with both LTD4 and SCF synergistically enhance each other’s responses via cross-talk between associated signaling leading to augmented inflammatory and proliferatory responses.
MCs are mediators of the early phase of allergic inflammation. MCs are released into the blood stream as progenitor cells, and are then recruited to the tissues where they undergo maturation (Gurish and Boyce, 2006; Kirshenbaum et al., 1999; Metcalfe et al., 1997). SCF is an important regulator of MC growth, differentiation, survival and chemotaxis (Grimbaldeston et al., 2006; Prussin and Metcalfe, 2006; Tkaczyk et al., 2006). SCF acts through c-Kit tyrosine kinase receptor which activates downstream signaling molecules such as PI3 Kinase and AKT to exert its effects on MCs (Ali et al., 2004; Moller et al., 2005). SCF was also shown to enhance allergen induced FCεRI mediated degranulation of MCs and release of inflammatory mediators (Gilfillan and Tkaczyk, 2006; Ito et al., 2012; Jensen et al., 2007). These inflammatory mediators may further activate MCs and boost inflammation.
Cys-LTs are produced by MCs and were also shown to activate MCs. We have previously shown that LAD2 cells express both CysLT1R and CysLT2R and that cys-LTs (LTC4, LTD4 and LTE4) induce calcium influx, c-fos expression and phosphorylation (Kondeti et al., 2013; Laidlaw et al., 2011; Paruchuri et al., 2008). Both c-Kit signaling and CysLTR signaling are shown to be mitogenic for MCs. In addition, CysLT1R has been shown to transactivate c-Kit receptor in hMCs (Jiang et al., 2006). Interestingly, patients with systemic mastocytosis showed significantly higher urinary excretion of cys-LTs than controls (Raithel et al., 2011). Further, LTC4 synthase knockout mice were unable to develop MC hyperplasia in the inflamed mucosal surface of the lung in a model of allergen-induced pulmonary inflammation (Kim et al., 2006). However, it is not known if these pro-inflammatory mediators act in concert with SCF. In the present study, though we did not observe any c-Kit phosphorylation or proliferation with LTD4 alone, stimulation of MCs with SCF induced phosphorylation as well as MC proliferation. Interestingly, c-Kit phosphorylation and MC proliferation was augmented in the presence of both SCF and LTD4 suggesting the existence of a cross-talk between their receptors. LTD4, but not LTC4 or LTE4, increased c-Kit phosphorylation by SCF, indicating a specific role for LTD4 in this cross-talk. We also showed that this potentiation of c-Kit is mediated through Cys-LT1R. Notably, we found that stimulating MCs with SCF prior to LTD4 enhanced LTD4-induced calcium flux. In contrast, SCF alone did not induce any calcium flux. Furthermore, we found that c-fos phosphorylation and expression, COX-2, TNF-α and MIP1β transcript expression, as well as MIP1β protein secretion are amplified by combined treatment with SCF and LTD4. Thus, our findings suggest that while LTD4 synergistically activates SCF-induced proliferative signals, SCF in turn potentiates LTD4-induced inflammatory signals in MCs. The concept of this bi-directional cross-talk between LTD4 (CysLT1R) and SCF (c-Kit) signaling is novel and may have important implications in targeting MC-mediated inflammatory responses. Importantly, our results suggest that the inflammatory microenvironment, but not a single molecule, dictate the inflammatory phenotype of MCs. SCF was previously shown to enhance FCεRI mediated responses in MCs (Gilfillan and Tkaczyk, 2006; Ito et al., 2012; Jensen et al., 2008; Jensen et al., 2007). However, recently it was shown that while acute treatment of SCF potentiated inflammatory phenotype of MCs, prolonged treatment of SCF inhibited PGE2 and allergen-induced MC degranulation (Gilfillan and Tkaczyk, 2006; Ito et al., 2012; Jensen et al., 2007). The underlying molecular mechanism could be alterations in the cytoskeleton by SCF-mediated regulation of src kinase, hck (Ito et al., 2012; Smrz et al., 2013).
In conclusion, our results suggest that the cross-talk between SCF and LTD4 induces MC proliferation, gene regulation, and cytokine production. SCF signaling through c-Kit may regulate baseline maintenance of MCs. However, locally derived cys-LTs produced at the site of inflammation enhance MC numbers by cross talking with c-Kit receptor. Similarly, this c-Kit and CysLT1R crosstalk also potentiates LTD4 effects enhancing MC inflammatory responses leading to enhanced pathological loop (Fig. 7). Although SCF-induced MC activation in allergen mediated AHR was shown to be dependent on leukotrienes (Oliveira et al., 2001), it is not known whether cys-LTs can modulate SCF receptor. Thus, our findings that LTD4 can enhance c-Kit-dependent proliferative response and SCF potentiate LTD4-mediated inflammatory responses are very intriguing and may provide basis for novel therapeutic targets for. asthma and allergic diseases
Supplementary Material
Acknowledgements
This work is supported by National Institutes of Health Grants (HL098953) and by James Foght Assistant Professor to S.P.
Footnotes
The authors have no conflict of interest.
Literature Cited
- Ali K, Bilancio A, Thomas M, Pearce W, Gilfillan AM, Tkaczyk C, Kuehn N, Gray A, Giddings J, Peskett E, Fox R, Bruce I, Walker C, Sawyer C, Okkenhaug K, Finan P, Vanhaesebroeck B. Essential role for the p110δ phosphoinositide 3-kinase in the allergic response. Nature. 2004;431(7011):1007–1011. doi: 10.1038/nature02991. [DOI] [PubMed] [Google Scholar]
- Blume-Jensen P, Ronnstrand L, Gout I, Waterfield MD, Heldin CH. Modulation of Kit/stem cell factor receptor-induced signaling by protein kinase C. J Biol Chem. 1994;269(34):21793–21802. [PubMed] [Google Scholar]
- Blume-Jensen P, Siegbahn A, Stabel S, Heldin CH, Ronnstrand L. Increased Kit/SCF receptor induced mitogenicity but abolished cell motility after inhibition of protein kinase C. Embo J. 1993;12(11):4199–4209. doi: 10.1002/j.1460-2075.1993.tb06104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campbell E, Hogaboam C, Lincoln P, Lukacs NW. Stem cell factor-induced airway hyperreactivity in allergic and normal mice. Am J Pathol. 1999;154(4):1259–1265. doi: 10.1016/S0002-9440(10)65377-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davidson AB, Lee TH, Scanlon PD, Solway J, McFadden ER, Jr, Ingram RH, Jr, Corey EJ, Austen KF, Drazen JM. Bronchoconstrictor effects of leukotriene E4 in normal and asthmatic subjects. Am Rev Respir Dis. 1987;135(2):333–337. doi: 10.1164/arrd.1987.135.2.333. [DOI] [PubMed] [Google Scholar]
- Drazen JM, Austen KF. Leukotrienes and airway responses. Am Rev Respir Dis. 1987;136(4):985–998. doi: 10.1164/ajrccm/136.4.985. [DOI] [PubMed] [Google Scholar]
- Duah E, Adapala RK, Al-Azzam N, Kondeti V, Gombedza F, Thodeti CK, Paruchuri S. Cysteinyl leukotrienes regulate endothelial cell inflammatory and proliferative signals through CysLT(2) and CysLT(1) receptors. Sci Rep. 2013;3:3274. doi: 10.1038/srep03274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galli SJ, Tsai M, Wershil BK, Tam SY, Costa JJ. Regulation of mouse and human mast cell development, survival and function by stem cell factor, the ligand for the c-kit receptor. Int Arch Allergy Immunol. 1995;107(1–3):51–53. doi: 10.1159/000236928. [DOI] [PubMed] [Google Scholar]
- Gilfillan AM, Tkaczyk C. Integrated signalling pathways for mast- cell activation. Nat Rev Immunol. 2006;6(3):218–230. doi: 10.1038/nri1782. [DOI] [PubMed] [Google Scholar]
- Grimbaldeston MA, Metz M, Yu M, Tsai M, Galli SJ. Effector and potential immunoregulatory roles of mast cells in IgE-associated acquired immune responses. Curr Opin Immunol. 2006;18(6):751–760. doi: 10.1016/j.coi.2006.09.011. [DOI] [PubMed] [Google Scholar]
- Gurish MF, Boyce JA. Mast cells: ontogeny, homing, and recruitment of a unique innate effector cell. J Allergy Clin Immunol. 2006;117(6):1285–1291. doi: 10.1016/j.jaci.2006.04.017. [DOI] [PubMed] [Google Scholar]
- Heise CE, O'Dowd BF, Figueroa DJ, Sawyer N, Nguyen T, Im DS, Stocco R, Bellefeuille JN, Abramovitz M, Cheng R, Williams DL, Jr, Zeng Z, Liu Q, Ma L, Clements MK, Coulombe N, Liu Y, Austin CP, George SR, O'Neill GP, Metters KM, Lynch KR, Evans JF. Characterization of the human cysteinyl leukotriene 2 receptor. J Biol Chem. 2000;275(39):30531–30536. doi: 10.1074/jbc.M003490200. [DOI] [PubMed] [Google Scholar]
- Hundley TR, Gilfillan AM, Tkaczyk C, Andrade MV, Metcalfe DD, Beaven MA. Kit and FcepsilonRI mediate unique and convergent signals for release of inflammatory mediators from human mast cells. Blood. 2004;104(8):2410–2417. doi: 10.1182/blood-2004-02-0631. [DOI] [PubMed] [Google Scholar]
- Iemura A, Tsai M, Ando A, Wershil BK, Galli SJ. The c-kit ligand, stem cell factor, promotes mast cell survival by suppressing apoptosis. Am J Pathol. 1994;144(2):321–328. [PMC free article] [PubMed] [Google Scholar]
- Ito T, Smrz D, Jung MY, Bandara G, Desai A, Smrzova S, Kuehn HS, Beaven MA, Metcalfe DD, Gilfillan AM. Stem cell factor programs the mast cell activation phenotype. J Immunol. 2012;188(11):5428–5437. doi: 10.4049/jimmunol.1103366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BM, Beaven MA, Iwaki S, Metcalfe DD, Gilfillan AM. Concurrent inhibition of kit- and FcepsilonRI-mediated signaling: coordinated suppression of mast cell activation. J Pharmacol Exp Ther. 2008;324(1):128–138. doi: 10.1124/jpet.107.125237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jensen BM, Metcalfe DD, Gilfillan AM. Targeting kit activation: a potential therapeutic approach in the treatment of allergic inflammation. Inflamm Allergy Drug Targets. 2007;6(1):57–62. doi: 10.2174/187152807780077255. [DOI] [PubMed] [Google Scholar]
- Jiang Y, Kanaoka Y, Feng C, Nocka K, Rao S, Boyce JA. Cutting edge: Interleukin 4-dependent mast cell proliferation requires autocrine/intracrine cysteinyl leukotriene-induced signaling. J Immunol. 2006;177(5):2755–2759. doi: 10.4049/jimmunol.177.5.2755. [DOI] [PubMed] [Google Scholar]
- Kanaoka Y, Boyce JA. Cysteinyl leukotrienes and their receptors: cellular distribution and function in immune and inflammatory responses. J Immunol. 2004;173(3):1503–1510. doi: 10.4049/jimmunol.173.3.1503. [DOI] [PubMed] [Google Scholar]
- Kim DC, Hsu FI, Barrett NA, Friend DS, Grenningloh R, Ho IC, Al-Garawi A, Lora JM, Lam BK, Austen KF, Kanaoka Y. Cysteinyl leukotrienes regulate Th2 cell- dependent pulmonary inflammation. J Immunol. 2006;176(7):4440–4448. doi: 10.4049/jimmunol.176.7.4440. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AS, Akin C, Wu Y, Rottem M, Goff JP, Beaven MA, Rao VK, Metcalfe DD. Characterization of novel stem cell factor responsive human mast cell lines LAD 1 and 2 established from a patient with mast cell sarcoma/leukemia; activation following aggregation of FcεRI or FcγRI. Leuk Res. 2003;27(8):677–682. doi: 10.1016/s0145-2126(02)00343-0. [DOI] [PubMed] [Google Scholar]
- Kirshenbaum AS, Goff JP, Semere T, Foster B, Scott LM, Metcalfe DD. Demonstration that human mast cells arise from a progenitor cell population that is CD34(+), c-kit(+), and expresses aminopeptidase N (CD13) Blood. 1999;94(7):2333–2342. [PubMed] [Google Scholar]
- Kondeti V, Duah E, Al-Azzam N, Thodeti CK, Boyce JA, Paruchuri S. Differential regulation of cysteinyl leukotriene receptor signaling by protein kinase C in human mast cells. PLoS One. 2013;8(8):e71536. doi: 10.1371/journal.pone.0071536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laidlaw TM, Steinke JW, Tinana AM, Feng C, Xing W, Lam BK, Paruchuri S, Boyce JA, Borish L. Characterization of a novel human mast cell line that responds to stem cell factor and expresses functional FcεRI. J Allergy Clin Immunol. 2011;127(3):815–822. e811–e815. doi: 10.1016/j.jaci.2010.12.1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lynch KR, O'Neill GP, Liu Q, Im DS, Sawyer N, Metters KM, Coulombe N, Abramovitz M, Figueroa DJ, Zeng Z, Connolly BM, Bai C, Austin CP, Chateauneuf A, Stocco R, Greig GM, Kargman S, Hooks SB, Hosfield E, Williams DL, Jr, Ford-Hutchinson AW, Caskey CT, Evans JF. Characterization of the human cysteinyl leukotriene CysLT1 receptor. Nature. 1999;399(6738):789–793. doi: 10.1038/21658. [DOI] [PubMed] [Google Scholar]
- Mani M, Venkatasubrahmanyam S, Sanyal M, Levy S, Butte A, Weinberg K, Jahn T. Wiskott-Aldrich syndrome protein is an effector of Kit signaling. Blood. 2009;114(14):2900–2908. doi: 10.1182/blood-2009-01-200733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor EA, Austen KF, Boyce JA. Cysteinyl leukotrienes and uridine diphosphate induce cytokine generation by human mast cells through an interleukin 4- regulated pathway that is inhibited by leukotriene receptor antagonists. J Exp Med. 2002;195(5):583–592. doi: 10.1084/jem.20020044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor EA, Frank N, Soler D, Hodge MR, Lora JM, Austen KF, Boyce JA. Expression of the type 2 receptor for cysteinyl leukotrienes (CysLT2R) by human mast cells: Functional distinction from CysLT1R. Proc Natl Acad Sci U S A. 2003;100(20):11589–11593. doi: 10.1073/pnas.2034927100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mellor EA, Maekawa A, Austen KF, Boyce JA. Cysteinyl leukotriene receptor 1 is also a pyrimidinergic receptor and is expressed by human mast cells. Proc Natl Acad Sci U S A. 2001;98(14):7964–7969. doi: 10.1073/pnas.141221498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metcalfe DD, Baram D, Mekori YA. Mast cells. Physiol Rev. 1997;77(4):1033–1079. doi: 10.1152/physrev.1997.77.4.1033. [DOI] [PubMed] [Google Scholar]
- Moller C, Alfredsson J, Engstrom M, Wootz H, Xiang Z, Lennartsson J, Jonsson JI, Nilsson G. Stem cell factor promotes mast cell survival via inactivation of FOXO3a-mediated transcriptional induction and MEK-regulated phosphorylation of the proapoptotic protein Bim. Blood. 2005;106(4):1330–1336. doi: 10.1182/blood-2004-12-4792. [DOI] [PubMed] [Google Scholar]
- Okayama Y, Kawakami T. Development, migration, and survival of mast cells. Immunol Res. 2006;34(2):97–115. doi: 10.1385/IR:34:2:97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oliveira SH, Hogaboam CM, Berlin A, Lukacs NW. SCF-induced airway hyperreactivity is dependent on leukotriene production. Am J Physiol Lung Cell Mol Physiol. 2001;280(6):L1242–L1249. doi: 10.1152/ajplung.2001.280.6.L1242. [DOI] [PubMed] [Google Scholar]
- Paruchuri S, Hallberg B, Juhas M, Larsson C, Sjolander A. Leukotriene D(4) activates MAPK through a Ras-independent but PKCε-dependent pathway in intestinal epithelial cells. J Cell Sci. 2002;115(Pt 9):1883–1893. doi: 10.1242/jcs.115.9.1883. [DOI] [PubMed] [Google Scholar]
- Paruchuri S, Jiang Y, Feng C, Francis SA, Plutzky J, Boyce JA. Leukotriene E4 activates peroxisome proliferator-activated receptor γand induces prostaglandin D2 generation by human mast cells. J Biol Chem. 2008;283(24):16477–16487. doi: 10.1074/jbc.M705822200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paruchuri S, Tashimo H, Feng C, Maekawa A, Xing W, Jiang Y, Kanaoka Y, Conley P, Boyce JA. Leukotriene E4-induced pulmonary inflammation is mediated by the P2Y12 receptor. J Exp Med. 2009;206(11):2543–2555. doi: 10.1084/jem.20091240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prussin C, Metcalfe DD. 5. IgE, mast cells, basophils, and eosinophils. J Allergy Clin Immunol. 2006;117(2 Suppl Mini-Primer):S450–S456. doi: 10.1016/j.jaci.2005.11.016. [DOI] [PubMed] [Google Scholar]
- Raithel M, Zopf Y, Kimpel S, Naegel A, Molderings GJ, Buchwald F, Schultis HW, Kressel J, Hahn EG, Konturek P. The measurement of leukotrienes in urine as diagnostic option in systemic mastocytosis. J Physiol Pharmacol. 2011;62(4):469–472. [PubMed] [Google Scholar]
- Smrz D, Bandara G, Beaven MA, Metcalfe DD, Gilfillan AM. Prevention of F-actin assembly switches the response to SCF from chemotaxis to degranulation in human mast cells. Eur J Immunol. 2013;43(7):1873–1882. doi: 10.1002/eji.201243214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tkaczyk C, Jensen BM, Iwaki S, Gilfillan AM. Adaptive and innate immune reactions regulating mast cell activation: from receptor-mediated signaling to responses. Immunol Allergy Clin North Am. 2006;26(3):427–450. doi: 10.1016/j.iac.2006.05.011. [DOI] [PubMed] [Google Scholar]
- Tsai M, Takeishi T, Thompson H, Langley KE, Zsebo KM, Metcalfe DD, Geissler EN, Galli SJ. Induction of mast cell proliferation, maturation, and heparin synthesis by the rat c-kit ligand, stem cell factor. Proc Natl Acad Sci U S A. 1991;88(14):6382–6386. doi: 10.1073/pnas.88.14.6382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wedemeyer J, Tsai M, Galli SJ. Roles of mast cells and basophils in innate and acquired immunity. Curr Opin Immunol. 2000;12(6):624–631. doi: 10.1016/s0952-7915(00)00154-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.