Abstract
Objective
To examine general and treatment-specific predictors of children's weight outcomes during a pediatric weight management trial.
Method
150 overweight children [69.3% female; M BMI z-score (z-BMI)=2.21±0.30] completed family-based behavioral weight loss treatment (FBT), followed by randomization to social facilitation maintenance treatment (SFM) addressing social support and body image; behavioral skills maintenance treatment (BSM) which extended FBT skills to maintenance; or a control condition with no maintenance treatment. Regression and mixed-effects repeated-measures ANCOVA examined child and parent anthropometric, demographic, and psychosocial variables in predicting relative weight outcomes over short- and long-term follow-ups.
Results
Among FBT completers, lower child baseline z-BMI and age, and greater parent BMI reductions during FBT and baseline self-efficacy predicted better child relative weight loss following FBT [F(6,137)=7.77; p<.001]. Higher child-reported post-FBT eating pathology predicted greater relative weight loss in SFM than BSM or control from post-FBT to 2-year follow-up [F(4,255.88)=3.48; p=.009], whereas higher parent-reported post-FBT social support predicted greater relative weight loss in BSM than control [F(2,141.65)=3.28; p=.04]. Lower parent-reported post-FBT behavioral problems predicted greater relative weight loss in SFM and BSM versus control [F(2,147.84)=7.37; p<.001]; higher problems predicted equivalent outcome across treatments.
Conclusion
SFM may improve weight outcomes for FBT completers with initially higher eating pathology, whereas extending FBT skills may be effective for those with higher familial support. These results suggest that certain pretreatment variables moderate the effectiveness of different pediatric weight control interventions. Further understanding these findings may help optimally match families to treatments.
Keywords: Pediatric obesity, family-based treatment, predictors, moderators, treatment outcome
Overweight and obesity affect 32% of children in the United States (Ogden, Carroll, Kit, & Flegal, 2012) and are associated with negative physical and psychosocial health sequelae (Puhl & Latner, 2007; Reilly et al., 2003). Family-based behavioral weight loss treatment (FBT), which targets both child and parent weight-related behaviors, is the current treatment of choice for pediatric obesity (United States Preventive Services Task Force, 2010; Wilfley, Tibbs, et al., 2007), and maintenance treatments (MTs) can extend its effects for up to two years (Wilfley, Stein, et al., 2007; Wilfley et al., 2010). Although children generally maintain relative weight changes achieved at 2-year follow-up over longer periods (Epstein, Valoski, Wing, & McCurley, 1994), initial non-response and relapse are challenges for many children (Wilfley, Tibbs, et al., 2007). In our current healthcare environment, it is essential to identify children who are most likely to benefit from interventions so as to inform the allocation of limited treatment resources. Gaining a better understanding of predictors of outcome can assist in this way, including targeting children who would most benefit from existing treatments, and modifying future interventions to help maximize effectiveness.
Examining general predictors can help identify patients who will respond more or less favorably to treatment, regardless of the type of treatment provided. Moderators are treatment-specific predictors of outcome that can help identify patients who may respond more favorably to one type of treatment than another (Kraemer, Wilson, Fairburn, & Agras, 2002). Although general predictors can assist with patient selection, treatment-specific moderators may have more clinical utility, such as when wanting to match patients to treatment.
To date, few studies have investigated predictors of FBT outcome. Some (Reinehr, Kleber, Lass, & Toschke, 2010; Sabin et al., 2007), but not all (Moens, Braet, & Van Winckel, 2010), studies have demonstrated that younger children are more likely to succeed than older children in weight control programs, perhaps because their weight-related habits are less ingrained and more amenable to change. Higher initial body weight also predicts better outcome (Braet, 2006; Goossens, Braet, Van Vlierberghe, & Mels, 2009; Moens et al., 2010), and some studies suggest that females have improved treatment response (Epstein, Koeske, Wing, & Valoski, 1986), although findings are mixed (Epstein, Valoski, et al., 1994). Potentially modifiable factors associated with better short- and long-term outcome across treatments include early treatment response (Goldschmidt et al., 2011; Jelalian et al., 2008), which may be a proxy for initial motivation or stronger engagement in treatment; better overall psychosocial functioning, including greater social support (Braet, 2006; Epstein, Valoski, et al., 1994; Moens et al., 2010) and lower impulsivity and food reinforcement (Best et al., 2012), all of which may facilitate adherence to treatment recommendations; and familial factors such as greater parent weight loss (Wrotniak, Epstein, Paluch, & Roemmich, 2004) and lower parental psychopathology (Epstein, Wisniewski, & Weng, 1994; Frohlich, Pott, Albayrak, Hebebrand, & Pauli-Pott, 2011; Moens et al., 2010), which could operate through parents' abilities to provide healthy modeling and support for behavior change. To date, there have been no studies examining predictors of outcome following MT. Moreover, there is an incomplete understanding of patient moderating variables predicting response among different approaches with demonstrated average efficacy across children (Epstein et al., 2012; Yildirim et al., 2011).
Our group previously tested two MT approaches designed to assist youth in maintaining their weight loss following standard FBT (Wilfley, Stein, et al., 2007). Behavioral skills maintenance (BSM) focused on helping families develop behavioral weight maintenance skills, including self-regulatory and relapse prevention strategies (e.g., enhancing efficacy to cope with situations presenting a high risk for behavioral lapses), whereas social facilitation maintenance (SFM) was designed to help families modify their social environment to support weight maintenance, emphasizing peer-related (e.g., lack of social support for healthy behaviors) and social self-perceptual (e.g., body image) barriers to long-term weight maintenance. Both MTs led to improved weight outcomes over two years when compared to a control condition that received no MT, and children with lower initial levels of social problems were found to particularly benefit from SFM, perhaps because they could more readily implement the basic SFM skills (Wilfley, Stein, et al., 2007). However, other predictors that could theoretically promote or inhibit weight change both throughout the duration of treatment, and within the specific MT conditions, were not examined in the context of our original trial, thus limiting the data's clinical applicability. Investigating a wider range of putative general and treatment-specific predictors could assist with treatment matching, as well as with designing or enhancing treatments to optimally facilitate weight change across the spectrum of overweight youth and their families.
The purpose of the current research was to expand upon the results of our randomized controlled trial (which reported on the outcome of MT interventions following standard FBT(Wilfley, Stein, et al., 2007) by identifying general predictors and treatment-specific moderators of outcome among FBT completers beyond the initially identified social problems effect. Based on the previous literature (Braet, 2006; Epstein et al., 1986; Epstein, Valoski, et al., 1994; Epstein, Wisniewski, et al., 1994; Frohlich et al., 2011; Goossens et al., 2009; Jelalian et al., 2008; Moens et al., 2010; Reinehr et al., 2010; Sabin et al., 2007; Wrotniak et al., 2004), we hypothesized that the following factors would be associated with improved child weight outcomes in both the short- and long-term, across MT treatments: being female; younger age, and higher z-BMI at initiation of FBT; greater parent weight change during FBT; and better child and parent psychosocial functioning, including general- and eating-related psychopathology, self-efficacy, and family support. We further expected that BSM would particularly benefit youth with lower initial self-efficacy as compared to SFM and control, since BSM specifically aimed to enhance self-efficacy in changing weight-related behaviors. Correspondingly, we expected SFM to particularly benefit those with poorer initial peer support and higher initial eating-related psychopathology (including shape and weight concerns) as compared to BSM and control, since SFM specifically sought to improve functioning in these domains.
Method
Participants
Participants were 150 overweight children (20–100% above their age- and sex-specific median BMI), aged 7–12, with at least one overweight parent (BMI≥25), who were involved in our randomized controlled trial of MT interventions following FBT (Wilfley, Stein, et al., 2007). Table 1 presents sample descriptive statistics. Participants were recruited through local media outlets, community organizations, and pediatric clinics in and around San Diego, California. Child and parent exclusion criteria included medical or psychiatric disturbances that would preclude treatment participation; use of appetite- and/or weight-affecting medications; and concurrent involvement in weight loss or psychological treatment.
Table 1.
Descriptive statistics for predictor and outcome variables at baseline and randomization [post-family-based weight loss treatment (FBT)]
| Baseline | Randomization | |||||
|---|---|---|---|---|---|---|
|
| ||||||
| Variable | Total N | M (SD) or % (n) | Sample range | Total N | M (SD) | Sample range |
| Demographics | ||||||
|
| ||||||
| Child age | 150 | 9.85 (1.30) | 6.95–12.21 | --- | --- | |
| Child sex, % female | 150 | 69.3 (104) | --- | --- | --- | |
| Child White/non-Hispanic | 68.7 (103) | --- | --- | --- | --- | |
| race/ethnicity, % White/Hispanic | 150 | 18.7 (28) | --- | --- | --- | --- |
| Black | 7.3 (11) | --- | --- | --- | --- | |
| Other | 5.3 (8) | --- | --- | --- | --- | |
| Family socioeconomic status | 150 | 47.33 (11.13) | 22.00 – 66.00 | --- | --- | --- |
|
| ||||||
| Anthropometries | ||||||
|
| ||||||
| Child z-BMI | 150 | 2.21 (0.30) | 1.11 – 2.77 | 150 | 1.99 (0.38) | 0.60 – 2.66 |
| Δ z-BMI from baseline to randomization | -- | -- | -- | 150 | −0.22 (0.17) | −0.89 – 0.08 |
| Parent BMI | 150 | 34.91 (6.33) | 21.82 – 57.11 | 150 | 32.94 (6.04) | 21.37 – 55.60 |
| Δ z-BMI from baseline to randomization | -- | -- | -- | 150 | −1.96 (1.75) | −7.74 – 1.50 |
|
| ||||||
| Child psychosocial measures | ||||||
|
| ||||||
| CDSS dietary self-efficacy | 148 | 5.56 (4.42) | −7.00 – 11.00 | 143 | 7.11 (3.50) | −4.00 – 11.00 |
| SESCPA overcoming barriers | 148 | 2.56 (1.22) | 0.00 – 4.00 | 140 | 2.76 (1.20) | 0.00 – 4.00 |
| SSEHS family participation | 141 | 27.68 (9.55) | 10.00 – 50.00 | 134 | 30.43 (9.59) | 10.00 – 50.00 |
| SSEHS family encouragement | 142 | 15.92 (4.86) | 5.00 – 25.00 | 134 | 19.02 (4.99) | 5.00 – 25.00 |
| ChEDE global severity | 148 | 0.92 (0.62) | 0.00 – 4.30 | 144 | 0.96 (0.67) | 0.00 – 3.53 |
| CBCL total problems | 149 | 54.00 (10.11) | 32.00 – 73.00 | 145 | 47.98 (9.52) | 32.00 – 73.00 |
|
| ||||||
| Parent psychosocial measures | ||||||
|
| ||||||
| EHCS eating self-efficacy | 147 | 3.86 (0.55) | 2.60 – 5.00 | 147 | 3.70 (0.70) | 2.00 – 5.00 |
| EHCS exercise self-efficacy | 142 | 3.88 (0.68) | 1.25 – 5.00 | 135 | 3.33 (0.87) | 1.00 – 5.00 |
| EHCS reducing calories self-efficacy | 146 | 4.27 (0.55) | 2.20 – 5.00 | 143 | 4.01 (0.66) | 2.40 – 5.00 |
| SSEHS family participation | 148 | 20.98 (7.05) | 10.00 – 40.00 | 135 | 23.45 (7.35) | 10.00 – 45.00 |
| SSEHS family encouragement | 148 | 11.36 (4.79) | 5.00 – 25.00 | 135 | 15.38 (4.98) | 5.00 – 25.00 |
| BSI global severity | 147 | 48.78 (10.67) | 33.00 – 72.00 | 146 | 46.00 (10.44) | 33.00 – 76.00 |
| EDE-Q global score | 145 | 2.21 (0.91) | 0.09 – 4.18 | 143 | 2.03 (0.81) | 0.18 – 4.18 |
Note: BMI=body mass index (kg/m2); CDSS=Child Dietary Self-Efficacy Scale (range=−15 to 15); SESCPA=Self-Efficacy Scale for Children's Physical Activity (range=0–4); SSEHS=Social Support for Eating/Exercise Habits Survey (range for family participation subscale=10–50; range for family encouragement subscale=5–25); ChEDE=Child Eating Disorder Examination (range=0 to 6); CBCL=Child Behavior Checklist (range=0 to 100); EHCS=Eating Habits Confidence Survey (range=1–5); BSI=Brief Symptom Inventory (range=0 to 80); EDE-Q=Eating Disorder Examination-Questionnaire (range=0 to 6).
Procedures
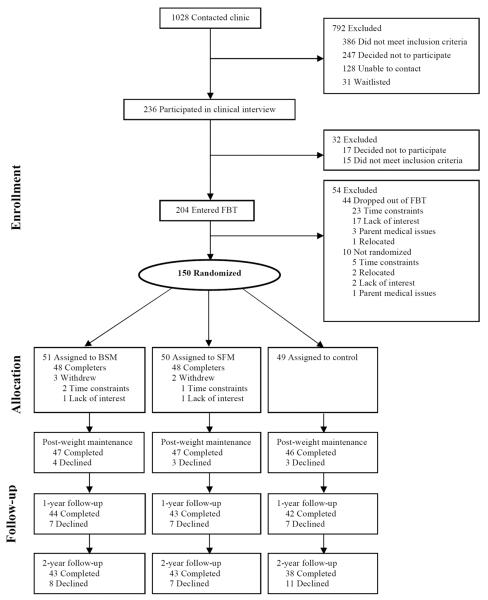
After completing FBT (described below), families were randomized to one of three MT conditions: BSM, SFM, or a no-MT control group. Psychosocial and anthropometric data were obtained at baseline (month 0; prior to initiating FBT), randomization (month 5; post-FBT), post-weight maintenance (month 9), 1-year follow-up (month 17; 1-year post-FBT), and 2-year follow-up (month 29; 2 years post-FBT). A total of 204 families entered FBT, and 150 were ultimately randomized to MT conditions. The present study analyses utilized the sample of 150 participants randomized to MT conditions. Due to attrition (and after linearly interpolating missing data based on observed values at immediately preceding and ensuing time points), weight data were available for 145 children at post-weight maintenance (96.7% of those randomized); 137 children at 1-year follow-up (91.3% of those randomized), and 124 children at 2-year follow-up (82.7% of those randomized; see Figure 1). Children and parents provided written informed assent and consent, respectively. The study received Institutional Review Board approval.
Figure 1.
Participant flowchart
Note: FBT=Family-based behavioral weight loss treatment; BSM=Behavioral skills maintenance; SFM=Social facilitation maintenance. Follow-up numbers listed as “completed” indicate the number of observed data points and do not include the number of participants available for analysis based on interpolation. The total number available for analyses is reported in Table 1. Adapted from “Efficacy of maintenance treatment approaches for childhood overweight: A randomized controlled trial,” by D. E. Wilfley, R. I. Stein, B. E. Saelens, D. S. Mockus, G. E. Matt, H. A. Hayden Wade… L. H. Epstein, 2007, Journal of the American Medical Association, 298, p. 1663. Copyright 2007 by the American Medical Association.
Interventions
FBT and both MTs consisted of 20-minute individual family (typically parent-child dyad) sessions, and 40-minute concurrent child and parent group sessions. Group FBT sessions focused on basic weight control strategies (see below for modality-specific MT content). Group session content was similar for parents and children, except that parents received additional information on effective parenting skills related to the content. Family sessions reinforced group session content and provided more individualized treatment (e.g., addressing barriers to compliance).
FBT consisted of 20 weekly sessions delivered over 5 months. FBT targets both parents and children and addresses dietary and activity-related change through the use of behavioral skills strategies for weight control in the family context (Goldfield & Epstein, 2002). Behavioral change strategies included self-monitoring of food intake, physical activity, and weight; parental modeling of healthy weight-related behaviors; positive reinforcement; and stimulus control (e.g., changing the home environment). The dietary component of treatment utilized the Traffic Light approach (Goldfield & Epstein, 2002) to reduce caloric intake, change taste preferences, and improve nutrient quality. Physical activity was mastery-based with an ultimate goal of 90 minutes of moderate to intense activity, at least 5 days/week.
The two MT approaches consisted of 16 weekly sessions delivered over 4 months. Although both MT approaches focused on achieving energy balance for weight maintenance, they were theoretically and procedurally distinct: BSM focused on helping families develop behavioral weight maintenance skills, and SFM focused on helping families change their social environment and body image to support weight maintenance. The control group received no further treatment contact after FBT.
Measures
Demographic Measures
Child and parent weight and height were measured by trained research assistants using a calibrated balance beam scale and stadiometer. Child z-BMI [body mass index (kg/m2) z-score] was calculated using age- and sex-specific CDC normative data (Kuczmarski et al., 2000). We chose to use z-BMI as a measure of relative weight because it not only considers the normative data based on child age and sex, but also the distribution of the normative reference data (Whitlock, O'Connor, Williams, Beil, & Lutz, 2008). Socioeconomic status (SES) was determined using the Hollingshead four-factor index (Hollingshead, 1975).
Self-Efficacy
Children self-reported their perceived efficacy in choosing healthy foods on the Child Dietary Self-Efficacy Scale (CDSS; Parcel et al., 1995; current study α=.83). The child-reported Self-Efficacy Scale for Children's Physical Activity (SESCPA; Saunders et al., 1997; current study KR-20=.75) assessed children's perceived efficacy in overcoming barriers to physical activity. Both measures have good reliability and validity in children (Parcel et al., 1995; Saunders et al., 1997).
The Eating/Exercise Habits Confidence Survey (EHCS; Sallis, Grossman, Pinski, Patterson, & Nader, 1988) measured parents' self-reported self-efficacy with regards to eating, exercise, and reducing calories. This measure has acceptable psychometric properties (Sallis et al., 1988; current study α=.88).
Social Support
Social support received for eating and physical activity were self-reported separately by children and parents using the Social Support for Eating/Exercise Habits Survey (SSEHS; Sallis, Grossman, Pinski, Patterson, & Nader, 1987). The family participation subscale, which measures familial involvement in physical activity with the respondent, was used in the present analyses, along with the family encouragement subscale, which measures perceived familial support for the respondent's healthy eating behaviors. The scale has good criterion and discriminant validity in adults (Sallis et al., 1987; current study α=.89) and has been successfully adapted for children (Pietrobelli, Leone, Heymsfield, & Faith, 1998; current study α=.88).
Psychological Functioning
The Child Eating Disorder Examination (ChEDE; Bryant-Waugh, Cooper, Taylor, & Lask, 1996) is a semi-structured interview used to assess eating disorder symptoms. The global severity score, which comprises an average of the dietary restraint, eating concern, weight concern, and shape concern subscales, was included in the present analyses. The ChEDE has good reliability and validity (Bryant-Waugh et al., 1996; Watkins, Frampton, Lask, & Bryant-Waugh, 2005; current study global score α=.85). The Child Behavior Checklist (CBCL; Achenbach, 1991) is a parent-reported measure of child competency and functioning in a range of behavioral domains. The CBCL has demonstrated good reliability and validity (Achenbach, 1991). For the purposes of the present study, only the total problems scale was examined (current study α unavailable; published scale α=.97).
The Global Severity Index of the self-reported Brief Symptom Inventory (BSI; Derogatis, 1991) was used to measure parent psychopathology. The BSI has good psychometric properties (Derogatis, 1991; current study α=.93). The global score of the Eating Disorder Examination-Questionnaire (EDE-Q; Fairburn & Beglin, 1994) measured severity of parent eating disorder symptoms. The EDE-Q has adequate reliability and validity (Berg, Peterson, Frazier, & Crow, 2012; current study α=.87).
Statistical Analysis
The present analyses included demographic and psychosocial data collected at baseline and randomization, and anthropometric data collected at all available time points. Correlations among the psychosocial measures were in the small to moderate range (absolute value .26 to .49). We first used bivariate correlations to identify whether any of the child or parent baseline demographic, anthropometric, or psychosocial variables, as well as parent BMI change from baseline to MT randomization, were associated with change in children's z-BMI from baseline to randomization. Significant correlates were then entered simultaneously into a hierarchical linear regression model to determine whether they were unique predictors of child z-BMI change from baseline to randomization.
Second, to determine long-term general predictors and treatment-specific moderators of outcome, we used mixed effects repeated measures analysis of covariance (ANCOVA) via the SPSS 21.0 MIXED procedure. This analytic approach is recommended for study designs in which assessments occur pre- and post-treatment, as well as through follow-up periods, but do not include assessments during treatment (Wolitzky-Taylor, Arch, Rosenfield, & Craske, 2012). In these analyses, change in child z-BMI from baseline to randomization and child z-BMI at randomization were entered as covariates. In order to enhance the precision of the parameter estimates and variances of the analyses (Singer & Willett, 2003), the time-variant dependent variable included all post-randomization time points (i.e., post-weight maintenance, 1- and 2-year follow-up). In cases in which there was a significant predictive or moderating effect, planned contrasts examined only the post-weight maintenance and 2-year follow-up time points. The potential predictor/moderator (hereafter referred to simply as “predictor”) measured at randomization, as well as the MT condition (i.e., BSM, SFM, or control), were entered as the between-subjects independent variables. A similar pattern of results was obtained whether using predictors measured at baseline or at randomization, with the exception that when examining baseline predictors, child eating pathology no longer significantly moderated MT outcome (although findings were in the same direction; p=.16). We chose to report results based on predictors measured at randomization since these variables are likely to change during FBT and, practically, a clinician would be more likely to use the most recent data to inform MT planning. The repeated measures ANCOVAs used maximum likelihood estimation, which involves an implicit form of imputation, to include all participants who had data at randomization and at one (or more) follow-up time points (i.e., post-weight maintenance; 1-year and/or 2-year follow-up). We specified a first-order autoregressive covariance matrix, which accounts for correlations among within-subjects error residuals across time points. Denominator degrees of freedom were calculated from the Satterthwaite approximation (Satterthwaite, 1946), which yields more precise values. A separate repeated measures ANCOVA was performed for each potential predictor, and each ANCOVA included all possible main effects (time, treatment condition, predictor), two-way interactions (predictor × time, predictor × treatment condition, treatment condition × time), and the three-way interaction (predictor × time × treatment condition). Given that we have previously reported the main outcomes of treatment group on weight maintenance over time (Wilfley, Stein, et al., 2007), in the current study, we only report significant effects of the predictor, predictor × time, predictor × treatment condition, and predictor × time × treatment condition. A significant predictor × treatment condition or predictor × treatment condition × time effect indicates moderation. Effect sizes (P2) for significant effects were determined by computing the proportion of the residual variance in time that was accounted for when including the significant effect versus excluding that effect from the model (Singer & Willett, 2003). P2 can be interpreted similarly to R2 in linear regression analysis (Wolitzky-Taylor et al., 2012)
We used model-based estimations of simple slopes and treatment group effects at low (−1 SD) and high (+1 SD) levels of the predictor to illustrate significant two- and three-way interactions (see Wolitzky-Taylor et al., 2012, for a discussion of this approach). In reporting these simple slopes, we provide the unstandardized beta (B), its 95% confidence interval and its significance value. When a higher-order interaction was significant (e.g., 3-way interaction), we do not report significant lower-order interactions (2-way interactions) or main effects. With a correlation between repeated z-BMI measures of approximately 0.85, the sample size provides at least 80% power to detect an effect size of .04 (either R2 or P2; Faul, Erdfelder, Lang, & Buchner, 2007).
Results
Preliminary Analyses
All variables were initially screened for departures from normality, and log10 transformations were applied to all variables with significant skew (i.e., skewness values≥|1|), which included: change in z-BMI from baseline to randomization; and baseline and randomization ChEDE global score. These transformed values were no longer skewed and were used in subsequent analyses. Furthermore, all continuous variables were standardized (M=0, SD=1) before analysis to aid interpretation of beta coefficients.
Predictors of Weight Loss Following FBT
Bivariate correlations revealed that the following baseline variables were significantly correlated with children's reduction in z-BMI from baseline to randomization, with negative correlations indicating that lower values of the construct are associated with greater reduction in z-BMI, and positive correlations indicating that higher values of the construct are associated with greater reductions in z-BMI: baseline z-BMI (r=−.19, p=.02), child age (r=−.17, p=.04), parents' self-reported encouragement from family (r=−.18, p=.03) and from friends (r=−.17, p=.04), and parents' self-reported efficacy to reduce calories (r=.27, p=.001). That is, lower child baseline z-BMI and age, lower baseline parent-reported encouragement, and higher baseline parent-reported self-efficacy were associated with greater z-BMI reductions from baseline to randomization. Additionally, parents' reduction in BMI from baseline to randomization was significantly correlated with children's z-BMI change from baseline to randomization, such that greater parent BMI reduction was related to greater child z-BMI reduction (r=.30, p<.001).
To determine the unique contributions of these variables to children's z-BMI change from baseline to randomization, we created a two-step hierarchical regression model in which the baseline predictors were entered in step 1 and parents' change in BMI from baseline to randomization was entered in step 2 (see Table 2). Overall, the regression model was significant [F(6,137)=7.77; p<.001] and explained 25% of the variance in children's z-BMI change from baseline to randomization. Higher baseline z-BMI and older child age predicted smaller z-BMI changes from baseline to randomization, whereas greater parent self-reported efficacy to reduce calories predicted greater z-BMI change in their children from baseline to randomization. Parents' reported family encouragement and friend encouragement were not significant predictors in this model (ps>.09). Parents' weight loss remained a significant predictor of their children's z-BMI change from baseline to randomization, accounting for 8% of the variance in children's z-BMI change during FBT after accounting for the baseline predictors.
Table 2.
Prediction of child z-BMI changes from baseline to randomization [post-family-based weight loss treatment (FBT)]
| Variable | B | SE | B | p value | Δ R 2 |
|---|---|---|---|---|---|
| Step 1: Baseline variables | .17 | ||||
| Child z-BMI | −.57 | .26 | −.18 | .029 | |
| Child age | −.16 | .06 | −.21 | .008 | |
| Parent SSEHS Family Encouragement | −.14 | .08 | −.14 | .094 | |
| Parent SSEHS Friend Encouragement | −.09 | .08 | −.09 | .292 | |
| Parent EHCS Efficacy to Reduce Calories | .21 | .08 | .22 | .006 | |
|
| |||||
| Step 2: Parent weight loss | .08 | ||||
| Parent reduction in BMI | .17 | .04 | .31 | <.001 | |
Note: BMI=body mass index (kg/m2); SSEHS=Social Support for Eating/Exercise Habits Survey; EHCS=Eating Habits Confidence Survey. Positive coefficients indicate that higher levels of the predictor are associated with greater z-BMI reduction.
Predictors and Moderators of Long-term Weight Maintenance
Two statistical outliers were identified, who showed extreme child z-BMI values at 2-year follow-up. As reported by Wilfley and colleagues (2007), both cases were highly influential on the primary outcome analysis, and therefore, were excluded from the present analysis. They were included in analyses examining predictors of FBT outcome because the extreme scores were evident at follow-up and not during FBT.
Anthropometrics and Weight Loss
Child z-BMI change from baseline to randomization interacted with time to predict weight maintenance from randomization through the follow-up periods [F(2,263.56)=11.12; p<.001; P2=4.2%; see Figure 2]. Children with initially greater reductions in z-BMI (from baseline to randomization) regained less relative weight from randomization to post-weight maintenance (B=−0.05; 95% CI=−0.09 to 0.003; p=.04) but regained more relative weight from randomization to 2-year follow-up (B=0.11; 95% CI=0.07 to 0.15; p<.001). Overall change in z-BMI (from baseline to 2-year follow-up) was substantially greater for children with greater initial (baseline to randomization) reductions in z-BMI than for children who initially lost less relative weight; however, those with greater initial z-BMI reductions regained more relative weight after randomization through 2-year follow-up.
Figure 2.
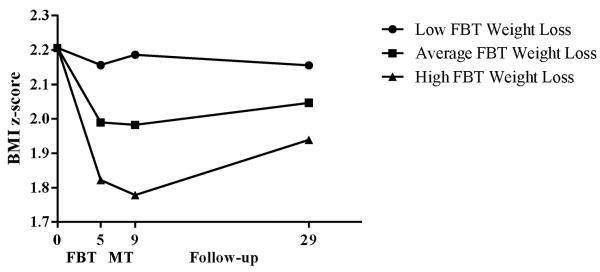
Children's weight loss during family-based behavioral weight loss treatment as a predictor of long-term weight maintenance
Note: FBT=Family-based behavioral weight loss treatment; MT=Maintenance treatment.
The two-way interaction between parent BMI at randomization and time was significant [F(2,263.85)=5.24; p=.002; P2=5.4%]. Higher parent BMI at randomization predicted greater relative weight regain in their children from randomization to 2-year follow-up (B=0.08; 95% CI=0.04 to 0.11; p<.001), but not from randomization to post-weight maintenance (p=.55). No other child or parent anthropometric variables or changes in these variables were significant predictors or moderators.
Demographics
No demographic variables were significant predictors or moderators.
Child Psychosocial Variables
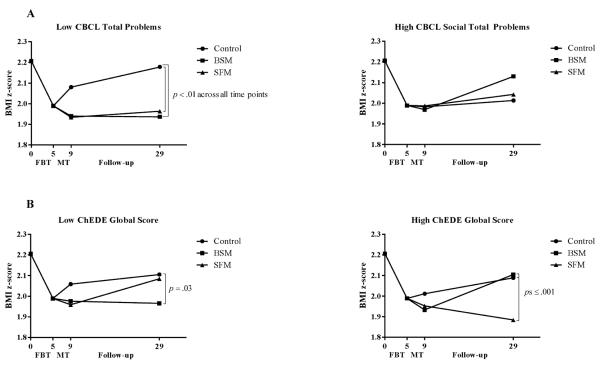
A significant CBCL child total problems × treatment condition interaction was observed for children's z-BMI change from randomization through the follow-up periods [F(2,147.84)=7.37; p<.001; P2=6.9%]. Among children with low total problems at randomization (−1 SD; left panel of Figure 3A), those assigned to the control condition regained more relative weight than did children in either BSM (B=0.18; 95% CI=0.06 to 0.29; p=.002) or SFM (B=0.19; 95% CI=0.08 to 0.30; p=.001) from randomization through follow-up. This pattern was similar across the post-weight maintenance and 2-year follow-up time points. Among children with high total problems at randomization (+1 SD; right panel of Figure 3A), there were no differences in relative weight regain among the treatment conditions (ps>.28). Thus, BSM and SFM were most effective among those with low initial total problems.
Figure 3.
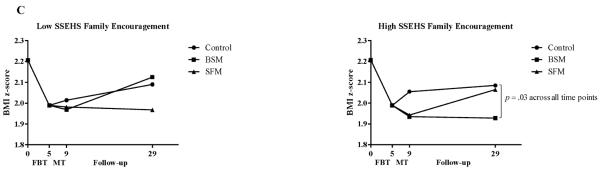
Child and parent moderators of long-term weight maintenance
Note: CBCL=Child Behavior Checklist; BSM=Behavioral skills maintenance; SFM=Social facilitation maintenance; FBT=Family-based behavioral weight loss treatment; MT=Maintenance treatment; ChEDE=Child Eating Disorder Examination; SSEHS=Social Support for Eating/Exercise Habits Survey. Z-BMI values are estimated from models that contain change in children's z-BMI during FBT (months 0 to 5) and children's z-BMI at randomization to MT (5 months) as covariates. Analyses were conducted on continuously-measured variables and with all available subjects included; categorical splits in the figures are for illustrative purposes only.
There was a significant ChEDE global score × time × treatment condition effect for children's z-BMI change from randomization through the follow-up periods [F(4,255.88)=3.48; p=.009; P2=3.7%]. Among children with low eating pathology at randomization (−1 SD), children in BSM regained less relative weight from randomization to 2-year follow-up compared to children in the control condition (B=−0.14; 95% CI=−0.27 to 0.01; p=.03) but did not differ from children in SFM (p=.07; see Figure 3B). Among children with high eating pathology at randomization (+1 SD), those assigned to the control condition (B=0.20; 95% CI=0.08 to 0.33; p<.001) and to BSM (B=0.22; 95% CI=0.09 to 0.35; p=.001) had regained more relative weight than had children in SFM from randomization to 2-year follow-up. No other child-reported psychosocial variables were significant predictors or moderators.
Parent Psychosocial Variables
We observed a significant SSEHS parent-reported family encouragement × treatment condition effect [F(2,141.65)=3.28; p=.04; P2=2.9%; see Figure 3C]. For children of parents who reported low family encouragement to engage in healthy eating behaviors at randomization (−1 SD), there were no differences in weight regain across the follow-up time points (ps>.12). However, among parents with high family encouragement at randomization (+1 SD), children in BSM showed less relative weight regain than did children in the control condition (B=−0.13; 95% CI=−0.24 to −0.02; p=.03) from randomization across the follow-up assessments, whereas children in SFM had intermediate relative weight regain and did not differ from children in the control condition (p=.20) or from children in BSM (p=.32).
Multiple Predictor Analysis
In a final analysis, we entered all significant moderators and their respective interaction terms, along with change in child z-BMI from baseline to randomization, into a single mixed model to determine unique associations. This model explained 17% of the residual variance in the repeated measure of z-BMI. The results of this model mirrored the findings above in that the following terms remained significant: child z-BMI change × time (p<.001); CBCL child total problems × treatment condition (p=.002); ChEDE global score × time × treatment condition (p=.05); and SSEHS parent-reported family encouragement × treatment condition (p=.04).
Discussion
This study aimed to investigate general and treatment-specific predictors of outcome in a pediatric weight control trial. Among FBT completers, children's initial change in z-BMI during FBT and parent's BMI at randomization, as well as certain psychosocial factors that were targeted by the maintenance treatments, appeared to have a significant impact on outcome across the 2-year follow-up period; other hypothesized predictors (e.g., self-efficacy) were not associated with outcome, perhaps because of overlap in some of the maintenance treatment targets (e.g., focus on achieving energy balance for weight maintenance). Our data have important clinical implications in terms of identifying youth who are likely to respond to treatment, and how to best match them to maintenance interventions.
We found that among FBT completers, lower initial child z-BMI and younger child age predicted better short-term weight loss, which replicates most of the existing literature (Braet, 2006; Epstein, Valoski, et al., 1994; Goossens et al., 2009; Reinehr et al., 2010; Sabin et al., 2007) and highlights the importance of early intervention as a way to capitalize on children's natural growth and weight-related habits that are less ingrained and more amenable to change (Goldschmidt, Wilfley, Paluch, Roemmich, & Epstein, 2013). Contradictory findings from previous studies that have found higher relative weight to predict better outcome may be related to differing methods of assessing relative body weight (e.g., using percent over median BMI versus z-BMI, which have different distributions); this speaks to the importance of standardizing measures of children's relative body weight across studies. Child relative weight change during FBT predicted long-term weight maintenance (from randomization to 2-year follow-up). Although completers who lost more weight during FBT experienced some weight regain over two years, their overall weight loss still exceeded that of children who initially responded less favorably to FBT. These results are consistent with previous work (Jelalian et al., 2008) indicating that initial response strongly predicts long-term outcome. Generally, findings suggest that clinicians should facilitate early behavior change to improve long-term outcome among those who have completed weight loss treatment. Indeed, given that parents' reported self-efficacy to reduce calories was a general predictor of FBT outcome, providers may wish to focus on promoting familial self-efficacy as a means to improve children's treatment outcome.
Parent BMI and BMI change were also important predictors of short- and long-term outcome among FBT completers. Consistent with previous findings (Wrotniak et al., 2004), parent weight change during FBT was associated with children's weight change during that same timeframe. This may reflect the importance of parenting changes such as modeling of healthy weight control behaviors. Further, children of parents with a higher BMI at randomization had greater long-term relative weight regain, which could indicate that heavier parents, like heavier children, had a more difficult time sustaining behavior changes in the long-term. These families may require more intensive or different support through maintenance in order to persist with weight control behaviors and establish lifestyle changes.
Important novel findings are that a socially-focused approach was particularly potent in sustaining children's weight status improvements in the long-term among those FBT completers who presented with initially higher impairments in some psychosocial domains (e.g., eating pathology; social problems as found in our previous study; Wilfley, Stein, et al., 2007). Most notably, children with higher eating pathology at randomization regained less relative weight following SFM than did similar children assigned to BSM or the control condition, perhaps because SFM promotes positive body image, which may be a barrier to physical activity among overweight children (Hayden-Wade et al., 2005). Conversely, SFM may reduce interpersonally-driven overeating (Elliott et al., 2010) via its focus on improving overall social functioning (e.g., positive responding to teasing, encouragement to engage in more social or peer-based activities). Overall, when considered in conjunction with our previous findings that children with low social problems particularly benefited from SFM, results cumulatively suggest that SFM capitalizes on existing skills in some areas, but also benefits children with impairments in others.
By contrast, BSM particularly benefited FBT completers whose parents reported higher familial encouragement for healthy weight-related behaviors. In other words, families that were able to accumulate adequate social support by the time they were assigned to BSM had better long-term child weight outcomes. This finding suggests that standard FBT should enhance its focus on building familial social support for behavior change in order to improve long-term weight maintenance in families receiving an extended form of behavioral treatment. Alternatively, as BSM introduced less new content than SFM given BSM's focus on extending previously-learned FBT skills to weight maintenance, it may be that this less “intensive” treatment is sufficient for children living within a more supportive social environment.
Interestingly, completers with lower parent-reported behavioral problems had poorer long-term weight outcomes when not receiving any MT, which may seem counterintuitive: one would expect children with fewer behavioral problems to show better weight maintenance despite having less prolonged support. It is possible that greater behavioral problems or psychopathology served as an indirect way to bring parents' attention to children's need for support around long-term weight maintenance. That is, parents of children with fewer behavioral problems may mistakenly assume that their children no longer need support for weight control following FBT if they are functioning well in other aspects of their lives. Conversely, this could have been a spurious finding, thus highlighting the need for replication.
Conclusion
This was the first study, to our knowledge, that examined predictors of children's weight outcomes following maintenance treatments designed to extend the effects of standard FBT. Our data provide important clinical information concerning how to optimally match children to maintenance treatments. Treatment completers with greater initial eating pathology may be better suited for MTs, such as SFM, that focus on enhancing social support and improving self-perceptual factors related to weight maintenance, including body image, whereas those with higher initial family support may be best served by treatments such as BSM, that focus on extending FBT skills to weight maintenance. Alternatively, an integrated intervention approach allowing for individualized tailoring might be suitable, given that FBT completers (on average) benefited from both maintenance treatments, but children with some presenting profiles might require a more intensive focus on certain skills than others; this should be tested in future studies. Moreover, those with fewer behavioral problems may need additional ongoing support around weight maintenance despite functioning quite well in other domains. In terms of general predictors of outcome, initial relative weight loss and parent weight status appear to be particularly important in minimizing children's weight regain. Overall, the current findings are strengthened by the large sample size, the use of well-validated measures, and the availability of long-term follow-up data with high retention. Indeed, this study makes an important contribution to the literature on predictors of children's weight loss treatment outcome, which has largely focused on short-term findings and has not included a MT component.
Nevertheless, our study had several limitations that warrant discussion. First, because there was no ongoing measurement of psychosocial factors during treatment, we were unable to explore mediators of treatment outcome, which are crucial to understanding the mechanisms by which FBT and MTs achieve their effects. We also could not examine interactions among predictors/moderators, which should be addressed in future study designs. Relatedly, we reported on moderators measured at post-FBT because randomization to maintenance treatment occurred at that time point; however, this may not be desirable for clinicians seeking to match patients to treatments at initial presentation. Results were similar whether examining moderators assessed at baseline or post-FBT, but future studies may wish to further explore the optimal time point at which to match patients to treatment based on personal characteristics. Second, a subset of families were lost to follow-up (most of whom were assessment non-completers or FBT dropouts and hence not randomized to a maintenance condition), thus it is unclear if results are generalizable to families who have fewer resources and/or less motivation to continue treatment past the standard five months. On a related note, although our sample was more demographically diverse than those included in many previous pediatric weight control studies, participants tended to be female (69.3%) and Caucasian (68.7%) and thus, results should be replicated with even more diverse samples. Finally, although theoretically-driven, a large number of analyses were undertaken. This approach was not unreasonable given that this was the first study, to our knowledge, exploring predictors of MT outcome and was meant to identify factors that could potentially be manipulated in future pediatric weight control trials; however, this again underscores the need for replication of our findings.
Tailoring interventions to personal characteristics (including children's behavioral problems and eating pathology, and parents' self-efficacy) may be one method for improving treatment outcome among overweight youth. Our data highlight several factors in both children and parents that may be easily measured and assist with treatment planning in order to minimize weight regain over the long-term. Future studies should seek to further enhance our understanding of the means by which these factors exert their influence.
Acknowledgements
This work was supported by NIH grants R01 HD036904, K24 MH070446, and T32 HL007456, and NCRR grants KL2 RR025000, KL2 RR024994, and UL1 RR024992
References
- Achenbach TM. Manual for the Child Behavior Checklist/4–18 and 1991 profile. University of Vermont Department of Psychiatry; Burlington, VT: 1991. [Google Scholar]
- Berg KC, Peterson CB, Frazier P, Crow SJ. Psychometric evaluation of the eating disorder examination and eating disorder examination-questionnaire: A systematic review of the literature. International Journal of Eating Disorders. 2012;45:428–438. doi: 10.1002/eat.20931. doi:10.1002/eat.20931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Best JR, Theim KR, Gredysa DM, Stein RI, Welch RR, Saelens BE, Wilfley DE. Behavioral economic predictors of overweight children's weight loss. Journal of Consulting and Clinical Psychology. 2012;80:1086–1096. doi: 10.1037/a0029827. doi:10.1037/a0029827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Braet C. Patient characteristics as predictors of weight loss after an obesity treatment for children. Obesity. 2006;14:148–155. doi: 10.1038/oby.2006.18. doi:10.1038/oby.2006.18. [DOI] [PubMed] [Google Scholar]
- Bryant-Waugh RJ, Cooper PJ, Taylor CL, Lask BD. The use of the Eating Disorder Examination with children: A pilot study. International Journal of Eating Disorders. 1996;19:391–397. doi: 10.1002/(SICI)1098-108X(199605)19:4<391::AID-EAT6>3.0.CO;2-G. doi:10.1002/(SICI)1098-108X(199605)19:4<391∷AID-EAT6>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- Derogatis LR. The Brief Symptom Inventory (BSI); Administration, Scoring and Procedures Manual-II. Clinical Psychometric Research Inc.; Baltimore, MD: 1991. [Google Scholar]
- Elliott CA, Tanofsky-Kraff M, Shomaker LB, Columbo KM, Wolkoff LE, Ranzenhofer LM, Yanovski JA. An examination of the interpersonal model of loss of control eating in children and adolescents. Behaviour Research and Therapy. 2010;48:424–428. doi: 10.1016/j.brat.2009.12.012. doi:10.1016/j.brat.2009.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Koeske R, Wing RR, Valoski A. The effect of family variables on child weight change. Health Psychology. 1986;5:1–11. doi: 10.1037//0278-6133.5.1.1. doi:10.1037/0278-6133.5.1.1. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Raja S, Daniel TO, Paluch RA, Wilfley DE, Saelens BE, Roemmich JN. The built environment moderates effects of family-based childhood obesity treatment over 2 years. Annals of Behavioral Medicine. 2012;44:248–258. doi: 10.1007/s12160-012-9383-4. doi:10.1007/s12160-012-9383-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein LH, Valoski AM, Wing RR, McCurley JJ. Ten-year outcomes of behavioral family-based treatment of childhood obesity. Health Psychology. 1994;13:373–383. doi: 10.1037//0278-6133.13.5.373. doi:10.1037/0278-6133.13.5.373. [DOI] [PubMed] [Google Scholar]
- Epstein LH, Wisniewski L, Weng R. Child and parent psychological problems influence child weight control. Obesity Research. 1994;2:509–515. doi: 10.1002/j.1550-8528.1994.tb00099.x. doi:10.1002/j.1550-8528.1994.tb00099.x. [DOI] [PubMed] [Google Scholar]
- Fairburn CG, Beglin SJ. Assessment of eating disorders: Interview or self-report questionnaire? International Journal of Eating Disorders. 1994;16:363–370. doi:10.1002/1098-108X(199412)16:4<363∷AID-EAT2260160405>3.0.CO;2-#. [PubMed] [Google Scholar]
- Faul F, Erdfelder E, Lang AG, Buchner A. G*Power 3: A flexible statistical power analysis program for the social, behavioral, and biomedical sciences. Behav Res Methods. 2007;39:175–191. doi: 10.3758/bf03193146. [DOI] [PubMed] [Google Scholar]
- Frohlich G, Pott W, Albayrak O, Hebebrand J, Pauli-Pott U. Conditions of long-term success in a lifestyle intervention for overweight and obese youths. Pediatrics. 2011;128:e779–785. doi: 10.1542/peds.2010-3395. doi:10.1542/peds.2010-3395. [DOI] [PubMed] [Google Scholar]
- Goldfield GS, Epstein LH. Management of obesity in children. In: Fairburn CG, Brownell KD, editors. Eating disorders and obesity: A comprehensive handbook. 2nd ed. Guilford Press; New York, NY: 2002. pp. 573–577. [Google Scholar]
- Goldschmidt AB, Stein RI, Saelens BE, Theim KR, Epstein LH, Wilfley DE. Importance of early weight change in a pediatric weight management trial. Pediatrics. 2011;128:e33–39. doi: 10.1542/peds.2010-2814. doi:10.1542/peds.2010-2814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldschmidt AB, Wilfley DE, Paluch RA, Roemmich JN, Epstein LH. Indicated prevention of adult obesity: How much weight change is necessary for normalization of weight status in children? Archives of Pediatrics and Adolescent Medicine. 2013;167:21–26. doi: 10.1001/jamapediatrics.2013.416. doi:10.1001/jamapediatrics.2013.416. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goossens L, Braet C, Van Vlierberghe L, Mels S. Weight parameters and pathological eating as predictors of obesity treatment outcome in children and adolescents. Eating Behaviors. 2009;10:71–73. doi: 10.1016/j.eatbeh.2008.10.008. doi:10.1016/j.eatbeh.2008.10.008. [DOI] [PubMed] [Google Scholar]
- Hayden-Wade HA, Stein RI, Ghaderi A, Saelens BE, Zabinski MF, Wilfley DE. Prevalence, characteristics, and correlates of teasing experiences among overweight children vs. non-overweight peers. Obesity Research. 2005;13:1381–1392. doi: 10.1038/oby.2005.167. doi:10.1038/oby.2005.167. [DOI] [PubMed] [Google Scholar]
- Hollingshead A. Four factor index of social status. Yale University; 1975. [Google Scholar]
- Jelalian E, Hart CN, Mehlenbeck RS, Lloyd-Richardson EE, Kaplan JD, Flynn-O'Brien KT, Wing RR. Predictors of attrition and weight loss in an adolescent weight control program. Obesity. 2008;16:1318–1323. doi: 10.1038/oby.2008.51. doi:10.1038/oby.2008.51. [DOI] [PubMed] [Google Scholar]
- Kraemer HC, Wilson GT, Fairburn CG, Agras WS. Mediators and moderators of treatment effects in randomized clinical trials. Archives of General Psychiatry. 2002;59:877–883. doi: 10.1001/archpsyc.59.10.877. doi:10.1001/archpsyc.59.10.877. [DOI] [PubMed] [Google Scholar]
- Kuczmarski RJ, Ogden CL, Grummer-Strawn LM, Flegal KM, Guo SS, Wei R, Johnson CL. CDC growth charts: United States. Advance Data. 2000;314:1–27. [PubMed] [Google Scholar]
- Moens E, Braet C, Van Winckel M. An 8-year follow-up of treated obese children: Children's, process and parental predictors of successful outcome. Behaviour Research and Therapy. 2010;48:626–633. doi: 10.1016/j.brat.2010.03.015. doi:10.1016/j.brat.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Ogden CL, Carroll MD, Kit BK, Flegal KM. Prevalence of obesity and trends in body mass index among US children and adolescents, 1999–2010. Journal of the American Medical Association. 2012;307:483–490. doi: 10.1001/jama.2012.40. doi:10.1001/jama.2012.40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parcel GS, Edmundson E, Perry CL, Feldman HA, O'Hara-Tompkins N, Nader PR, Stone EJ. Measurement of self-efficacy for diet-related behaviors among elementary school children. Journal of School Health. 1995;65:23–27. doi: 10.1111/j.1746-1561.1995.tb03335.x. doi:10.1111/j.1746-1561.1995.tb03335.x. [DOI] [PubMed] [Google Scholar]
- Pietrobelli A, Leone MA, Heymsfield SB, Faith MS. Association of physical-activity-teasing with reported activity and activity-attitudes in pediatiric sample. Paper presented at the Eighth International Congress on Obesity; Paris, France. 1998. [Google Scholar]
- Puhl RM, Latner JD. Stigma, obesity, and the health of the nation's children. Psychological Bulletin. 2007;133:557–580. doi: 10.1037/0033-2909.133.4.557. doi:10.1037/0033-2909.133.4.557. [DOI] [PubMed] [Google Scholar]
- Reilly JJ, Methven E, McDowell ZC, Hacking B, Alexander D, Stewart L, Kelnar CJ. Health consequences of obesity. Archives of Disease in Childhood. 2003;88:748–752. doi: 10.1136/adc.88.9.748. doi:10.1136/adc.88.9.748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinehr T, Kleber M, Lass N, Toschke AM. Body mass index patterns over 5 y in obese children motivated to participate in a 1-y lifestyle intervention: Age as a predictor of long-term success. American Journal of Clinical Nutrition. 2010;91:1165–1171. doi: 10.3945/ajcn.2009.28705. doi:10.3945/ajcn.2009.28705. [DOI] [PubMed] [Google Scholar]
- Sabin MA, Ford A, Hunt L, Jamal R, Crowne EC, Shield JP. Which factors are associated with a successful outcome in a weight management programme for obese children? Journal of Evaluation in Clinical Practice. 2007;13:364–368. doi: 10.1111/j.1365-2753.2006.00706.x. doi:10.1111/j.1365-2753.2006.00706.x. [DOI] [PubMed] [Google Scholar]
- Sallis JF, Grossman RB, Pinski RM, Patterson TL, Nader PR. The development of self-efficacy scales for health-related diet and exercise behaviors. Health Education Research. 1988;3:283–292. doi:10.1093/her/3.3.283. [Google Scholar]
- Sallis JF, Grossman RM, Pinski RB, Patterson TL, Nader PR. The development of scales to measure social support for diet and exercise behaviors. Preventive Medicine. 1987;16:825–836. doi: 10.1016/0091-7435(87)90022-3. doi:10.1016/0091-7435(87)90022-3. [DOI] [PubMed] [Google Scholar]
- Satterthwaite FE. An approximate distribution of estimates of variance components. Biometrics Bulletin. 1946;2:110–114. doi:10.2307/3002019. [PubMed] [Google Scholar]
- Saunders RP, Pate RR, Felton G, Dowda M, Weinrich MC, Ward DS, Baranowsky T. Development of questionnaires to measure psychosocial influences on children's physical activity. Preventive Medicine. 1997;26:241–247. doi: 10.1006/pmed.1996.0134. doi:10.1006/pmed.1996.0134. [DOI] [PubMed] [Google Scholar]
- Singer JD, Willett JB. Applied longitudinal data analysis: Modeling change and event occurrence. Oxford University Press; New York, NY: 2003. [Google Scholar]
- United States Preventive Services Task Force Screening for obesity in children and adolescents: U.S. Preventive Services Task Force Recommendation Statement. Pediatrics. 2010;125:361–367. doi: 10.1542/peds.2009-2037. doi: 10.1542/peds.2009-2037. [DOI] [PubMed] [Google Scholar]
- Watkins B, Frampton I, Lask B, Bryant-Waugh R. Reliability and validity of the child version of the Eating Disorder Examination: A preliminary investigation. International Journal of Eating Disorders. 2005;38:183–187. doi: 10.1002/eat.20165. doi:10.1002/eat.20165. [DOI] [PubMed] [Google Scholar]
- Whitlock EP, O'Connor EA, Williams SB, Beil TL, Lutz KW. Effectiveness of weight management programs in children and adolescents. Agency for Healthcare Research and Quality; Rockville, MD: 2008. [PubMed] [Google Scholar]
- Wilfley DE, Stein RI, Saelens BE, Mockus DS, Matt GE, Hayden-Wade HA, Epstein LH. Efficacy of maintenance treatment approaches for childhood overweight: A randomized controlled trial. Journal of the American Medical Association. 2007;298:1661–1673. doi: 10.1001/jama.298.14.1661. doi:10.1001/jama.298.14.1661. [DOI] [PubMed] [Google Scholar]
- Wilfley DE, Tibbs TL, Van Buren DJ, Reach KP, Walker MS, Epstein LH. Lifestyle interventions in the treatment of childhood overweight: A meta-analytic review of randomized controlled trials. Health Psychology. 2007;26:621–532. doi: 10.1037/0278-6133.26.5.521. doi:10.1037/0278-6133.26.5.521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilfley DE, Van Buren DJ, Theim KR, Stein RI, Saelens BE, Ezzet F, Epstein LH. The use of biosimulation in the design of a novel multilevel weight loss maintenance program for overweight children. Obesity. 2010;18:91–98. doi: 10.1038/oby.2009.437. doi:10.1038/oby.2009.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolitzky-Taylor KB, Arch JJ, Rosenfield D, Craske MG. Moderators and Non-Specific Predictors of Treatment Outcome for Anxiety Disorders: A Comparison of Cognitive Behavioral Therapy to Acceptance and Commitment Therapy. Journal of Consulting and Clinical Psychology. 2012 doi: 10.1037/a0029418. doi:10.1037/a0029418. [DOI] [PubMed] [Google Scholar]
- Wrotniak BH, Epstein LH, Paluch RA, Roemmich JN. Parent weight change as a predictor of child weight change in family-based behavioral obesity treatment. Archives of Pediatrics and Adolescent Medicine. 2004;158:342–347. doi: 10.1001/archpedi.158.4.342. doi:10.1001/archpedi.158.4.342. [DOI] [PubMed] [Google Scholar]
- Yildirim M, van Stralen MM, Chinapaw MJ, Brug J, van Mechelen W, Twisk JW, Te Velde SJ. For whom and under what circumstances do school-based energy balance behavior interventions work? Systematic review on moderators. International Journal of Pediatric Obesity. 2011;6:e46–57. doi: 10.3109/17477166.2011.566440. doi:10.3109/17477166.2011.566440. [DOI] [PMC free article] [PubMed] [Google Scholar]