Abstract
Although chronic inflammation is believed to contribute to the pathology of age-related macular degeneration (AMD), knowledge regarding the events that elicit the change from para-inflammation to chronic inflammation in the pathogenesis of AMD is lacking. We propose here that lipocalin-2 (LCN2), a mammalian innate immunity protein that is trafficked to the lysosomes, may contribute to this process. It accumulates significantly with age in retinal pigment epithelial (RPE) cells of Cryba1 conditional knockout (cKO) mice, but not in control mice. We have recently shown that these mice, which lack βA3/A1-crystallin specifically in RPE, have defective lysosomal clearance. The age-related increase in LCN2 in the cKO mice is accompanied by increases in chemokine (C-C motif) ligand 2 (CCL2), reactive gliosis, and immune cell infiltration. LCN2 may contribute to induction of a chronic inflammatory response in this mouse model with AMD-like pathology.
Keywords: age-related macular degeneration; Cryba1 cKO mice, inflammation; lipocalin-2; lysosomes; retinal pigment epithelium
AMD is one of the leading causes of blindness in developed countries. Several studies support the hypothesis that the immune system is involved in the pathogenesis of AMD, in concert with, or in addition to, other factors (Ambati et al., 2013; Whitcup et al., 2013). One factor that has captured our interest is βA3/A1-crystallin, which has been reported to be present in human drusen (Crabb et al., 2002), a precursor to AMD. We have recently shown in the Nuc1 rat (a spontaneous mutation in the Cryba1 gene) and in mice lacking βA3/A1-crystallin (cKO) specifically in the RPE (Sinha et al., 2008; Valapala et al., 2014), impaired lysosomal clearance and decreased autophagy and phagocytosis. This leads to RPE cell degeneration, subretinal lesions in the posterior pole, and deposits between the RPE and Bruch's membrane, all characteristic changes in AMD (Zigler et al., 2011; Kaarniranta et al., 2013). We now report further degenerative changes in the RPE of aging cKO mice. Transmission electron microscopy (TEM) of the RPE from 12-month-old mice revealed RPE degeneration, including loss of basal infoldings, prominent intracellular vacuoles, undigested melanosomes and photoreceptor outer segments, and widening of adherens junctions in cKO mice compared to age-matched Cryba1fl/fl controls (Fig.1A–D). We also observed a significant increase in cell death in the cKO RPE compared to controls (Fig.1E).
Figure 1.
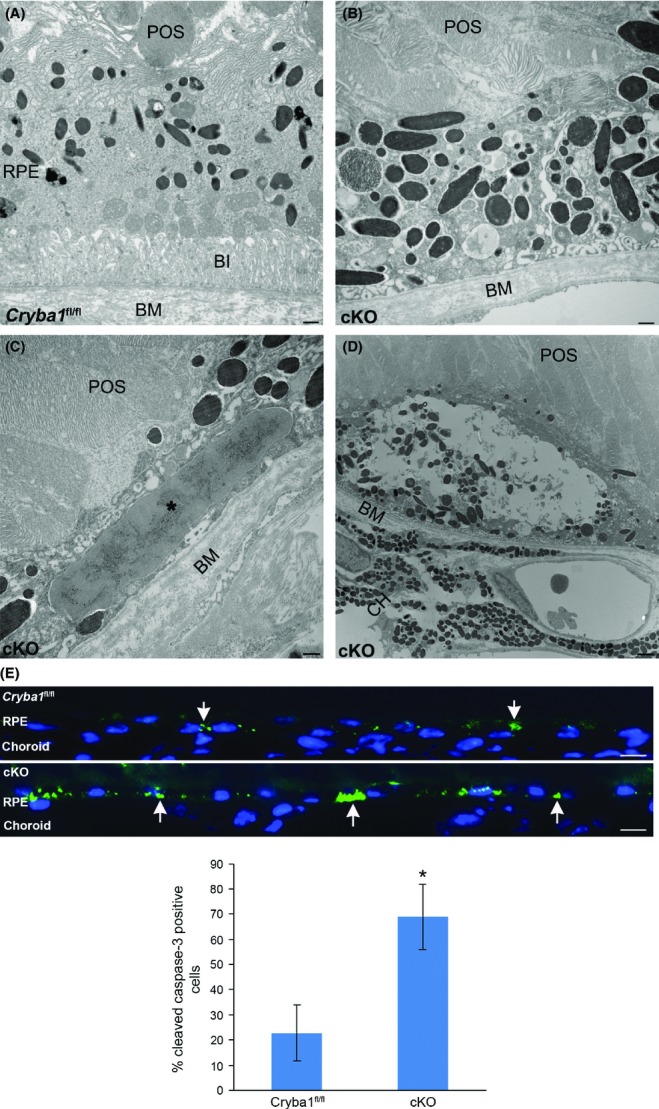
Degenerative changes in aging cKO retina: TEM (panels A–D) shows loss and truncation of basal infoldings (BI) and accumulation of undigested materials in 12-month-old cKO RPE (panel B), as compared to Cryba1 fl/fl (panel A). Additional degenerative changes in cKO RPE include large intracellular deposits (* in panel C) and large vacuoles (panel D). Bar = 500 nm. E shows immunostaining of 12-month-old Cryba1 fl/fl and cKO retinal sections with anticleaved caspase-3 antibody. A significant increase in cleaved caspase-3 positive cells (green, arrows) is seen in the cKO RPE compared to control. Nuclei stained with DAPI (blue). The histogram shows the percentage of total cells that are caspase-3-positive. Bar = 10 μm. BI: basal infoldings; BM: Bruch's membrane; POS: photoreceptor outer segments; CH: choroid (*P ≤ 0.05).
A recent study reported that aberrant lysosomal function can elicit innate immune responses (Hasan et al., 2013). In various tissues, the downstream consequences of lysosomal dysfunction usually include inflammation, resulting in pathogenesis (Lee et al., 2010). It is likely that disturbances in the homeostasis of RPE cells, due to accumulation of undegraded intracellular material in the cKO mice, trigger a para-inflammatory response in an attempt to restore normal RPE function. Para-inflammation is intermediate between the basal homeostatic state and a chronic inflammatory response.
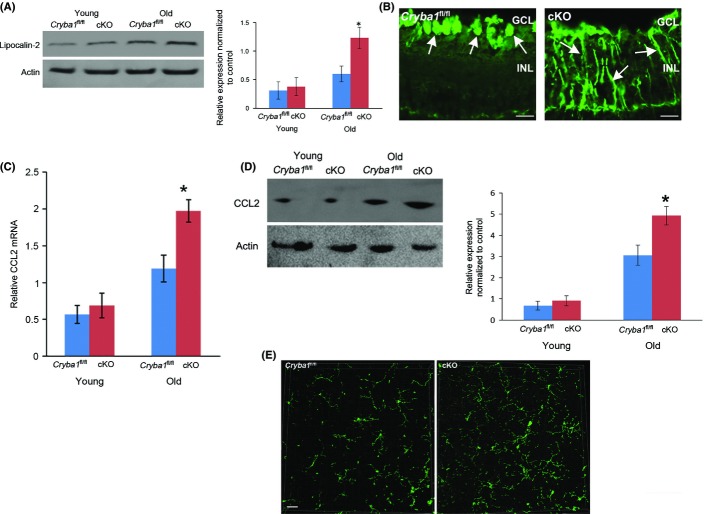
Dysregulated para-inflammation leading to a chronic inflammatory state has been proposed to play an important role in other diseases, including type 2 diabetes, cardiovascular disease as well as in age-related retinal diseases (Medzhitov, 2008; Xu et al., 2009). Adipokines are involved in the pathogenesis of obesity, diabetes, and hypertension. We wondered whether they might also be acting in concert with impaired autophagy and phagocytosis to convert the para-inflammatory response to a chronic inflammatory response in cKO mice. To test this hypothesis, we performed an adipokine array and found that LCN2 protein was elevated in RPE cells from cKO mice relative to Cryba1 fl/fl. Western analysis confirmed this finding in RPE cells from aging mice, but found no difference in younger mice (Fig.2A). LCN2 has been shown to be markedly elevated in serum and tissues during inflammation (Flo et al., 2004). As, in macrophages (Halaas et al., 2010), LCN2 is initially endocytosed into early endosomes and subsequently sorted to lysosomes, one would expect it to accumulate in cKO RPE with age, as lysosomal-mediated clearance is impaired in these mice. Interestingly, the mRNA expression of LCN2 is also upregulated in the RPE from aging cKO mice (Fig. S1). We have postulated that βA3/A1-crystallin regulates the mechanistic target of rapamycin, complex 1 (mTORC1) signaling in the lysosomes of RPE cells (Valapala et al., 2014). It is now accepted that mTORC1 is also a key regulator of gene transcription (Laplante & Sabatini, 2013). Thus, LCN2-mediated induction of inflammatory stimuli in the cKO RPE could result from increased LCN2 transcription as well as reduced LCN2 degradation.
Figure 2.
Evidence of LCN2-mediated inflammation in RPE of cKO mice: (A) Western analysis shows significant upregulation of lipocalin-2 protein in older cKO mice compared to age-matched controls. No significant change was found in younger animals. (B) In 12-month-old retina sections stained with GFAP antibody (green), staining is limited to the GCL in Cryba1 fl/fl retina, characteristic of astrocytes (left panel, arrows). In the cKO retina (right panel), additional staining of Muller glia processes (arrows) indicates Muller cell activation. Bar = 10 μm. (C) qRT–PCR shows significant upregulation of CCL2 mRNA in older cKO mice (12 months) compared to the younger animals (4 month). (D) Consistent with the PCR results, protein levels of CCL2 were upregulated in older cKO mice compared to age-matched controls. (E) Retinal flat mounts stained with Iba1 antibody appear denser with extended processes in the cKO mice compared to Cryba1fl/fl. Bar = 30 μm (*P ≤ 0.05).
To confirm that an inflammatory response occurs in the aging retinas of cKO mice, we stained 12-month-old Cryba1fl/fl and cKO retinal sections with an antibody against GFAP (glial-fibrillary acidic protein). GFAP staining of Müller cell processes indicates the cells are activated (Gehlbach et al., 2006), a condition associated with reactive gliosis and an inflammatory response. Our data revealed the presence of GFAP-expressing activated Müller cells in the retina of the cKO mice, but not in the Cryba1fl/fl controls (Fig.2B). In the control retina, GFAP staining was only observed in the ganglion cell layer (Fig.2B), where astrocytes, for which GFAP is used as a marker, reside.
One of the molecules known to be produced during inflammation is the chemokine CCL2, which is upregulated in AMD (Raoul et al., 2010; Suzuki et al., 2012). Our results show a significant induction of CCL2 mRNA in the 12-month-old cKO mice compared to controls (Fig.2C). In contrast, at 4 months of age, levels of CCL2 mRNA were similar in Cryba1fl/fl and cKO samples. These data are further supported by Western analysis of protein extracts from RPE/choroid preparations. The CCL2 protein levels were significantly higher (,65% increase) in the older cKO animals compared to controls, while in younger animals, they were not significantly different (Fig.2D). Interestingly, in LCN2 KO mice (Berger et al., 2006), GFAP levels in the RPE are similar to control, while CCL2 levels are lower than control (Figs S2 and S3), indicating that loss of LCN2 does not induce inflammation. The retinas of LCN2 KO mice appear to be anatomically normal (data not shown).
Tissue homeostasis is monitored by resident tissue macrophages that remain in a basal homeostatic state. Para-inflammation acts to maintain tissue homeostasis and to restore tissue function (Medzhitov, 2008). However, when tissue malfunction is excessive, additional macrophages are recruited, inducing chronic inflammation. Our data show that Iba1+ microglial cells, while present in both Cryba1fl/fl and cKO retinas (Fig.2E), appear denser in cKO samples with extended processes. Interestingly, the infiltration of microglia into the cKO retina does not appear to be as dense as might be expected from a chronic inflammatory response. However, this is likely misleading due to the highly dynamic nature of the cellular interactions in the abnormal tissue and the fact that LCN2 has diverse functions and is known to participate in the self-regulatory elimination of activated microglia in vivo (Lee et al., 2007).
A variety of animal models have been developed over the years that simulate different aspects of AMD (Pennesi et al., 2012). Our mouse model exhibits a slowly progressing form of AMD-like pathology associated with inefficient lysosomal clearance. This could switch the para-inflammatory response to a chronic inflammatory immune response as the mice age. In summary, this study provides intriguing findings relevant to AMD, suggesting a linkage of LCN2 with chronic inflammatory responses.
Acknowledgments
We thank Dr. Morton F. Goldberg for critical reading and discussion of the manuscript. DS is a recipient of the Carolyn K. McGillvray memorial award for Macular Degeneration Research from BrightFocus Foundation and the Sybil B. Harrington Special Scholar award for Macular Degeneration from Research to Prevent Blindness. Supported by National Institutes of Health: EY019037-S (DS), EY14005 (JTH), EY019904 (JTH), BrightFocus Foundation (DS) and Research to Prevent Blindness (an unrestricted grant to The Wilmer Eye Institute).
Funding
Supported by National Institutes of Health: EY019037-S (DS), EY14005 (JTH), EY019904 (JTH), BrightFocus Foundation (DS) and Research to Prevent Blindness (an unrestricted grant to The Wilmer Eye Institute).
Conflict of interest
None declared.
Supporting Information
Additional Supporting Information may be found in the online version of this article at the publisher's web-site.
Data S1 Experimental methods.
Fig. S1 Quantitative reverse transcriptase (qRT) PCR using Taqman expression probes shows that lipocalin-2 message is significantly increased in the old cKO mice compared to age matched floxed controls (*P ≤ 0.05).
Fig. S2 Left panel shows western blot for GFAP in RPE from wild type and LCN2-KO mice at 2 months and 9 months of age.
Fig. S3 Left panel shows western blot for CCL2 in RPE from 2 month and 9 month old wild type and LCN2-KO mice.
References
- Ambati J, Atkinson JP, Gelfand BD. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013;13:438–451. doi: 10.1038/nri3459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berger T, Togawa A, Duncan GS, Elia AJ, You-Ten A, Wakeham A, Fong HE, Cheung CC, Mak TW. Lipocalin-2-deficient mice exhibit increased sensitivity to Escherichia coli infection but not to ischemia-reperfusion injury. Proc. Natl Acad. Sci. USA. 2006;103:1834–1839. doi: 10.1073/pnas.0510847103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crabb JW, Miyagi M, Gu X, Shadrach K, West KA, Sakaguchi H, Kamei M, Hasan A, Yan L, Rayborn ME, Salomon RG, Hollyfield JG. Drusen proteome analysis: an approach to the etiology of age-related macular degeneration. Proc. Natl Acad. Sci. USA. 2002;99:14682–14687. doi: 10.1073/pnas.222551899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flo TH, Smith KD, Sato S, Rodriguez DJ, Holmes MA, Strong RK, Akira S, Aderem A. Lipocalin 2 mediates an innate immune response to bacterial infection by sequestrating iron. Nature. 2004;432:917–921. doi: 10.1038/nature03104. [DOI] [PubMed] [Google Scholar]
- Gehlbach P, Hose S, Lei B, Zhang C, Cano M, Arora M, Neal R, Barnstable C, Goldberg MF, Zigler JS, Jr, Sinha D. Developmental abnormalities in the Nuc1 rat retina: a spontaneous mutation that affects neuronal and vascular remodeling and retinal function. Neuroscience. 2006;137:447–461. doi: 10.1016/j.neuroscience.2005.08.084. [DOI] [PubMed] [Google Scholar]
- Halaas O, Steigedal M, Haug M, Awuh JA, Ryan L, Brech A, Sato S, Husebye H, Cangelosi GA, Akira S, Strong RK, Espevik T, Flo TH. Intracellular Mycobacterium aiuvm intersect transferring in the Rab11+ recycling endocytic pathway and avoid Lipocalin 2 trafficking the lysosomal pathway. J. Infect. Dis. 2010;201:783–792. doi: 10.1086/650493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasan M, Koch J, Rakheja D, Pattnaik AK, Brugarolas J, Dozmorov I, Levine B, Wakeland EK, Lee-Kirsch MA, Yan N. Trex1 regulates lysosomal biogenesis and interferon-independent activation of antiviral genes. Nat. Immunol. 2013;14:61–71. doi: 10.1038/ni.2475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaarniranta K, Sinha D, Blasiak J, Kauppinen A, Vereb Z, Salminen A, Bourlton ME, Petrovski G. Autophagy and heterophagy dysregulation leads to retinal pigment epithelium dysfunction and development of age-related macular degeneration. Autophagy. 2013;9:973–984. doi: 10.4161/auto.24546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laplante M, Sabatini DM. Regulation of mTORC1 and its impact on gene expression at a glance. J. Cell Sci. 2013;126:1713–1719. doi: 10.1242/jcs.125773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S, Lee J, Kim S, Park J-Y, Lee W-H, Mori K, Kim S-H, Kim IK, Suk K. A dual role of Lipocalin 2 in the apoptosis and deramification of activated microglia. J. Immunol. 2007;179:3231–3241. doi: 10.4049/jimmunol.179.5.3231. [DOI] [PubMed] [Google Scholar]
- Lee JH, Yu WH, Kumar A, Lee S, Mohan PS, Peterhoff CM, Wolfe DM, Martinez-Vicente M, Massey AC, Sovak G, Uchiyama Y, Westaway D, Cuervo AM. Lysosomal proteolysis and autophagy require presenilin 1 and are disrupted by Alzheimer-related PS1 mutations. Cell. 2010;141:1146–1158. doi: 10.1016/j.cell.2010.05.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medzhitov R. Origin and physiological roles of inflammation. Nature. 2008;454:428–435. doi: 10.1038/nature07201. [DOI] [PubMed] [Google Scholar]
- Pennesi ME, Neuringer M, Courtney RJ. Animal models of age related macular degeneration. Mol. Aspects Med. 2012;33:487–509. doi: 10.1016/j.mam.2012.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raoul W, Auvynet C, Camelo S, Guillonneau X, Feumi C, Combadiere C, Sennlaub F. CCL2/CCR2 and CX3CL1/CX3CR1 chemokine axes and their possible involvement in age-related macular degeneration. J. Neuroinflammation. 2010;7:87. doi: 10.1186/1742-2094-7-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinha D, Klise A, Sergeev Y, Hose S, Bhutto IA, Hackler L, Jr, Malpic-Llanos T, Samtani S, Grebe R, Goldberg MF, Hejtmancik JF, Nath A, Zack DJ, Fariss RB, McLeod DS, Sundin O, Broman KW, Lutty GA, Zigler JSJ. βA3/A1-crystallin in astroglial cells regulates retinal vascular remodeling during development. Mol. Cell. Neurosci. 2008;37:85–95. doi: 10.1016/j.mcn.2007.08.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki M, Tsujikawa M, Itabe H, Du Z-J, Xie P, Matsumura N, Fu X, Zhang R, Sonoda KH, Egasgura K, Hazen SL, Kamei M. Chronic photo-oxidative stress and subsequent MCP-1 activation as causative factors for age-related macular degeneration. J. Cell Sci. 2012;125:2407–2415. doi: 10.1242/jcs.097683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valapala M, Wilson C, Hose S, Bhutto IA, Grebe R, Dong A, Greenbaum S, Gu L, Sengupta S, Cano M, Hackett S, Xu G, Lutty GA, Dong L, Sergeev Y, Handa JT, Campochiaro P, Wawrousek E, Zigler JS, Jr, Sinha D. Lysosomal-mediated waste clearance in retinal pigmented epithelial cells is regulated by CRYBA1/βA3/A1-crystallin via V-ATPase-MTORC1 signaling. Autophagy. 2014;10:480–496. doi: 10.4161/auto.27292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whitcup SM, Sodhi A, Atkinson JP, Holers M, Sinha D, Rohrer B, Dick AD. The role of the immune response in age-related macular degeneration. Int. J. Inflam. 2013;2013:348092. doi: 10.1155/2013/348092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu H, Chen M, Forrester JV. Para-inflammation in the aging retina. Prog. Retin. Eye Res. 2009;28:348–368. doi: 10.1016/j.preteyeres.2009.06.001. [DOI] [PubMed] [Google Scholar]
- Zigler JS, Jr, Zhang C, Grebe R, Sehrawat G, Hackler L, Jr, Adhya S, Hose S, McLeod DS, Bhutto I, Barbour W, Parthasarathy G, Zack DJ, Sergeev Y, Lutty GA, Handa JT, Sinha D. Mutation in the betaA3/A1-crystallin gene impairs phagosome degradation in the retinal pigmented epithelium of the rat. J. Cell Sci. 2011;124:523–531. doi: 10.1242/jcs.078790. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data S1 Experimental methods.
Fig. S1 Quantitative reverse transcriptase (qRT) PCR using Taqman expression probes shows that lipocalin-2 message is significantly increased in the old cKO mice compared to age matched floxed controls (*P ≤ 0.05).
Fig. S2 Left panel shows western blot for GFAP in RPE from wild type and LCN2-KO mice at 2 months and 9 months of age.
Fig. S3 Left panel shows western blot for CCL2 in RPE from 2 month and 9 month old wild type and LCN2-KO mice.