Abstract
Despite numerous studies on mammalian fertilization, the mechanisms of fertilization—including the timing of acrosome reaction—remain largely unknown; more accurately described, the classical theory built upon years of layered experimental data is being challenged by recent conflicting evidence provided by gene-manipulated animals. Although in vitro fertilization remains our central research tool, the classical theory’s decline reminds us of the importance of in vivo observations. Here, I describe the essential roles of gene-manipulated animals in elucidating the mechanism of fertilization and the pitfalls of in vitro fertilization studies trapping many researchers.
Keywords: acrosome reaction, fertilization, gene-manipulated animals, live imaging, sperm-egg fusion
Introduction
The role of spermatozoa is to fertilize eggs. However, mammalian spermatozoa produced in large numbers compared to eggs are incapable of fertilizing upon ejaculation. They must first undergo a physiological change called capacitation and a subsequent morphological change known as the acrosome reaction [59]. Both of these events occur in the female reproductive tract, with the sperm number ultimately decreasing to one, as a single spermatozoon fertilizes one egg. Thus the study of fertilization has an intrinsic disadvantage of small cell numbers to be examined. Fertilization was originally studied for the purpose of establishing an in vitro fertilization system [60]. Initially, the system required the female factors, but soon a defined medium was reported in which spermatozoa could be capacitated, acrosome reacted and eggs fertilized. This in vitro fertilization system was utilized by many researchers to analyze the mechanism of fertilization, with numerous factors contributing to sperm-egg interaction identified and classical models for the mechanisms of fertilization formed [46].
Problems Involved in the inVitro Fertilization System
Although the in vitro fertilization (IVF) system produced findings and clinical applications (such as the Nobel-recognized human in vitro fertilization), it has an inherent scientific flaw. Table 1 lists our in-house in vitro fertilization results using frozen BALB/C spermatozoa. As indicated, the fertilization rate using the same spermatozoa fluctuates from 12 to 98%. In the in vitro fertilization system, the same spermatozoa show very different fertilizing abilities when different media are used. In other words, in vitro fertilization is susceptible to external influences and therefore cannot offer an ideal examination of factors impacting fertilization. The fertilizing ability of the same spermatozoa can be evaluated in many ways depending on the condition of the medium. Moreover, a larger number of spermatozoa are required for fertilization in vitro than in vivo. We must keep in mind that the in vitro fertilization system is very different from natural fertilization.
Table 1. A significant difference found in fertilizing ability in different media.
| Meida | Fertilization | Number of eggs | ||||||
|---|---|---|---|---|---|---|---|---|
| Sperm preincubation | Fertilization | (%) | sum | 2 cells | Unfertilized | Polyspermy | ||
| mHTF | mHTF | 12 | 49 | 6 | 43 | 0 | ||
| FERTIUP | mHTF | 10 | 63 | 6 | 57 | 0 | ||
| mHTF | CARD Medium 1* | 41 | 47 | 19 | 27 | 1 | ||
| FERTIUP | CARD Medium 1* | 71 | 44 | 30 | 12 | 2 | ||
| FERTIUP | CARD Medium 2** | 98 | 63 | 57 | 1 | 5 | ||
Frozen BALB/C spermatozoa were thawed and then preincubated in mHTF or FERTIUP media and were added to eggs in various media to analyze fertilizing ability (fertilization was observed 24 hr after insemination). * with 15 μl B solution. ** with 30 μl B solution. From in-house data of the Animal Resource Center for Infectious Diseases, Research Institute for Microbial Diseases, Osaka University.
Most of the Fertilization-related Factors Found Using the inVitro Fertilization System were Shown to be Inessential in Gene-disruption Experiments
Fertilization related factors identified using the in vitro fertilization system are indicated in Table 2 together with the fertilizing ability of the mice in which these genes were disrupted. As indicated, most of the factors predicted to be involved in sperm-egg interaction were found to be inessential in gene disruption experiments. One of the exceptions is the OBF13 antigen. This antigen, named IZUMO1, is the only known fusion-related factor on spermatozoa as of today [26]. Adam2, as a subunit of fertilin, turned out to be essential but not with the initially postulated role in fusion.
Table 2. Sperm proteins involved in sperm-egg interaction indicated by biochemical means.
| Genes | Predicted roles | No. of pups/litter (before vs. after gene disruption) |
References |
|---|---|---|---|
| Acr (acrosin) | zona penetration | 10.0 vs. 12.5 | (Baba et al., 1994) [3] |
| β4galt1 (GalTase) | sperm-zona binding | fertile in vivo | (Lu and Shur, 1997) [36] |
| Spam1 (Ph-20, hyaluronidase) | sperm-zona binding | 13.8 vs. 12.2 | (Baba et al., 2002) [2] |
| Cd46 | sperm-egg fusion | 9.0 vs. 8.9 | (Inoue et al., 2003) [27] |
| Sed1 | sperm-zona binding | 9.3 vs. 3.3 or | (Ensslin and Shur, 2003) [11] |
| *fertile in vivo | *(Hanayama et al., 2004) [18] | ||
| Izumo1 (OBF13) | sperm-egg fusion | 8.8 vs. 0.0 | (Inoue et al., 2005) [26] |
| Adam1a/b (Fertilin) | sperm-egg fusion | 9.9 vs. 9.3 | (Kim et al., 2006) [33] |
| Crisp1 | sperm-egg fusion | 7.3 vs. 6.5 | (Da Ros et al., 2008) [10] |
| Pkdrej | sperm-zona binding | 8.8 vs. 7.1 | (Sutton et al., 2008) [51] |
| Zan (zonadhesin) | sperm-zona binding | 5.5 vs. 6.5 | (Tardif et al.,2010) [52] |
| Zp3r (Sp56) | sperm-zona binding | 8.6 vs. 9.4 | (Muro et al., 2012) [40] |
The results shown in Table 2 startled researchers in this field. Without evaluating these factors using gene-manipulated animals, researchers would never realize that fertilization could be accomplished without them. Thus, the gene-disruption experiment is a very powerful tool in investigating fertilization, but we must be cautious in interpreting the knockout results.
Before introducing the power of gene-disruption experiments, I will elaborate on the drawbacks of gene-disruption experiments. When MRF4, one of the members of a basic helix-loop-helix myogenic regulatory factor (MRF) family, was disrupted, Arnold’s group declared that the mice die at birth, Olson’s group indicated that the mice survive, and Wold’s group said that the mice die occasionally. Afterward, it was found that insertion of a neo gene was detrimental to the neighboring Myf5 gene and Mrf4 disruption was not the cause of the neonatal death [44]. A similar case was reported in prion disruption. Some groups reported an ataxia phenotype, whereas other groups reported no phenotype. The difference was that when some of the targeting vectors were used, it caused an exon skip and connect the prion gene to the neighboring doppel gene to express an aberrant fusion protein ectopically [47]. Another caution is also required, as disrupting the gene may unexpectedly eliminate miRNA (s) in the modified area [45]. In all of these cases, however, the gene disruption may result in a false positive phenotype. If we see no phenotype after gene disruption, one may conclude that the gene’s elimination does not greatly affect the mice.
However, when some genes pair with others to form an essential gene set, a single gene disruption may not result in an apparent phenotype. Moreover, if the knockout of one factor is compensated for by existing redundant factors, we may not be able to observe the phenotypes. Could these cases be applicable to the genes listed in Table 2? I will leave that for the readers to consider.
Table 2 presents an interesting reflection on the history of fertilin disruption. Fertilin is an ADAM1b/ADAM2 heterodimer, a sperm-specific protein depicted by many textbooks as a sperm-egg fusion protein [7]. Many researchers support its involvement in sperm-egg fusion. To support this notion, egg integrins have emerged as a fertilin counterpart on egg [1]. However, the first evidence that this theory might be crumbling followed the integrin-disruption experiment. Integrin-deficient eggs were found to fuse with spermatozoa [21, 38]. Meanwhile the elimination of Adam2, resulting in the loss of fertilin from spermatozoa, caused infertility in males. Therefore, fertilin was thought to be an essential protein for fertilization [9]. However, differing from expectation, the phenotype was not in sperm-egg fusion but rather impaired sperm-zona interaction [9]. Moreover, when fertilin-null spermatozoa were made by disrupting the Adam1b gene (ADAM1b being the second subunit of the fertilin heterodimer), the males remained fertile, despite the disappearance of fertilin [33]. The reason for the infertility in the Adam2-deficient/fertilin knockout mice was later discovered. ADAM2 had a function in testis to form a dimer with testicular ADAM1a (not with sperm ADAM1b), which functions to present ADAM3 on spermatozoa [42]. (ADAM3 is a protein that is required for spermatozoa to exhibit fertilization competency [49].)
Various Essential Factors in Fertilization are Emerging Serendipitously through Gene-disruption Experiments
Genes essential for fertilization have emerged from gene disruption experiments. Surprisingly, more than 10 gene-disrupted mouse lines shared common phenotypes, with i) no migration into the oviduct in vivo and ii) aberrant zona binding ability in vitro. We refer to this group as Class I; all spermatozoa from this group were shown to lack ADAM3 (or have aberrant ADAM3). Since the Adam3-disrupted male mice are infertile and produce spermatozoa with a Class I phenotype [49] ADAM3 was considered to function as an ultimately essential factor in all gene-disrupted mouse lines with Class I phenotype. The gene-disrupted mouse lines with Class I phenotype are listed in Table 3.
Table 3. Gene-disrupted mouse lines with Class I phenotype.
| Gene | localization | ADAM3 on spermatozoa |
zona binding ability | References |
|---|---|---|---|---|
| Clgn (calmegin) | ER membrane | disappeared | impaired | (Ikawa et al., 1997) [24] |
| Adam2 | sperm surface | disappeared | impaired | (Cho et al., 1998) [7] |
| Ace (angiotensin converting enzyme) | Sperm surface | aberrantly localized | impaired | (Hagaman et al., 1998) [17] (Yamaguchi et al., 2006) [58] |
| Adam3 | sperm surface | disappeared | impaired | (Shamsadin et al., 1999; [49] Yamaguchi et al., 2009) [57] |
| Adam1a | sperm surface | disappeared | impaired | (Nishimura et al., 2004) [42] |
| Calr3 (calsperin) | ER lumen | disappeared | impaired | (Ikawa et al., 2011) [25] |
| Tpst2 | Acrosomal cap → equatorial segment | disappeared | impaired | (Marcello et al., 2011) [37] |
| Pdilt | ER membrane | disappeared | impaired | (Tokuhiro et al., 2012) [53] |
| Pmis-2 | sperm surface | disappeared | impaired | (Yamaguchi et al., 2012) [56] |
| RNase10 | epididymis | disappeared | impaired | (Krutskikh et al., 2012) [34] |
| Tex101 | spermatid | disappeared | impaired | (Fujihara et al., 2013) [14] |
| Prss37 | Spermatid/Sperm | disappeared | impaired | (Shen et al., 2013) [50] |
| Ly6K | Testicular germ cells | intact | impaired | (Fujihara et al., 2014) [13] |
Class I: impaired sperm migration into oviduct in vivo and aberrant zona binding in vitro. ADAM3 seems to be a key molecule in mouse fertilization because all of the gene disrupted infertile males have ADAM3 impaired spermatozoa (except newly reported Ly6k). However, ADAM3 is a pseudo gene in human. Since many other genes are preserved in human, a key factor may remain missing and replace the position of ADAM3. The characteristic nature of Ly6k KO, in which ADAM3 on spermatozoa seems to remain intact, may imply the existence of an undiscovered factor which might commonly exist in mouse and human.
In mice, the uterus and oviduct meet in a structure called the uterotubal junction (UTJ), which significantly reduces the number of spermatozoa reaching eggs.
Sperm migration is restricted by factors other than just the narrow opening of the UTJ. We produced chimeric mice that ejaculate both wild-type spermatozoa and GFP-tagged calmegin-disrupted spermatozoa and mated them with females. We found that only wild-type spermatozoa migrated into the oviduct, while the equally motile calmegin-disrupted spermatozoa remained in the uterus. Therefore, some unknown recognition system between individual spermatozoa and the UTJ is at work, restricting entry into the oviduct.
The “Zona-binding Ability” of Spermatozoa Must be Reconsidered
When we mix spermatozoa with cumulus-free eggs, we can observe many spermatozoa binding to the zona pellucida. However, they cannot bind to the zonae of 2-cell-stage eggs. Fertilization was therefore considered to involve a sperm-zona binding step, with the loss of zona-binding ability explaining infertility in Class I knockout mouse lines.
However, recent results with gene-manipulated animals indicated that we must abandon or significantly revise this assumption. As listed in Table 3, Pdilt-disrupted mice produce spermatozoa with impaired zona binding ability [53]. We crossed this mouse line with another transgenic mouse line with fluorescent protein-tagged sperm [20] for visibility in the female reproductive tract. After observing impaired zona binding and impaired sperm migration into the oviduct, we deposited the same spermatozoa directly into the ampulla to bypass the UTJ migration. Despite the lack of zona binding ability, the spermatozoa fertilized eggs under this condition [53]. Likewise, it is reported that spermatozoa from infertile Adam1a−/− mice fertilized eggs covered by cumulus cells [42]. These observations indicate that the “sperm-zona binding” reported by many researchers may not reflect the real sperm-egg interaction required for fertilization in vivo.
Zona-induced Acrosome Reaction Must be Reconsidered
Spermatozoa have a huge lysosome-like organelle called acrosome on the tip of their head containing various lysosomal enzymes. These enzymes are considered to be released by exocytosis and required for the penetration of egg investments (cumulus cell layers and the zona pellucida) [12]. It was therefore assumed that acrosome reaction must occur upon final approach to eggs. More specifically, many researchers thought that it was induced upon contact with the zona pellucida, and that spermatozoa undergoing acrosome reaction before zona contact had no fertilizing ability [6]. This idea was supported by reports that the addition of solubilized zona pellucida can induce acrosome reaction [12], and that a partial ZP3 sequence can accomplish the same effect [22]. Based on this context, zona binding proteins were assumed to initiate the signaling cascade leading to acrosome reaction [16] and various zona binding proteins were purified from spermatozoa. However, the gene disruption of these factors did not show a significant phenotype in fertilization as indicated in Table 2. In order to observe the moment of acrosome reaction in living spermatozoa, we created a transgenic mouse line with GFP targeted in acrosome. Upon reaction, fluorescence disappeared from the acrosome in these spermatozoa.
Despite the widely accepted idea that sperm binding to zona induces acrosome reaction, zona-binding spermatozoa had intact acrosome and did not acrosome react by binding to zona [4, 41]. This was reinforced by a recent study observing the fertilization process in vitro using spermatozoa having acrosomal GFP. According to Jin et al., most of the fertilizing spermatozoa were acrosome-reacted before reaching the zona pellucida in the in vitro fertilization process [31].
Another report using gene-manipulated animals also questioned the zona-induced acrosome reaction theory. Izumo1 [26] and CD9 disrupted mouse lines [32, 35, 39] are known to make infertile males and females, respectively. Their spermatozoa and eggs have no fusing ability with the gametes of the other sex. Therefore, if we collect eggs from the oviducts of mated animals, we can find many spermatozoa accumulated inside the zona pellucida, as sperm-egg fusion does not occur in these animals and the zona remain intact to receive many spermatozoa. In this manner, many zona-penetrated spermatozoa, which are all acrosome-reacted, were flushed out from perivitelline space and added to fresh, cumulus-enclosed, zona-intact eggs. In the past, when spermatozoa acrosome reacted before binding to zona, it was called premature acrosome reaction and these spermatozoa were thought to have no fertilizing ability. However, the spermatozoa recovered from the perivitelline space could penetrate egg investments for the second time and fertilize eggs [30].
These results indicated that the timing of acrosome reaction is flexible, as indicated long ago in rabbit [54]. It also suggests that meaningful “sperm-zona binding” must occur between the acrosome-reacted spermatozoa and zona pellucida, while most of the classical “sperm-zona binding” assays observed binding between acrosome-intact spermatozoa and zona pellucida [15]. This experiment could never be achieved without gene-manipulated animals because, unlike rabbit, mouse zona-penetrated spermatozoa fuse with eggs rather promptly and have few extra spermatozoa in the perivitelline space. This is a good example of the essential role of gene-manipulated animals in the study of fertilization.
Sperm-egg Fusion
The first fusion-related factor functioning on egg was found purely by accident using gene-manipulated animals. Although the role of CD9 in fusion was suggested by the researchers in fertilization field [8], a tetraspanin protein CD9 was initially disrupted by the researchers in other fields to clarify its role in immunology, and was unexpectedly found to play an important role in sperm-egg fusion on eggs [32, 33, 39]. On the sperm side, a gene named Izumo1 emerged from analysis of an antigen reacting to monoclonal antibody OBF13 which inhibits sperm egg fusion. Izumo1−/− mice produce normal-looking spermatozoa which are completely infertile. As mentioned in an earlier section, the Izumo1-disrupted spermatozoa can penetrate cumulus layers and the ZP normally, but fail to fuse with eggs [26].
CD9 on the egg and IZUMO1 on spermatozoa are the only two essential factors for fusion described to date. However, interaction between the two factors has not been observed. This indicates that these two factors do not correlate directly in sperm-egg fusion events. Since no fusogenic domain was found in either of the factors, an additional factor (s) for fusion must exist. Namely, while Izumo1−/− males are completely sterile, the infertility of Cd9−/− females is not complete. There should be an IZUMO1-interacting protein on egg. However the recent identification and disruption of an IZUMO1-associating protein (angiotensin converting enzyme-3; Ace3) [28] and an alleged partner Igsf8 [29] resulted in fertile mice. Discovery of an IZUMO1-interacting factor (s) on egg which functions in sperm-egg fusion is yet to be made.
Live Imaging of fertilization
Historically, scientific observation progressed from use of the naked eye to the microscope, the electron microscope, the monoclonal antibody, and beyond. Now, gene-manipulated animals have taken us to the next level, offering us the newest tool for observation. In terms of spermatozoa, gene-manipulated IZUMO1 (fusing fluorescent protein sequent to the Izumo1 gene) provided a powerful means to investigate the role of IZUMO1 in fusion.
Such transgenic mouse spermatozoa could indicate the localization of IZUMO1 in live spermatozoa without the staining procedure. Since we did not know how IZUMO1 migrated from acrosomal membrane to outer plasma membrane, RFP-tagged IZUMO1-bearing mice were used to study the dynamic movement of IZUMO1 during acrosome reaction. It was found that a very swift migration of IZUMO1 took place during acrosome reaction. The dynamic movement of IZUMO1 at fusion is also observed using the same spermatozoa. IZUMO1 mainly localized on the equatorial segment of acrosome-reacted spermatozoa was found to disperse on egg surface after sperm-egg fusion. However, some of the IZUMO1 remained on inner acrosomal membrane and was incorporated into the egg cytoplasm together with the inner acrosomal membrane structure. These movements of IZUMO1 were recorded in movies [48].
Conclusion
A modern view largely based on in vivo observations of gene-manipulated animals for fertilization is postulated in Fig. 1. Classical theories are not indicated in the figure. In any field, the results obtained in in vitro study sometimes mislead the scientists. I was involved in producing more than 300 gene-disrupted mouse lines in the past and learned that more than 50% of these gene-disrupted mice showed negligible—or unexpected—phenotypes. This indicates that it is very difficult to predict the role of a given gene. I would recommend that, in any field of research, molecules of interest must be validated in gene-manipulated animals to examine their effect (if they are a direct product of the genes). Fertilization may be one of the most suitable research fields incorporating use of gene-manipulated animals, as the mysterious behavior of gametes is seemingly difficult to reproduce in vitro.
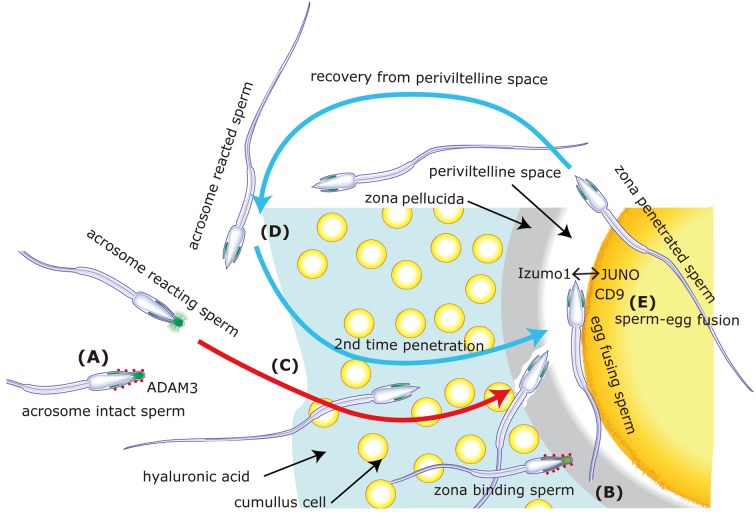
Fig. 1.
The mechanism of fertilization from a modern viewpoint. A) As shown in Table 3, disruption of any of more than 10 genes is know to cause a similar male infertility based on the loss of sperm migrating activity into oviduct. The impairment of sperm surface ADAM3 (red dots) was evident in each case. Basically, ADAM3 is considered to enable spermatozoa to migrate into oviduct. However, the recent Ly6k disruption experiment [13] might suggest that some unknown alternative factor (s) may function during sperm migration into oviduct. B) Many spermatozoa are found to bind to zona pellucida when mixed with cumulus-free oocytes [19]. The expression of ADAM3 (or some alternative factor (s)) on sperm surface was considered to be essential for sperm fertilizing ability in terms of enabling spermatozoa to bind to zona [23]. However, it was found that this binding ability was dispensable. The spermatozoa which lost the so-called “zona-binding” ability still able to fertilize eggs in vivo once the oviduct migration step was bypassed [53]. C) Spermatozoa must undergo a morphological change called acrosome reaction. Acrosome contains various hydrolytic enzymes and the exocytosis upon acrosome reaction was considered to assist spermatozoa to penetrate the egg investments [59]. Taking observations in B) into account, real acrosome reaction was considered to be elicited when the spermatozoa bind to zona [55]. However, recent observation indicates that fertilizing spermatozoa are acrosome reacted before contact with zona pellucida [31]. D) The modern view of the timing of acrosome reaction which take place independent to zona binding was strengthened by experiments demonstrating that acrosome reacted spermatozoa recovered from the perivitelline space could penetrate zona pellucida for the second time and fertilize eggs [30]. E) Only acrosome reacted spermatozoa can fuse with eggs. IZUMO1 on spermatozoa is essentially required for fusion [26]. IZUMO1 is hidden under the plasma membrane in intact spermatozoa. One of the reasons that acrosome reaction is required for sperm-egg fusion could be that IZUMO1 hidden under plasma membrane migrates out to the sperm surface upon acrosome reaction [48]. CD9 on egg is playing an important role in fertilization [32, 35, 39], but CD9 appears not to interact directly with IZUMO1. The finding of a real counterpart is awaited to elucidate the mechanism of sperm-egg fusion. This figure is modified from [43]. Recently, JUNO was identified as a IZUMO1 interacting factor [5].
References
- 1.Almeida E.A., Huovila A.P., Sutherland A.E., Stephens L.E., Calarco P.G., Shaw L.M., Mercurio A.M., Sonnenberg A., Primakoff P., Myles D.G., White J.M.1995. Mouse egg integrin alpha 6 beta 1 functions as a sperm receptor. Cell 81: 1095–1104. doi: 10.1016/S0092-8674(05)80014-5 [DOI] [PubMed] [Google Scholar]
- 2.Baba D., Kashiwabara S., Honda A., Yamagata K., Wu Q., Ikawa M., Okabe M., Baba T.2002. Mouse sperm lacking cell surface hyaluronidase PH-20 can pass through the layer of cumulus cells and fertilize the egg. J. Biol. Chem. 277: 30310–30314. doi: 10.1074/jbc.M204596200 [DOI] [PubMed] [Google Scholar]
- 3.Baba T., Azuma S., Kashiwabara S., Toyoda Y.1994. Sperm from mice carrying a targeted mutation of the acrosin gene can penetrate the oocyte zona pellucida and effect fertilization. J. Biol. Chem. 269: 31845–31849. [PubMed] [Google Scholar]
- 4.Baibakov B., Gauthier L., Talbot P., Rankin T.L., Dean J.2007. Sperm binding to the zona pellucida is not sufficient to induce acrosome exocytosis. Development 134: 933–943. doi: 10.1242/dev.02752 [DOI] [PubMed] [Google Scholar]
- 5.Bianchi E., Doe B., Goulding D., Wright G.J.2014. Juno is the egg Izumo receptor and is essential for mammalian fertilization. Nature 508: 483–487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bleil J.D., Wassarman P.M.1983. Sperm-egg interactions in the mouse: sequence of events and induction of the acrosome reaction by a zona pellucida glycoprotein. Dev. Biol. 95: 317–324. doi: 10.1016/0012-1606(83)90032-5 [DOI] [PubMed] [Google Scholar]
- 7.Blobel C.P., Wolfsberg T.G., Turck C.W., Myles D.G., Primakoff P., White J.M.1992. A potential fusion peptide and an integrin ligand domain in a protein active in sperm-egg fusion. Nature 356: 248–252. doi: 10.1038/356248a0 [DOI] [PubMed] [Google Scholar]
- 8.Chen M.S., Tung K.S., Coonrod S.A., Takahashi Y., Bigler D., Chang A., Yamashita Y., Kincade P.W., Herr J.C., White J.M.1999. Role of the integrin-associated protein CD9 in binding between sperm ADAM 2 and the egg integrin alpha6beta1: implications for murine fertilization. Proc. Natl. Acad. Sci. USA 96: 11830–11835. doi: 10.1073/pnas.96.21.11830 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cho C., Bunch D.O., Faure J.E., Goulding E.H., Eddy E.M., Primakoff P., Myles D.G.1998. Fertilization defects in sperm from mice lacking fertilin beta. Science 281: 1857–1859. doi: 10.1126/science.281.5384.1857 [DOI] [PubMed] [Google Scholar]
- 10.Da Ros V.G., Maldera J.A., Willis W.D., Cohen D.J., Goulding E.H., Gelman D.M., Rubinstein M., Eddy E.M., Cuasnicu P.S.2008. Impaired sperm fertilizing ability in mice lacking Cysteine-RIch Secretory Protein 1 (CRISP1). Dev. Biol. 320: 12–18. doi: 10.1016/j.ydbio.2008.03.015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ensslin M.A., Shur B.D.2003. Identification of mouse sperm SED1, a bimotif EGF repeat and discoidin-domain protein involved in sperm-egg binding. Cell 114: 405–417. doi: 10.1016/S0092-8674(03)00643-3 [DOI] [PubMed] [Google Scholar]
- 12.Florman, H.M., Ducibella, T.2006.. Fertilization in mammals. Knbil and Neill’s Physiology of Reproduction 1 (3rd ed): 55–112. [Google Scholar]
- 13.Fujihara, Y, Okabe, M, Ikawa, M.2014.. GPI-anchored protein complex, LY6K/TEX101, is required for sperm migration into the oviduct and male fertility in mice. Biol Reprod 90: 60. [DOI] [PubMed] [Google Scholar]
- 14.Fujihara Y., Tokuhiro K., Muro Y., Kondoh G., Araki Y., Ikawa M., Okabe M.2013. Expression of TEX101, regulated by ACE, is essential for the production of fertile mouse spermatozoa. Proc. Natl. Acad. Sci. USA 110: 8111–8116. doi: 10.1073/pnas.1222166110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gahlay G., Gauthier L., Baibakov B., Epifano O., Dean J.2010. Gamete recognition in mice depends on the cleavage status of an egg’s zona pellucida protein. Science 329: 216–219. doi: 10.1126/science.1188178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gong X., Dubois D.H., Miller D.J., Shur B.D.1995. Activation of a G protein complex by aggregation of beta-1,4-galactosyltransferase on the surface of sperm. Science 269: 1718–1721. doi: 10.1126/science.7569899 [DOI] [PubMed] [Google Scholar]
- 17.Hagaman J.R., Moyer J.S., Bachman E.S., Sibony M., Magyar P.L., Welch J.E., Smithies O., Krege J.H., O’Brien D.A.1998. Angiotensin-converting enzyme and male fertility. Proc. Natl. Acad. Sci. USA 95: 2552–2557. doi: 10.1073/pnas.95.5.2552 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Hanayama R., Tanaka M., Miyasaka K., Aozasa K., Koike M., Uchiyama Y., Nagata S.2004. Autoimmune disease and impaired uptake of apoptotic cells in MFG-E8-deficient mice. Science 304: 1147–1150. doi: 10.1126/science.1094359 [DOI] [PubMed] [Google Scholar]
- 19.Hartmann J.F., Gwatkin R.B., Hutchison C.F.1972. Early contact interactions between mammalian gametes in vitro: evidence that the vitellus influences adherence between sperm and zona pellucida. Proc. Natl. Acad. Sci. USA 69: 2767–2769. doi: 10.1073/pnas.69.10.2767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hasuwa H., Muro Y., Ikawa M., Kato N., Tsujimoto Y., Okabe M.2010. Transgenic mouse sperm that have green acrosome and red mitochondria allow visualization of sperm and their acrosome reaction in vivo. Exp. Anim. 59: 105–107. doi: 10.1538/expanim.59.105 [DOI] [PubMed] [Google Scholar]
- 21.He Z.Y., Brakebusch C., Fässler R., Kreidberg J.A., Primakoff P., Myles D.G.2003. None of the integrins known to be present on the mouse egg or to be ADAM receptors are essential for sperm-egg binding and fusion. Dev. Biol. 254: 226–237. doi: 10.1016/S0012-1606(02)00043-X [DOI] [PubMed] [Google Scholar]
- 22.Hinsch K.D., Hinsch E., Meinecke B., Töpfer-Petersen E., Pfisterer S., Schill W.B.1994. Identification of mouse ZP3 protein in mammalian oocytes with antisera against synthetic ZP3 peptides. Biol. Reprod. 51: 193–204. doi: 10.1095/biolreprod51.2.193 [DOI] [PubMed] [Google Scholar]
- 23.Ikawa M., Inoue N., Benham A.M., Okabe M.2010. Fertilization: a sperm’s journey to and interaction with the oocyte. J. Clin. Invest. 120: 984–994. doi: 10.1172/JCI41585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ikawa M., Wada I., Kominami K., Watanabe D., Toshimori K., Nishimune Y., Okabe M.1997. The putative chaperone calmegin is required for sperm fertility. Nature 387: 607–611. doi: 10.1038/42484 [DOI] [PubMed] [Google Scholar]
- 25.Ikawa M., Yamaguchi R., Inoue N., Okabe M.2008. Haploid-specific chaperone calspelin (Calr3) is required for sperm fertility. Biol. Reprod. 578. 78, 190. [Google Scholar]
- 26.Inoue N., Ikawa M., Isotani A., Okabe M.2005. The immunoglobulin superfamily protein Izumo is required for sperm to fuse with eggs. Nature 434: 234–238. doi: 10.1038/nature03362 [DOI] [PubMed] [Google Scholar]
- 27.Inoue N., Ikawa M., Nakanishi T., Matsumoto M., Nomura M., Seya T., Okabe M.2003. Disruption of mouse CD46 causes an accelerated spontaneous acrosome reaction in sperm. Mol. Cell. Biol. 23: 2614–2622. doi: 10.1128/MCB.23.7.2614-2622.2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Inoue N., Kasahara T., Ikawa M., Okabe M.2010. Identification and disruption of sperm-specific angiotensin converting enzyme-3 (ACE3) in mouse. PLoS ONE 5: e10301. doi: 10.1371/journal.pone.0010301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Inoue N., Nishikawa T., Ikawa M., Okabe M.2012. Tetraspanin-interacting protein IGSF8 is dispensable for mouse fertility. Fertil. Steril. 98: 465–470. doi: 10.1016/j.fertnstert.2012.04.029 [DOI] [PubMed] [Google Scholar]
- 30.Inoue N., Satouh Y., Ikawa M., Okabe M., Yanagimachi R.2011. Acrosome-reacted mouse spermatozoa recovered from the perivitelline space can fertilize other eggs. Proc. Natl. Acad. Sci. USA 108: 20008–20011. doi: 10.1073/pnas.1116965108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jin M., Fujiwara E., Kakiuchi Y., Okabe M., Satouh Y., Baba S.A., Chiba K., Hirohashi N.2011. Most fertilizing mouse spermatozoa begin their acrosome reaction before contact with the zona pellucida during in vitro fertilization. Proc. Natl. Acad. Sci. USA 108: 4892–4896. doi: 10.1073/pnas.1018202108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kaji K., Oda S., Shikano T., Ohnuki T., Uematsu Y., Sakagami J., Tada N., Miyazaki S., Kudo A.2000. The gamete fusion process is defective in eggs of Cd9-deficient mice. Nat. Genet. 24: 279–282. doi: 10.1038/73502 [DOI] [PubMed] [Google Scholar]
- 33.Kim E., Yamashita M., Nakanishi T., Park K.E., Kimura M., Kashiwabara S., Baba T.2006. Mouse sperm lacking ADAM1b/ADAM2 fertilin can fuse with the egg plasma membrane and effect fertilization. J. Biol. Chem. 281: 5634–5639. doi: 10.1074/jbc.M510558200 [DOI] [PubMed] [Google Scholar]
- 34.Krutskikh, A., Poliandri, A., Cabrera-Sharp, V., Dacheux, J.L., Poutanen, M., and Huhtaniemi, I2012. Epididymal protein Rnase10 is required for post-testicular sperm maturation and male fertility. FASEB J. 26:4198–4209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Le Naour F., Rubinstein E., Jasmin C., Prenant M., Boucheix C.2000. Severely reduced female fertility in CD9-deficient mice. Science 287: 319–321. doi: 10.1126/science.287.5451.319 [DOI] [PubMed] [Google Scholar]
- 36.Lu Q., Shur B.D.1997. Sperm from beta 1,4-galactosyltransferase-null mice are refractory to ZP3-induced acrosome reactions and penetrate the zona pellucida poorly. Development 124: 4121–4131. [DOI] [PubMed] [Google Scholar]
- 37.Marcello M.R., Jia W., Leary J.A., Moore K.L., Evans J.P.2011. Lack of tyrosylprotein sulfotransferase-2 activity results in altered sperm-egg interactions and loss of ADAM3 and ADAM6 in epididymal sperm. J. Biol. Chem. 286: 13060–13070. doi: 10.1074/jbc.M110.175463 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Miller B.J., Georges-Labouesse E., Primakoff P., Myles D.G.2000. Normal fertilization occurs with eggs lacking the integrin alpha6beta1 and is CD9-dependent. J. Cell Biol. 149: 1289–1296. doi: 10.1083/jcb.149.6.1289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Miyado K., Yamada G., Yamada S., Hasuwa H., Nakamura Y., Ryu F., Suzuki K., Kosai K., Inoue K., Ogura A., Okabe M., Mekada E.2000. Requirement of CD9 on the egg plasma membrane for fertilization. Science 287: 321–324. doi: 10.1126/science.287.5451.321 [DOI] [PubMed] [Google Scholar]
- 40.Muro Y., Buffone M.G., Okabe M., Gerton G.L.2012. Function of the acrosomal matrix: zona pellucida 3 receptor (ZP3R/sp56) is not essential for mouse fertilization. Biol. Reprod. 86: 1–6. doi: 10.1095/biolreprod.111.095877 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nakanishi T., Ikawa M., Yamada S., Parvinen M., Baba T., Nishimune Y., Okabe M.1999. Real-time observation of acrosomal dispersal from mouse sperm using GFP as a marker protein. FEBS Lett. 449: 277–283. doi: 10.1016/S0014-5793(99)00433-0 [DOI] [PubMed] [Google Scholar]
- 42.Nishimura H., Kim E., Nakanishi T., Baba T.2004. Possible function of the ADAM1a/ADAM2 Fertilin complex in the appearance of ADAM3 on the sperm surface. J. Biol. Chem. 279: 34957–34962. doi: 10.1074/jbc.M314249200 [DOI] [PubMed] [Google Scholar]
- 43.Okabe M.2013. The cell biology of mammalian fertilization. Development 140: 4471–4479. doi: 10.1242/dev.090613 [DOI] [PubMed] [Google Scholar]
- 44.Olson E.N., Arnold H.H., Rigby P.W., Wold B.J.1996. Know your neighbors: three phenotypes in null mutants of the myogenic bHLH gene MRF4. Cell 85: 1–4. doi: 10.1016/S0092-8674(00)81073-9 [DOI] [PubMed] [Google Scholar]
- 45.Osokine I., Hsu R., Loeb G.B., McManus M.T.2008. Unintentional miRNA ablation is a risk factor in gene knockout studies: a short report. PLoS Genet. 4: e34. doi: 10.1371/journal.pgen.0040034 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Primakoff P., Myles D.G.2002. Penetration, adhesion, and fusion in mammalian sperm-egg interaction. Science 296: 2183–2185. doi: 10.1126/science.1072029 [DOI] [PubMed] [Google Scholar]
- 47.Rossi D., Cozzio A., Flechsig E., Klein M.A., Rülicke T., Aguzzi A., Weissmann C.2001. Onset of ataxia and Purkinje cell loss in PrP null mice inversely correlated with Dpl level in brain. EMBO J. 20: 694–702. doi: 10.1093/emboj/20.4.694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Satouh Y., Inoue N., Ikawa M., Okabe M.2012. Visualization of the moment of mouse sperm-egg fusion and dynamic localization of IZUMO1. J. Cell Sci. 125: 4985–4990. doi: 10.1242/jcs.100867 [DOI] [PubMed] [Google Scholar]
- 49.Shamsadin R., Adham I.M., Nayernia K., Heinlein U.A., Oberwinkler H., Engel W.1999. Male mice deficient for germ-cell cyritestin are infertile. Biol. Reprod. 61: 1445–1451. doi: 10.1095/biolreprod61.6.1445 [DOI] [PubMed] [Google Scholar]
- 50.Shen C., Kuang Y., Liu J., Feng J., Chen X., Wu W., Chi J., Tang L., Wang Y., Fei J., Wang Z.2013. Prss37 is required for male fertility in the mouse. Biol. Reprod. 88: 123. doi: 10.1095/biolreprod.112.107086 [DOI] [PubMed] [Google Scholar]
- 51.Sutton K.A., Jungnickel M.K., Florman H.M.2008. A polycystin-1 controls postcopulatory reproductive selection in mice. Proc. Natl. Acad. Sci. USA 105: 8661–8666. doi: 10.1073/pnas.0800603105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Tardif S., Wilson M.D., Wagner R., Hunt P., Gertsenstein M., Nagy A., Lobe C., Koop B.F., Hardy D.M.2010. Zonadhesin is essential for species specificity of sperm adhesion to the egg zona pellucida. J. Biol. Chem. 285: 24863–24870. doi: 10.1074/jbc.M110.123125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Tokuhiro K., Ikawa M., Benham A.M., Okabe M.2012. Protein disulfide isomerase homolog PDILT is required for quality control of sperm membrane protein ADAM3 and male fertility [corrected]. Proc. Natl. Acad. Sci. USA 109: 3850–3855. doi: 10.1073/pnas.1117963109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Valdivia M., Sillerico T., De Ioannes A., Barros C.1999. Proteolytic activity of rabbit perivitelline spermatozoa. Zygote 7: 143–149. doi: 10.1017/S0967199499000507 [DOI] [PubMed] [Google Scholar]
- 55.Wassarman P.M., Litscher E.S.2008. Mammalian fertilization: the egg’s multifunctional zona pellucida. Int. J. Dev. Biol. 52: 665–676. doi: 10.1387/ijdb.072524pw [DOI] [PubMed] [Google Scholar]
- 56.Yamaguchi R., Fujihara Y., Ikawa M., Okabe M.2012. Mice expressing aberrant sperm-specific protein PMIS2 produce normal-looking but fertilization-incompetent spermatozoa. Mol. Biol. Cell 23: 2671–2679. doi: 10.1091/mbc.E11-12-1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Yamaguchi R., Muro Y., Isotani A., Tokuhiro K., Takumi K., Adham I., Ikawa M., Okabe M.2009. Disruption of ADAM3 impairs the migration of sperm into oviduct in mouse. Biol. Reprod. 81: 142–146. doi: 10.1095/biolreprod.108.074021 [DOI] [PubMed] [Google Scholar]
- 58.Yamaguchi R., Yamagata K., Ikawa M., Moss S.B., Okabe M.2006. Aberrant distribution of ADAM3 in sperm from both angiotensin-converting enzyme (Ace)- and calmegin (Clgn)-deficient mice. Biol. Reprod. 75: 760–766. doi: 10.1095/biolreprod.106.052977 [DOI] [PubMed] [Google Scholar]
- 59.Yanagimachi R.1994Mammalian Fertilization (Raven Press, LTD., New York) second Ed pp 189–317. [Google Scholar]
- 60.Yanagimachi R., Chang M.C.1963. Fertilization of hamster eggs in vitro. Nature 200: 281–282. doi: 10.1038/200281b0 [DOI] [PubMed] [Google Scholar]