Abstract
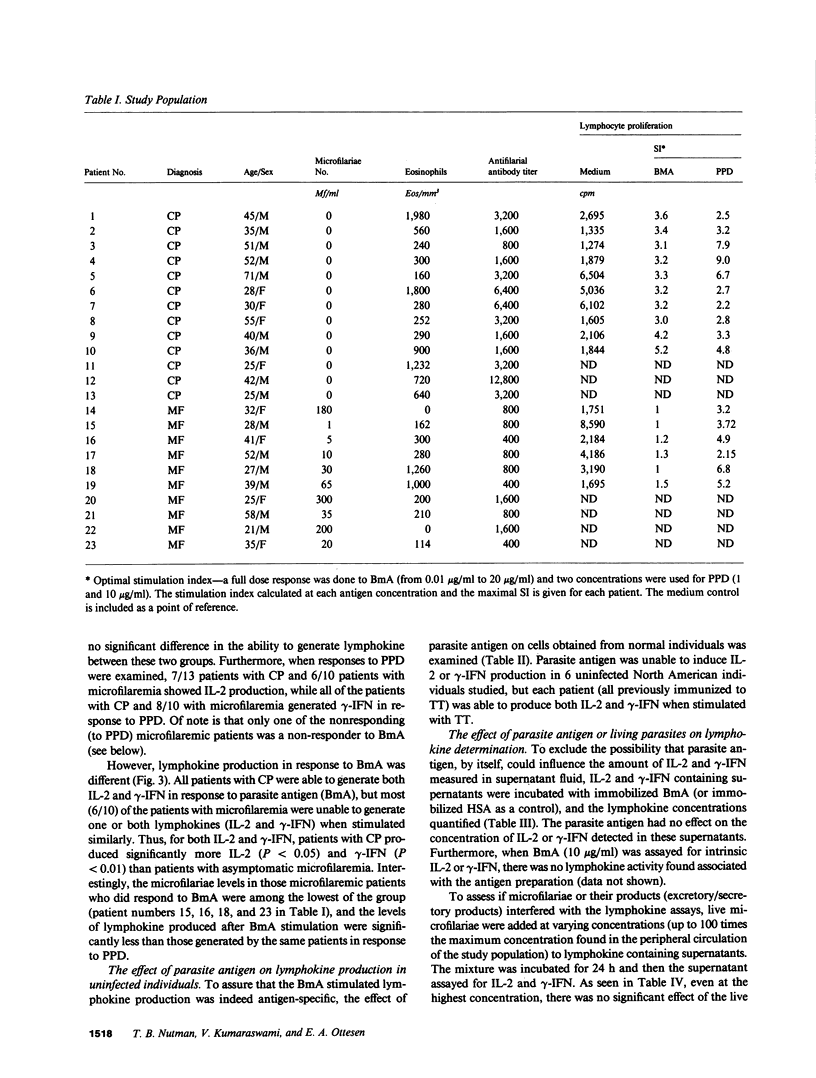
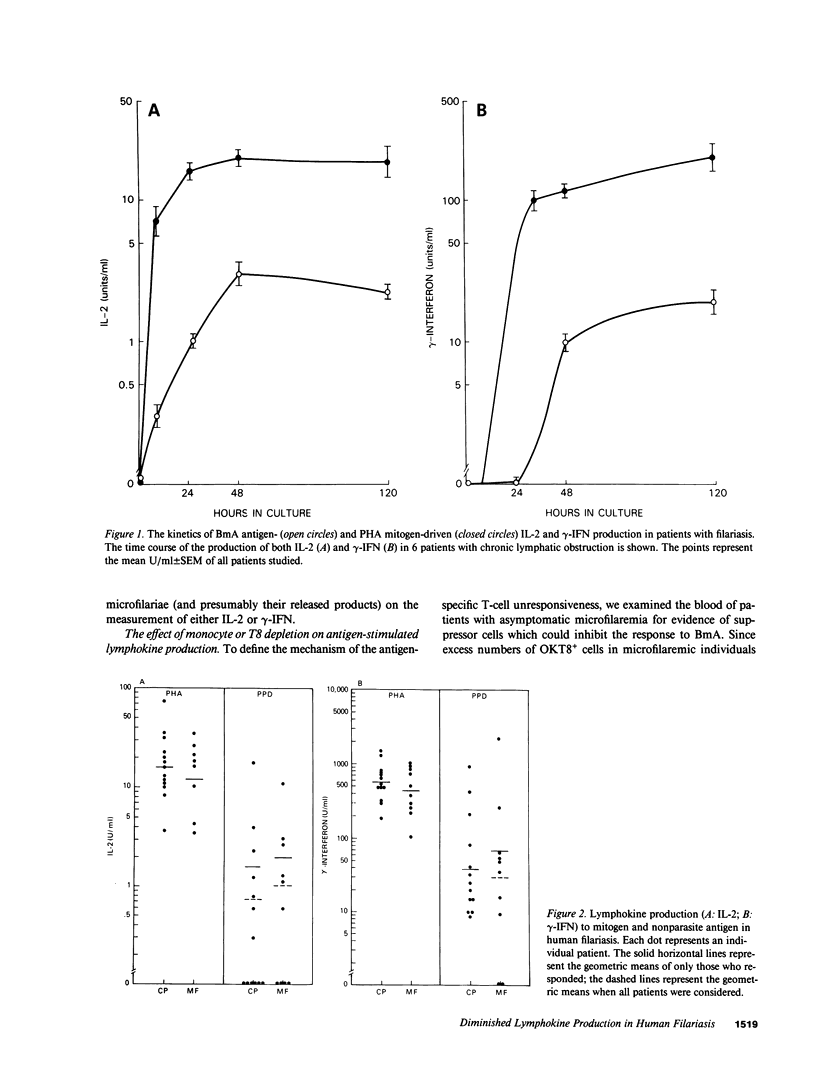
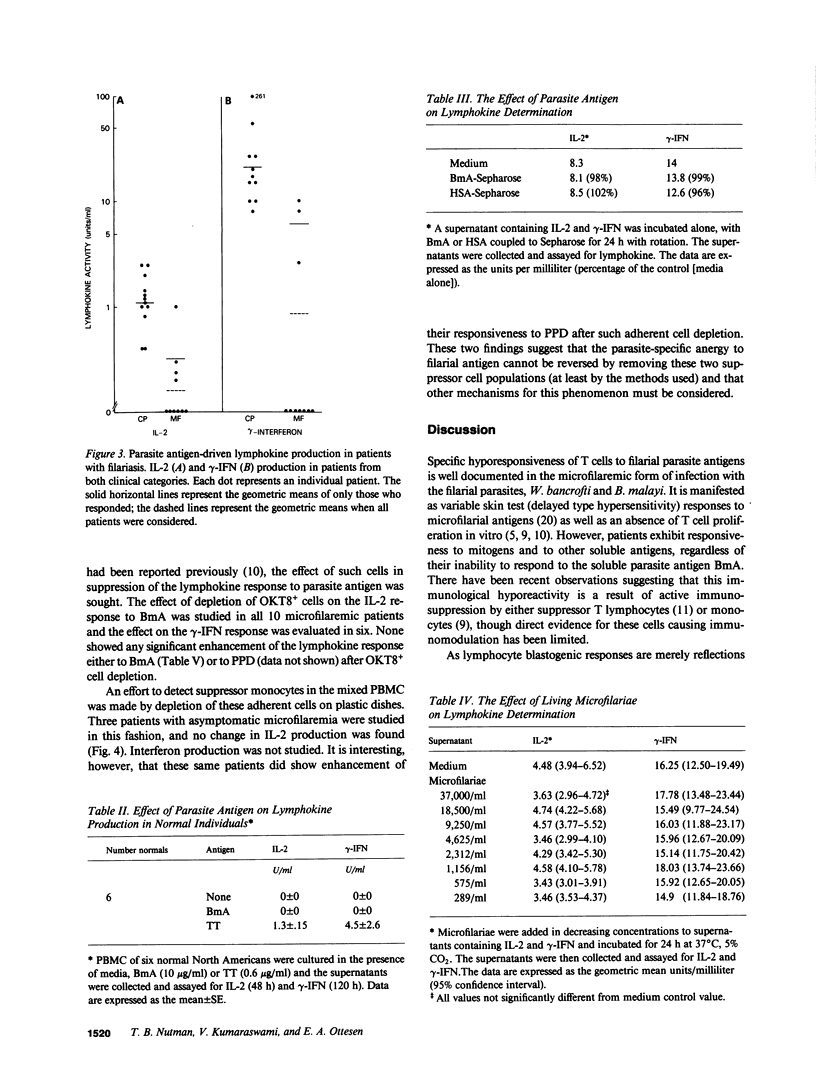
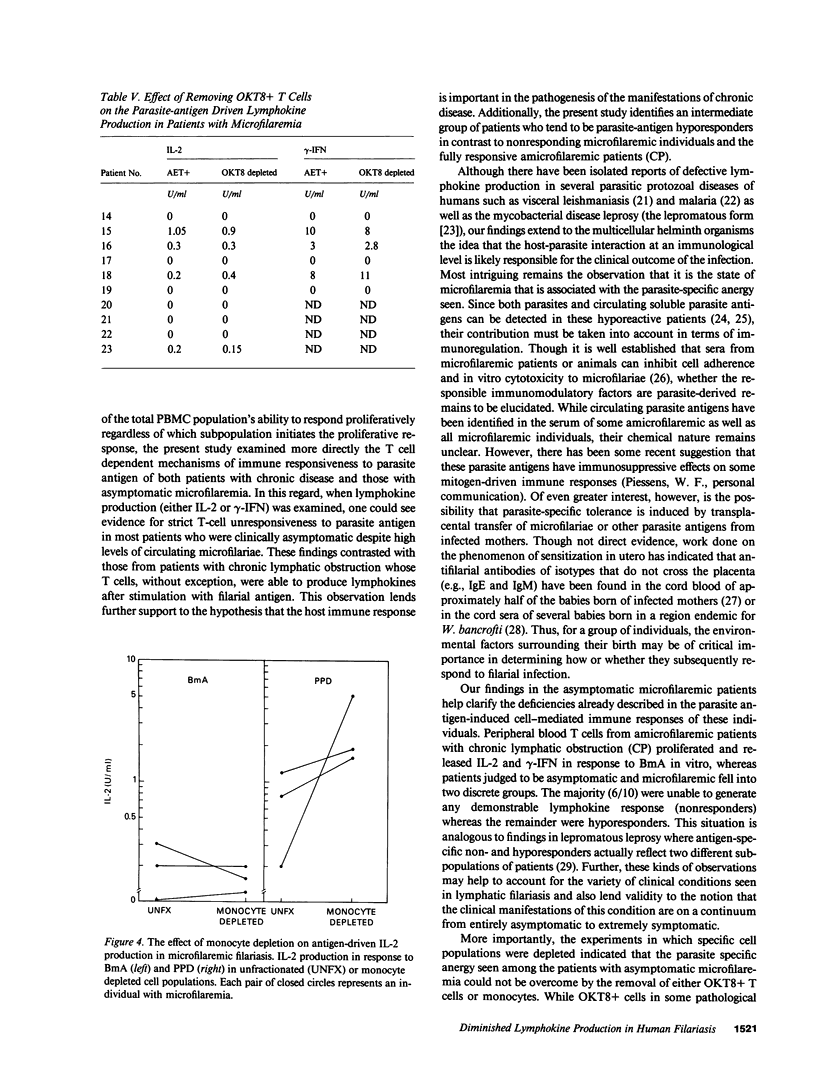
The antigen-specific immune unresponsiveness seen in bancroftian filariasis was studied by examining lymphokine production in peripheral blood mononuclear cells (PBMC) or PBMC subpopulations from 10 patients with asymptomatic microfilaremia, 13 patients with elephantiasis and 6 normal North Americans. In each group of patients, the kinetics of the lymphokine response and the response to mitogens and nonparasite antigens did not differ significantly. In marked contrast, when antigen-induced lymphokine production was examined, most patients with microfilaremia were unable to produce either interleukin 2 (IL-2) or gamma-interferon (i.e., were nonresponders), and the few who could (hyporesponders, generally with quite low microfilaremia levels) did so at levels significantly less than those of patients with elephantiasis, all of whom showed strong responses to parasite antigen. Removal of neither adherent cells or T8+ cells affected the parasite-specific anergy seen in those with microfilaremia, suggesting a state of T cell tolerance to the parasite in patients with this most common clinical manifestation of bancroftian filariasis.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carvalho E. M., Badaró R., Reed S. G., Jones T. C., Johnson W. D., Jr Absence of gamma interferon and interleukin 2 production during active visceral leishmaniasis. J Clin Invest. 1985 Dec;76(6):2066–2069. doi: 10.1172/JCI112209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dissanayake S., de Silva L. V., Ismail M. M. IgM antibody to filarial antigens in human cord blood: possibility of transplacental infection. Trans R Soc Trop Med Hyg. 1980;74(4):542–544. doi: 10.1016/0035-9203(80)90075-9. [DOI] [PubMed] [Google Scholar]
- Falkoff R. M., Peters M., Fauci A. S. T cell enrichment and depletion of human peripheral blood mononuclear cell preparations. Unexpected findings in the study of the functional activities of the separated populations. J Immunol Methods. 1982;50(1):39–49. doi: 10.1016/0022-1759(82)90302-7. [DOI] [PubMed] [Google Scholar]
- Fanning M. M., Kazura J. W. Genetic association of murine susceptibility to Brugia malayi microfilaraemia. Parasite Immunol. 1983 May;5(3):305–316. doi: 10.1111/j.1365-3024.1983.tb00746.x. [DOI] [PubMed] [Google Scholar]
- Forsyth K. P., Spark R., Kazura J., Brown G. V., Peters P., Heywood P., Dissanayake S., Mitchell G. F. A monoclonal antibody-based immunoradiometric assay for detection of circulating antigen in Bancroftian filariasis. J Immunol. 1985 Feb;134(2):1172–1177. [PubMed] [Google Scholar]
- Hamilton R. G., Hussain R., Ottesen E. A. Immunoradiometric assay for detection of filarial antigens in human serum. J Immunol. 1984 Oct;133(4):2237–2242. [PubMed] [Google Scholar]
- Hussain R., Hamilton R. G., Kumaraswami V., Adkinson N. F., Jr, Ottesen E. A. IgE responses in human filariasis. I. Quantitation of filaria-specific IgE. J Immunol. 1981 Oct;127(4):1623–1629. [PubMed] [Google Scholar]
- Kaplan G., Weinstein D. E., Steinman R. M., Levis W. R., Elvers U., Patarroyo M. E., Cohn Z. A. An analysis of in vitro T cell responsiveness in lepromatous leprosy. J Exp Med. 1985 Sep 1;162(3):917–929. doi: 10.1084/jem.162.3.917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb J. R., Skidmore B. J., Green N., Chiller J. M., Feldmann M. Induction of tolerance in influenza virus-immune T lymphocyte clones with synthetic peptides of influenza hemagglutinin. J Exp Med. 1983 May 1;157(5):1434–1447. doi: 10.1084/jem.157.5.1434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Linker-Israeli M., Bakke A. C., Quismorio F. P., Jr, Horwitz D. A. Correction of interleukin-2 production in patients with systemic lupus erythematosus by removal of spontaneously activated suppressor cells. J Clin Invest. 1985 Feb;75(2):762–768. doi: 10.1172/JCI111758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lê thi Bich-Thuy, Lane H. C., Fauci A. S. Human B-cell proliferation in response to recombinant interleukin 2 is not due to T-cell help. Cell Immunol. 1985 Sep;94(2):353–359. doi: 10.1016/0008-8749(85)90259-x. [DOI] [PubMed] [Google Scholar]
- McGreevy P. B., Ratiwayanto S., Tuti S., McGreevy M. M., Dennis D. T. Brugia malayi: relationship between anti-sheath antibodies and amicrofilaremia in natives living in an endemic area of South Kalimantan, Borneo. Am J Trop Med Hyg. 1980 Jul;29(4):553–562. doi: 10.4269/ajtmh.1980.29.553. [DOI] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Mohagheghpour N., Gelber R. H., Larrick J. W., Sasaki D. T., Brennan P. J., Engleman E. G. Defective cell-mediated immunity in leprosy: failure of T cells from lepromatous leprosy patients to respond to Mycobacterium leprae is associated with defective expression of interleukin 2 receptors and is not reconstituted by interleukin 2. J Immunol. 1985 Aug;135(2):1443–1449. [PubMed] [Google Scholar]
- Morimoto C., Letvin N. L., Distaso J. A., Aldrich W. R., Schlossman S. F. The isolation and characterization of the human suppressor inducer T cell subset. J Immunol. 1985 Mar;134(3):1508–1515. [PubMed] [Google Scholar]
- Nutman T. B., Ottesen E. A., Fauci A. S., Volkman D. J. Parasite antigen-specific human T cell lines and clones. Major histocompatibility complex restriction and B cell helper function. J Clin Invest. 1984 Jun;73(6):1754–1762. doi: 10.1172/JCI111384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ojo-Amaize E. A., Salimonu L. S., Williams A. I., Akinwolere O. A., Shabo R., Alm G. V., Wigzell H. Positive correlation between degree of parasitemia, interferon titers, and natural killer cell activity in Plasmodium falciparum-infected children. J Immunol. 1981 Dec;127(6):2296–2300. [PubMed] [Google Scholar]
- Ottesen E. A. Immunological aspects of lymphatic filariasis and onchocerciasis in man. Trans R Soc Trop Med Hyg. 1984;78 (Suppl):9–18. doi: 10.1016/0035-9203(84)90309-2. [DOI] [PubMed] [Google Scholar]
- Ottesen E. A., Mendell N. R., MacQueen J. M., Weller P. F., Amos D. B., Ward F. E. Familial predisposition to filarial infection--not linked to HLA-A or-B locus specificities. Acta Trop. 1981 Sep;38(3):205–216. [PubMed] [Google Scholar]
- Ottesen E. A., Weller P. F., Heck L. Specific cellular immune unresponsiveness in human filariasis. Immunology. 1977 Sep;33(3):413–421. [PMC free article] [PubMed] [Google Scholar]
- Ottesen E. A., Weller P. F., Lunde M. N., Hussain R. Endemic filariasis on a Pacific Island. II. Immunologic aspects: immunoglobulin, complement, and specific antifilarial IgG, IgM, and IgE antibodies. Am J Trop Med Hyg. 1982 Sep;31(5):953–961. [PubMed] [Google Scholar]
- Piessens W. F., Hoffman S. L., Ratiwayanto S., Piessens P. W., Partono F., Kurniawan L., Marwoto H. A. Opposing effects of filariasis and chronic malaria on immunoregulatory T lymphocytes. Diagn Immunol. 1983;1(3):257–260. [PubMed] [Google Scholar]
- Piessens W. F., McGreevy P. B., Piessens P. W., McGreevy M., Koiman I., Saroso J. S., Dennis D. T. Immune responses in human infections with Brugia malayi: specific cellular unresponsiveness to filarial antigens. J Clin Invest. 1980 Jan;65(1):172–179. doi: 10.1172/JCI109648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piessens W. F., Partono F., Hoffman S. L., Ratiwayanto S., Piessens P. W., Palmieri J. R., Koiman I., Dennis D. T., Carney W. P. Antigen-specific suppressor T lymphocytes in human lymphatic filariasis. N Engl J Med. 1982 Jul 15;307(3):144–148. doi: 10.1056/NEJM198207153070302. [DOI] [PubMed] [Google Scholar]
- Piessens W. F., Partono F., Hoffman S. L., Ratiwayanto S., Piessens P. W., Palmieri J. R., Koiman I., Dennis D. T., Carney W. P. Antigen-specific suppressor T lymphocytes in human lymphatic filariasis. N Engl J Med. 1982 Jul 15;307(3):144–148. doi: 10.1056/NEJM198207153070302. [DOI] [PubMed] [Google Scholar]
- Sim B. K., Kwa B. H., Mak J. W. The presence of blocking factors in Brugia malayi microfilaraemic patients. Immunology. 1984 Jul;52(3):411–416. [PMC free article] [PubMed] [Google Scholar]
- Watson J. Continuous proliferation of murine antigen-specific helper T lymphocytes in culture. J Exp Med. 1979 Dec 1;150(6):1510–1519. doi: 10.1084/jem.150.6.1510. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil G. J., Hussain R., Kumaraswami V., Tripathy S. P., Phillips K. S., Ottesen E. A. Prenatal allergic sensitization to helminth antigens in offspring of parasite-infected mothers. J Clin Invest. 1983 May;71(5):1124–1129. doi: 10.1172/JCI110862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weller P. F., Ottesen E. A. Failure of diethylcarbamazine as a provocative test in subperiodic Wuchereria bancrofti filariasis. Trans R Soc Trop Med Hyg. 1978;72(1):31–32. doi: 10.1016/0035-9203(78)90293-6. [DOI] [PubMed] [Google Scholar]
- Weller P. F., Ottesen E. A., Heck L. Immediate and delayed hypersensitivity skin test responses to the Dirofilaria immitis filarial skin test (Sawada) antigen in Wuchereria bancrofti filariasis. Am J Trop Med Hyg. 1980 Sep;29(5):809–814. doi: 10.4269/ajtmh.1980.29.809. [DOI] [PubMed] [Google Scholar]



