Abstract
Determining the causes and evolution of reproductive barriers to gene flow between populations, speciation, is the key to understanding the origin of diversity in nature. Many species manifest hybrid breakdown when they intercross, characterized by increasingly exacerbated problems in later generations of hybrids. Recently, Caenorhabditis nematodes have emerged as a genetic model for studying speciation, and here we investigate the nature and causes of hybrid breakdown between C. remanei and C. latens. We quantify partial F1 hybrid inviability and extensive F2 hybrid inviability; the ~75% F2 embryonic arrest occurs primarily during gastrulation or embryonic elongation. Moreover, F1 hybrid males exhibit Haldane’s rule asymmetrically for both sterility and inviability, being strongest when C. remanei serves as maternal parent. We show that the mechanism by which sterile hybrid males are incapable of transferring sperm or a copulatory plug involves defective gonad morphogenesis, which we hypothesize results from linker cell defects in migration and/or cell death during development. This first documented case of partial hybrid male sterility in Caenorhabditis follows expectations of Darwin’s corollary to Haldane’s rule for asymmetric male fitness, providing a powerful foundation for molecular dissection of intrinsic reproductive barriers and divergence of genetic pathways controlling organ morphogenesis.
INTRODUCTION
Separated populations evolve reproductive barriers as a consequence of selection and genetic drift that drives genetic differentiation and divergence between them, to then further restrict gene flow. A completed process of speciation requires genetically encoded extrinsic (environment- or context-dependent) and/or intrinsic (context independent) barriers to genetic exchange. Extrinsic and intrinsic pre-zygotic barriers to reproduction play crucial roles in speciation (Coyne and Orr 2004), but here we focus on understanding intrinsic hybrid inviability and sterility that act after fertilization as post-zygotic barriers to gene flow. Negative epistatic interactions in hybrids, Dobzhansky-Muller incompatibilities (commonly referred to as DMIs), provide a well-supported mechanism underlying intrinsic post-zygotic reproductive isolation (Dobzhansky 1936; Muller 1942; Coyne and Orr 2004). Dominant allele interactions in hybrids manifest DMIs in the F1 generation, but fitness will break down only in F2 and later generations when DMIs involve recessive allele interactions. An important contribution of recessive DMIs motivates the dominance theory as a rationale for the involvement of sex chromosomes as an explanation for the pervasiveness of Haldane’s rule (disproportionate hybrid dysfunction in the heterogametic sex) because individuals of the heterogametic sex will reveal sex-linked recessive incompatibility phenotypes even in the F1 generation (Haldane 1922; Turelli and Orr 1995). These models have overwhelming empirical support by genetic analysis from a broad diversity of organisms (Coyne and Orr 2004; Presgraves 2010). The faster male theory provides a complementary model for disproportionate sterility in hybrid males: sterility factors may evolve faster in males than in females owing to either higher inherent sensitivity of spermatogenesis to genetic and developmental perturbations or to greater sexual selection on male specific traits (Wu and Davis 1993; Wu et al. 1996; Schilthuizen et al. 2011).
Reciprocal hybrid crosses often differ in their degree of hybrid sterility or inviability. Such asymmetries in post-zygotic isolation have long been documented, from plants to fungi, insects, and vertebrates (Tiffin et al. 2001; Bolnick et al. 2008). However, such asymmetry has only recently been modeled theoretically and termed Darwin’s corollary to Haldane’s rule (Turelli and Moyle 2007). Uniparentally inherited genetic factors involved in DMIs can induce asymmetries, including cyto-nuclear incompatibilities involving mitochondria or chloroplasts. Asymmetries could also arise from differences in the number and magnitude of X-linked incompatibility loci between species, from maternal-zygotic incompatibilities, or from asymmetric chromosome marking. Empirical tests of the causes of asymmetry are few, although differential rates of cytoplasmic and autosomal evolution can predict the directionality of the asymmetry in fish (Bolnick et al. 2008) and epigenetic maternal-zygotic effects appear to operate in some systems (Brown and O'Neill 2010). Additional heterogeneity in hybrid function can derive from within-species genetic variation, as has been documented in diverse organisms (Cutter 2012).
The genetic underpinnings to hybrid incompatibility have been studied most extensively in genetic model organisms, most notably Drosophila (Presgraves 2010; Maheshwari and Barbash 2011). However, Caenorhabditis nematodes largely have been a dormant player in speciation research, despite the breadth of their application to other topics in developmental biology and evolution (Cutter et al. 2009; Baird and Seibert 2013). Historically, high interspecies divergence for a paucity of species known to science, coupled with no species pairs capable of yielding fertile hybrid progeny, has hampered genetic analysis of species barriers in this group. Nevertheless, embryonic arrest phenotypes and rare viable (but sterile) larvae and adults in crosses between C. elegans, C. briggsae, C. remanei and C. brenneri demonstrated that Haldane’s rule is upheld and that sexual transformation during development contributes to it (Baird et al. 1992; Baird and Yen 2000; Baird 2002). Research on reproductive isolation in this genus gained new momentum with the discovery of fertile F1 female hybrids resulting from crosses between the self-fertile C. briggsae and outbreeding C. nigoni (Woodruff et al. 2010; Baird and Stonesifer 2012; Kozlowska et al. 2012; Yan et al. 2012; Baird and Seibert 2013), where C. nigoni was formerly known as C. sp. 9 (Felix et al. 2014). This species pair also conforms to Haldane’s rule, with F1 male hybrids being rare and sterile, and also suffering reduced viability compared to female hybrids such that males occur with appreciable frequency only among the offspring derived from C. nigoni mothers (i.e. an asymmetry following Darwin’s corollary) (Woodruff et al. 2010; Kozlowska et al. 2012). Further, Kozlowska et al. (2012) reported significant heritable variation within both parental species for their reproductive isolation to each other, implicating polymorphic incompatibility loci (Cutter 2012). Another species pair with incomplete reproductive isolation was discovered more recently (C. remanei and C. latens), but the details of their partial isolation are largely unknown (Dey et al. 2012). On the continuum from i) full compatibility of individuals in a population, ii) partial incompatibilities between populations or incipient species, iii) substantial incompatibilities of recently-diverged species, to iv) total reproductive isolation between very distant species, molecular evolutionary evidence and preliminary cross analysis indicate that C. remanei and C. latens lie in regime (iii) (Dey et al. 2012).
To test for the presence and nature of Haldane’s rule, here we characterize hybrid breakdown between C. remanei and C. latens (Dey et al. 2012), where C. latens was formerly known as C. sp. 23 (Felix et al. 2014). Both species are gonochoristic (=dioecious, with male and female sexes), unlike the divergent mating systems of C. briggsae and C. nigoni. Thus, C. remanei and C. latens circumvent the complication of a transition in reproductive mode on top of speciation. We show that both Haldane’s rule and Darwin’s corollary apply. Moreover, we find asymmetric F1 hybrid male sterility such that nearly all hybrid males with C. remanei mothers are sterile whereas nearly all hybrid males with C. latens mothers are fertile. We then demonstrate how defects in gonad formation underlie this hybrid male sterility.
MATERIALS AND METHODS
Strain Stocks and Maintenance
Population genetic divergence as well as experimental crosses confirmed C. latens as a distinct species closely related to C. remanei (Dey et al. 2012). We performed hybrid and back crosses using 12 strains of C. remanei (PB213, PB214, PB219, MY219, MY202, MY223, NIC148, NIC222, NIC225, VX0003, VX0016, QG549), three strains of C. latens (VX0081, VX0084, and VX0088) and strain JU724 (putatively C. latens). The C. remanei PB strains are isofemale lines derived from a population in woods on the Wright State University campus, Ohio, USA (courtesy S. Baird); NIC strains from Berne, Switzerland (courtesy C. Braendle); MY strains from Bohnhusen, Germany (courtesy H. Schulenburg); VX strains from Ontario, Canada; QG strain from Okinawa, Japan (courtesy M. Rockman). The JU724 strain is from Jiangsu, China (courtesy M.-A. Felix) and the collection of C. latens strains are isofemale lines derived from a population in Wuhan, Hubei province in China (Dey et al. 2012).
Worms were cultured, maintained and crossed at 25°C on Petri dishes containing NGM-Lite agar media seeded with Escherichia coli strain OP50. Strains were cleaned from bacterial and fungal contamination by standard bleaching protocol prior to use in crosses (Stiernagle 1999. We performed crosses on 35 mm Petri dishes with a single ~1cm diameter bacterial spot in the center.
Hybrid viability
To quantify viability of F1 and F2 hybrids, we performed interspecies crosses and compared them with intraspecies control crosses. In each cross, a single fourth stage larval (L4, virgin) female was placed with six males for 24 hours to mature and mate, after which the males were removed. We transferred maternal parents to a new plate every 24 hours until egg laying ceased. For each replicate, F1 progeny were allowed to grow to adulthood, after which they were placed at 4°C to halt development for counting to quantify the total lifetime F1 progeny from each cross and the number of each sex to determine the sex ratio. As the first batch of F1 grew to adulthood, we isolated individual L4 females with six L4 males to initiate F1×F1 sibling crosses. We then quantified F2 offspring as for the F1s. These crosses focused on a single representative strain of C. remanei (PB219) and C. latens (VX0088).
Further, we tested for variation among genetic backgrounds in the extent of F1 reproductive isolation by crossing males of C. latens (VX0088) with 12 distinct iso-female lines of C. remanei. The C. remanei genetic backgrounds originated from five geographic localities: Ohio, USA (PB213, PB214, and PB219); Ontario, Canada (VX0003, and VX0016); Japan (QG549); Germany (MY202, MY219, and MY223); and Switzerland (NIC148, NIC222, and NIC225). Statistical analyses of hybrid progeny production were performed on log10(x+1) transformed values of 11 to 38 replicates (depending on the cross), although figures show original or back-transformed values for ease of interpretation.
Finally, we quantified embryonic inviability of F1 and F2 hybrids (and parental strains) in a hatching assay. For each of the 4 cross combinations, we allowed 3 mated females to lay eggs for 4 hours on an NGM-lite agar dish without bacteria (6 replicate dishes per treatment). After removing the maternal animals, we immediately counted the eggs and then re-counted unhatched eggs after 1 day to calculate hatching success. Strains PB219 and VX0088 were used in all crosses, and sib matings were subsequently conducted between F1 animals. The total number of eggs per dish ranged from 53 to 108 for F1 eggs, and from 8 to 121 for F2 eggs (F2 eggs derived from the PB219 grand-maternal hybrid cross were uncommon, owing to the strong hybrid breakdown, with a mean of just 31.8 per dish; mean egg counts for all other treatments were >60 and <100 per dish). Statistical analysis used arcsin square-root transformed values of the proportion hatching, although figures show untransformed values for ease of interpretation.
Arrested developmental profiles of dead embryos
To identify terminal phenotypes of dead F2 embryos, we observed unhatched eggs (after 2 days post-laying at 25°C) with differential interference contrast (DIC) microscopy (400X magnification) after mounting them in M9 buffer on 5% agarose pads on microscope slides. Arrested profiles of 237 F2 embryos were recorded for both reciprocal crosses and categorized as gastrulation and post-gastrulation arrested embryos. We observed no embryonic arrest preceding gastrulation. Eggs identified as post gastrulation arrested embryos were further classified into a) arrested before elongation and b) elongation arrest which includes embryos in bean stage, comma stage, 2-fold plum stage, and 3-fold pretzel stage (Baird and Yen 2000).
Hybrid fertility
We performed backcrosses to each parent species and F1 sib-matings to determine the fertility of the F1 hybrids between C. remanei and C. latens. Crosses were set up as described for hybrid viability, using a single genetic background for each species (PB219, VX0088). We scored females as fertile if any progeny were observed on the plate. In a similar fashion, we initiated crosses with individual virgin F1 hybrid males and tested their fertility when presented with six virgin females of each parental species (or sibs) over the course of 4 days. We repeated this for each direction of parental cross, and for intraspecies control crosses, with 24 replicates on average. Male or female fertility was summarised as the fraction of worms that yielded at least some progeny. Plates wherein the hybrid worm being assayed fled or died prematurely were censored.
To test for the ability to transfer gonad contents during copulation, we quantified the ability of males to successfully deposit a copulatory plug. In Caenorhabditis, males transfer from their vas deferens the mucin-like protein PLG-1 in their seminal fluid during ejaculation, which forms a visible gelatinous mass over the vulva of the mated female (Barker 1994; Hodgkin and Doniach 1997; Palopoli et al. 2008). We placed a single F1 hybrid male (or C. remanei PB219 male as a positive control) with two female C. remanei (PB219) on small 3.5cm diameter mating dishes, and then scored the females for presence of a copulatory plug after 4 hr.
Finally, we examined adult male gonad morphology to identify any developmental defects in gonad formation. Specifically, we quantified the incidence of partial gonads with DIC microscopy among 97 F1 hybrid males with C. remanei (PB219) maternal parents, as well as 11 C. remanei control males. We categorized defects according to whether the migration of the gonad arm exhibited a “meandering” morphology, a “premature turn” to the posterior end of the animal (i.e. gonad turn occurs >1/3 body length away from the mouth of the worm), a “failure to turn” posteriorly, presence of “vacuolization”, “absence of spermatids” from the gonad, and presence of a bulbous “gonad mass” that obviously failed to connect to the cloaca.
RESULTS
Asymmetric hybrid breakdown between C. remanei and C. latens
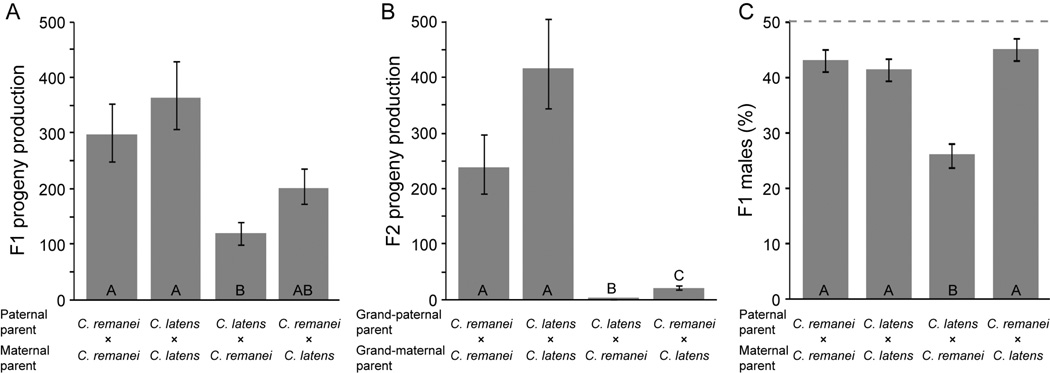
We observed a significant reduction in the production of F1 hybrid progeny compared to the control intraspecies crosses, and hybrid breakdown was particularly pronounced in the F2 generation (Figure 1). Maternal species exerted a significant effect on the number of F1 progeny (log10 transformed), in addition to the stronger effect of the type of cross (hybrid versus intraspecific), indicating asymmetry in the magnitude of reproductive isolation between cross directions (cross type F1,39 = 20.4, P < 0.0001; maternal species F1,39 = 4.81, P = 0.034; cross type × maternal species interaction P > 0.05). The C. remanei ♀×C. latens ♂ hybrid cross yielded the fewest viable F1 progeny and is significantly different from both control crosses (Tukey-Kramer HSD test; Figure 1A). Moreover, sib-mated F1 hybrids from a C. remanei (PB219) mother produced the fewest F2 progeny (log10 back-transformed mean 1.5 progeny vs. 30 progeny; maternal species F1,38 = 60.0, P<0.0001; cross type × maternal species interaction F1,38 = 25.4, P < 0.0001; Figure 1B).
Figure 1.
Interspecies hybrid crosses between C. remanei (PB219) and C. latens (VX0088) yield significantly fewer F1 offspring (A), F2 offspring (B) and males (C) than do intraspecies crosses. Within each panel, bars sharing the same letter are not significantly different (Tukey – Kramer HSD post-hoc tests) following two-way ANOVA (A, model F3,39=8.45, P=0.0002; B, model F3,38=152.67, P<0.0001; C, model F4,38=155.13, P<0.0001). Reproductive output in (B) shows the number of F2 offspring produced by matings of F1 siblings from the indicated crosses. Conspecific crosses in (A) and (B) are independent; although conspecific crosses used a single isofemale strain, residual heterozygosity might contribute to the nominally different means in these separate control experiments. Statistical analysis in (A) and (B) was performed on log10-transformed values; back-transformed values are shown for clarity. Error bars indicate standard error of the mean. Dashed line in (C) indicates the expected 1:1 sex ratio.
Genetic variation for reproductive isolation
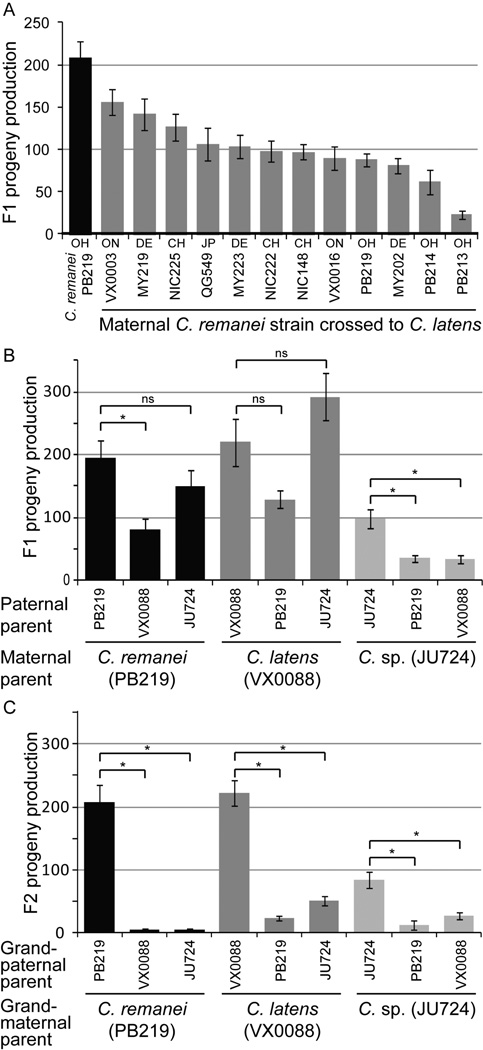
C. remanei is distributed across North America, Europe and parts of Asia, with modest but significant population subdivision among distinct geographic locations (Dey et al. 2012). To test for heritable variation in reproductive isolation (Cutter 2012), we performed crosses of 12 distinct maternal genetic backgrounds of C. remanei (isofemale lines) to males of C. latens (strain VX0088). We uncovered a 6-fold range of variation in the production of F1 hybrids, implicating substantial polymorphism for reproductive isolation (one-way ANOVA F11,190=10.38, P<0.0001, Figure 2A). Interestingly, however, we observed no strong coupling of the magnitude of reproductive isolation with the geographic origins of the strains (Figure 2A). This suggests segregation within populations, rather than differentiation among them, for much of the genetic differences that affect the propensity of C. remanei females to produce hybrids, although it remains unclear what role human-mediated dispersal could have in shaping population structure of these species. From these data alone, we are unable to discern the cause of the variable reproductive isolation, as it could potentially follow either from post-zygotic incompatibility or pre-zygotic isolation, for example, from differential female sensitivity to harmful heterospecific sperm (Ting et al. 2014).
Figure 2.
Genetic backgrounds differ in the magnitude of reproductive isolation. (A) Twelve maternal backgrounds of C. remanei differ significantly in reproductive output when crossed inter-specifically to males from a single genetic background of C. latens (VX0088) (ANOVA F11,190=10.4, P<0.0001). Geographical origin of strains is indicated with two-letter abbreviations (CH Switzerland, DE Germany, JP Japan, OH Ohio USA, ON Ontario Canada). (B) F1 reproductive output and (C) F2 reproductive output indicates hybrid breakdown between all combinations of C. remanei, C. latens, and strain JU724. DNA sequences show JU724 to be most similar to strains of C. latens (Dey et al. 2012). Significant Tukey – Kramer HSD post-hoc tests for contrasts within each maternal background in (B) and (C) are indicated with asterisks above the corresponding comparison. Statistical analyses were performed on log10-transformed values, but untransformed values are shown for clarity. Error bars indicate standard error of the mean.
Strain JU724, the sole isofemale line collected from Jiangsu, China, was previously inferred to correspond to C. latens based on its geographic origin and patterns of nucleotide sequence distance (Dey et al. 2012). However, JU724 shows F2 hybrid breakdown in crosses with canonical strains of both C. remanei and C. latens (one-way ANOVA F2,37=19.9, P<0.0001; Figure 2C). Moreover, F1 progeny production from JU724 females was significantly lower in crosses to males from canonical strains of C. remanei and C. latens than in crosses to JU724 strains (Figure 2B). In the case of strain JU724 crossed to C. latens, we actually observe a trend toward excess of hybrid F1 production when C. latens is the maternal parent and a significant deficit of F1s in the reciprocal cross (Figure 2B). These findings potentially implicate JU724 as a representative of yet another cryptic biological species within this group of Caenorhabditis, providing an additional avenue to explore the evolution of reproductive isolation in these nematodes. However, the alternative possibility of JU724 being derived from hybridization of C. remanei and C. latens, or from introgression of portions of the C. remanei genome into C. latens, should first be ruled out by genomic analysis.
Haldane’s rule: Male inviability in F1 hybrids
We next investigated whether hybrid inviability could involve male biased mortality, as would be predicted under Haldane’s rule (Haldane 1922; Schilthuizen et al. 2011). Indeed, we observed significantly fewer F1 male progeny for the C. remanei ♀×C. latens ♂ cross compared to the reciprocal and control crosses (log10 transformed progeny counts in 2-way ANOVA F4,38 = 155.13, P < 0.0001; cross type effect F1,38 = 8.39, P = 0.006; maternal species effect F1,38 = 15.34, P = 0.0004; Tukey – Kramer HSD post-hoc tests; Figure 1C). Specifically, only 26% of the surviving F1 hybrid progeny in the C. remanei ♀×C. latens ♂ crosses are male, significantly below the expected level of 50% (χ2 = 199.004, P < 0.0001). By contrast, the mean percentage of males in the other three types of crosses averaged ~43%. We have observed such a slight female bias in sex ratio within C. remanei and C. latens, as well as C. brenneri, in previous experiments (unpublished observations). This subtle female biased sex ratio could potentially reflect an assay bias, owing to more vigorous male dispersal tendencies (Lipton et al. 2004) that leads to male-biased dessication mortality as they crawl up the sides of assay plates. Alternatively, the slight fertilization advantage of X-bearing sperm in C. briggsae that yields hermaphrodite-biased broods could prove general to these other species of Caenorhabditis (LaMunyon and Ward 1997). In sum, Haldane’s rule from hybrid male inviability occurs and it shows a cross-direction asymmetry consistent with Darwin’s corollary.
Embryonic inviability
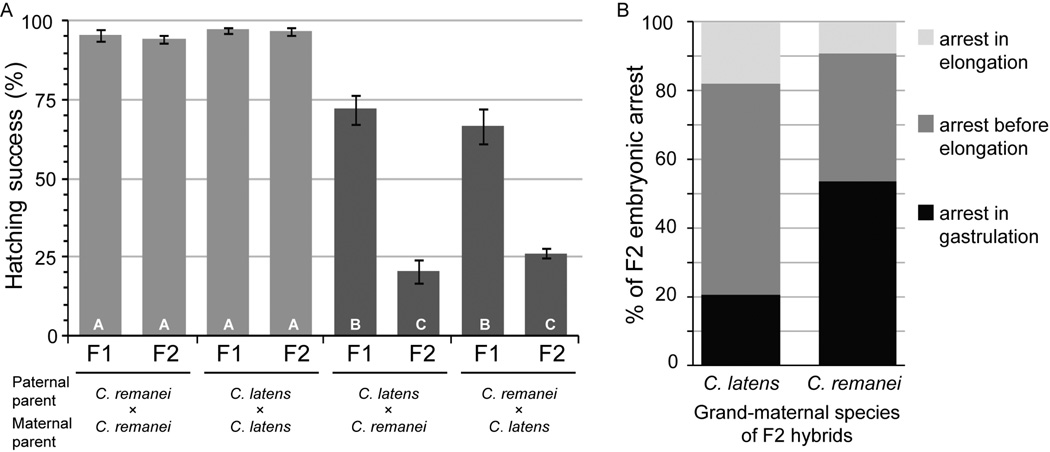
We quantified the incidence of unhatched, dead eggs to determine the extent of embryonic mortality in hybrid crosses. Intra-species crosses did not differ in hatching success between C. remanei and C. latens, with >94% hatching success of both F1 and F2 eggs (Figure 3A). By contrast, F1 hatching success was significantly lower for hybrid eggs (71.5% C. remanei maternal, 66.6% for C. latens maternal), and F2 egg hatching success was even lower for hybrid embryos (20.5% for C. remanei grandmaternal, 26.3% for C. latens grandmaternal) (Figure 3A). We observed no significant parent-of-origin effect on overall embryonic inviability for F1 or F2 eggs.
Figure 3.
Embryonic mortality in F1 and F2 hybrids. (A) F1 hybrid embryos show significantly lower hatching success than pure-species crosses, and F2 embryonic mortality is even more extreme (arcsin square-root transformed proportion hatching model F7,40=84.8, P<0.0001; F1 vs. F2 embryo generation F1,40=73.81, P<0.0001; cross type nested within generation F6,40=86.67, P<0.0001). Within (A), bars sharing the same letter are not significantly different (Tukey – Kramer HSD post-hoc tests); bars show non-transformed values for clarity. Error bars indicate standard error of the mean. In F2 embryos (B), 20.8% of developmental arrest occurs during gastrulation when the grandmaternal species is C. latens (n=183) whereas 53.7% of embryos arrest in gastrulation when C. remanei is the grandmaternal species (n=54; Fisher’s exact test P<0.0001). C. remanei strain is PB219; C. latens strain is VX0088.
To further dissect embryonic mortality, we identified terminal developmental stage phenotypes of arrested F2 hybrid embryos left unhatched, as in previous studies of crosses between more distantly-related Caenorhabditis species (Baird et al. 1992; Baird and Yen 2000). When we analyzed the embryos derived from reciprocal parental crosses, we observed striking asymmetry in the stages at which F2 hybrid embryos arrested development. We found gastrulation stage arrest in ~21% of F2 embryos that came from C. latens maternal crosses with the remaining embryonic lethality occurring later in development, whereas ~54% of embryonic arrest occurred in gastrulation when C. remanei was the maternal parent (Fisher’s exact test P<0.0001; Figure 3B). These included embryos arrested at the onset as well as further into gastrulation, although the exact cell stages were not classified in more detail. The developmental arrest profiles of F2 embryos derived from a C. latens maternal genetic background are qualitatively similar to those obtained by Baird and Yen (2000) for F1 hybrid embryos of C. briggsae/C. brenneri, C. remanei/C. brenneri, C. briggsae/C. remanei in that most embryonic arrest occured post-gastrulation. However, our observations implicate more severe, earlier-acting incompatibilities in C. remanei/C. latens hybrid embryos that receive a maternal contribution from C. remanei.
Haldane’s rule: Sterility of F1 hybrids
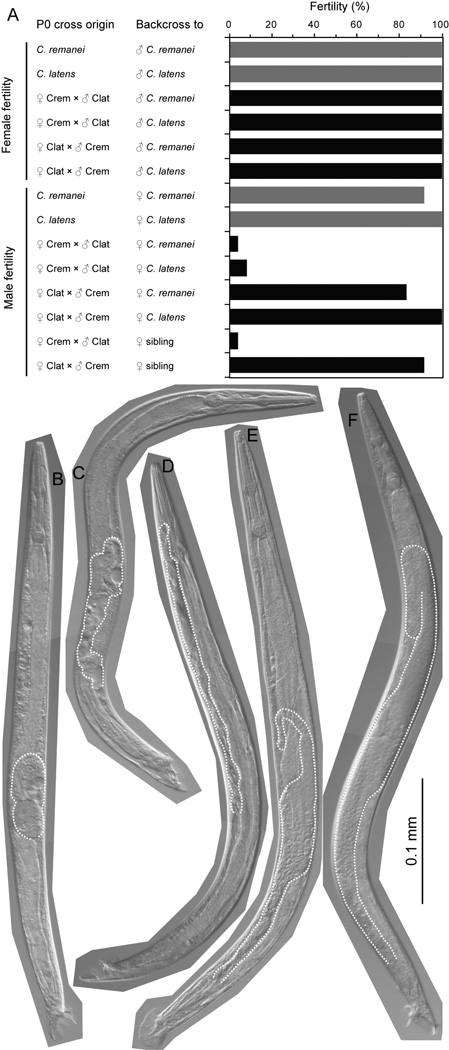
Even when several males mated to a single female, we observed that many F1×F1 crosses yielded no offspring in our assays of hybrid breakdown: could this be due to hybrid male sterility? To address this question, we tested the fertility of individual F1 males or females in backcross and sib-matings, quantified as the fraction of matings that yielded at least some viable progeny. We found that only 4–8% of F1 hybrid males derived from C. remanei mothers were fertile, regardless of whether the F1 hybrid males were mated to C. remanei females (1 out of 24 males fertile), to C. latens females (2 out of 24), or to F1 sibling females (1 of 24). In contrast, 100% of their F1 hybrid female sisters were fertile when mated to males of C. remanei or C. latens (C. remanei n=23; C. latens n=21; Fisher’s exact test, P < 0.0001; Figure 4A). This shows strong Haldane’s rule for hybrid male sterility in F1 males derived from the C. remanei ♀ × C. latens ♂ cross.
Figure 4.
Most F1 hybrid males are sterile when they derive from C. remanei mothers. (A) Females from both intra-species crosses (dark bars) and inter-species crosses (light bars) are fully fertile. Male offspring from intra-species crosses (dark bars) are mostly fertile, as are hybrid males (light bars) derived from C. latens mothers, regardless of the genetic background of tester females used in mating assays of hybrid male fertility. However, hybrid males (light bars) derived from C. remanei mothers are rarely fertile (Fisher’s exact test, P = 6.4×10−24). C. remanei strain is PB219; C. latens strain is VX0088; fertility assayed as the proportion of successful matings of single males with 6 females or single females with 6 males. (B-E) Examples of defective gonads of hybrid males derived from C. remanei mothers, compared to normal C. remanei male gonads (F). Defects include masses of gonad tissue (B), extensive vacuolization and abnormal tail morphology (C) and migration defects, such as lack of a turn toward the posterior (D) and posteriorly-biased extent of the gonad (E). Worm images are overlays of representative digital micrographs taken at 400X with differential interference contrast; gonad tissue is outlined with stipples.
To test whether Haldane’s rule for sterility also is obeyed in F1 hybrid males derived from the reciprocal cross (C. latens ♀ × C. remanei ♂), we mated these F1 hybrid males in the same way. Their fertility was >80% (C. remanei female mates: 20 of 24 males fertile; C. latens females: 24 of 24; F1 sibling females: 22 of 24), which is not significantly different from the 100% fertility observed for their sisters (Fisher’s exact test, P = 0.117) (Figure 4A), indicating that most hybrids of both sexes from this cross direction appear fertile and do not strongly obey Haldane’s rule. Consequently, the parent-of-origin asymmetry indicates that Darwin’s corollary to Haldane’s rule applies to F1 hybrid male sterility (Turelli and Moyle 2007).
We observed F1 males with C. remanei mothers to engage in normal courtship behavior, including spicule insertion during copulation (Garcia et al. 2007). Consequently, we hypothesized that hybrid male sterility might involve failure to transfer sperm and seminal fluid properly. Copulatory plugs are composed primarily of the mucin protein PLG-1 in C. elegans, which is synthesized in secretory cells of the somatic gonad of males and deposited on the vulva of female mating partners after sperm transfer (Palopoli et al. 2008). In an assay of insemination success, we found that copulatory plugs were deposited on the vulva of virgin females by only 4 of the 15 hybrid males with C. remanei mothers (27%). By contrast, 100% of 15 C. remanei males deposited plugs in the same 4 hr mating assay. Moreover, only 2 of the hybrid males (13%) induced egg laying in females compared to all 15 of the C. remanei males; sperm and the male-derived signaling protein MSP act as triggers of oocyte production in Caenorhabditis (Hill and L'Hernault 2001; Miller et al. 2001). Thus, hybrid male sterility is associated with the inability to properly transfer seminal products.
Finally, we visualized the gonad morphology of hybrid males with DIC microscopy. We observed that 95% of F1 hybrid males derived from C. remanei mothers had unusual gonad morphology by at least one criterion, which we observed in none of the control C. remanei males. Most commonly, the hybrid males had small gonads with the turn of their gonad arm located abnormally posterior within the animal (63% of hybrid males) or had unusual vacuolization within the gonad (44%) (Figure 4B–F). We also frequently observed that the hybrid male gonad traced a meandering path through the animal (28%), had gonad tissue balled up in a bulbous mass (21%), or, in one case, extended too far anteriorly so as to terminate next to the pharynx (Figure 4B–F). We observed some instances of defects in the morphology of the male tail, and spermatids also were often not visible within the gonad (29%).
DISCUSSION
Here we demonstrate reproductive isolation between the nematodes C. remanei and C. latens is comprised of moderate F1 hybrid inviability, strong F2 hybrid inviability, and strong F1 hybrid sterility. This provides the first case of partial hybrid male sterility documented in Caenorhabditis and allows deep genetic and developmental analysis of the evolution of barriers to reproduction during speciation. The stronger inviability acting in later generations is consistent with an important role for recessive incompatibility alleles, rendered homozygous in the F2 generation, being involved in DMIs (Muller 1940; Orr 1993). Further, the stronger F1 male sterility than F1 inviability is consistent with, albeit not exclusive to, a ‘faster male’ model for the evolution of post-zygotic reproductive isolation. These observations also conform to both Haldane’s rule, with stronger adverse effects on the male heterogametic sex, and to Darwin’s corollary, with strikingly asymmetric parent-of-origin effects on hybrid fitness implicating uniparental inheritance of incompatibility factors. Our observations of substantial strain-specific variation in the magnitude of effects indicates that some genetic factors contributing to asymmetry in reproductive incompatibility remain polymorphic within C. remanei. Moreover, the developmental defects in the gonad responsible for hybrid male sterility present a clear research program for future dissection of its underlying molecular mechanism.
Hybrid male sterility
This first case of partial hybrid male sterility in Caenorhabditis establishes a foundation for developmental genetic dissection of this important reproductive isolation barrier. In contrast to the total sterility of the rare hybrid males produced by crosses between C. briggsae and C. nigoni (Woodruff et al. 2010; Kozlowska et al. 2012), the presence of some fertile hybrid males of C. remanei and C. latens permits both F2 and backcross analysis to dissect the genetic underpinnings of reproductive incompatibility. Hybrid males suffer elevated rates of both inviability and sterility, specifically, for hybrid males derived from C. remanei mothers. The asymmetric sterility of F1 hybrid males is striking: only ~5% of hybrid males with C. remanei mothers are fertile, whereas nearly all of the hybrid males from the reciprocal cross are fertile. Hybrid male sterility occurs despite an absence of gross defects in either male tail mating structures or mating behavior of most animals, instead reflecting problems of gonad development that appear to render them incapable of transferring sperm and seminal fluid. The sterile male hybrids from a related species pair, C. briggsae and C. nigoni, also show problems in gonad development that can preclude sperm production (Woodruff et al. 2010).
Among hybrid males produced by C. remanei and C. latens, we observed severe gonadogenesis defects (Figure 4). In C. elegans males, normal gonad development involves a U-shaped elongation path, led by the linker cell first anterior from the ventral mid-body and then turning posterior before reaching the cloaca at the male tail (Klass et al. 1976; Kimble and White 1981). This gonad morphogenesis occurs primarily during mid- to late-larval development (L2 to L4), and requires appropriate timing of the death of the linker cell for successful completion and for adult male fertility. Linker cell death in C. elegans depends on the heterochronic zinc finger transcription factor LIN-29, the polyglutamine-repeat protein PQN-41, the mitogen-activated protein kinase SEK-1, and the microRNA gene let-7, whereas proper linker cell migration requires the metalloproteases MIG-17 and GON-1, the HIM-4 extracellular matrix protein and the nuclear receptors DAF-12 and NHR-67 (Blelloch and Kimble 1999; Blelloch et al. 1999; Euling et al. 1999; Nishiwaki et al. 2000; Vogel and Hedgecock 2001; Abraham et al. 2007; Kato and Sternberg 2009; Blum et al. 2012). Additionally, dorso-ventral migration of the linker cell is affected by unc-5, unc-6, and unc-40 (Hedgecock et al. 1990). In parallel with lin-29, the X-linked sek-1 controls expression of autosomal pqn-41 at the onset of linker cell death (Blum et al. 2012). Premature death of the linker cell results in severe defects in gonad elongation (Kimble and White 1981), whereas persistent linker cell survival in spite of normal gonad migration blocks the exit of sperm and seminal fluid from the reproductive system (Abraham et al. 2007).
We hypothesize that disrupted migration or, perhaps less likely given the observed gonad defects, programmed cell death of the linker cell plays an important role in sterility of hybrid males of C. remanei and C. latens, as well as the inability of sterile hybrid males to produce a copulatory plug from seminal fluid. Consistent with this, we commonly observed ‘floating’ masses of gonad tissue that appear to fail to connect to the cloaca in male hybrids. Hybrid male gonads typically did not extend as far anterior as normal, suggestive of premature turning during linker cell migration in L2/L3, although it could also be a byproduct of the small size of the hybrid male gonads. The small gonads also suggest that future work may reveal additional sources of disrupted gonad development. Spermatids were seen in many gonads, despite unusual gonad morphology, indicating that germ cell division appears capable of proceeding properly and therefore suggests that distal tip cells are competent to function properly, as observed in C. elegans linker cell ablation experiments (Kimble and White 1981). Hybrid male gonads also commonly had extensive vacuolization, suggesting the occurrence of some kind of cell death process, necrosis, or perhaps over-accumulation of seminal fluid components that are known to include proteases or to accumulate in vacuoles (Palopoli et al. 2008; Smith and Stanfield 2011). The known role for small RNAs in C. elegans sperm fertility (Abraham et al. 2007; Conine et al. 2009) suggests the intriguing mechanism of maternal transmission of small RNAs as a potential source of the asymmetric parent-of-origin sterility of male hybrids. However, more traditional Dobzhansky-Muller incompatibilities based on mito-nuclear or X-autosome genetic interactions also are plausible explanations for the production of asymmetric hybrid male sterility owing to gonadogenesis defects.
Antagonistic coevolution as a result of sperm competition can drive selection for increased sperm competitiveness in males and ovum defensiveness in females, leading to asymmetries in fertilization success (Martin-Coello et al. 2009). Genes with male-biased expression evolve fast in many species (Ellegren and Parsch 2007), as is the case for sperm-associated genes and genes involved in sex determination in Caenorhabditis (Haag et al. 2002; Cutter and Ward 2005; Artieri et al. 2008; Hill and Haag 2009). Rapid evolution of such genes forms a tantalizing hypothesis for the formation of male-specific DMIs responsible for asymmetric hybrid male sterility like that observed here (Howard et al. 2009).
Embryonic hybrid inviability
Embryonic mortality is a major source of hybrid inviability in this system. Our analysis of terminal developmental phenotypes of unhatched, dead eggs revealed that most embryonic arrest occurs during or after gastrulation. This concurs with embryonic arrest phenotypes of hybrid embryos between more divergent species pairs in Caenorhabditis (Baird et al. 1992; Baird and Yen 2000). Given that gastrulation marks a point in development at which maternal transcript abundance transitions to strong activation of zygotic gene expression (Baugh et al. 2003; Levin et al. 2012), this suggests that DMIs acting later in embryogenesis could exact a particularly strong barrier to reproductive isolation. This does not preclude a role for maternal × zygotic interactions, and also raises the question of whether early stage events, like spindle dynamics in the first cell divisions (Riche et al. 2013), could presage hybrid embryonic arrest. More detailed examination of the defects in the arrested embryos will help to identify whether particular cells, molecules and pathways consistently compromise hybrid embryos (Bao et al. 2006; Zhao et al. 2008). Among many possibilities, for instance, it has been hypothesized that misregulation of the actin cytoskeleton in embryonic compaction and elongation could play a role in hybrid embryonic arrest (Baird and Yen 2000; Baird and Seibert 2013). It is plausible that the higher embryonic mortality observed for hybrids with C. remanei mothers could be a primary source of the elevated inviability of the male sex in this cross, although it remains to be tested whether the disproportionate male inviability occurs primarily in embryo or in larval stages of development. Testing for transcriptome disruption in hybrids provides one means of generating further hypotheses, as in such studies for Drosophila that have identified sex-biased expression disruption (Michalak and Noor 2003; Ranz et al. 2004).
Variable Reproductive Isolation
Heritable variation among different genotypes within a species can cause varying degrees of hybrid viability in interspecies crosses (Martin and Willis 2010; Cutter 2012). Here we identify such variable reproductive isolation among different female C. remanei genetic backgrounds when crossed to C. latens males. The C. remanei variation in hybrid F1 production does not associate obviously with geographic origin, consistent with gene flow occuring readily among C. remanei populations (Dey et al. 2012). Moreover, we found that strain JU724 shows F2 hybrid breakdown in crosses with canonical strains of both C. remanei and C. latens, despite its relatively close geographic origin and genetic distance to C. latens (Dey et al. 2012). This finding suggests that ongoing species discovery in Caenorhabditis is likely to uncover additional morphological cryptic species with relatively short genetic distances, which will provide further systems for genetic dissection of reproductive isolation (Kiontke et al. 2011). The mechanism responsible for heritable variation in reproductive isolation remains to be determined. It could involve allelic variation in post-zygotic DMI loci, but also could conceivably involve differential sensitivity of females to gametic reproductive isolation (Ting et al. 2014). We also have not yet explored non-heritable contributions to hybrid inviability and sterility. For example, the manifestation of Haldane’s rule can be sensitive to rearing temperatures (Hutter 1997; Wade et al. 1999; Koevoets et al. 2012) and extrinsic factors can be important in reproductive isolation more generally (Coyne and Orr 2004). Consequently, the total reproductive isolation between C. remanei and C. latens is likely underestimated here, as both extrinsic factors and intrinsic pre-mating and post-mating pre-zygotic factors not considered in this study would provide exacerbating barriers to gene flow. Future investigation of heritable, environmental, and genotype × environment effects will prove valuable in dissecting the molecular and developmental bases for reproductive isolation in this system.
Asymmetric reproductive isolation and Darwin’s corollary
In addition to Haldane’s rule, F1 hybrid males of C. remanei and C. latens exhibit asymmetry in inviability and sterility depending upon the parent of origin. Qualitatively similar asymmetries are observed for hybrids derived from C. briggsae and C. nigoni (Table 1) (Woodruff et al. 2010; Kozlowska et al. 2012). F1 asymmetries in reciprocal crosses, so-called Darwin’s corollary to Haldane’s rule, can be caused by uniparentally inherited Dobzhansky-Muller incompatibilities (Turelli and Moyle 2007). For example, they can result from i) X-linked incompatibilities that occur in different numbers and magnitude in each species and might not have equal fitness when combined in hybrids with the other species’ autosomes, ii) cyto-nuclear interactions in which products from the cytoplasmic genome of one species (mitochondria, chloroplasts) and nuclear genes derived from the second species interact non-reciprocally, or iii) epigenetically inherited maternal gene regulatory machinery (e.g. transcription factor proteins, small RNAs, chromosome marks) that act to improperly regulate paternally derived genes in the zygote or through differential imprinting (Turelli and Moyle 2007). Any or all of the above explanations could contribute to the reciprocal cross asymmetry in hybrid sterility and inviability in hybrids of C. remanei and C. latens. Recent evidence suggests spermatogenesis genes in C. elegans are regulated by small RNA based mechanisms, several of which are transmitted maternally and are critical for maintenance of male fertility (Batista et al. 2008; Wang and Reinke 2008; Conine et al. 2009; Wu et al. 2010; Johnson and Spence 2011). Drosophila also shows misregulation of small RNAs in hybrids (Kelleher et al. 2012). Discriminating among these possible explanations for asymmetry in hybrid fitness (Darwin’s corollary to Haldane’s rule), for which empirical data are scarce (Turelli and Moyle 2007; Vrana 2007; Bolnick et al. 2008; Campbell et al. 2013), will help establish the causes of this major unsolved problem in speciation.
Table 1.
Comparison of reproductive isolation (RI) factors for two pairs of inter-species hybrids.
| RI feature | C. remanei × C. latens a | C. briggsae × C. nigoni b |
|---|---|---|
| Extrinsic isolation (temperature-sensitivity) |
n.d. | Present |
| Gametic isolation | n.d. | Strong |
| F1 male inviability | Weak/Moderate | Strong |
| F1 female inviability | Weak/Moderate | Strong |
| F1 male sterility | ~10% or ~95% | ~100% |
| F1 female sterility | ~0% | ~40% or ~60% |
| F2 male inviability | Strong | n.a. c |
| F2 female inviability | Strong | n.a. c |
| Haldane’s rule | Sterility and inviability | Sterility and inviability |
| Darwin’s corollary | Stronger RI for maternal C. remanei |
Stronger RI for maternal C. briggsae |
| Heritable VRI d | Present | Present |
this study;
F2s cannot be produced owing to complete F1 male sterility;
genetic variability within species for reproductive isolation between species
ACKNOWLEDGEMENTS
We are grateful to Christian Braendle, Matthew Rockman and Hinrich Schulenburg for sharing strains of C. remanei for this study. A.D.C. is supported by funds from the Natural Sciences and Engineering Research Council of Canada, a Canada Research Chair, and the National Institutes of Health.
REFERENCES
- Abraham MC, Lu Y, Shaham S. A morphologically conserved nonapoptotic program promotes linker cell death in Caenorhabditis elegans. Dev. Cell. 2007;12:73–86. doi: 10.1016/j.devcel.2006.11.012. [DOI] [PubMed] [Google Scholar]
- Artieri CG, Haerty W, Gupta BP, Singh RS. Sexual selection and maintenance of sex: evidence from comparisons of rates of genomic accumulation of mutations and divergence of sex-related genes in sexual and hermaphroditic species of Caenorhabditis. Mol. Biol. Evol. 2008;25:972–979. doi: 10.1093/molbev/msn046. [DOI] [PubMed] [Google Scholar]
- Baird SE. Haldane's Rule by sexual transformation in Caenorhabditis. Genetics. 2002;161:1349–1353. doi: 10.1093/genetics/161.3.1349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird SE, Seibert SR. Reproductive isolation in the Elegans-Group of Caenorhabditis. Natural Science. 2013;5:18–25. [Google Scholar]
- Baird SE, Stonesifer R. Reproductive isolation in Caenorhabditis briggsae: Dysgenic interactions between maternal- and zygotic-effect loci result in a delayed development phenotype. Worm. 2012;1:189–195. doi: 10.4161/worm.23535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird SE, Sutherlin ME, Emmons SW. Reproductive isolation in Rhabditidae (Nematoda, Secernentea): mechanisms that isolate 6 species of 3 genera. Evolution. 1992;46:585–594. doi: 10.1111/j.1558-5646.1992.tb02067.x. [DOI] [PubMed] [Google Scholar]
- Baird SE, Yen W-C. Reproductive isolation in Caenorhabditis: terminal phenotypes of hybrid embryos. Evol. Dev. 2000;2:9–15. doi: 10.1046/j.1525-142x.2000.00031.x. [DOI] [PubMed] [Google Scholar]
- Bao ZR, Murray JI, Boyle T, Ooi SL, Sandel MJ, Waterston RH. Automated cell lineage tracing in Caenorhabditis elegans. Proc. Natl. Acad. SciU.SA. 2006;103:2707–2712. doi: 10.1073/pnas.0511111103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DM. Copulatory plugs and paternity assurance in the nematode Caenorhabditis elegans. Anim. Behav. 1994;48:147–156. [Google Scholar]
- Batista PJ, Ruby JG, Claycomb JM, Chiang R, Fahlgren N, Kasschau KD, Chaves DA, Gu W, Vasale JJ, Duan S, Conte D, Jr, Luo S, Schroth GP, Carrington JC, Bartel DP, Mello CC. PRG-1 and 21U-RNAs interact to form the piRNA complex required for fertility in C. elegans. Mol. Cell. 2008;31:67–78. doi: 10.1016/j.molcel.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baugh LR, Hill AA, Slonim DK, Brown EL, Hunter CP. Composition and dynamics of the Caenorhabditis elegans early embryonic transcriptome. Development. 2003;130:889–900. doi: 10.1242/dev.00302. [DOI] [PubMed] [Google Scholar]
- Blelloch R, Kimble J. Control of organ shape by a secreted metalloprotease in the nematode Caenorhabditis elegans. Nature. 1999;399:586–590. doi: 10.1038/21196. [DOI] [PubMed] [Google Scholar]
- Blelloch R, Santa Anna-Arriola S, Gao DL, Li YJ, Hodgkin J, Kimble J. The gon-1 gene is required for gonadal morphogenesis in Caenorhabditis elegans. Dev. Biol. 1999;216:382–393. doi: 10.1006/dbio.1999.9491. [DOI] [PubMed] [Google Scholar]
- Blum ES, Abraham MC, Yoshimura S, Lu Y, Shaham S. Control of nonapoptotic developmental cell death in Caenorhabditis elegans by a polyglutamine-repeat protein. Science. 2012;335:970–973. doi: 10.1126/science.1215156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bolnick DI, Turelli M, Lopez-Fernandez H, Wainwright PC, Near TJ. Accelerated mitochondrial evolution and "Darwin's corollary": asymmetric viability of reciprocal F1 hybrids in Centrarchid fishes. Genetics. 2008;178:1037–1048. doi: 10.1534/genetics.107.081364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown JD, O'Neill RJ. Chromosomes, conflict, and epigenetics: chromosomal speciation revisited. Annu. Rev. Genomics Hum. Genet. 2010;11:291–316. doi: 10.1146/annurev-genom-082509-141554. [DOI] [PubMed] [Google Scholar]
- Campbell P, Good JM, Nachman MW. Meiotic sex chromosome inactivation is disrupted in sterile hybrid male house mice. Genetics. 2013;193:819–828. doi: 10.1534/genetics.112.148635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conine CC, Batista PJ, Gu W, Claycomb JM, Chaves DA, Shirayama M, Mello CC. Argonautes ALG-3 and ALG-4 are required for spermatogenesis-specific 26G-RNAs and thermotolerant sperm in Caenorhabditis elegans. Proc. Natl. Acad. Sci. U.S.A. 2009;107:3588–3593. doi: 10.1073/pnas.0911685107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coyne JA, Orr HA. Speciation. Sunderland, MA: Sinauer; 2004. [Google Scholar]
- Cutter AD. The polymorphic prelude to Bateson-Dobzhansky-Muller incompatibilities. Trends Ecol. Evol. 2012;27:209–218. doi: 10.1016/j.tree.2011.11.004. [DOI] [PubMed] [Google Scholar]
- Cutter AD, Dey A, Murray RL. Evolution of the Caenorhabditis elegans genome. Mol. Biol. Evol. 2009;26:1199–1234. doi: 10.1093/molbev/msp048. [DOI] [PubMed] [Google Scholar]
- Cutter AD, Ward S. Sexual and temporal dynamics of molecular evolution in C. elegans development. Mol. Biol. Evol. 2005;22:178–188. doi: 10.1093/molbev/msh267. [DOI] [PubMed] [Google Scholar]
- Dey A, Jeon Y, Wang G-X, Cutter AD. Global population genetic structure of Caenorhabditis remanei reveals incipient speciation. Genetics. 2012;191:1257–1269. doi: 10.1534/genetics.112.140418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobzhansky T. Studies on hybrid sterility. II. Localization of sterility factors in Drosophila pseudoobscura hybrids. Genetics. 1936;21:113–135. doi: 10.1093/genetics/21.2.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ellegren H, Parsch J. The evolution of sex-biased genes and sex-biased gene expression. Nat. Rev. Genet. 2007;8:689–698. doi: 10.1038/nrg2167. [DOI] [PubMed] [Google Scholar]
- Euling S, Bettinger JC, Rougvie AE. The LIN-29 transcription factor is required for proper morphogenesis of the Caenorhabditis elegans male tail. Dev. Biol. 1999;206:142–156. doi: 10.1006/dbio.1998.9063. [DOI] [PubMed] [Google Scholar]
- Felix MA, Braendle C, Cutter AD. A streamlined system for species diagnosis in Caenorhabditis (Nematoda: Rhabditidae) with name designations for 15 distinct biological species. PLoS ONE. 2014;9:e94723. doi: 10.1371/journal.pone.0094723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia LR, LeBoeuf B, Koo P. Diversity in mating behavior of hermaphroditic and male-female Caenorhabditis nematodes. Genetics. 2007;175:1761–1771. doi: 10.1534/genetics.106.068304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haag ES, Wang SP, Kimble J. Rapid coevolution of the nematode sex-determining genes fem-3 and tra-2. Curr. Biol. 2002;12:2035–2041. doi: 10.1016/s0960-9822(02)01333-7. [DOI] [PubMed] [Google Scholar]
- Haldane JBS. Sex ratio and unisexual sterility in hybrid animals. J. Genet. 1922;12:101–109. [Google Scholar]
- Hedgecock EM, Culotti JG, Hall DH. The unc-5, unc-6, and unc-40 genes guide circumferential migrations of pioneer axons and mesodermal cells on the epidermis in C. elegans. Neuron. 1990;4:61–85. doi: 10.1016/0896-6273(90)90444-k. [DOI] [PubMed] [Google Scholar]
- Hill KL, L'Hernault SW. Analyses of reproductive interactions that occur after heterospecific matings within the genus Caenorhabditis. Dev. Biol. 2001;232:105–114. doi: 10.1006/dbio.2000.0136. [DOI] [PubMed] [Google Scholar]
- Hill RC, Haag ES. A sensitized genetic background reveals evolution near the terminus of the Caenorhabditis germline sex determination pathway. Evol. Dev. 2009;11:333–342. doi: 10.1111/j.1525-142X.2009.00340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgkin J, Doniach T. Natural variation and copulatory plug formation in Caenorhabditis elegans. Genetics. 1997;146:149–164. doi: 10.1093/genetics/146.1.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard DJ, Palumbi SR, Birge LM, Manier MK. Sperm and speciation. In: Birkhead TR, Hosken DJ, Pitnick S, editors. Sperm Biology: An Evolutionary Perspective. Boston: Academic Press; 2009. [Google Scholar]
- Hutter P. Genetics of hybrid inviability Drosophila. Adv. Genet. 1997;36:157–185. doi: 10.1016/s0065-2660(08)60309-0. [DOI] [PubMed] [Google Scholar]
- Johnson CL, Spence AM. Epigenetic licensing of germline gene expression by maternal RNA in C. elegans. Science. 2011;333:1311–1314. doi: 10.1126/science.1208178. [DOI] [PubMed] [Google Scholar]
- Kato M, Sternberg PW. The C. elegans tailless/Tlx homolog nhr-67 regulates a stage-specific program of linker cell migration in male gonadogenesis. Development. 2009;136:3907–3915. doi: 10.1242/dev.035477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelleher ES, Edelman NB, Barbash DA. Drosophila interspecific hybrids phenocopy piRNA-pathway mutants. PLoS Biol. 2012;10 doi: 10.1371/journal.pbio.1001428. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimble JE, White JG. On the control of germ-cell development in Caenorhabditis elegans. Dev. Biol. 1981;81:208–219. doi: 10.1016/0012-1606(81)90284-0. [DOI] [PubMed] [Google Scholar]
- Kiontke K, Felix M-A, Ailion M, Rockman M, Braendle C, Penigault J-B, Fitch D. A phylogeny and molecular barcodes for Caenorhabditis, with numerous new species from rotting fruits. BMC Evol. Biol. 2011;11:339. doi: 10.1186/1471-2148-11-339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klass M, Wolf N, Hirsh D. Development of male reproductive-system and sexual transformation in nematode Caenorhabditis elegans. Dev. Biol. 1976;52:1–18. doi: 10.1016/0012-1606(76)90002-6. [DOI] [PubMed] [Google Scholar]
- Koevoets T, van de Zande L, Beukeboom LW. Temperature stress increases hybrid incompatibilities in the parasitic wasp genus Nasonia. J. Evol. Biol. 2012;25:304–316. doi: 10.1111/j.1420-9101.2011.02424.x. [DOI] [PubMed] [Google Scholar]
- Kozlowska JL, Ahmad AR, Jahesh E, Cutter AD. Genetic variation for post-zygotic reproductive isolation between Caenorhabditis briggsae and Caenorhabditis sp. 9. Evolution. 2012;66:1180–1195. doi: 10.1111/j.1558-5646.2011.01514.x. [DOI] [PubMed] [Google Scholar]
- LaMunyon CW, Ward S. Increased competitiveness of nematode sperm bearing the male X chromosome. Proc. Natl. Acad. Sci. U.S.A. 1997;94:185–189. doi: 10.1073/pnas.94.1.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levin M, Hashimshony T, Wagner F, Yanai I. Developmental milestones punctuate gene expression in the Caenorhabditis embryo. Dev. Cell. 2012;22:1101–1108. doi: 10.1016/j.devcel.2012.04.004. [DOI] [PubMed] [Google Scholar]
- Lipton J, Kleemann G, Ghosh R, Lints R, Emmons SW. Mate searching in Caenorhabditis elegans : a genetic model for sex drive in a simple invertebrate. J. Neurosci. 2004;24:7427–7434. doi: 10.1523/JNEUROSCI.1746-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maheshwari S, Barbash DA. The genetics of hybrid incompatibilities. Annu. Rev. Genet. 2011;45:331–355. doi: 10.1146/annurev-genet-110410-132514. [DOI] [PubMed] [Google Scholar]
- Martin-Coello J, Benavent-Corai J, Roldan ERS, Gomendio M. Sperm competition promotes asymmetries in reproductive barriers between closely related species. Evolution. 2009;63:613–623. doi: 10.1111/j.1558-5646.2008.00585.x. [DOI] [PubMed] [Google Scholar]
- Martin NH, Willis JH. Geographical variation in postzygotic isolation and its genetic basis within and between two Mimulus species. Philos. TransRSoc. B. 2010;365:2469–2478. doi: 10.1098/rstb.2010.0030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Michalak P, Noor MAF. Genome-wide patterns of expression in Drosophila pure species and hybrid males. Mol. Biol. Evol. 2003;20:1070–1076. doi: 10.1093/molbev/msg119. [DOI] [PubMed] [Google Scholar]
- Miller MA, Nguyen VQ, Lee M-H, Kosinski M, Schedl T, Caprioli RM, Greenstein D. A sperm cytoskeletal protein that signals oocyte meiotic maturation and ovulation. Science. 2001;291:2144–2147. doi: 10.1126/science.1057586. [DOI] [PubMed] [Google Scholar]
- Muller HJ. Bearings of the Drosophila work on systematics. In: Huxley J, editor. New Systematics. Oxford: Clarendon Press; 1940. [Google Scholar]
- Muller HJ. Isolating mechanisms, evolution and temperature. Biol. Symp. 1942;6:71–125. [Google Scholar]
- Nishiwaki K, Hisamoto N, Matsumoto K. A metalloprotease disintegrin that controls cell migration in Caenorhabditis elegans. Science. 2000;288:2205–2208. doi: 10.1126/science.288.5474.2205. [DOI] [PubMed] [Google Scholar]
- Orr HA. Haldane rule has multiple genetic causes. Nature. 1993;361:532–533. doi: 10.1038/361532a0. [DOI] [PubMed] [Google Scholar]
- Palopoli MF, Rockman MV, Tinmaung A, Ramsay C, Curwen S, Aduna A, Laurita J, Kruglyak L. Molecular basis of the copulatory plug polymorphism in Caenorhabditis elegans. Nature. 2008;454:1019–1022. doi: 10.1038/nature07171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Presgraves DC. Darwin and the origin of interspecific genetic incompatibilities. Am. Nat. 2010;176(Suppl 1):S45–S60. doi: 10.1086/657058. [DOI] [PubMed] [Google Scholar]
- Presgraves DC. The molecular evolutionary basis of species formation. Nat. Rev. Genet. 2010;11:175–180. doi: 10.1038/nrg2718. [DOI] [PubMed] [Google Scholar]
- Ranz JM, Namgyal K, Gibson G, Hartl DL. Anomalies in the expression profile of interspecific hybrids of Drosophila melanogaster and Drosophila simulans. Genome Res. 2004;14:373–379. doi: 10.1101/gr.2019804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riche S, Zouak M, Argoul Fo, Arneodo A, Pecreaux J, Delattre M. Evolutionary comparisons reveal a positional switch for spindle pole oscillations in Caenorhabditis embryos. J. Cell Biol. 2013;201:653–662. doi: 10.1083/jcb.201210110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schilthuizen M, Giesbers MCWG, Beukeboom LW. Haldane's rule in the 21st century. Heredity. 2011;107:95–102. doi: 10.1038/hdy.2010.170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith JR, Stanfield GM. TRY-5 is a sperm-activating protease in Caenorhabditis elegans seminal fluid. PLoS Genet. 2011;7 doi: 10.1371/journal.pgen.1002375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stiernagle TL. Maintenance of C. elegans. In: Hope IA, editor. C. elegans: A Practical Approach. New York: Oxford University Press; 1999. [Google Scholar]
- Tiffin P, Olson MS, Moyle LC. Asymmetrical crossing barriers in angiosperms. ProcRSoc. B. 2001;268:861–867. doi: 10.1098/rspb.2000.1578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ting JJ, Woodruff GC, Leung G, Shin N-R, Cutter AD, Haag ES. Intense sperm-mediated sexual conflict promotes gametic isolation in Caenorhabditis nematodes. PLoS Biol. 2014;12:e1001915. doi: 10.1371/journal.pbio.1001915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M, Moyle LC. Asymmetric postmating isolation: Darwin's corollary to Haldane's rule. Genetics. 2007;176:1059–1088. doi: 10.1534/genetics.106.065979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turelli M, Orr HA. The dominance theory of Haldane's rule. Genetics. 1995;140:389–402. doi: 10.1093/genetics/140.1.389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogel BE, Hedgecock EM. Hemicentin, a conserved extracellular member of the immunoglobulin superfamily, organizes epithelial and other cell attachments into oriented line-shaped junctions. Development. 2001;128:883–894. doi: 10.1242/dev.128.6.883. [DOI] [PubMed] [Google Scholar]
- Vrana PB. Genomic imprinting as a mechanism of reproductive isolation in mammals. J. Mammal. 2007;88:5–23. [Google Scholar]
- Wade MJ, Johnson NA, Toquenaga Y. Temperature effects and genotype-by-environment interactions in hybrids: Haldane's rule in flour beetles. Evolution. 1999;53:855–865. doi: 10.1111/j.1558-5646.1999.tb05379.x. [DOI] [PubMed] [Google Scholar]
- Wang G, Reinke V. A C. elegans Piwi, PRG-1, regulates 21U-RNAs during spermatogenesis. Curr. Biol. 2008;18:861–867. doi: 10.1016/j.cub.2008.05.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodruff GC, Eke O, Baird SE, Felix MA, Haag ES. Insights into species divergence and the evolution of hermaphroditism from fertile interspecies hybrids of Caenorhabditis nematodes. Genetics. 2010;186:997–1012. doi: 10.1534/genetics.110.120550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu CI, Davis AW. Evolution of postmating reproductive isolation: the composite nature of Haldane rule and its genetic bases. Am. Nat. 1993;142:187–212. doi: 10.1086/285534. [DOI] [PubMed] [Google Scholar]
- Wu CI, Johnson NA, Palopoli MF. Haldane's rule and its legacy: Why are there so many sterile males? Trends Ecol. Evol. 1996;11:281–284. doi: 10.1016/0169-5347(96)10033-1. [DOI] [PubMed] [Google Scholar]
- Wu TF, Nera B, Chu DS, Shakes DC. Elucidating gene regulatory mechanisms for sperm function through the integration of classical and systems approaches in C. elegans. Syst. Biol. Reprod. Med. 2010;56:222–235. doi: 10.3109/19396361003749986. [DOI] [PubMed] [Google Scholar]
- Yan C, Bi Y, Yin D, Zhao Z. A method for rapid and simultaneous mapping of genetic loci and introgression sizes in nematode species. PLoS ONE. 2012;7:e43770. doi: 10.1371/journal.pone.0043770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Z, Boyle TJ, Bao Z, Murray JI, Mericle B, Waterston RH. Comparative analysis of embryonic cell lineage between Caenorhabditis briggsae and Caenorhabditis elegans. Dev. Biol. 2008;314:93–99. doi: 10.1016/j.ydbio.2007.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]