Abstract
Using a versatile synthetic approach, a new class of potential ester prodrugs of highly potent, but systemically too toxic, platinum–acridine anticancer agents was generated. The new hybrids contain a hydroxyl group, which has been masked with a cleavable lipophilic acyl moiety. Both butanoic (butyric) and bulkier 2-propanepentanoic (valproic) esters were introduced. The goals of this design were to improve the drug-like properties (e.g., logD) and to reduce the systemic toxicity of the pharmacophore. Two distinct pathways by which the target compounds undergo effective ester hydrolysis, the proposed activating step, have been confirmed: platinum-assisted, self-immolative ester cleavage in a low-chloride environment (LC-ESMS, NMR spectroscopy) and enzymatic cleavage by human carboxylesterase-2 (hCES-2) (LC-ESMS). The valproic acid ester derivatives are the first example of a metal-containing agent cleavable by the pro-drug-converting enzyme. They show excellent chemical stability and reduced systemic toxicity. Preliminary results from screening in lung adenocarcinoma cell lines (A549, NCI-H1435) suggest that the mechanism of the valproic esters may involve intracellular deesterification.
Keywords: anticancer drugs, carboxylesterase, lipophilicity, lung cancer, toxicity, valproic acid
Introduction
Traditional chemotherapies often suffer from high systemic toxicity and a narrow therapeutic window. To improve the pharmacokinetics (PK) and toxicity profiles of anticancer drugs, various avenues are being pursued, such as nano-sized delivery platforms, receptor-targeted conjugates, and prodrug designs.[1, 2] The rationale behind the latter approach is to generate a precursor molecule, which is converted post administration to the bioactive form of the drug either enzymatically or in response to a chemical stimulus. Bioactivation may occur during absorption, circulation, or at the tumor site.[3] Potential benefits of lipophilic prodrugs include efficient retention in, and absorption from, circulation, as well as improved penetration of membranes and accumulation in target tissues.[1]
Compound 1 (PT-ACRAMTU, Figure 1; ACRAMTU =1-[2-(acridin-9-ylamino)ethyl]-1,3-dimethylthiourea) represents the prototype of a class of DNA-targeted platinum–acridine hybrid agents, which have shown exquisite potency in several solid tumor models.[4] Non-small cell lung cancer (NSCLC) cells prove to be particularly sensitive to this pharmacophore, with the newer derivatives showing IC50 values in NSCLC cell lines in the low-nanomolar range and activity in tumor xenografts.[4, 5] Unlike cisplatin [cis-diamminedichloridoplatinum(II)] and its analogues, platinum–acridines derived from PT-ACRAMTU do not cross-link DNA bases but produce structurally unique hybrid adducts that are an intrinsically more severe form of DNA damage than the former bifunctional adducts.[6–8] The classical structure–activity relationship (SAR) approach based on modular library screening has previously been used to tune the chemical stability and reduce the off-target reactivity of the pharmacophore.[9] The desired improvements were achieved essentially by modifying the ligand and donor sets around the electrophilic metal.[4] These efforts have led to the development of a derivative (1‴, Figure 1) that shows three orders of magnitude higher potency than cisplatin (the IC50 values for 1‴ in NCI-H460 and A549 lung cancer cells were 1.3 and 3.9 nM, respectively; see the Supporting Information for dose–response curves).
Figure 1.

General structure of first- and second-generation platinum–acridine hybrid agents.
Despite their promising cell-kill properties in chemoresistant, intractable cancer cells, platinum–acridines show unfavorable ADME[1] (absorption, distribution, metabolism, and excretion) properties, which hamper their preclinical development. Compound 1′, for instance, while inhibiting the growth of xenografted NCI-H460 tumors in mice, showed signs of severe toxicity in the test animals, resulting in a low maximum tolerated dose (MTD).[5] Mice, which were necropsied after treatment with 1′, showed high levels of platinum in normal tissues, but insufficient accumulation in tumors, as well as discoloration of the kidneys, a possible sign of hepatotoxicity or nephrotoxicity.[5] To improve the pharmacological properties of platinum–acridines and to potentially open the therapeutic window for systemic treatment with these agents, we have now designed lipophilic ester-based derivatives that can be tuned to specifically undergo enzymatic hydrolysis. This concept has resulted in the first case of a platinum-containing agent that is recognized as a substrate by human carboxyl-esterase-2 (hCES-2), a key enzyme involved in the activation of several anticancer prodrugs.[1]
Results and Discussion
Design and chemistry
Platinum–acridines show excellent cytotoxicity but unfavorable drug-like properties. The most cytotoxic derivatives exist in their fully protonated [pKa(9-aminoacridine) ≈ 9.5–10], dicationic form.[4 Because these hybrids are too hydrophilic, they show poor tissue distribution and are most likely removed too rapidly from circulation via renal clearance.[10] Thus, the goal of the structural modifications introduced here was to increase the lipophilicity of the agents while maintaining good water solubility. The alkyl residue of the amidine donor group (residue R, for Y =NH, see Figure 1) was chosen as the site of attachment for the carboxylic acid ester groups. The design involved introduction of a hydroxymethyl (MeOH) or an extended 3-hydroxy-propyl (Pr3-OH) group as R in place of simple Me and Et in 1′–1‴ and masking of the terminal OH function as lipophilic esters (see Scheme 1). A primary carboxylic acid, butanoic (butyric) acid, and a bulkier, branched derivative, 2-propylpen-tanoic (valproic) acid, were introduced as acyl components (see Scheme 1). Use of the latter residue was inspired by an analogous valproic amide-based prodrug of the anticancer drug gemcitabine, which is activated by hCES-2.[11]
Scheme 1.

Synthesis of target compounds. i) AgNO3, DMF, RT; ii) Appropriate nitrile-ester (11 a–d, see the Supporting Information), DMF, 60 °C, 4 h, iii) 1) 13, DMF, 4 °C, 2) 1 M HNO3. Abbreviations: en = ethane-1,2-diamine, pn =propane-1,3-diamine.
Two distinct mechanisms of ester cleavage have to be considered: chemical and enzymatic hydrolysis. The former mechanism has previously been observed in chemically related carboxylic acid-modified platinum–acridines containing a reversed ester linkage (Pt-linker-C(O)OR′, instead of Pt-linker-OC(O)R′ used in this study).[12] It is dominated by platinum-assisted ester cleavage in a chloride-ion concentration dependent manner. Low intracellular chloride favors aquation of platinum, which serves as a Lewis-acidic metallohydrolase and accelerates ester cleavage. The second mode of activation involves carboxylesterase isoenzymes, in particular hCES-2, which is not only expressed at high levels in the gastrointestinal tract and the liver, but also in tumor tissue.[1] The choice of hCES-2 rather than hCES-1 as the target enzyme was based on the well-documented substrate selectivity of the two forms, according to which hCES-2 preferentially recognizes esters containing bulky alcohols (here, the hydroxyl-modified hybrid agent itself) in combination with relatively smaller acyl moieties.[13]
A total of seven ester-protected hybrids were synthesized. Structural diversity in this set of compounds was achieved by varying the chain length, n, and the nature of the acyl residue, R′ (Scheme 1). In addition, different non-leaving amines (L) were introduced to tune the reactivity of the platinum moiety (see Discussion section below). Compounds 2–8 were generated from the platinum precursors (12a–g) containing the appropriate ester-modified nitrile ligands (11 a–g, for synthetic details see the Supporting Information). The ligand substitution reactions leading to the precursor complexes containing short-chain nitrile esters (n =1, 12a–d) produced significantly lower yields than reactions performed with the long-chain derivatives (n =3, 12e–g) (20–30 vs 80–90 %). This previously observed effect can be attributed to the high CH acidity and reactivity of the nitrile ligands in the former set of derivatives.[12] (Attempts to generate analogous derivatives with n =2 failed due to unexpected β-elimination of the hydroxyl group; data not shown.) The final step affording hybrid agents 2–8 (yield >75 %, analytical purity >95 %) involved addition of the NHMe group in N-(acridin-9-yl)-N′-methylethane-1,2-diamine (13) across the metal-activated nitrile triple bond, [14] producing the desired amidine linkage, and subsequent protonation to generate the dinitrate salts.
An anomaly in the stereochemistry of the addition reactions leading to hybrids 2–5 was observed. The extended-chain ester derivatives (6, 7, and 8) and the prototype (1′) almost exclusively (>90 %) exist as E isomers in which the platinum moiety and amidine-NMe group adopt a trans configuration with respect to the N(imino)=C double bond, as typically observed in amidine ligands formed from secondary amines.[15] By contrast, hybrids 2–5 form a relatively high amount of the Z isomer (>25 %, based on 1 D and 2 D NMR analysis, see the Supporting Information). We attribute this outcome to the increased steric hindrance produced by the short-chain (n =1) acyl groups around the nitrile group. Intramolecular hydrogen bonding between the imino proton and the ester group (NH···O=C–O) may also contribute to this effect (Supporting Information).[16]
All newly synthesized hybrids maintain excellent solubility of >10 mg mL−1 in relevant aqueous media. To demonstrate the effect of the pendant ester groups on the hydrophilicity/lipophilicity balance of the compounds, we studied the partitioning of selected derivatives between octanol and phosphate-buffered saline (PBS) (expressed as log[octanol]/[PBS] =logD, the distribution coefficient for protonable pharmacophores[3]). The experiment was performed in PBS at pH 7.4 rather than water to suppress complex aquation and platinum-mediated ester hydrolysis, which is described in the following section. This setup also takes into consideration the pH dependence of logD to faithfully mimic conditions in plasma. For the unmodified, hydrophilic agent, 1‴, a logD of −0.98 (±0.19) was determined. By contrast, compound 8, the presumably most lipophilic derivative (n =3, valproic ester, L =pn), preferentially partitions into the octanol phase with a logD of 0.73 (±0.06), which reflects an increase in lipophilicity by 50-fold relative to compound 1′. An intermediate logD of −0.31 (±0.06) was determined for compound 7 (n =3, L =en, butyric ester). The logD value generated for this compound has to be interpreted with caution because of minor ester hydrolysis, which was unavoidable under the conditions of the experiment (<10 %, see the following section).
Metal-assisted ester hydrolysis
One of the proposed mechanisms of activation of compounds 2–8 as prodrugs involves platinum-promoted ester cleavage. To mimic the chloride ion concentration differential that exists between serum during circulation and after uptake into target cells, compounds were incubated at 37 °C in PBS (≈150 mM NaCl, pH 7.4) and in phosphate buffer (PB, pH 7.4), respectively. The reaction mixtures were analyzed at appropriate time points by in-line high-performance liquid chromatography-electrospray mass spectrometry (LC-ESMS). Reaction products were identified as 1+ or 2+ charged molecular ions in mass spectra recorded in positive-ion mode and quantified by integrating UV-visible HPLC traces at an acridine-specific wavelength (see the Supporting Information for a complete set of data).
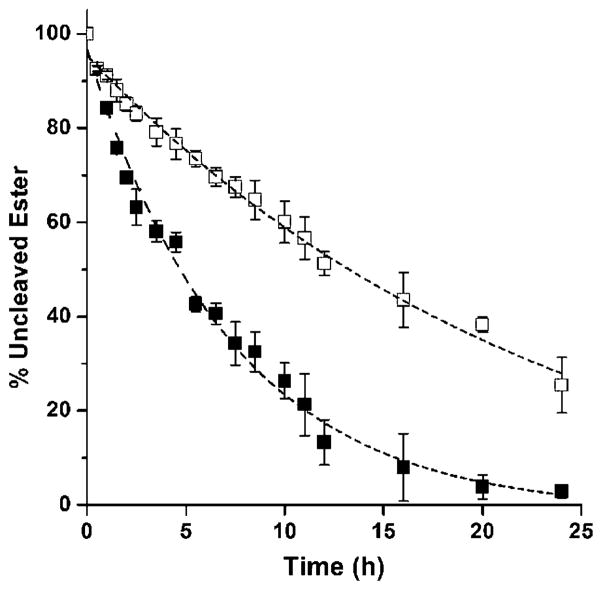
The time course of the ester hydrolysis yielding hydroxyl-modified platinum–acridine and butyric/valproic acid is summarized in Figure 2 for both media. Generally, in sets of analogues characterized by common spacers linking the platinum and ester moieties, (CH2)n, the valproic esters show significantly slower conversion than the butyric esters, or no conversion at all (3–5 vs 2, and 6, 8 vs 7). In phosphate-buffered solution in the absence of chloride (Figure 2A), the most extensive hydrolysis is observed for butyric ester-based compounds 2 (n =1) and 7 (n =3), with the former producing approximately twofold higher levels of cleaved product after 36 h of continuous incubation. Hydrolytic activity is also observed for the valproic ester derivatives 3, 4, and 5 (all n =1), but at a much slower rate. Most strikingly, hybrids 6 and 8, which contain the same secondary acyl moiety but on an extended linker (n =3), are completely resistant to cleavage under these conditions. When incubations were performed in buffer supplemented with physiological chloride, a major reduction in ester hydrolysis of up to 75 % was observed (Figure 2B) compared to reactions in chloride-free media, consistent with the notion that (reversible) aquation of the platinum moiety plays a role in the cleavage mechanism.
Figure 2.
Cleavage of ester moieties in compounds 2–8 monitored by quantitative HPLC for hydrolysis reactions in phosphate buffer, PB, pH 7.4 (panel A), phosphate-buffered saline, PBS, pH 7.4 (panel B), and in PBS in the presence of hCES-2 (panel C). Plotted data are the mean of three incubations ±standard deviations. Yields of conversion for compound 7 in panel C represent the sum of chemical (minor) and enzymatic (major) cleavage, which produced indistinguishable products. Reactions were performed at 37 °C.
The LC-ESMS profiles of compounds 2–5 share common features and support the proposed mechanism of platinum-mediated ester cleavage. We have chosen compound 2, which was also suitable for a kinetic study by 1H NMR spectroscopy, for a detailed discussion (for complete sets of LC-ESMS data for all other analogues, see the Supporting Information). The only hydrolysis product formed in incubations of compound 2 in PB was identified as a chelate in which the chloro leaving group has been replaced with the unprotected hydroxyl oxygen of the cleaved butyric ester (see peak labeled “a” in Figure 3A and the corresponding mass spectrum/structure in Figure 3D/ E, respectively). A minor amount of chemically unchanged 2 and a product resulting from substitution of chloride by phosphate containing intact ester are also observed after 36 h of incubation (peaks b and c). The same array of products was observed when compound 2 was incubated in the presence of sodium chloride in PBS, but ester cleavage and chelate formation were significantly suppressed based on the relative abundances of each species (Figure 3B). The absence of an opened-chelate form in this high-chloride environment attests to the inertness of the five-membered amidine-N/hydroxyl-O chelate. This was also confirmed in incubations with biological nitrogen and sulfur model nucleophiles, in which this product was completely unreactive (N-acetylcysteine, 2′-deoxyguanosine; data not shown). Unlike compounds 2–5 (n =1), compound 7 (n = 3) exclusively forms hydrolysis products containing a dangling hydroxyl group, confirming that a seven-membered, presumably less stable, chelate does not form (Supporting Information).
Figure 3.
Reverse-phase HPLC traces for the separation of reaction products resulting from ester cleavage in compound 2 in PB (A), in PBS (B), and by hCES-2 (C). Mass spectra recorded in positive-ion mode for fractions labeled a–d and the corresponding structures and m/z values of the molecular ions are given in panels D and E, respectively.
The dramatic effect of chloride ion on the kinetics of ester hydrolysis was confirmed for compound 2 in arrayed 1H NMR experiments (for an analysis of 1H NMR spectra see the Supporting Information). Cleavage of the butyric ester follows a (pseudo-) first-order rate law with rate constants of k =3.9 ± 10−5 s−1 in PB and k =1.2 ±10−5 s−1 in PBS, which corresponds to half-lives of 5 and 16 h, respectively (Figure 4). Thus, chloride slows cleavage of the pendant ester in complex 2 by approximately 70 %.
Figure 4.
Kinetics of ester hydrolysis in compound 2 monitored by 1H NMR spectroscopy [D2O, pH* 7.0, 2 mM Pt in 10 mM PB (solid squares) or PBS (open squares)] at 37 °C for 24 h. The data sets were fitted to the first-order exponential decay function y = y0 + A e−x/t, where t−1 is the rate constant, k [h−1], and x is the reaction time, t [h]. Data points represent means of at least three integrated signal intensities ±standard deviations. The experiment was performed in duplicate with similar results.
On the basis of the above product analysis, a mechanism of platinum-mediated, intramolecular ester cleavage is proposed (Scheme 2). Cleavage is triggered by (reversible) aquation of the platinum complex. The Lewis-acidic metal assists in the de-protonation of the aqua to a hydroxo ligand, which undergoes a nucleophilic attack on the acyl carbon to promote cleavage of the ester linkage. Cleavage results in the loss of the acyl protecting group as carboxylic acid and in a dangling or chelated alcohol/alkoxide moiety. High chloride concentrations shift the aquation reaction toward the chloro-substituted hybrid, which quenches ester cleavage. An intramolecular attack by platinum-associated hydroxide is also supported by the following observations: 1) Hydrolysis of ester is approximately twice as efficient for complex 4 bearing ammine (NH3) non-leaving groups than for the en-substituted analogue 3. This effect is consistent with the lower pKa value of the aqua ligands in Pt–ammine than in Pt–en complexes, [17] which produces higher concentrations of the more reactive hydroxo ligand. 2) For derivatives containing the same acyl moiety, ester cleavage is dramatically reduced for n =3 versus n =1. This effect can be rationalized with the fact that internal nucleophilic attack by the hydroxo ligand in the former compound requires formation of a 9-membered, thermodynamically less favorable, macrocyclic intermediate.
Scheme 2.
Proposed mechanisms of chemical and enzymatic ester cleavage.
Ester cleavage by human carboxylesterase-2 (hCES-2)
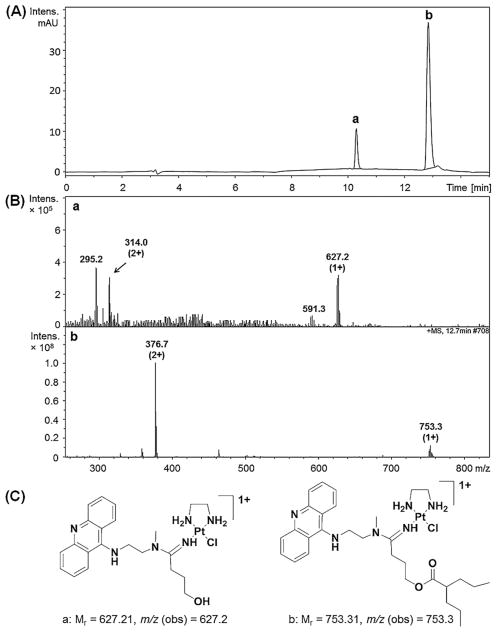
To determine if the compounds, especially those not undergoing chemical ester cleavage (6 and 8), are susceptible to deesterification by a pharmacologically relevant prodrug-converting enzyme, we also performed incubations with recombinant human carboxylesterse-2 (hCES-2). The particular engineered form of the protein used was sufficiently robust to provide enzymatic activity over long reaction times (>30 min), which allows for identification of cleavage products for non-classical, slowly metabolized substrates.[18] Reactions were performed in a buffer supplemented with chloride to suppress non-enzymatic hydrolysis and monitored for a relatively short period of time to minimize the effects of loss of enzyme activity over time and chemical hydrolysis. Unavoidable contributions from the latter pathway have been subtracted, where possible, from the data presented in Figure 2C. LC-ESMS analysis of the reaction mixtures shows minimal or no enzyme-mediated cleavage of ester for compounds 2–5, respectively, which contain short spacers (n =1). By contrast, derivatives 6–8 (n =3) show deesterification under these conditions with yields of ≈20 % at the 10 h time point. Importantly, the bulky valproic esters in compounds 6 and 8, which are completely resistant to chemical hydrolysis, are efficiently cleaved by the enzyme. The HPLC trace recorded for compound 2 after 10 h of incubation with enzyme (Figure 3C) shows the same reaction products formed in PB and PBS, as well as an additional peak (d, ≈5 %) not observed for chemical hydrolysis. Positive-ion mass spectra unequivocally identified this product as the platinum-chloro complex containing a dangling hydroxyl group (Figure 3D, E), consistent with a non-metal-mediated cleavage mechanism.
The fact that no opened chelate was detected for chemical hydrolysis of derivatives with n =1, but only five-membered N,O-chelate, can be taken as evidence that the former must be an enzyme-specific product. The synthesis of cisplatin derivatives containing chelated aminoalcoholato ligands requires basic conditions to help deprotonate the alcohol, while opening of such chelates only occurs under acidic conditions.[19] This is in agreement with the observation that the two forms (peaks a and d, Figure 3C) do not inter-convert under the conditions and on the time scale of the assay. Unlike the butyric ester derivative 7, which shows dual cleavage reactivity resulting in the dangling hydroxyl form of the hybrid agent, the valproic ester derivatives 6 and 8 only undergo enzyme-mediated cleavage to produce the corresponding deesterified form (shown for compound 6 in Figure 5). It can be concluded that only esters installed on an extended linker are viable substrates for hCES-2 and that only the chemically robust valproic ester confers true selectivity for enzymatic cleavage.
Figure 5.
Reverse-phase HPLC traces for the separation of reaction products resulting from ester cleavage in compound 6 by hCES-2 (A). Mass spectra recorded in positive-ion mode for fractions labeled a and b and the corresponding structures and m/z values of the molecular ions are given in panels B and C, respectively.
Cytotoxicity studies
The anticancer properties of the newly generated compounds 2–8 were assessed using a colorimetric cell proliferation assay and compared to that of prototype 1′ (chloride salt). (Note: attempts to synthesize the deesterified, proposed active hydroxyl forms of 2–8 in pure form for screening in this assay have been unsuccessful due to the chemical instability of the platinum-nitrile precursor complexes.) Two NSCLC adenocarcinoma cell lines were screened for chemosensitivity: A549, which responds well to treatment with platinum–acridines, and NCI-H1435, a DNA repair-proficient and highly chemoresistant form of this cancer.[20] The latter cell line has also been shown to express significantly higher levels (three–fourfold) of hCES-2 enzyme than A549 and other lung adenocarcinomas.[21] Thus, cytotoxicity levels observed in the two cell lines may also provide hints about potential intracellular prodrug activation. Both cell lines were dosed with hybrid agents for 72 h at three selected concentrations, which were based on relative chemo-sensitivities established for compound 1′. A549 was dosed at 5.0, 50, and 500 nM, whereas 20-fold higher concentrations, 0.1, 1.0, and 10 μM, were chosen for NCI-H1435 to account for the relatively higher resistance observed in the latter cell line. The dose-response data resulting from the chemosensitivity screen are presented in Figure 6.
Figure 6.
Results of the chemosensitivity screen of compounds 1′–8 in A549 (A) and NCI-H1435 (B) cancer cells. Relative cell viabilities are means of two independent experiments performed in triplicate ±the standard deviation.
As a general trend, the ester-modified derivatives show reduced cytotoxicity levels compared to the unmodified hybrid, 1′, except for compound 7, which shows a response similar to that of the prototype in both cell lines. Because the butyrate-protected derivative is efficiently cleaved both chemically and enzymatically on a time scale relevant to the cell culture assay (see previous section), it is proposed that the hydroxyl form of this agent is the major contributor to the cell kill. It also suggests that the nPr3-OH residue in deesterified 7 in place of the Et residue in 1′ does not compromise the potency of the pharmacophore, possibly pointing to a similar mechanism at the DNA level. By contrast, the derivatives containing bulky valproic ester show greatly reduced activity. Compound 6, for instance, which would generate the same active hydroxyl form as compound 7, and compound 8 show no cell kill in A549 at the highest dose and significantly reduced activity in NCI-H1435. This observation is in agreement with the less efficient intracellular cleavage of the bulkier valproic esters 6 and 8, which, in their intact form, are less cytotoxic, possibly due to their reduced reactivity with cellular DNA. The same trend is observed for compounds 3–5, which proved to be relatively resistant to chemical and enzymatic hydrolysis. For compounds sharing the same linkers and ester moieties, the nature of the non-leaving group had only minor or no effects on the activity. Finally, compound 2, although cleaved very efficiently in chloride-free media (see previous section), showed activity inferior to the prototype. This outcome was expected because of the inertness of the 5-membered chelate generated in the process.
Compounds 6 and 8, which are cleaved by hCES-2, show a more pronounced enhancement in activity relative to compound 1′ in NCI-H1435 (high hCES-2) than in A549 (low hCES-2). The opposite is the case for compound 7. As a preliminary measure of chemo-selectivity, we defined the selectivity index, S: S = [CVi,NCI-H1435/CV1′,NCI-H1435]/[CVi,A549/CV1′,A549], where CVi and CV1′ are the % viabilities of the ester-based compounds and compound 1′ for their highest doses, respectively. CV1′ was introduced to normalize for differences in chemosensitivities between the two cell lines. Assuming that CV1′ is the highest cell kill that can be achieved with any of the cleaved esters, S values smaller than “1” would indicate a relative advantage of a given derivative in NCI-H1435, and vice versa. For compounds 6, 7, and 8, S values of 0.6, 1.4, and 0.5 were calculated, respectively. It can be concluded that the esters that are not cleaved chemically (6, 8) but show hCES-2-mediated cleavage perform relatively better in NCI-H1435. Conversely, compound 7, which efficiently converts to its active form via an enzyme-independent pathway, appears to have a relative advantage in A549. These findings may indicate that compounds 6 and 8 act as prodrugs at high levels of hCES-2, which confers sensitivity to NCI-H1435 cells. It should be noted, however, that a direct comparison of cytotoxic responses between two different cell lines has to be interpreted with caution. Because cellular uptake and efflux, among other factors, may also contribute to the observed differences, additional experiments in hCES-2 knockdown or hCES-2 transfected cells[11] of the same type are necessary.
Conclusion
Lipophilic, ester-modified derivatives of platinum–acridines have been synthesized and evaluated with the goal of improving the drug-like properties of these highly cytotoxic anticancer agents. On the basis of their physicochemical properties, chemical reactivities, their ability to serve as hCES-2 substrates, and their performance in cell lines, compounds 6 and 8 can be considered candidates for slow cleavage by hCES-2. Compound 7 holds promise of providing a dual mode of cleavage, in which chemical activation may be necessary for applications requiring extended circulation of precursor in plasma and self-immolative activation in cells lacking hCES-2. By contrast, the more lipophilic and chemically less reactive analogues 6 and 8, which should achieve even longer half-lives in circulation, would require activation by target enzyme expressed in the liver (similar to the anticancer prodrug irinotecan) or in tumor tissue. Whether the butyric and valproic esters are prone to hydrolysis by other ubiquitous serum esterases remains to be determined. To this end, incubations of 2–8 with fetal bovine serum (FBS, Thermo Scientific HyClone), which is commonly used as a model for human serum, [23] have shown no evidence for this unwanted reactivity (data not reported).
Another potential advantage of bulky prodrugs of platinum–acridines may be their poor DNA-binding properties. If ester cleavage occurs primarily intracellularly in tumor tissue, differential DNA recognition by the ester-protected (inactive) and hydroxo (active) forms of the hybrid agent might confer pro-drug selectivity to cancer cells while sparing normal cells. In addition to an improved ADME profile, lipophilic prodrugs also have the potential benefit of improving drug safety.[1] To test if this supposition holds for our prodrug design, dose escalation studies were performed with the chemically robust derivative 8 in Swiss Webster mice. Mice tolerated this analogue without showing signs of toxicity and weight loss when injected intra-peritoneally (i.p.) once a day for five consecutive days (qd × 5) at a dose of 1.6 mg kg−1 (Supporting Information). For comparison, compound 1′ was significantly more toxic and showed an MTD of 0.1 mg kg−1 when the same dosing schedule was applied.[5]
In conclusion, we have developed a versatile platform for tuning the pharmacological parameters of potent platinum–acridines and have demonstrated for the first time that a metal-based pharmacophore is compatible with the classical concept of carboxylesterase-mediated prodrug activation. This feature may provide a strategy of improving the ADME and safety of platinum–acridines and other metallopharmacophores.
Experimental Section
Materials, general procedures, and instrumentation
All reagents were used as obtained from commercial sources without further purification unless indicated otherwise. Compound 1′ [5] (chloride salt) and N-(acridin-9-yl)-N′-methylethane-1,2-diamine[22] (13) were prepared according to published procedures. The platinum-nitrile precursors (12 a–g) were synthesized from the corresponding nitriles (11a–d) and silver-ion activated diam(m)inedichloridoplatinum(II) complexes (Supporting Information). 1H NMR spectra of the target compounds and intermediates were recorded on Bruker Advance DRX-500 and 300 MHz instruments. Proton-decoupled 13C NMR spectra were recorded on a Bruker DRX-500 instrument operating at 125.8 MHz. 2 D 1H-13C gradient-selected Heteronuclear Multiple Bond Coherence (gsHMBC) experiments and temperature-dependent spectra were acquired on a Bruker DRX-500 instrument equipped with a TBI probe and a variable-temperature unit. 2 D HMBC spectra were collected with 2048 pts in t2(sw =6510 Hz), 256 pts in t1 (sw = 27 670 Hz), 128 scans per t1 increment, and a recycle delay (d1) of 1.5 s. Chemical shifts (δ) are given in parts per million (ppm) relative to internal standard tetramethylsilane (TMS). 1H NMR data is reported in the conventional form including chemical shift (δ, ppm), multiplicity (s =singlet, d =doublet, t =triplet, q =quartet, m =multiplet, br =broad), coupling constants (Hz), and signal integrations. 13C NMR data are reported as chemical shift listings (δ, ppm). The NMR spectra were processed and analyzed using the MestReNova software package. HPLC-grade solvents were used for all HPLC and mass spectrometry experiments. LC-ESMS analysis was performed on an Agilent 1100LC/MSD ion trap mass spectrometer equipped with an atmospheric pressure electrospray ionization source. Eluent nebulization was achieved with a N2 pressure of 50 psi and solvent evaporation was assisted by a flow of N2 drying gas (350 °C). Positive-ion mass spectra were recorded with a capillary voltage of 2800 V and a mass-to-charge scan range of 150 to 2200 m/z. To establish the purity of target compounds, samples were diluted in methanol containing 0.1 % formic acid and separated using a 4.6 mm × 150 mm reverse-phase Agilent ZORBAX SB-C18 (5 mm) analytical column at 25 °C, by using the following solvent system: solvent A, optima water, and solvent B, methanol/0.1 % formic acid, with a flow rate of 0.5 mL min−1 and a gradient of 95 to 5 % A over 30 min. HPLC traces were recorded with a monitoring wavelength range of 363–463 nm. Peak integration was done using the LC/ MSD Trap Control 4.0 data analysis software. Analytical purity of greater than 95 % was confirmed this way for all target compounds prior to analytical and biological experiments (for analytical data and details of the NMR spectral assignments, including atom numbering schemes, see the Supporting Information).
Synthesis of [PtCl(en)C22H27N4O2](NO3)2 (2)
Precursor complex 12a (240 mg, 0.5 mmol) was dissolved in 10 mL of anhydrous DMF and the solution was cooled to −20 °C. Acridine-amine 13 (138 mg, 0.55 mmol) was added to the solution, and the suspension was stirred at 4 °C for 24 h. The reaction mixture was added dropwise into 200 mL of anhydrous ethyl ether, and the resulting yellow slurry was vigorously stirred for 30 min. The precipitate was recovered by membrane filtration, dried in a vacuum overnight, and dissolved in 20 mL of methanol containing 1 equivalent of HNO3. After removal of the solvent by rotary evaporation, the crude product was recrystallized from hot ethanol, affording 2 as a 3:1 mixture of E and Z isomers. Yield: 238 mg (71 %). 1H NMR (300 MHz, [D4]MeOH) δ=8.61–8.51 (m, 2 H, acridine-H1/8), 8.13–7.75 (m, 4 H, acridine-H3–6), 7.75–7.45 (m, 2 H, acridine-H2/7), 6.87–6.57 (m, 1 H, Pt-NH=CCH2O-), 5.50 (s, 1.5 H, Pt-NH= CCH2O-, E-isomer), 5.49–5.39 (m, 4 H, -NH2CH2CH2NH2-), 4.76 (s, 0.5 H, Pt-NH=CCH2O-, Z-isomer), 4.59 (m, 0.5 H, -NHCH2CH2N(CH3)-, Z-isomer), 4.45 (t, J =6.3 Hz, 1.5 H, -NHCH2CH2N(CH3)-, E-isomer), 4.02 (t, J =6.3 Hz, 1.5 H, -NHCH2CH2N(CH3)-, E-isomer), 3.56–3.39 (m, 0.5 H, -NHCH2CH2N(CH3), Z-isomer), 3.14 (s, 2.25 H, -NHCH2CH2N-(CH3)-, E-isomer), 2.61–2.57(m, 4 H, -NH2CH2CH2NH2-), 2.38–2.06 (m, 2 H, -COCH2CH2CH3), 1.76–1.40 (m, 2 H, -COCH2CH2CH3), 1.02–0.75 ppm (m, 3 H, -COCH2CH2CH3); 13C NMR (75 MHz, [D4]MeOH) δ=172.50 (-COCH2CH2CH3, E-isomer), 171.76 (-COCH2CH2CH3, Z-isomer), 165.14 (Pt-NH=CCH2O-, Z-isomer), 163.38 (Pt-NH=CCH2O-, E-isomer), 158.48 (acridine-C9), 139.87 (acridine-11C/13C), 135.21(acridine-C3/6), 124.99 (acridine-C1/8), 124.02 (acridine-C2/7), 118.28 (acridine-C4/5), 112.63 (acridine-C10/12), 63.55 (Pt-NH=CCH2O-, E-isomer), 61.87 (Pt-NH=CCH2O-, Z-isomer), 35.03 (-COCH2CH2CH3), 17.69 (-COCH2CH2CH3), 12.43 ppm (-COCH2CH2CH3); MS (ESI, positive-ion mode): m/z: calcd for C24H35ClN6O2Pt [M–H] +: 669.22; found: 669.3.
Synthesis of [PtCl(en)C26H35N4O2](NO3)2 (3)
Compound 3 was prepared according to the procedure described for 2 from precursor 12 b with a yield of 69 %. 1H NMR (300 MHz, [D4]MeOH) δ = 8.82–8.40 (m, 2 H, acridine-H1/8), 8.11–7.78 (m, 4H, acridine-H3–6), 7.77–7.50 (m, 2 H, acridine-H2/7), 5.52 (s, 1.5 H, Pt-NH=CCH2O-, E-isomer), 5.42–5.13 (m, 4 H, -NH2CH2CH2NH2-), 4.64–4.54 (m, 0.5 H, NHCH2CH2N(CH3)-, Z-isomer), 4.48 (t, J = 6.5 Hz, 1.5 H, NHCH2CH2N(CH3)-, E-isomer), 4.03 (t, J = 6.4 Hz, 1.5 H, -NHCH2CH2N-(CH3)-, E-isomer), 3.15 (s, 2.25 H, -NHCH2CH2N(CH3)-, E-isomer), 2.75–2.47 (m, 4 H, -NH2CH2CH2CH2NH2-), 2.44–2.11 (m, 1 H, -COCH(CH2CH2CH3)2), 1.54–1.13 (m, 8 H, -COCH(CH2CH2CH3)2), 0.97–0.71 ppm (m, 6 H, -COCH(CH2CH2CH3)2); 13C NMR (75 MHz, [D4]MeOH) δ=176.65 (-CO(CH2CH2CH3)2, E-isomer), 176.01 (-COCH(CH2CH2CH3)2, Z-isomer), 167.30 (Pt-NH=CCH2O-, Z-isomer), 164.85 (Pt-NH=CCH2O-, E-isomer), 159.90 (acridine-C9), 141.38 (acridine-C11/13), 136.77 (acridine-C3/6), 126.49 (acridine-C1/8), 125.61 (acridine-C2/7), 119.88 (acridine-C4/5), 114.12 (acridine-C10/12), 66.90 (Pt-NH=CCH2O-, E-isomer), 65.27 (Pt-NH=CCH2O-, Z-isomer), 49.87 (-NH2CH2CH2NH2-), 48.16 (-NHCH2CH2N(CH3)-), 46.35 (-COCH(CH2CH2CH3)2), 35.48 (-COCH(CH2CH2CH3)2), 21.58 (-COCH(CH2CH2CH3)2), 14.31 ppm (-COCH(CH2CH2CH3)2); MS (ESI, positive-ion mode): m/z: calcd for C28H43ClN6O2Pt [M–H] +: 724.28; found: 724.4.
Synthesis of [PtCl(NH3)2C26H35N4O2](NO3)2 (4)
Compound 4 was prepared according to the procedure described for 2 from precursor 12 c with a yield of 74 %. 1H NMR (300 MHz, [D4]MeOH) δ = 8.56 (d, J = 8.7, 2 H, acridine-H1/8), 8.03 (ddd, J = 9.2, 5.8, 1.8 Hz, 2 H, acridine-H3/6), 7.95–7.79 (m, 2 H, acridine-H4/5), 7.75–7.51 (m, 2 H, acridine-H2/7), 5.56 (s, 1.5 H, Pt-NH=CCH2O-, E-isomer), 4.67–4.54 (s, 0.5 H, -NHCH2CH2N(CH3)-, Z-isomer), 4.48 (t, J =6.5 Hz, 1.5 H, -NHCH2CH2N(CH3)-, E-isomer), 4.18 (bs, 3 H, NH3), 4.02 (t, J =6.5 Hz, 1.5 H, NHCH2CH2N(CH3)-, E-isomer), 3.93 (bs, 3 H, NH3), 3.14 (s, 2.25, H-NHCH2CH2N(CH3)-, E-isomer), 2.36–2.16 (m, 1 H, -COCH(CH2CH2CH3)2), 1.56–1.03 (m, 8 H, -COCH(CH2CH2CH3)2), 0.90–0.68 ppm (m, 6 H, -COCH(CH2CH2CH3)2); 13C NMR (75 MHz, [D4]MeOH) δ=176.67 (-CO(CH2CH2CH3)2, E-isomer), 176.07 (-COCH(CH2CH2CH3)2, Z-isomer), 164.74 (Pt-NH=CCH2O-, E-isomer), 159.89 (acridine-C9), 141.39 (acridine-C11/13), 136.76 (acridine-C3/6), 126.77 (acridine-C1/8), 125.60 (acridine-C2/7), 119.88 (acridine-C4/5), 114.20 (acridine-C10/12), 65.32 (Pt-NH=CCH2O-, E-isomer), 46.14 (-COCH(CH2CH2CH3)2), 35.49 (-COCH(CH2CH2CH3)2) 21.58 (COCH(CH2CH2CH3)2), 14.29 ppm (-COCH(CH2CH2CH3)2); MS (ESI, positive-ion mode): m/z: calcd for C26H41ClN6O2Pt [M–H] +: 699.26; found: 699.3.
Synthesis of [PtCl(pn)C26H35N4O2](NO3)2 (5)
Compound 5 was prepared according to the procedure described for 2 from precursor 12 d with the yield of 77 %. 1H NMR (300 MHz, [D4]MeOH) δ=8.61 (dd, J =8.8, 4.5 Hz, 2 H, acridine-H1/8), 8.14–7.95 (m, 2 H, acridine-H3/6), 7.87 (dd, J =9.0, 4.2 Hz, 2 H, acridine-H4/5), 7.75–7.53 (m, 2 H, acridine-H2/7), 6.83–6.73 (m, 1 H, Pt-NH= CCH2O-), 5.53 (s, 1.5 H, Pt-NH=CCH2O-, E-isomer), 5.29–4.88 (m, 4 H, -NH2CH2CH2CH2NH2-), 4.68–4.58 (m, 0.5 H, -NHCH2CH2N(CH3), Z-isomer), 4.55 (t, J =6.6 Hz, 1.5 H, -NHCH2CH2N(CH3)-, E-isomer), 4.03 (t, J =6.4 Hz, 1.5 H, -NHCH2CH2N(CH3)-, E-isomer), 3.68–3.44 (m, 0.5 H, m, 0.5 H, - NHCH2CH2N(CH3)-, Z-isomer), 3.15 (s, 2.25 H, -NHCH2CH2N(CH3)-, E-isomer), 2.95–2.52 (m, 4 H, -NH2CH2CH2CH2NH2-), 2.41–2.07 (m, 1 H, -COCH(CH2CH2CH3)2), 1.92–1.69 (m, 2 H, -NH2CH2CH2CH2NH2-), 1.59–0.98 (m, 8 H, -COCH(CH2CH2CH3)2), 0.98–0.64 ppm (m, 6 H, -COCH(CH2CH2CH3)2); 13C NMR (75 MHz, [D4]MeOH) δ=176.68 (-CO(CH2CH2CH3)2, E-isomer), 176.22 (-COCH(CH2CH2CH3)2, Z-isomer), 164.89 (Pt-NH= CCH2O-, E-isomer), 159.90 (acridine-C9), 141.41 (acridine-C11/13), 136.76 (acridine-C3/6), 126.56 (acridine-C1/8), 125.60 (acridine-C2/7), 119.88 (acridine-C4/5), 113.48 (acridine-C10/12), 46.29 (-COCH(CH2CH2CH3)2), 44.42 (-NH2CH2CH2CH2NH2-), 43.63 (-NH2CH2CH2CH2NH2-), 35.54 (-COCH(CH2CH2CH3)2), 29.39 (-NH2CH2CH2CH2NH2-), 21.60 (-COCH(CH2CH2CH3)2), 14.31 ppm (-COCH(CH2CH2CH3)2); MS (ESI, positive-ion mode): m/z : calcd for C29H45ClN6O2Pt [M–H] +: 739.29; found: 739.4.
Synthesis of [PtCl(en)C28H39N4O2](NO3)2 (6)
Compound 6 was prepared according to the procedure described for 2 from precursor 12 e with the yield of 83 %. 1H NMR (300 MHz, [D4]MeOH) δ = 8.46 (dd, J = 8.5, 4.3 Hz, 2 H, acridine-H1/8), 7.91 (m, 2 H, acridine-H3/6), 7.77 (dd, J =9.0, 4.2 Hz, 2 H, acridine-H4/5), 7. 54 (m, 2 H, acridine-H2/7), 6.08 (s, 1 H, Pt-NH=CCH2CH2CH2O-), 5.36–5.11 (m, 4 H, -NH2CH2CH2NH2-), 4.33 (t, J = 6.6 Hz, 2 H, -NHCH2CH2N-(CH3)-), 4.03 (t, J = 6.4 Hz, 2 H, -NHCH2CH2N(CH3)-), 3.88 (m, 2 H, Pt-NH=CCH2CH2CH2O-), 3.03 (s, 3 H, -NHCH2CH2N(CH3)-), 2.97 (m, 2 H, Pt-NH=CCH2CH2CH2O-), 2.55–2.37 (m, 4 H, -NH2CH2CH2NH2-), 2.10–2.03 (m, 3 H, Pt-NH=CCH2CH2CH2O-,-COCH(CH2CH2CH3)2), 1.59–0.98 (m, 8 H, -COCH(CH2CH2CH3)2), 0.98–0.64 ppm (m, 6 H, -COCH(CH2CH2CH3)2); 13C NMR (75 MHz, [D4]MeOH) δ=178.08 (-CO(CH2CH2CH3)2), 170.02 (Pt-NH=CCH2O-), 159.98 (acridine-C9), 141.37 (acridine-C11/13), 136.71 (acridine-C3/6), 126.52 (acridine-C1/8), 125.53 (acridine-C2/7), 119.85 (acridine-C4/5), 114.10 (acridine-C10/12), 64.83 (Pt-NH=CCH2CH2CH2O-), 46.53 (-COCH(CH2CH2CH3)2), 35.81 (-COCH(CH2CH2CH3)2), 29.39 (-NH2CH2CH2CH2NH2-), 27.15 (Pt-NH=CCH2CH2CH2O-), 21.67 (-COCH(CH2CH2CH3)2), 14.34 ppm (-COCH(CH2CH2CH3)2); MS (ESI, positive-ion mode): m/z calcd for C30H47ClN6O2Pt [M–H] +: 752.31; found: 752.4.
Synthesis of [PtCl(en)C24H31N4O2](NO3)2 (7)
Compound 7 was prepared according to the procedure described for 2 from precursor 12 f with the yield of 89 %. 1H NMR (300 MHz, [D4]MeOH) δ = 8.72–8.46 (m, 2 H, acridine-1/8), 8.01 (ddd, J = 8.1, 6.9, 1.0 Hz, 2 H, acridine-H3/6), 7.92–7.80 (m, 2 H, acridine-H4/5), 7.64 (ddd, J =8.3, 6.9, 1.2 Hz, 2 H, acridine-H2/7), 6.16 (s, 1 H, Pt-NH=CCH2CH2CH2O-), 5.44–5.22 (m, 4 H,-NH2CH2CH2NH2-), 4.42 (t, J = 6.5 Hz, 2 H, -NHCH2CH2N(CH3)-), 4.18 (t, J = 6.5 Hz, 2 H, -NHCH2CH2N-(CH3)-), 3.98 (t, J = 6.5 Hz, 2 H, Pt-NH=CCH2CH2CH2O-), 3.12–3.02 (m, 5 H, Pt-NH=CCH2CH2CH2O-,-NHCH2CH2N(CH3)-), 2.74–2.46 (m, 4 H, -NH2CH2CH2NH2-), 2.34–1.99 (m, 4 H, Pt-NH=CCH2CH2CH2O-, -COCH2CH2CH3), 1.58 (d, J = 7.3 Hz, 2 H, -COCH2CH2CH3), 0.90 ppm (t, J =7.4 Hz, 3 H, -COCH2CH2CH3); 13C NMR (75 MHz, [D4]MeOH) δ = 175.33 (-COCH2CH2CH3), 170.11 (Pt-NH=CCH2CH2CH2O-), 160.04 (acridine-C9), 141.38 (acridine-C11/13), 136.69 (acridine-C3/6), 126.43 (acridine-C1/8), 125.50 (acridine-C2/7), 119.87 (acridine-C4/5), 114.12 (acridine-C10/12), 64.7 (Pt-NH=CCH2CH2CH2O-), 36.84 (-COCH2CH2CH3), 32.07 (Pt-NH=CCH2CH2CH2O-), 27.10 (Pt-NH= CCH2CH2CH2O-), 19.37 (-COCH2CH2CH3), 13.94 ppm (-COCH2CH2CH3); MS (ESI, positive-ion mode): m/z: calcd for C26H39ClN6O2Pt [M–H] +: 697.25; found: 697.3.
Synthesis of [PtCl(pn)C28H39N4O2](NO3)2 (8)
Complex 8 was prepared according to the procedure described for 2 from precursor 12 g with the yield of 76 %. 1H NMR (300 MHz, [D4]MeOH) δ = 8.54 (d, J = 8.6 Hz, 2 H, acridine-H1/8), 8.10–7.76 (m, 4 H, acridine-H3–6), 7.59 (t, J =7.4 Hz, 2 H, acridine-H2/7), 4.40 (t, J = 6.1 Hz, 2 H, -NHCH2CH2N(CH3)-), 4.17 (t, J = 6.2 Hz, 2 H, -NHCH2CH2N-(CH3)-), 3.97 (t, J = 6.0 Hz, 2 H, Pt-NH=CCH2CH2CH2O-), 3.21–2.98 (m, 5 H, Pt-NH=CCH2CH2CH2O-,-NHCH2CH2N(CH3)-), 2.94–2.54 (m, 4 H, -NH2CH2CH2CH2NH2-), 2.40–1.95 (m, 3 H, Pt-NH=CCH2CH2CH2O-,-COCH(CH2CH2CH3)2), 1.79 (m, 2 H, -NH2CH2CH2CH2NH2-), 1.56–0.99 (m, 8 H, -COCH(CH2CH2CH3)2), 0.92–0.68 ppm (m, 6 H, -COCH(CH2CH2CH3)2); 13C NMR (75 MHz, [D4]MeOH) δ=178.02 (-CO(CH2CH2CH3)2), 169.87 (Pt-NH=CCH2CH2CH2O-), 159.85 (acridine-C9), 141.33 (acridine-C11/13), 136.67 (acridine-C3/6), 126.61 (acridine-C1/8), 125.50 (acridine-C2/7), 119.87 (acridine-C4/5), 114.13 (acridine-C10/12), 64.86 (Pt-NH=CCH2CH2CH2O-), 46.56 (-COCH(CH2CH2CH3)2), 44.31 (-NH2CH2CH2CH2NH2-), 43.63 (-NH2CH2CH2CH2NH2-), 35.83 (-COCH(CH2CH2CH3)2), 29.29 (-NH2CH2CH2CH2NH2-), 26.76 (Pt-NH=CCH2CH2CH2O-), 21.69 (-COCH(CH2CH2CH3)2), 14.37 ppm (-COCH(CH2CH2CH3)2); MS (ESI, positive-ion mode): m/z: calcd for C31H49ClN6O2Pt [M–H] +: 767.33; found: 767.4.
Time-dependent NMR spectroscopy
NMR spectra in arrayed experiments were collected at 37 °C on a Bruker 500 DRX spectrometer equipped with a triple-resonance broadband inverse probe and a variable temperature unit. Reactions were performed with 2 mM platinum complex dissolved in either 600 μL of 10 mM phosphate buffer (PB, D2O, pH* = 7.0) or in 600 mL of phosphate-buffered saline (1 × PBS, D2O, pH* = 7.0). The 1 D NMR kinetics experiments were carried out as a standard Bruker arrayed 2 D experiment using a variable-delay list. Each incremented spectrum was processed using the same procedure, and suitable signals of the ester moiety were integrated. Data were processed with MestReNova NMR software. The concentrations of platinum complex at each time point were deduced from relative peak intensities, averaged over multiple signals to account for differences in proton relaxation, and fitted to a first-order exponential decay function in Origin 8.0 (OriginLab, Northampton, MA).
Chemical hydrolysis assay
The ester hydrolysis study of compounds 2–8 was carried out by incubating 1 mM of each test compound in 10 mM phosphate buffer (pH 7.4) or 1 × PBS containing ≈150 mM NaCl at 37 °C. At various time points samples were withdrawn from the reaction mixture and analyzed by in-line LC-ESMS. Chromatographic separations were performed with a 4.6 × 150 mm reverse-phase Agilent ZORBAX SB-C18 (5 μm) analytical column with the column temperature maintained at 25 °C. The following solvent system was used: solvent A, optima water, and solvent B, methanol/0.1 % formic acid, at a flow rate of 0.5 mL min−1 and a gradient of 95 % A to 5 % A over 15 min.
Enzymatic cleavage assay
To study ester cleavage in compounds 2–8 by recombinant human carboxylesterase-2 (rhCES-2), 30 μM of each compound was incubated with 400 μg mL−1 hCES-2 (BD Biosciences, San Jose, CA, USA) at 37 °C in 1 × PBS. Aliquots were withdrawn at various time points, quenched in an equal volume of methanol, and centrifuged for 5 min at 10 000 g to denature and precipitate protein. The supernatant was collected and subjected to product separation and analysis using in-line LC-ESMS. Chromatography was performed on a 4.6 × 150 mm reverse-phase Agilent ZORBAX SB-C18 (5 μm) analytical column with the column temperature maintained at 25 °C. The following solvent system was used: solvent A, optima water, and solvent B, methanol/0.1 % formic acid, at a flow rate of 0.5 mL min−1 and a gradient of 95 % A to 5 % A over 15 min.
Determination of partition coefficients (logD)
To obtain octanol-saturated water and water-saturated octanol, 100 mL of PBS was stirred with 100 mL of octanol for 24 h, followed by centrifugation for 5 min. The platinum complexes were dissolved in 1.0 mL of octanol-saturated PBS to a typical concentration of 0.1 mM and then mixed with 1.0 mL water-saturated octanol. Triplicates of each experiment were mixed in a multi-tube vortexer incubator for 16 h at room temperature and then centrifuged for 5 min. The layers were separated carefully, and the content of platinum–acridines was determined spectrophotometrically at 413 nm (with ε413 = 10 000 M −1 cm−1 in octanol-saturated PBS and ε413 = 8600 M −1 cm−1 in PBS-saturated octanol). The partition coefficients (D) of the samples were then determined as the quotient of the concentration of compound in octanol and the concentration in the aqueous layer. Reported logD values are the mean ± standard deviations of three determinations.
Cell culture maintenance
The human non-small cell lung cancer cell lines, NCI-H1435 and A549 (adenocarcinomas) were obtained from the American Type Culture Collection (Rockville, MD, USA). A549 cells were cultured in HAM’s F12K media (Gibco) supplemented with 10 % fetal bovine serum (FBS), 10 % penstrep (P&S), 10 % L-glutamine, and 1.5 g L−1 NaHCO3. NCI-H1435 cells were cultured in serum-free 1:1 DMEM/ F12 media (Gibco) containing 2.436 g L−1 NaHCO3, 0.02 mg mL−1 insulin, 0.01 mg mL−1 transferrin, 25 nM sodium selenite, 50 nM hydrocortisone, 1 ng mL−1 epidermal growth factor, 0.01 mM ethanol-amine, 0.01 mM phosphorylethanolamine, 100 pM triiodothyronine, 0.5 % (w/v) bovine serum albumin (BSA), 10 mM HEPES, 0.5 mM sodium pyruvate, and an extra 2 mM L-glutamine (final concentration 4.5 mM). Cells were incubated at a constant temperature at 37°C in a humidified atmosphere containing 5 % CO2 and were subcultured every 2–3 days in order to maintain cells in logarithmic growth, except for slowly proliferating NCI-H1435, which was subcultured every seven days.
Cytotoxicity assay
The cytotoxicity studies were carried out according to a standard protocol using the Celltiter 96 aqueous nonradioactive cell proliferation assay kit (Promega, Madison, WI). Stock solutions (5–10 mM) of 1′–8 were made in DMF and serially diluted with media prior to incubation with cancer cells. All drugs and controls were tested at the indicated concentrations in triplicate wells on duplicate plates. Incubations were carried out for 72 h and cell viabilities were determined by comparing drug-treated wells with control cells.
Supplementary Material
Acknowledgments
The authors thank Tiffany K. West for assistance with cell culture maintenance. This work was supported by the National Institutes of Health/NCI (Grant CA101880) and the Product Innovation and Commercialization Services of Wake Forest University through a SPARK award.
Footnotes
Supporting information for this article is available on the WWW under http://dx.doi.org/10.1002/chem.201404675.
References
- 1.Huttunen KM, Raunio H, Rautio J. Pharmacol Rev. 2011;63:750–771. doi: 10.1124/pr.110.003459. [DOI] [PubMed] [Google Scholar]
- 2.Lammers T, Kiessling F, Hennink WE, Storm G. J Controlled Release. 2012;161:175–187. doi: 10.1016/j.jconrel.2011.09.063. [DOI] [PubMed] [Google Scholar]
- 3.Kerns EH, Di L. Drug Discovery Today. 2003;8:316–323. doi: 10.1016/s1359-6446(03)02649-7. [DOI] [PubMed] [Google Scholar]
- 4.Suryadi J, Bierbach U. Chem Eur J. 2012;18:12926–12934. doi: 10.1002/chem.201202050. [DOI] [PubMed] [Google Scholar]
- 5.Ma Z, Choudhury JR, Wright MW, Day CS, Saluta G, Kucera GL, Bierbach U. J Med Chem. 2008;51:7574–7580. doi: 10.1021/jm800900g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheung-Ong K, Song KT, Ma Z, Shabtai D, Lee AY, Gallo D, Heisler LE, Brown GW, Bierbach U, Giaever G, Nislow C. ACS Chem Biol. 2012;7:1892–1901. doi: 10.1021/cb300320d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Qiao X, Zeitany AE, Wright MW, Essader AS, Levine KE, Kucera GL, Bierbach U. Metallomics. 2012;4:645–652. doi: 10.1039/c2mt20031g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Smyre CL, Saluta G, Kute TE, Kucera GL, Bierbach U. ACS Med Chem Lett. 2011;2:870–874. doi: 10.1021/ml2001888. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ma Z, Rao L, Bierbach U. J Med Chem. 2009;52:3424–3427. doi: 10.1021/jm900451y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Lullmann H, Mohr K, Ziegler A, Bieger D. Color Atlas of Pharmacology. 2. Thieme; Stuttgart: 2000. pp. 32–42. [Google Scholar]
- 11.Pratt SE, Durland-Busbice S, Shepard RL, Heinz-Taheny K, Iversen PW, Dantzig AH. Clin Cancer Res. 2013;19:1159–1168. doi: 10.1158/1078-0432.CCR-12-1184. [DOI] [PubMed] [Google Scholar]
- 12.Graham LA, Suryadi J, West TK, Kucera GL, Bierbach U. J Med Chem. 2012;55:7817–7827. doi: 10.1021/jm300879k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Imai T, Taketani M, Shii M, Hosokawa M, Chiba K. Drug Metab Dispos. 2006;34:1734–1741. doi: 10.1124/dmd.106.009381. [DOI] [PubMed] [Google Scholar]
- 14.Ding S, Qiao X, Kucera GL, Bierbach U. J Med Chem. 2012;55:10198–10203. doi: 10.1021/jm301278c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Natile G, Intini FP, Bertani R, Michelin RA, Mozzon M, Sbovata SM, Venzo A, Seraglia R. J Organomet Chem. 2005;690:2121–2127. [Google Scholar]
- 16.Michelin RA, Sgarbossa P, Sbovata SM, Gandin V, Marzano C, Bertani R. Chem Med Chem. 2011;6:1172–1183. doi: 10.1002/cmdc.201100150. [DOI] [PubMed] [Google Scholar]
- 17.Hochreuther S, Puchta R, van Eldik R. Inorg Chem. 2011;50:8984–8996. doi: 10.1021/ic201151h. [DOI] [PubMed] [Google Scholar]
- 18.Wang J, Williams ET, Bourgea J, Wong YN, Patten CJ. Drug Metab Dispos. 2011;39:1329–1333. doi: 10.1124/dmd.111.039628. [DOI] [PubMed] [Google Scholar]
- 19.Valiahdi SM, Egger AE, Miklos W, Jungwirth U, Meelich K, Nock P, Berger W, Hartinger CG, Galanski M, Jakupec MA, Keppler BK. J Biol Inorg Chem. 2013;18:249–260. doi: 10.1007/s00775-012-0970-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Weaver DA, Crawford EL, Warner KA, Elkhairi F, Khuder SA, Willey JC. Mol Cancer. 2005;4:18. doi: 10.1186/1476-4598-4-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Barthel BL, Zhang Z, Rudnicki DL, Coldren CD, Polinkovsky M, Sun H, Koch GG, Chan DC, Koch TH. J Med Chem. 2009;52:7678–7688. doi: 10.1021/jm900694z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Martins ET, Baruah H, Kramarczyk J, Saluta G, Day CS, Kucera GL, Bierbach U. J Med Chem. 2001;44:4492–4496. doi: 10.1021/jm010293m. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Z, Hatta H, Tanabe K, Nishimoto S. Pharm Res. 2005;22:381–389. doi: 10.1007/s11095-004-1875-x. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.