Abstract
Picro-Sirius red is a routine diagnostic stain intended for the histological visualization of collagen fibers (fibrosis) in tissue. Multi-label immunohistochemistry is a powerful tool used by researchers to visualize different cell types and their location within a tissue specimen, and to observe co-localization of antigens. Combining the specificity of immunodetection with the simplicity of Sirius red staining will allow researchers to visualize multi-antigen detection in relation to fibrosis, a common histological feature of injury in many chronic diseases. Pre-treatment of formalin-fixed, paraffin-embedded tissue (FFPE) specimens with antigen retrieval is essential for the work-up of most commercially available antibodies. The most common form of antigen retrieval involves boiling tissue specimens in buffer to break the cross-linkages caused by formalin fixation. However, this method causes tissue modification and collagen fiber shrinkage leading to suboptimal results when counterstaining for Sirius red. Reduced heat and enzymatic digestion are antigen retrieval methods compatible with Sirius red counterstaining. This paper will discuss the difficulties faced when combining these two staining methods, and provide a detailed method for the simultaneous detection of antigen and Sirius red in FFPE tissues.
Keywords: Immunohistochemistry, histology, Sirius red, antigen retrieval, HIAR
Introduction
Diagnostic histochemistry has been in practice since the late 19th century, and is used in pathology laboratories worldwide. Special stains utilized for the interpretation of liver biopsies include H&E, Perls’ iron stain and Picro-Sirius red. Sirius red specifically stains collagen types I, II and III and is used to highlight and quantify liver fibrosis (Junqueira et al. 1979; Standish et al., 2006). Immunolabelling of patient biopsies is used for diagnostic purposes and in research laboratories. Advances in multi-antibody immunolabelling of tissue sections allows for the detailed phenotypic analysis of specific cell populations and their locations within tissue specimens. Furthermore, it saves tissue when limited specimens are available and provides confidence when reporting co-localization, which may be problematic with serial sectioning.
These advances in immunolabelling are due in part to increased specificity and the quality of primary antibodies and detection systems. However, it was the simple technique of boiling formalin-fixed, paraffin-embedded (FFPE) tissue sections in water, first published in 1991 (Shi et al. 1991) that augmented the quality and quantity of the immunohistological repertoire. The mechanism behind heat-induced antigen retrieval (HIAR) involves unmasking antigens by loosening or breaking the cross-linkages caused by formalin fixation (Shi et al. 1991). These advances in antigen retrieval immunostaining are not, however, standardized across antibodies or tissue samples. Some surface antigens are sensitive to regular antigen retrieval methods (10 min boiling in a microwave), which can cause protein denaturation and reduced intensity of immunostaining. Reduced heating time and non-heating methods, such as enzymatic digestion, which utilizes proteolytic enzymes to digest excess aldehyde linkages and expose the antigen, can also be used. The effectiveness of antigen retrieval methods will vary depending on tissue type and antibody selection and must be optimized for each tissue/antibody combination.
An interest in the relationship between infiltrating immune cells, ductular structures and fibrosis in chronic liver disease prompted the combination of immunolabelling and histochemical techniques. Simply performing routine immunohistochemistry and applying a Sirius red counterstain produced substandard results. It was hypothesized that HIAR may be responsible for altered Sirius red staining and, in the absence of published methodology, the optimization of the simultaneous detection of antigen and Sirius red in FFPE tissues was required. This paper will describe for the first time the challenges faced when combining these two staining methods and provide a detailed approach for optimum immunohistochemical/Sirius red staining.
Materials & Methods
Influence of HIAR on Collagen Fiber Staining
Serial sections of FFPE tissue were subjected to varying degrees of HIAR to identify the influence of increasing temperature on Sirius red collagen fiber staining. Sections were immersed in 400 ml of Tris-EDTA antigen retrieval buffer and microwaved for 3, 5 or 10 min. Baseline Sirius red reactivity was obtained by staining immediately following section preparation, avoiding HIAR. A detailed protocol describing optimal Sirius red staining in FFPE specimens is described below. Briefly, sections were stained in Sirius red solution for 1 hr, washed in acetic acid, quickly dehydrated, and then mounted in a resinous medium. The extent of reactivity for Sirius red was quantified using image analysis software (ImageJ, NIH; Bethesda, MD) and reported as percent of positive pixels per high-power field. Image analysis parameters were set up using the baseline control samples and applied to all remaining test sections.
Antibody Optimization
Two antibodies requiring different antigen retrieval methods and antibody incubation times were selected for the development of this method. Wide-spectrum keratin used to label ductular structures (CK-WSS; Dako; Carpinteria, CA) requires HIAR during routine immunohistochemical analysis. Sections were brought to the boil in a Tris-EDTA pH 9.0 buffer (~3 min) and then left to simmer for a further 10 min. In this study, CK-WSS was tested utilizing reduced HIAR to assess antigen exposure and the potential compatibility with a Sirius red counterstain. The compatibility of a second antibody that required enzymatic digestion for antigen retrieval was also tested. In order to visualize tissue macrophages, sections underwent trypsin digestion for 10 min at 37C and were labelled with F4/80 (AbD Serotec; Oxford, UK), before overnight antibody incubation.
Optimized Procedure for Immunolabelling FFPE Specimens with Sirius Red Counterstain
Section preparation: FFPE sections were dewaxed by heating at 50C for 30 min and then cleared in 3 × 5 min passes through a solution of xylene. Sections were then rehydrated through 2 min washes of decreasing grades of ethanol (100%, 100%, 100%, 95%, 95%, 70%) and brought to water.
- Antigen retrieval:
- For antibodies suited to reduced HIAR, sections were placed in a microwaveable dish and immersed in 400 ml of Tris-EDTA buffer (10 mM Tris-base/1 mM EDTA solution, pH 9.0). The lid was placed loosely onto the dish and the samples microwaved on high (850W) for 2 min. The dish was removed, swirled to distribute the heat evenly, and then microwavef for a further 1 min. Sections were then washed immediately under tap water and incubated in a solution of TBS/Tween-20.
- For antibodies suited to enzymatic digestion, sections were incubated at 37C with pre-heated Carezyme Trypsin (Biocare Medical) diluted 1:3 trypsin with buffer, with ample solution applied to the sections to ensure that the samples were adequately covered. Slides were then placed at 37C for 10 min, washed thoroughly under tap water, and incubated in a solution of TBS/Tween-20.
Chromogenic immunodetection: Endogenous peroxidase activity was quenched with a peroxidase block consisting of 0.03% hydrogen peroxide for 15 min. Specimens were then incubated with primary antibody for at least 1 hr at room temperature (individual antibody concentration and incubation times were optimized for every antigen). The slides were then rinsed thoroughly under tap water and placed in a solution of TBS/Tween-20. Slides were then incubated with horseradish peroxidase-labelled polymer for 30 min, rinsed thoroughly, and developed for 15 min using 3,3’-diaminobenzidine (DAB) substrate-chromogen to produce a brown precipitate at the antigen site. Sections were again rinsed and the slides left to run under the tap water for 3 min to ensure all residual DAB was removed.
Counterstaining with Sirius red: Slides were immersed in 1% Sirius red/saturated picric acid solution (0.5 g Sirius red F3B/500ml saturated aqueous solution picric acid) for 1 hr and then passed through two washes with 0.5% acetic acid.
Mounting slides: Slides were dehydrated through increasing grades of ethanol (70%, 100%, 100%) and cleared in xylene. This step was performed quickly, as prolonged ethanol washes will reduce the contrast between Sirius red staining and picric acid. The slides were mounted using a permanent mounting medium (DPX or Permount) for the long-term storage of slides.
Results
Heat-induced Antigen Retrieval Alters Sirius Red Collagen Staining
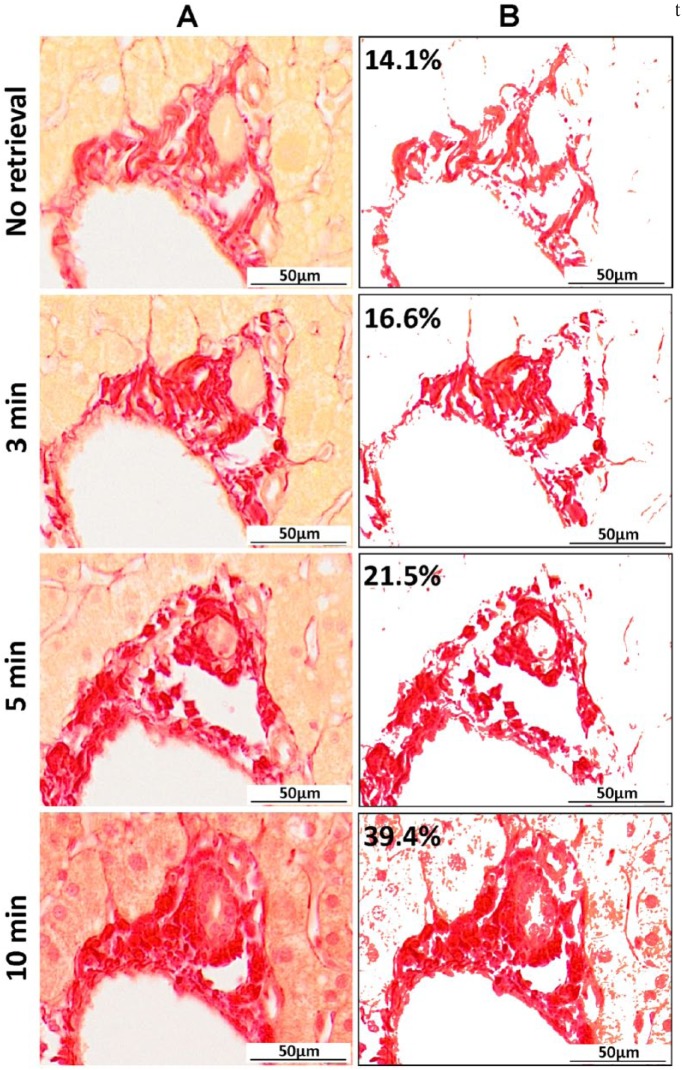
Under bright-field microscopy, collagen fibers should appear bright pink/red on a pale yellow background (Fig. 1A). However, the quality of this histochemical stain was greatly compromised after sections had been exposed to HIAR conditions for the immunodetection of CK-WSS. In a time- and heat-dependent manner, the stark contrast between collagen fibers and tissue was lost and non-specific nuclear staining was observed (Fig. 1). Image analysis performed on a specific region identified in each serial section revealed a significant increase in the amount of positive Sirius red staining with increased exposure to HIAR (14.1% baseline to 39.4% with full HIAR) (Fig. 1).
Figure 1.
Altered Sirius red staining on formalin-fixed paraffin-embedded (FFPE) tissue sections following exposure to heat-induced antigen retrieval (HIAR) conditions. (A) Sirius red single histochemical stains performed on serial FFPE tissue specimens under optimal conditions (no retrieval) and following exposure to higher temperatures under HIAR conditions (3, 5 and 10 min). (B) Image analysis of a representative portal region on serial sections highlights how increasing temperature alters the affinity of Sirius red staining and results in false-positive collagen expression (percentage of total analyzed area). Scale, 50 µm.
Sirius Red Counterstain Is Compatible with Antibodies that Require Reduced HIAR and Enzymatic Digestion
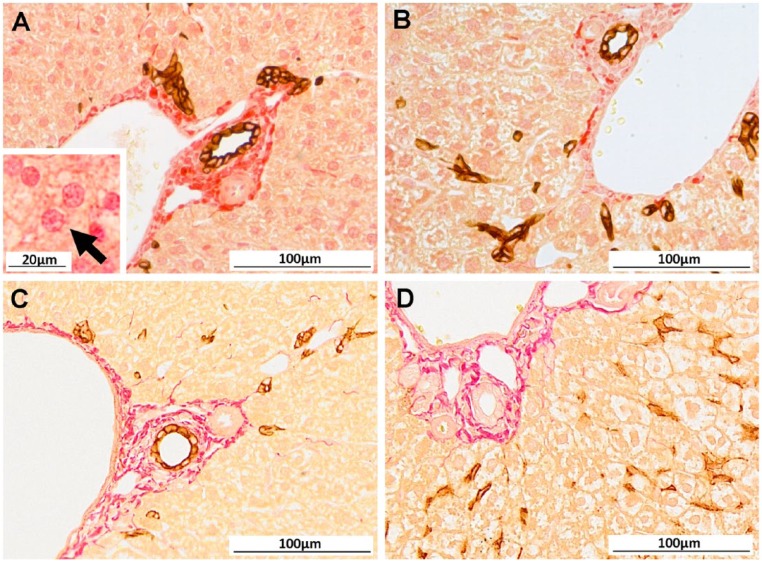
In order to combine immunodetection methods with a Sirius red counterstain, it was apparent that sections could not undergo prolonged HIAR. Inconsistencies in Sirius red positivity were observed across serial sections, with regions displaying non-specific nuclear positivity and background tissue staining (Fig. 2A). More importantly, in some regions, collagen stained with Sirius red appeared globular, and the delicate fibrous septa and spurs were lost (Fig. 2B). CK-WSS immunostaining was trialed utilizing other antigen retrieval methods. Non-heating methods and enzymatic digestion were insufficient for unmasking the epitope; however, significantly shortening the heating time during HIAR produced strong, positive DAB staining comparable to that seen with routine, single immunodetection of this antigen. Furthermore, after Sirius red counterstaining, it was clear that reduced HIAR also protected the collagen fibers from denaturation and shrinkage (Fig. 2C). Antigens that require enzymatic digestion (such as F4/80) were also found to be suitable for this technique, as the temperature of the specimens during antigen retrieval was not raised above 37C and the collagen fibers remained intact (Fig. 2D).
Figure 2.
Compatibility of Sirius red counterstain following immunodetection is dependent on antigen retrieval methods. (A) After full heat-induced antigen retrieval (HIAR) for immunolabelling of wide-spectrum keratin (CK-WSS; brown), Sirius red staining displayed widespread non-specific background and nuclear staining (Inset: higher magnification (×460) with arrow indicating positive hepatocyte nuclei). In other regions, collagen fibers appeared globular with a distinct loss of delicate fibrous spurs (B). Optimized immunohistochemical staining for CK-WSS and F4/80 (brown) with Sirius red counterstain utilizing reduced HIAR (C) or trypsin digest (D), respectively. Scale, 100 µm; Inset in (A), 20 µm.
Discussion
Antigen retrieval is an essential process used to unmask hidden epitopes during tissue immunolabelling; however, standard HIAR conditions significantly reduce the quality of routine Picro-Sirius red staining. The current study was undertaken to overcome this issue and provide a detailed approach for optimum immunolabelling with a Sirius red counterstain. We show that antibodies displaying the same affinity following reduced HIAR or antibodies that require enzymatic digestion are suited to this technique.
Many alterations in Sirius red staining have been observed following HIAR, including non-specific background and nuclear staining, loss of contrast between collagen fibers and the surrounding tissue, and, most importantly, destruction of collagen fibers. The denaturation or shrinkage of collagen significantly increases with high temperatures (>50C) and is a likely explanation for the staining patterns observed in our liver specimens following HIAR (Rossmann et al. 2014). Heat-induced changes to Sirius red staining have previously been observed and used to analyze the effects of thermal alterations in corneal tissues following laser thermokeratoplasty (Asiyo-Vogel et al. 1997). Non-specific nuclear staining is a common problem reported during the work-up of immunolabelling and histochemical staining protocols. Processes required for optimum immunohistochemical staining, such as blocking and HIAR, modify the physical properties of a tissue section, including tissue permeability, which causes tissues to be stained more rapidly (Suvarna et al. 2012). The loss of contrast between collagen fibers and the surrounding tissue as well as any non-specific nuclear staining could lead to a misinterpretation of the results and difficulties when performing image analysis, as highlighted in these studies.
Antigen retrieval methods are not equally applicable to all antibodies. If reduced HIAR or enzymatic digestion fails to retrieve antigen sites, consider adapting this technique with a combined antigen retrieval approach. Frost et al. (2000) compared the efficacy of various antigens utilizing standard HIAR conditions or a combination of low-temperature antigen retrieval with trypsin pre-treatment in order to reduce tissue loss. Equal or stronger staining was reported for three of the five antigens tested utilizing the low temperature antigen retrieval combination. Similar combinatorial methods proposed by Ezaki (2000) reduced non-specific background and false-positive staining when compared to HIAR conditions. Where available, Sirius red staining can also be performed on frozen tissue specimens (Kumar et al. 2011). Frozen sections are generally exempt from undergoing antigen retrieval during immunolabelling and may be used as an alternative to avoid collagen degradation during HIAR.
Double immunolabelling with a Sirius red counterstain was not trialed in the current study; however, it is likely this could be achieved using a variety of multi-target chromogenic immunolabelling techniques, provided the tissue specimens are not raised above 50C (van der Loos 2008, Kim et al. 2012). It is important to note that optimizing the staining conditions for each primary antibody is essential before trialing any multi-label techniques. These will serve as controls to ensure the quality of each stain when adjusting antigen retrieval methods for Sirius red counterstaining.
Performing multi-label immunostaining is a powerful tool with many benefits, including visualization of different cell types and their location within a tissue specimen. It can help identify individual cell populations and provide phenotypic and functional information. The simultaneous detection of antigen and Sirius red staining will provide users with an additional level of visual and quantifiable data for their studies.
Footnotes
Declaration of Conflicting Interests: The author declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author received no financial support for the research, authorship, and/or publication of this article.
References
- Asiyo-Vogel MN, Brinkmann R, Notbohm H, Eggers R, Lubatschowski H, Laqua H, Vogel A. (1997). Histologic analysis of thermal effects of laser thermokeratoplasty and corneal ablation using Sirius-red polarization microscopy. J Cataract Refract Surg 23:515-526. [DOI] [PubMed] [Google Scholar]
- Ezaki T. (2000). Antigen retrieval on formaldehyde-fixed paraffin sections: its potential drawbacks and optimization for double immunostaining. Micron 31:639-649. [DOI] [PubMed] [Google Scholar]
- Frost AR, Sparks D, Grizzle WE. (2000). Methods of antigen recovery vary in their usefulness in unmasking specific antigens in immunohistochemistry. Appl Immunohistochem Mol Morphol 8:236-243. [DOI] [PubMed] [Google Scholar]
- Junqueira LC, Bignolas G, Brentani RR. (1979). Picrosirius staining plus polarization microscopy, a specific method for collagen detection in tissue sections. Histochem J 11:447-455. [DOI] [PubMed] [Google Scholar]
- Kim M, Soontornniyomkij V, Ji B, Zhou X. (2012). System-wide immunohistochemical analysis of protein co-localization. PLOS One 7:e32043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar A, Yamauchi J, Girgenrath T, Girgenrath M. (2011). Muscle-specific expression of insulin-like growth factor 1 improves outcome in Lama2Dy-w mice, a model for congenital muscular dystrophy type 1A. Hum Mol Genet 20:2333-2343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossmann C, Garrett-Mayer E, Rattay F, Haemmerich D. (2014). Dynamics of tissue shrinkage during ablative temperature exposures. Physiol Meas 35:55-67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi SR, Key ME, Kalra KL. (1991). Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem 39:741-748. [DOI] [PubMed] [Google Scholar]
- Standish RA, Cholongitas E, Dhillon A, Burroughs AK, Dhillon A. 2006. An appraisal of the histopathological assessment of liver fibrosis. Gut 55:569-578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suvarna KS, Layton C, Bancroft JD. (2012). Bancroft’s Theory and Practice of Histological Techniques, UK: Elsevier Health Sciences. [Google Scholar]
- van der Loos CM. (2008). Multiple immunoenzyme staining: methods and visualizations for the observation with spectral imaging. J Histochem Cytochem 56:313-328. [DOI] [PMC free article] [PubMed] [Google Scholar]