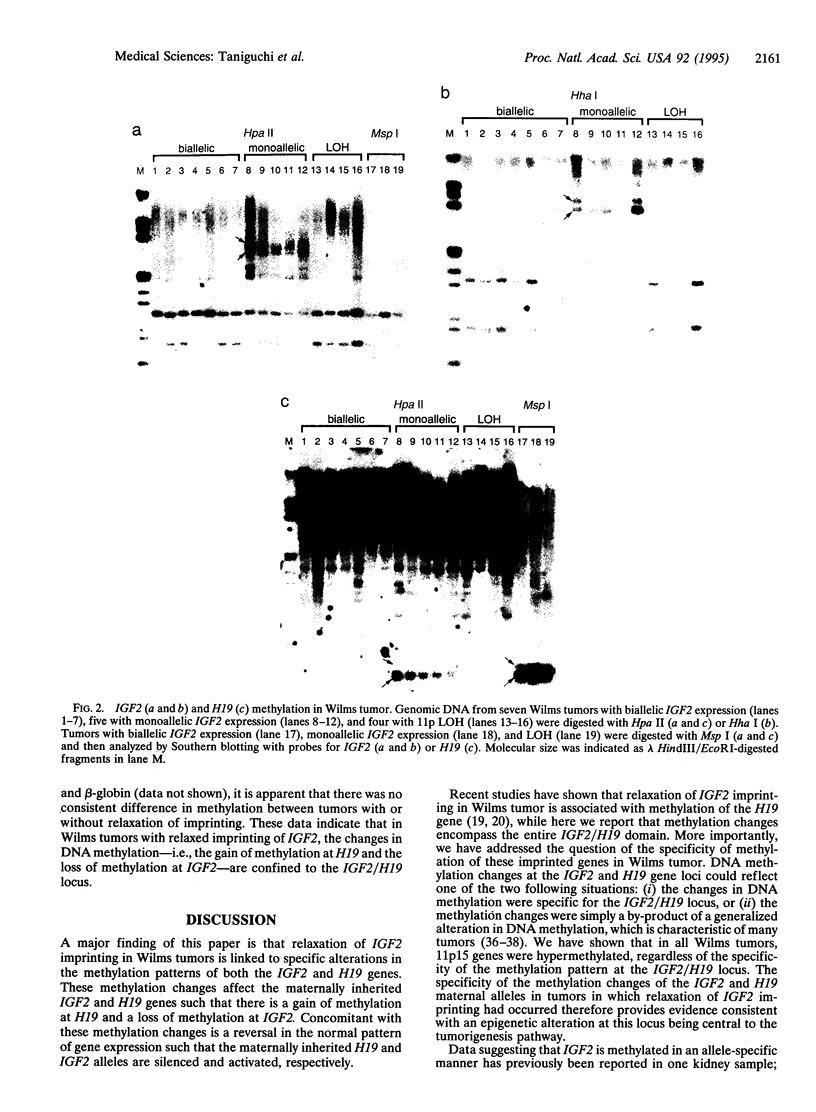
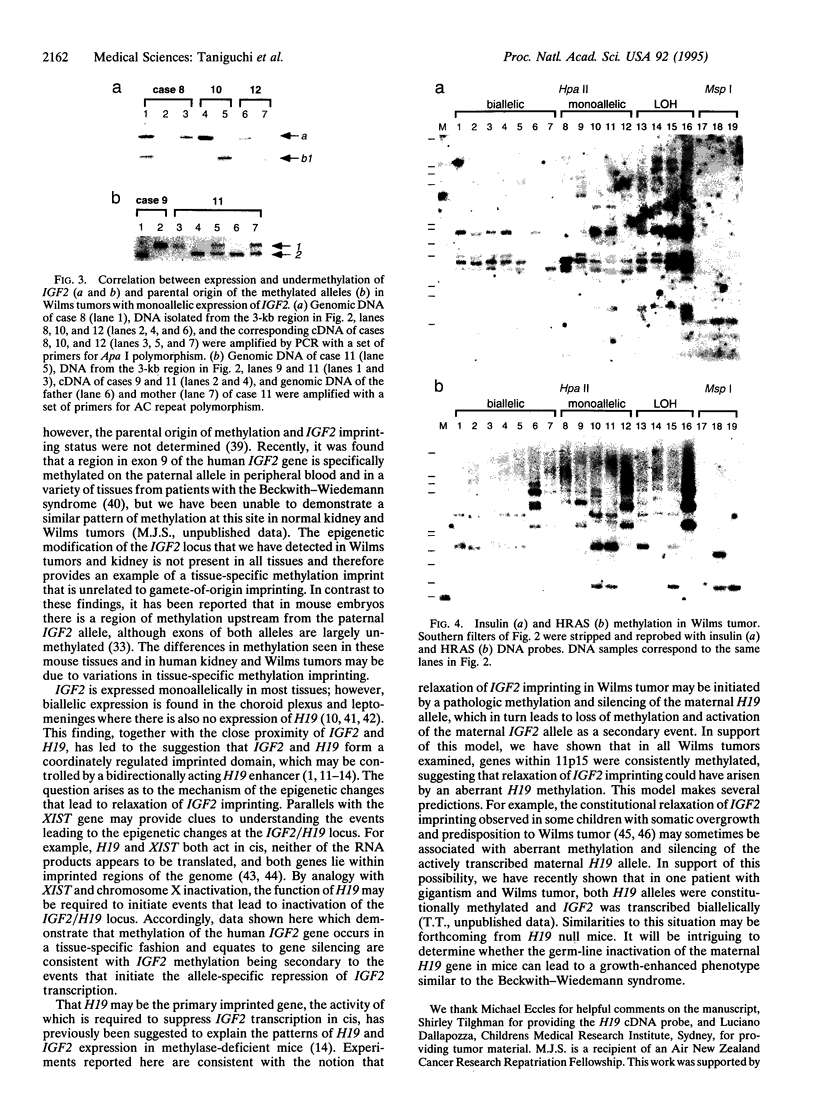
Abstract
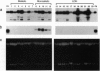
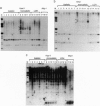
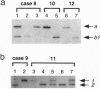
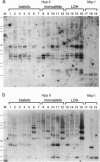
In most tissues IGF2 is expressed from the paternal allele while H19 is expressed from the maternal allele. We have previously shown that in some Wilms tumors the maternal IGF2 imprint is relaxed such that the gene is expressed biallelically. We have now investigated this subset of tumors further and found that biallelic expression of IGF2 was associated with undetectable or very low levels of H19 expression. The relaxation of IGF2 imprinting in Wilms tumors also involved a concomitant reversal in the patterns of DNA methylation of the maternally inherited IGF2 and H19 alleles. Furthermore, the only specific methylation changes that occurred in tumors with relaxation of IGF2 imprinting were solely restricted to the maternal IGF2 and H19 alleles. These data suggest that there has been an acquisition of a paternal epigenotype in these tumors as the result of a pathologic disruption in the normal imprinting of the IGF2 and H19 genes.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartolomei M. S., Webber A. L., Brunkow M. E., Tilghman S. M. Epigenetic mechanisms underlying the imprinting of the mouse H19 gene. Genes Dev. 1993 Sep;7(9):1663–1673. doi: 10.1101/gad.7.9.1663. [DOI] [PubMed] [Google Scholar]
- Bell G. I., Gerhard D. S., Fong N. M., Sanchez-Pescador R., Rall L. B. Isolation of the human insulin-like growth factor genes: insulin-like growth factor II and insulin genes are contiguous. Proc Natl Acad Sci U S A. 1985 Oct;82(19):6450–6454. doi: 10.1073/pnas.82.19.6450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bell G. I., Merryweather J. P., Sanchez-Pescador R., Stempien M. M., Priestley L., Scott J., Rall L. B. Sequence of a cDNA clone encoding human preproinsulin-like growth factor II. 1984 Aug 30-Sep 5Nature. 310(5980):775–777. doi: 10.1038/310775a0. [DOI] [PubMed] [Google Scholar]
- Brannan C. I., Dees E. C., Ingram R. S., Tilghman S. M. The product of the H19 gene may function as an RNA. Mol Cell Biol. 1990 Jan;10(1):28–36. doi: 10.1128/mcb.10.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cedar H. DNA methylation and gene activity. Cell. 1988 Apr 8;53(1):3–4. doi: 10.1016/0092-8674(88)90479-5. [DOI] [PubMed] [Google Scholar]
- Coppes M. J., Bonetta L., Huang A., Hoban P., Chilton-MacNeill S., Campbell C. E., Weksberg R., Yeger H., Reeve A. E., Williams B. R. Loss of heterozygosity mapping in Wilms tumor indicates the involvement of three distinct regions and a limited role for nondisjunction or mitotic recombination. Genes Chromosomes Cancer. 1992 Nov;5(4):326–334. doi: 10.1002/gcc.2870050408. [DOI] [PubMed] [Google Scholar]
- DeChiara T. M., Robertson E. J., Efstratiadis A. Parental imprinting of the mouse insulin-like growth factor II gene. Cell. 1991 Feb 22;64(4):849–859. doi: 10.1016/0092-8674(91)90513-x. [DOI] [PubMed] [Google Scholar]
- Efstratiadis A. Parental imprinting of autosomal mammalian genes. Curr Opin Genet Dev. 1994 Apr;4(2):265–280. doi: 10.1016/s0959-437x(05)80054-1. [DOI] [PubMed] [Google Scholar]
- Feinberg A. P., Gehrke C. W., Kuo K. C., Ehrlich M. Reduced genomic 5-methylcytosine content in human colonic neoplasia. Cancer Res. 1988 Mar 1;48(5):1159–1161. [PubMed] [Google Scholar]
- Ferguson-Smith A. C., Cattanach B. M., Barton S. C., Beechey C. V., Surani M. A. Embryological and molecular investigations of parental imprinting on mouse chromosome 7. Nature. 1991 Jun 20;351(6328):667–670. doi: 10.1038/351667a0. [DOI] [PubMed] [Google Scholar]
- Giannoukakis N., Deal C., Paquette J., Goodyer C. G., Polychronakos C. Parental genomic imprinting of the human IGF2 gene. Nat Genet. 1993 May;4(1):98–101. doi: 10.1038/ng0593-98. [DOI] [PubMed] [Google Scholar]
- Goshen R., Rachmilewitz J., Schneider T., de-Groot N., Ariel I., Palti Z., Hochberg A. A. The expression of the H-19 and IGF-2 genes during human embryogenesis and placental development. Mol Reprod Dev. 1993 Apr;34(4):374–379. doi: 10.1002/mrd.1080340405. [DOI] [PubMed] [Google Scholar]
- Hao Y., Crenshaw T., Moulton T., Newcomb E., Tycko B. Tumour-suppressor activity of H19 RNA. Nature. 1993 Oct 21;365(6448):764–767. doi: 10.1038/365764a0. [DOI] [PubMed] [Google Scholar]
- Hendrich B. D., Brown C. J., Willard H. F. Evolutionary conservation of possible functional domains of the human and murine XIST genes. Hum Mol Genet. 1993 Jun;2(6):663–672. doi: 10.1093/hmg/2.6.663. [DOI] [PubMed] [Google Scholar]
- Jones P. A., Rideout W. M., 3rd, Shen J. C., Spruck C. H., Tsai Y. C. Methylation, mutation and cancer. Bioessays. 1992 Jan;14(1):33–36. doi: 10.1002/bies.950140107. [DOI] [PubMed] [Google Scholar]
- Kay G. F., Penny G. D., Patel D., Ashworth A., Brockdorff N., Rastan S. Expression of Xist during mouse development suggests a role in the initiation of X chromosome inactivation. Cell. 1993 Jan 29;72(2):171–182. doi: 10.1016/0092-8674(93)90658-d. [DOI] [PubMed] [Google Scholar]
- Lawn R. M., Fritsch E. F., Parker R. C., Blake G., Maniatis T. The isolation and characterization of linked delta- and beta-globin genes from a cloned library of human DNA. Cell. 1978 Dec;15(4):1157–1174. doi: 10.1016/0092-8674(78)90043-0. [DOI] [PubMed] [Google Scholar]
- Lee J. E., Tantravahi U., Boyle A. L., Efstratiadis A. Parental imprinting of an Igf-2 transgene. Mol Reprod Dev. 1993 Aug;35(4):382–390. doi: 10.1002/mrd.1080350411. [DOI] [PubMed] [Google Scholar]
- Li E., Beard C., Jaenisch R. Role for DNA methylation in genomic imprinting. Nature. 1993 Nov 25;366(6453):362–365. doi: 10.1038/366362a0. [DOI] [PubMed] [Google Scholar]
- Moulton T., Crenshaw T., Hao Y., Moosikasuwan J., Lin N., Dembitzer F., Hensle T., Weiss L., McMorrow L., Loew T. Epigenetic lesions at the H19 locus in Wilms' tumour patients. Nat Genet. 1994 Jul;7(3):440–447. doi: 10.1038/ng0794-440. [DOI] [PubMed] [Google Scholar]
- Nicholls R. D. New insights reveal complex mechanisms involved in genomic imprinting. Am J Hum Genet. 1994 May;54(5):733–740. [PMC free article] [PubMed] [Google Scholar]
- Ogawa O., Becroft D. M., Morison I. M., Eccles M. R., Skeen J. E., Mauger D. C., Reeve A. E. Constitutional relaxation of insulin-like growth factor II gene imprinting associated with Wilms' tumour and gigantism. Nat Genet. 1993 Dec;5(4):408–412. doi: 10.1038/ng1293-408. [DOI] [PubMed] [Google Scholar]
- Ogawa O., Eccles M. R., Szeto J., McNoe L. A., Yun K., Maw M. A., Smith P. J., Reeve A. E. Relaxation of insulin-like growth factor II gene imprinting implicated in Wilms' tumour. Nature. 1993 Apr 22;362(6422):749–751. doi: 10.1038/362749a0. [DOI] [PubMed] [Google Scholar]
- Ohlsson R., Hedborg F., Holmgren L., Walsh C., Ekström T. J. Overlapping patterns of IGF2 and H19 expression during human development: biallelic IGF2 expression correlates with a lack of H19 expression. Development. 1994 Feb;120(2):361–368. doi: 10.1242/dev.120.2.361. [DOI] [PubMed] [Google Scholar]
- Ohlsson R., Nyström A., Pfeifer-Ohlsson S., Töhönen V., Hedborg F., Schofield P., Flam F., Ekström T. J. IGF2 is parentally imprinted during human embryogenesis and in the Beckwith-Wiedemann syndrome. Nat Genet. 1993 May;4(1):94–97. doi: 10.1038/ng0593-94. [DOI] [PubMed] [Google Scholar]
- Pal N., Wadey R. B., Buckle B., Yeomans E., Pritchard J., Cowell J. K. Preferential loss of maternal alleles in sporadic Wilms' tumour. Oncogene. 1990 Nov;5(11):1665–1668. [PubMed] [Google Scholar]
- Rainier S., Johnson L. A., Dobry C. J., Ping A. J., Grundy P. E., Feinberg A. P. Relaxation of imprinted genes in human cancer. Nature. 1993 Apr 22;362(6422):747–749. doi: 10.1038/362747a0. [DOI] [PubMed] [Google Scholar]
- Reeve A. E., Eccles M. R., Wilkins R. J., Bell G. I., Millow L. J. Expression of insulin-like growth factor-II transcripts in Wilms' tumour. Nature. 1985 Sep 19;317(6034):258–260. doi: 10.1038/317258a0. [DOI] [PubMed] [Google Scholar]
- Sasaki H., Jones P. A., Chaillet J. R., Ferguson-Smith A. C., Barton S. C., Reik W., Surani M. A. Parental imprinting: potentially active chromatin of the repressed maternal allele of the mouse insulin-like growth factor II (Igf2) gene. Genes Dev. 1992 Oct;6(10):1843–1856. doi: 10.1101/gad.6.10.1843. [DOI] [PubMed] [Google Scholar]
- Schneid H., Seurin D., Vazquez M. P., Gourmelen M., Cabrol S., Le Bouc Y. Parental allele specific methylation of the human insulin-like growth factor II gene and Beckwith-Wiedemann syndrome. J Med Genet. 1993 May;30(5):353–362. doi: 10.1136/jmg.30.5.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schroeder W. T., Chao L. Y., Dao D. D., Strong L. C., Pathak S., Riccardi V., Lewis W. H., Saunders G. F. Nonrandom loss of maternal chromosome 11 alleles in Wilms tumors. Am J Hum Genet. 1987 May;40(5):413–420. [PMC free article] [PubMed] [Google Scholar]
- Scott J., Cowell J., Robertson M. E., Priestley L. M., Wadey R., Hopkins B., Pritchard J., Bell G. I., Rall L. B., Graham C. F. Insulin-like growth factor-II gene expression in Wilms' tumour and embryonic tissues. Nature. 1985 Sep 19;317(6034):260–262. doi: 10.1038/317260a0. [DOI] [PubMed] [Google Scholar]
- Shih C., Weinberg R. A. Isolation of a transforming sequence from a human bladder carcinoma cell line. Cell. 1982 May;29(1):161–169. doi: 10.1016/0092-8674(82)90100-3. [DOI] [PubMed] [Google Scholar]
- Steenman M. J., Rainier S., Dobry C. J., Grundy P., Horon I. L., Feinberg A. P. Loss of imprinting of IGF2 is linked to reduced expression and abnormal methylation of H19 in Wilms' tumour. Nat Genet. 1994 Jul;7(3):433–439. doi: 10.1038/ng0794-433. [DOI] [PubMed] [Google Scholar]
- Surani M. A. Genomic imprinting: control of gene expression by epigenetic inheritance. Curr Opin Cell Biol. 1994 Jun;6(3):390–395. doi: 10.1016/0955-0674(94)90031-0. [DOI] [PubMed] [Google Scholar]
- Tadokoro K., Fujii H., Inoue T., Yamada M. Polymerase chain reaction (PCR) for detection of ApaI polymorphism at the insulin like growth factor II gene (IGF2). Nucleic Acids Res. 1991 Dec 25;19(24):6967–6967. doi: 10.1093/nar/19.24.6967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tilghman S. M. DNA methylation: a phoenix rises. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):8761–8762. doi: 10.1073/pnas.90.19.8761. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weksberg R., Shen D. R., Fei Y. L., Song Q. L., Squire J. Disruption of insulin-like growth factor 2 imprinting in Beckwith-Wiedemann syndrome. Nat Genet. 1993 Oct;5(2):143–150. doi: 10.1038/ng1093-143. [DOI] [PubMed] [Google Scholar]
- Williams J. C., Brown K. W., Mott M. G., Maitland N. J. Maternal allele loss in Wilms' tumour. Lancet. 1989 Feb 4;1(8632):283–284. doi: 10.1016/s0140-6736(89)91300-7. [DOI] [PubMed] [Google Scholar]
- Zemel S., Bartolomei M. S., Tilghman S. M. Physical linkage of two mammalian imprinted genes, H19 and insulin-like growth factor 2. Nat Genet. 1992 Sep;2(1):61–65. doi: 10.1038/ng0992-61. [DOI] [PubMed] [Google Scholar]
- Zhang Y., Shields T., Crenshaw T., Hao Y., Moulton T., Tycko B. Imprinting of human H19: allele-specific CpG methylation, loss of the active allele in Wilms tumor, and potential for somatic allele switching. Am J Hum Genet. 1993 Jul;53(1):113–124. [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Tycko B. Monoallelic expression of the human H19 gene. Nat Genet. 1992 Apr;1(1):40–44. doi: 10.1038/ng0492-40. [DOI] [PubMed] [Google Scholar]
- de Bustros A., Nelkin B. D., Silverman A., Ehrlich G., Poiesz B., Baylin S. B. The short arm of chromosome 11 is a "hot spot" for hypermethylation in human neoplasia. Proc Natl Acad Sci U S A. 1988 Aug;85(15):5693–5697. doi: 10.1073/pnas.85.15.5693. [DOI] [PMC free article] [PubMed] [Google Scholar]