Abstract
Because of the difficulty to exclude possible involvement of nuclear DNA mutations, it has been a controversial issue whether pathogenic mutations in mitochondrial DNA (mtDNA) and the resultant respiration defects are involved in tumor development. To address this issue, our previous study generated transmitochondrial mice (mito-mice-ND613997), which possess the nuclear and mtDNA backgrounds derived from C57BL/6J (B6) strain mice except that they carry B6 mtDNA with a G13997A mutation in the mt-Nd6 gene. Because aged mito-mice-ND613997 simultaneously showed overproduction of reactive oxygen species (ROS) in bone marrow cells and high frequency of lymphoma development, current study examined the effects of administrating a ROS scavenger on the frequency of lymphoma development. We used N-acetylcysteine (NAC) as a ROS scavenger, and showed that NAC administration prevented lymphoma development. Moreover, its administration induced longevity in mito-mice-ND613997. The gene expression profiles in bone marrow cells indicated the upregulation of the Fasl gene, which can be suppressed by NAC administration. Given that natural-killer (NK) cells mediate the apoptosis of various tumor cells via enhanced expression of genes encoding apoptotic ligands including Fasl gene, its overexpression would reflect the frequent lymphoma development in bone marrow cells. These observations suggest that continuous administration of an antioxidant would be an effective therapeutics to prevent lymphoma development enhanced by ROS overproduction.
Keywords: antioxidant, lymphoma prevention, mouse mtDNA mutation, ROS overproduction
Introduction
Mitochondrial DNA (mtDNA) mutations that induce mitochondrial respiration defects have been proposed to be involved in aging and age-associated disorders including tumor development [13, 14, 18, 23, 24]. Moreover, mitochondrial respiration defects caused by nuclear DNA mutations and the resultant enhanced glycolysis under normoxic conditions, i.e. the Warburg effect, are reported to be responsible for tumor development [4, 7, 16, 19]. Therefore, it is also possible that mitochondrial respiration defects caused by an age-associated accumulation of mtDNA mutations induce the Warburg effect via compensatory upregulation of aerobic glycolysis, resulting in tumor development. In fact, somatic mutations were preferentially accumulated in tumor mtDNA [6, 10, 21]. However, there has been no direct evidence for the involvement of mtDNA mutations in the Warburg effect and in tumor development, because of the difficulty to exclude possible involvement of nuclear DNA mutations in these processes [2].
Our previous studies [1, 11, 12] resolved this issue by the use of intercellular mtDNA transfer technology between mouse cells expressing different phenotypes related to tumors. While mtDNA introduced from tumor cells into normal cells did not induce tumorigenicity [1], mtDNA exchange between poorly and highly metastatic lung carcinoma cells provided convincing evidence that a somatic G13997A mtDNA mutation in the mt-Nd6 gene, which encodes subunit 6 of respiration complex I (NADH dehydrogenase), reversibly controls development of highly metastatic potentials [11]. In this case, the induction of high metastasis was not due to the Warburg effect, but to overproduction of the reactive oxygen species (ROS) [12]. Subsequently, G13997A mtDNA was transferred from highly metastatic carcinoma cells into mouse female germ line [26] to examine the effect of the G13997A mtDNA on tumor-related phenotypes. The resultant transmitochondrial mice possessing only G13997A mtDNA, named mito-mice-ND613997, showed high frequently of lymphoma formation with aging [9], providing convincing evidence for the involvement of the mtDNA mutations in tumor development.
Based on these observations, this study examined whether continuous administration of an antioxidant prevents lymphoma development, and corresponds to an effective therapeutics to protect lymphoma development in mito-mice-ND613997. We also examined the mechanisms of how ROS induce lymphoma formation in mito-mice-ND613997.
Materials and Methods
Ethical statement
All animal experiments were performed in accordance with protocols approved by the experimental animal committee of the University of Tsukuba, Japan.
Mice
Old inbred C57BL/6J Jcl (B6) mice were obtained from CLEA Japan. Mito-mice-ND613997 were generated in our previous work [26]. We maintained B6 mice and mito-mice-ND613997 sharing a common nuclear DNA background by repeated backcrossing of their females with B6 males. Animals were housed in groups of up to 5 in individually ventilated cages under standard conditions (22°C, 12 h light–dark cycle) receiving food and water ad libitum. Male mice were used in the experiments, and were monitored everyday for general health and signs of tumor burden such as hunched postures, ruffled coats and respiratory distress were humanely killed. Moribund mice were euthanized by cervical dislocation under general anesthesia (Avertin, 1.25%, 0.2 ml/20 g body weight, intraperitoneally).
NAC administration
At 15 months old, sixteen mito-mice-ND613997 were divided at random into two groups. One group was given drinking water containing 10 g/l of NAC (SIGMA), and another group was given regular water. NAC supplemented water and regular water were prepared fresh everyday.
Histological analyses
Formalin-fixed, paraffin-embedded serial sections (10 µm) were used for histological analyses. Hematoxylin-and-eosin–stained sections were used for histopathological analysis to identify tumor tissues. The immunohistological analysis was performed with antibody to CD45 to determine whether the tumor tissues originated from leukocytes, and subsequently with antibodies to B220 and CD3 to determine whether the tumor tissues were of B-cell or T-cell origin, respectively. Deparaffinized slides were boiled for antigen retrieval, then incubated with rat anti-mouse CD45 (clone 30-F11; BD Biosciences, Cat. No. 550539) at a dilution of 1:200 or rat anti-mouse B220 (clone RA3-6B2; BD Biosciences, Cat. No. 553085) at a dilution of 1:50 or goat anti-mouse CD3 (clone M-20; Santa Cruz, Cat. No. sc-1127) at a dilution of 1:50. Biotin-conjugated goat anti-rat IgG (BD Biosciences, Cat. No. 559286) and rabbit anti-goat IgG (Vector Laboratories, Cat. No. BA-5000) were used as secondary antibody at a dilution of 1:50 and 1:500, respectively. Detection of CD45, B220 and CD3 were performed using avidin-biotin complex methodologies (Vectastain Elite ABC Kit, Vector Laboratories) with DAB staining (Anti-Ig HRP Detection Kit, BD Pharmingen). Sections were counterstained with hematoxylin.
PCR array analysis
Mice were euthanized after 4-week administration of NAC and bone marrow cells were isolated from mice thighbones and shinbones. Total RNA was extracted by ISOGEN (Nippon Gene) from mouse bone marrow cells. RNA samples were subjected to DNase I treatment (Invitrogen) to eliminate DNA contaminants and reverse transcribed using Oligo (dT)12–18 primer, 10 mM dNTP Mix, 0.1 M DTT, RNase Out Recombinant Ribonuclease Inhibitor, and SuperScript II-Reverse Transcriptase (Invitrogen). cDNA samples were subjected to RNase H treatment (Invitrogen), and applied according to the manufacturer’s protocol to a RT2 profiler PCR array real-time PCR reaction. Real-time monitoring PCR was performed with SYBR Green PCR Master Mix (QIAGEN) and an ABI PRISM 7900HT sequence detection system (Applied Biosystems). Mouse Cancer Pathway Finder RT2 Profiler PCR Array PAMM-033Z (SABiosciences) was performed to profile the expression of 84 genes related to the cancer pathway (n=3). The expression profiling was performed using ΔΔCt methods according to manufacturer’s protocols. The relative expression level for each gene was represented as cycle threshold (Ct). Normalized expression level was calculated as ΔCt=Ct (Gene of interest) − Ct (control). The average ΔCt from three mice was calculated as the relative expression level of each gene. Differential expression was calculated as ΔΔCt=Ave ΔCt (sample mice) − Ave ΔCt (control mice). Fold change was calculated as 2-ΔΔCt. The P values are calculated based on a Student t-test of the replicate 2-ΔCt values for each gene in the control group and treatment groups.
Statistical analysis
We analyzed data with the Student’s t-test. Kaplan-Meier curves were assessed with the log-rank test. Values with P<0.05 were considered significant.
Results
This study examined the idea that the frequent lymphoma development in the aged male mito-mice-ND613997 could be prevented by the administration of an antioxidant, if ROS overproduction is responsible for the lymphoma development. Since most mito-mice-ND613997 began to develop lymphoma more than 18 months after the birth [9], we used 15-month-old males for continuous administration of N-acetylcysteine (NAC) as a ROS scavenger. Eight of 16 mito-mice-ND613997 were treated with NAC from 15 months after the birth by oral administration based on the procedure reported previously [5]. The remaining eight mito-mice-ND613997 were not treated with NAC. Seven 15-month-old B6 males untreated with NAC were used as controls.
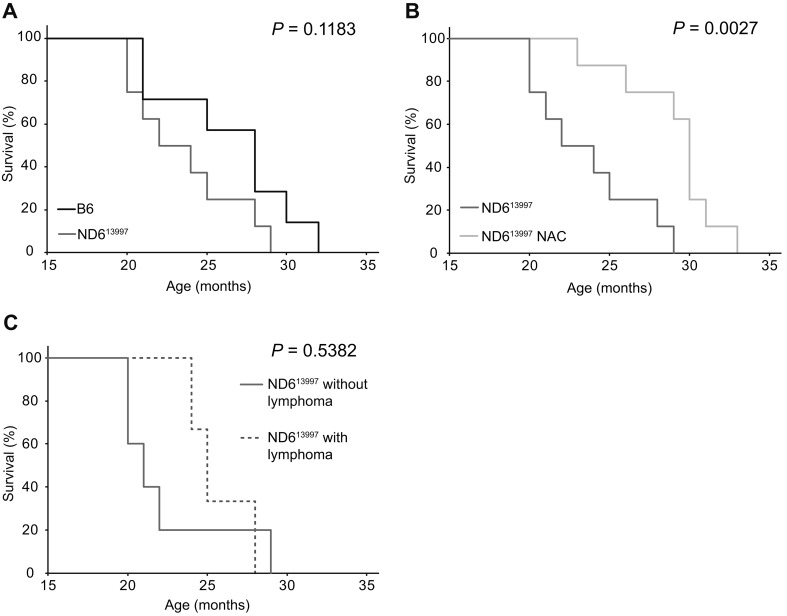
First, we examined whether the NAC treatment would affect the frequency of lymphoma development and the lifespan. Median survival times of NAC-treated mito-mice-ND613997, untreated mito-mice-ND613997 and untreated B6 mice were 29.3, 22.5 and 25.8 months, respectively (Figs. 1A and B). No statistical significance of median survival times was observed between untreated mito-mice-ND613997 and untreated B6 mice (Fig.1A). These results are consistent with our previous observations [9]. In contrast, NAC-treated mito-mice-ND613997 showed longer survival times than untreated mito-mice-ND613997 with statistical significance (Fig. 1B), while no statistical significance was present between NAC-treated mito-mice-ND613997 and untreated B6 mice (Figs. 1A and B). The median survival times of untreated mito-mice-ND613997 with and without lymphoma-like abnormalities were 24.5 and 20.5, respectively, and were not different with statistical significance (Fig. 1C).
Fig. 1.
Kaplan-Meier survival curves of mito-miceND613997. (A) Comparison of lifespans between B6 mice and mito-miceND613997. Median survival times of B6 mice (n=7) and mito-miceND613997(n=8) were 25.8 and 22.0 months, respectively. (B) Comparison of lifespans of between NAC-treated and untreated mito-miceND613997. NAC administration was started from 15 months after the birth to the end of their lives. Median survival times of mito-miceND613997(n=8) and mito-miceND613997 treated with NAC (n=8) were 22.0 and 29.3 months, respectively. (C) Comparison of lifespans between mito-miceND613997 that died with and without detectable lymphoma. Median survival times of mito-miceND613997 with detectable lymphoma (n=3) and mito-miceND613997 without detectable lymphoma (n=5) were 24.5 and 20.5 months, respectively.
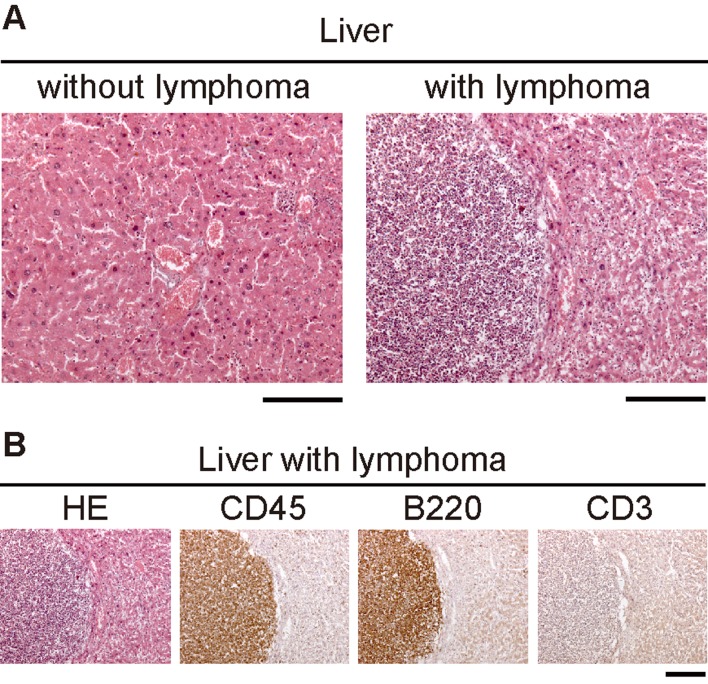
Gross necropsy of all dead or euthanized moribund mice showed that three of eight mito-mice-ND613997(38%) and one of seven B6 mice (14%) had macroscopic lymphoma-like abnormalities, including enlarged spleen, liver, and nodular tumors, but none of eight NAC-treated mito-mice-ND613997 had these abnormalities (Table1). Histological analyses of abnormal tissues revealed that all were hematopoietic neoplasms and positive for the pan-leukocyte marker CD45 (Table 1 and Fig. 2 ). These observations suggest that these hematological neoplasms may correspond to lymphoma cells, although current study did not examine copy-number variations (CNVs) in nuclear genomes to show chromosomal instability [9]. Three tumors were of B-cell origin, expressing the B-cell maker B220, while one mito-mouse-ND613997 developed T-cell lymphoma staining positive for the T-cell marker CD3 (Table 1). These data indicate that NAC administration is an effective therapeutics to prevent lymphoma development caused by ROS overproduction. However, further works using more numbers of animals than those used in this study are required to confirm this idea.
Table 1. Frequencies of lymphoma development in mice.
| Mouse strains | No. of mice | No. of mice with tumor | Tissues with tumor | Histological analysis | Cell lineage | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Spleen | Liver | Lymph node | CD45 | B220 | CD3 | |||||||
| B6 | 7 | 1 | + | + | + | + | + | - | B-cell | |||
| Mito-mice-ND613997 | 8 | 3 | + | - | + | + | + | - | B-cell | |||
| + | - | - | + | - | + | T-cell | ||||||
| + | + | + | + | + | - | B-cell | ||||||
| Mito-mice-ND613997 (NAC-treated) | 8 | 0 | ||||||||||
Fig. 2.
Histological analyses for identification of lymphoma cells in the liver from mito-miceND613997. (A) Hematoxylin and eosin (HE) staining of the liver sections from mito-miceND613997. HE staining of the liver sections was carried out to identify tumor-like abnormalities in the liver. Left and right panels represent normal liver and liver with tumor-like abnormalities, respectively. (B) Histological analysis of serial sections of the liver with tumor-like abnormalities. HE, hematoxylin-eosin staining to show tumor formation; CD45, immunohistochemistry using antibody to CD45 to detect leukocytes; B220, immunohistochemistry using antibody to B220 to detect B cells; CD3, immunohistochemistry using antibody to CD3 to detect T cells. Because this tissue was stained positively with CD45 and B220, but not with CD3, the results represent abnormal growth of B cell-lymphoma, but not T cell-lymphoma, in the liver. (Scale bars, 200 µm).
Then, we examined the mechanism of frequent lymphoma development, which can be prevented by administration of an antioxidant to mito-mouse-ND613997. Our previous study showed that no tumors other than lymphoma were developed, and that ROS were overproduced in bone marrow cells but not in splenocytes [9]. It is therefore likely that the ROS overproduction in the bone marrow cells of mito-mouse-ND613997 is crucial for lymphoma development. Thus, we used bone marrow cells to identify genes that would be responsible for frequent lymphoma development in mito-mice-ND613997, and compared the expression of the genes related to cancer pathway (transformation and tumorigenesis) between mito-mice-ND613997 and B6 mice using PCR array (Mouse Cancer Pathway Finder RT2 Profiler PCR Array). Because most mito-mice-ND613997 began to develop lymphoma 18 months after the birth, we used 15-month-old male mice for this experiment to exclude the influence of the developed lymphoma cells.
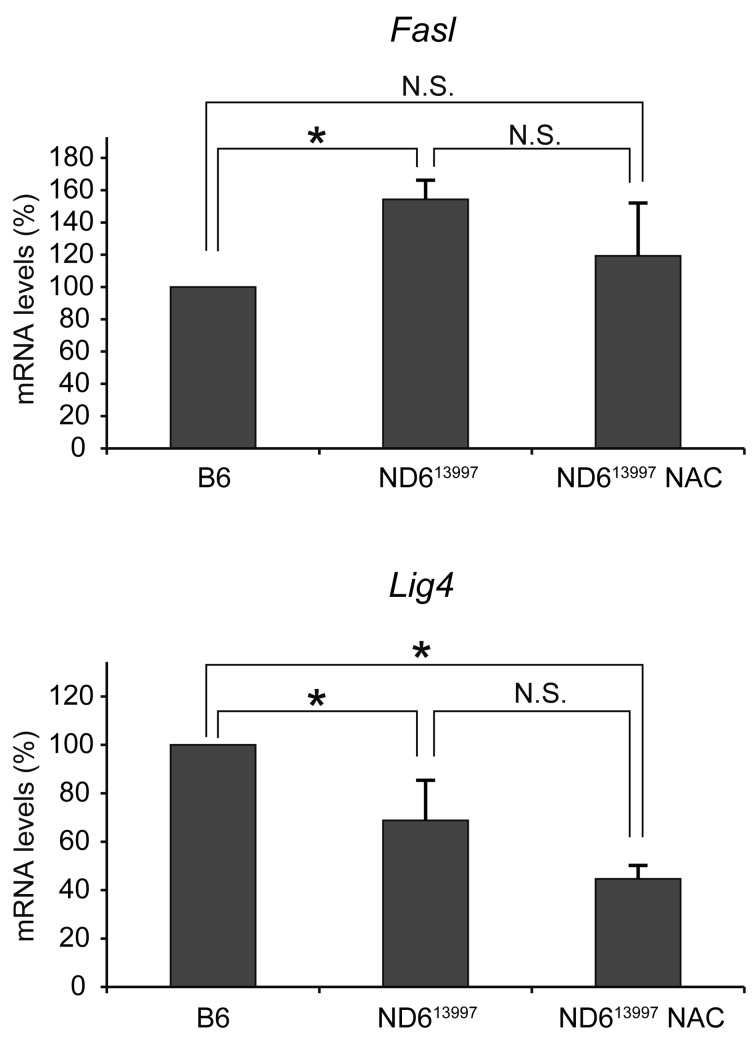
Out of 84 cancer pathway-related genes represented on Mouse Cancer Pathway Finder RT2 Profiler PCR Array (Supplemental table 1), only two genes, Fasl and Lig4 related to apoptosis and DNA repair, respectively, were selected as genes being differentially expressed between bone marrow cells of B6 and mito-mice-ND613997(Fig. 3). Then, we examined whether expression levels of these genes can be changed to the levels of B6 mice by administration of NAC for 4 weeks to mito-mice-ND613997. The results showed that Fasl overexpression in mito-mice-ND613997 was suppressed to B6 levels by NAC administration, while suppressed expression of Lig4 levels did not restore to B6 levels (Fig. 3). These results indicated the association of Fasl overexpression to frequent development of lymphoma in mito-mice-ND613997.
Fig. 3.
Cancer related gene expression profiles of bone marrow cells. RT2 Profiler mouse cancer pathway finder PCR array was performed on bone marrow cells. Two genes of 84 cancer pathway-related genes, Fasl and Lig4, were differentially expressed between bone marrow cells of B6 mice and mito-mice-ND613997. However, only the Fasl gene expression was reversibly regulated to normal levels by administration of NAC. Values were normalized to a pool of housekeeping genes on the array by ΔΔCt and are reported as the fold change in gene expression relative to healthy B6 bone marrow cells. Data are represented as mean values with SD (n=3). *, P<0.05.
Discussion
This study addressed two important issues of whether treatment of the mito-mice-ND613997 with an antioxidant could be an effective therapeutics to prevent lymphoma development mito-mice-ND613997, and how ROS overproduction caused by G13997A mtDNA introduced from highly metastatic lung carcinoma cells induces lymphoma development in mito-mice-ND613997.
Concerning the first issue on the therapeutics of lymphoma development, the results in this study showed that administration of NAC, one of the frequently used antioxidants, is very effective to prevent lymphoma development in mito-mice-ND613997. Our previous study proposed the idea that ROS overproduction, but not lactate overproduction, is responsible for frequent development of lymphoma in mito-mice-ND613997 with G13997A mtDNA based on the observations that lymphoma was not developed in different mito-mice-COI6589 that showed lactate overproduction but did not show ROS overproduction [9]. If this idea is correct, it can be predicted that administration of the antioxidants would prevent lymphoma development in mito-mice-ND613997. Current study examined this prediction and showed that NAC administration is effective to prevent lymphoma development caused by ROS in mito-mice-ND613997. However, further works are required to show that NAC administration prevents ROS overproduction in bone marrow cells of mito-mice-ND613997.
While NAC administration prevents lymphoma development (Table 1), it also induced longevity in mito-mice-ND613997 (Fig. 1B). Median survival times of B6 mice, mito-mice-ND613997, and NAC-treated mito-mice-ND613997 were 25.8, 22.0, and 29.3, respectively (Figs. 1A and B). The longer median survival times of NAC-treated mito-mice-ND613997 would not be due to the prevention of lymphoma formation by NAC administration, because the median survival times of untreated mito-mice-ND613997 with and without lymphoma were 24.5 and 20.5, respectively, and were not different with statistical significance (Fig. 1C). Since it appears to be controversial whether oxidative stress suppresses longevity or not [20, 25], we could not at present explain why NAC administration induced longevity in mito-mice-ND613997. To examine this issue, we have to carry out NAC administration not only to mito-mice-ND613997 but also to B6 mice using more numbers of animals.
Concerning the second issue of how ROS overproduction caused by G13997A mtDNA induced lymphoma development, this study showed that overexpression of Fasl is related the process, because its overexpression in mito-mice-ND613997 was suppressed to normal levels by NAC administration (Fig. 3). Given that natural-killer (NK) cells mediate the apoptosis of various tumor cells via the expression of genes encoding tumor necrosis factor-family ligands including the Fasl gene [22], it would not be conceivable that its overexpression is responsible for lymphoma development. Probably, overexpression of the Fasl gene in NK cells simply reflects the progress of the frequent lymphoma development in bone marrow cells of mito-mice-ND613997. Therefore, we could not at present explain the mechanisms of how ROS overproduction induces frequent lymphoma development in aged mito-mice-ND613997.
Then, a question is why mito-mice-ND613997 preferentially develop lymphoma [9] (Table 1), even though ROS induces various types of cellular damages, leading to genomic instability that can result in the development of various types of tumors [15]. One answer to this question would be that nuclear genetic background of B6 strain we used for generation of mito-mice-ND613997 is prone to develop lymphoma [3, 8, 17]. Based on these observations, we would like to propose an idea that G13997A mtDNA alone would not induce lymphoma development in the absence of B6 nuclear genetic background. To examine this idea, we are going to generate new mito-mice-ND613997 carrying G13997A mtDNA but carrying different nuclear genetic background derived from different mouse strains that are not prone to develop lymphoma by backcrossing female mito-mice-ND613997 to males from different mouse strains.
Supplementary
Acknowledgments
This work was supported by Grants-in-Aid for Scientific Research A 25250011 (to J.-I.H.), Scientific Research A 23240058 (to K.N.), and Scientific Research on Innovative Areas 24117503 (to J.-I.H.) from the Japan Society for the Promotion of Science. This work was supported also by the World Premier International Research Center Initiative; Ministry of Education, Culture, Sports, Science and Technology–Japan (to K.N. and J.-I.H.).
References
- 1.Akimoto M., Niikura M., Ichikawa M., Yonekawa H., Nakada K., Honma Y., Hayashi J.2005. Nuclear DNA but not mtDNA controls tumor phenotypes in mouse cells. Biochem. Biophys. Res. Commun. 327: 1028–1035. doi: 10.1016/j.bbrc.2004.12.105 [DOI] [PubMed] [Google Scholar]
- 2.Augenlicht L.H., Heerdt B.G.2001. Mitochondria: integrators in tumorigenesis? Nat. Genet. 28: 104–105. doi: 10.1038/88800 [DOI] [PubMed] [Google Scholar]
- 3.Balmain A., Nagase H.1998. Cancer resistance genes in mice: models for the study of tumour modifiers. Trends Genet. 14: 139–144. doi: 10.1016/S0168-9525(98)01422-X [DOI] [PubMed] [Google Scholar]
- 4.Baysal B.E., Ferrell R.E., Willett-Brozick J.E., Lawrence E.C., Myssiorek D., Bosch A., van der Mey A., Taschner P.E., Rubinstein W.S., Myers E.N., Richard C.W., 3rd, Cornelisse C.J., Devilee P., Devlin B.2000. Mutations in SDHD, a mitochondrial complex II gene, in hereditary paraganglioma. Science 287: 848–851. doi: 10.1126/science.287.5454.848 [DOI] [PubMed] [Google Scholar]
- 5.De Flora S., D’Agostini F., Masiello L., Giunciuglio D., Albini A.1996. Synergism between N-acetylcysteine and doxorubicin in the prevention of tumorigenicity and metastasis in murine models. Int. J. Cancer 67: 842–848. doi: [DOI] [PubMed] [Google Scholar]
- 6.Fliss M.S., Usadel H., Caballero O.L., Wu L., Buta M.R., Eleff S.M., Jen J., Sidransky D.2000. Facile detection of mitochondrial DNA mutations in tumors and bodily fluids. Science 287: 2017–2019. doi: 10.1126/science.287.5460.2017 [DOI] [PubMed] [Google Scholar]
- 7.Gottlieb E., Tomlinson I.P.2005. Mitochondrial tumour suppressors: a genetic and biochemical update. Nat. Rev. Cancer 5: 857–866. doi: 10.1038/nrc1737 [DOI] [PubMed] [Google Scholar]
- 8.Harvey M., McArthur M.J., Montgomery C.A., Jr, Bradley A., Donehower L.A.1993. Genetic background alters the spectrum of tumors that develop in p53-deficient mice. FASEB J. 7: 938–943. [DOI] [PubMed] [Google Scholar]
- 9.Hashizume O., Shimizu A., Yokota M., Sugiyama A., Nakada K., Miyoshi H., Itami M., Ohira M., Nagase H., Takenaga K., Hayashi J.2012. Specific mitochondrial DNA mutation in mice regulates diabetes and lymphoma development. Proc. Natl. Acad. Sci. USA 109: 10528–10533. doi: 10.1073/pnas.1202367109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.He Y., Wu J., Dressman D.C., Iacobuzio-Donahue C., Markowitz S.D., Velculescu V.E., Diaz L.A., Jr, Kinzler K.W., Vogelstein B., Papadopoulos N.2010. Heteroplasmic mitochondrial DNA mutations in normal and tumour cells. Nature 464: 610–614. doi: 10.1038/nature08802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ishikawa K., Takenaga K., Akimoto M., Koshikawa N., Yamaguchi A., Imanishi H., Nakada K., Honma Y., Hayashi J.2008. ROS-generating mitochondrial DNA mutations can regulate tumor cell metastasis. Science 320: 661–664. doi: 10.1126/science.1156906 [DOI] [PubMed] [Google Scholar]
- 12.Ishikawa K., Hashizume O., Koshikawa N., Fukuda S., Nakada K., Takenaga K., Hayashi J.2008. Enhanced glycolysis induced by mtDNA mutations does not regulate metastasis. FEBS Lett. 582: 3525–3530. doi: 10.1016/j.febslet.2008.09.024 [DOI] [PubMed] [Google Scholar]
- 13.Jacobs H.T.2003. The mitochondrial theory of aging: dead or alive? Aging Cell 2: 11–17. doi: 10.1046/j.1474-9728.2003.00032.x [DOI] [PubMed] [Google Scholar]
- 14.Khrapko K., Vijg J.2009. Mitochondrial DNA mutations and aging: devils in the details? Trends Genet. 25: 91–98. doi: 10.1016/j.tig.2008.11.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Klaunig J.E., Kamendulis L.M., Hocevar B.A.2010. Oxidative stress and oxidative damage in carcinogenesis. Toxicol. Pathol. 38: 96–109. doi: 10.1177/0192623309356453 [DOI] [PubMed] [Google Scholar]
- 16.Koppenol W.H., Bounds P.L., Dang C.V.2011. Otto Warburg’s contributions to current concepts of cancer metabolism. Nat. Rev. Cancer 11: 325–337. doi: 10.1038/nrc3038 [DOI] [PubMed] [Google Scholar]
- 17.Krupke D.M., Begley D.A., Sundberg J.P., Bult C.J., Eppig J.T.2008. The Mouse Tumor Biology database. Nat. Rev. Cancer 8: 459–465. doi: 10.1038/nrc2390 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Loeb L.A., Wallace D.C., Martin G.M.2005. The mitochondrial theory of aging and its relationship to reactive oxygen species damage and somatic mtDNA mutations. Proc. Natl. Acad. Sci. USA 102: 18769–18770. doi: 10.1073/pnas.0509776102 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Niemann S., Müller U.2000. Mutations in SDHC cause autosomal dominant paraganglioma, type 3. Nat. Genet. 26: 268–270. doi: 10.1038/81551 [DOI] [PubMed] [Google Scholar]
- 20.Pérez V.I., Bokov A., Van Remmen H., Mele J., Ran Q., Ikeno Y., Richardson A.2009. Is the oxidative stress theory of aging dead? Biochim. Biophys. Acta 1790: 1005–1014. doi: 10.1016/j.bbagen.2009.06.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Polyak K., Li Y., Zhu H., Lengauer C., Willson J.K., Markowitz S.D., Trush M.A., Kinzler K.W., Vogelstein B.1998. Somatic mutations of the mitochondrial genome in human colorectal tumours. Nat. Genet. 20: 291–293. doi: 10.1038/3108 [DOI] [PubMed] [Google Scholar]
- 22.Smyth M.J., Hayakawa Y., Takeda K., Yagita H.2002. New aspects of natural-killer-cell surveillance and therapy of cancer. Nat. Rev. Cancer 2: 850–861. doi: 10.1038/nrc928 [DOI] [PubMed] [Google Scholar]
- 23.Taylor R.W., Turnbull D.M.2005. Mitochondrial DNA mutations in human disease. Nat. Rev. Genet. 6: 389–402. doi: 10.1038/nrg1606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wallace D.C.1999. Mitochondrial diseases in man and mouse. Science 283: 1482–1488. doi: 10.1126/science.283.5407.1482 [DOI] [PubMed] [Google Scholar]
- 25.Yang W., Hekimi S.2010. A mitochondrial superoxide signal triggers increased longevity in Caenorhabditis elegans. PLoS Biol. 8: e1000556. doi: 10.1371/journal.pbio.1000556 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Yokota M., Shitara H., Hashizume O., Ishikawa K., Nakada K., Ishii R., Taya C., Takenaga K., Yonekawa H., Hayashi J.2010. Generation of trans-mitochondrial mito-mice by the introduction of a pathogenic G13997A mtDNA from highly metastatic lung carcinoma cells. FEBS Lett. 584: 3943–3948. doi: 10.1016/j.febslet.2010.07.048 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.