Abstract
Although the Epstein-Barr virus (EBV) has spread to all populations in the world, EBV-associated nasopharyngeal carcinoma (NPC) is prevalent only in South China and Southeast Asia. The role of EBV in the malignant transformation of nasopharyngeal epithelium is the main focus of current researches. Radiotherapy and chemoradiotherapy have been successful in treating early stage NPC, but the recurrence rates remain high. Unfortunately, local relapse and metastasis are commonly unresponsive to conventional treatments. These recurrent and metastatic lesions are believed to arise from residual or surviving cells that have the properties of cancer stem cells. These cancer stem-like cells (CSCs) have the ability to self-renew, differentiate, and sustain propagation. They are also chemo-resistant and can form spheres in anchorage-independent environments. This review summarizes recent researches on the CSCs in EBV-associated NPC, including the findings regarding cell surface markers, stem cell-related transcription factors, and various signaling pathways. In particular, the review focuses on the roles of EBV latent genes [latent membrane protein 1 (LMP1) and latent membrane protein 2A (LMP2A)], cellular microRNAs, and adenosine triphosphate (ATP)-binding cassette chemodrug transporters in contributing to the properties of CSCs, including the epithelial-mesenchymal transition, stem-like transition, and chemo-resistance. Novel therapeutics that enhance the efficacy of radiotherapy and chemoradiotherapy and inhibitors that suppress the properties of CSCs are also discussed.
Keywords: Cancer stem-like cells, Epstein-Barr virus, nasopharyngeal carcinoma, microRNA, signalling pathway
Epstein-Barr virus (EBV), a human gamma-herpes virus, is an infectious agent associated with several lymphoproliferative disorders, including Burkitt's lymphoma and Hodgkin's disease, and non-lymphoproliferative malignancies, such as nasopharyngeal carcinoma (NPC) and gastric cancer[1],[2]. Among these EBV-associated malignancies, nonkeratinizing NPC constitutes a significant health burden in South China due to its high incidence and early-age onset.
Nonkeratinizing NPC is a unique epithelial carcinoma in the head and neck region with peculiar histological and molecular characteristics, including intensive infiltration of lymphocytes, undifferentiated or poorly differentiated appearance, absence of p53 mutations, and consistent association with EBV infection. In contrast to its high incidence in South China and Southeast Asia, nonkeratinizing NPC is rarely found in western countries. Genetic susceptibility and environmental factors in the high-risk regions may contribute to the distinctive geographic and ethnic prevalence of the disease. Tumors arising in the post-nasal cavity were first reported in the early 1900s[3]. However, the histological variations in the tumors were not brought to research attention, and their causes remained obscure for over half a century. Following the discovery of EBV by Epstein et al.[4], several groups provided serologic and molecular evidence of the association of EBV with a certain type of post-nasal space carcinoma[5]. Later, this EBV-associated carcinoma was demonstrated to be a nonkeratinizing histological subtype of NPC[6]. In almost all nonkeratinizing NPCs, the EBV genome and its gene products for type II latency were detected in each tumor cell. Most importantly, the clonal origin of EBV infection in NPC was demonstrated by analyzing the terminal repeats in EBV episomes from NPC and premalignant lesions. These findings imply that NPC is derived from the expansion of a single EBV-infected nasopharyngeal epithelial cell. The critical roles of EBV infection in the initiation and progression of NPC are also supported by the oncogenic properties of various latent gene products [e.g., latent membrane protein 1 (LMP1), latent membrane protein 2A (LMP2A), and Epstein-Barr virus BamHI-A rightward transcripts (BARTs)] expressed in the cancer. We also revealed that the persistent latent EBV infection of these cells was dependent on the presence of specific genetic lesions, such as allelic loss of 3p, p16 inactivation, or CCND1 (Cyclin D1) amplification. The accumulation of additional genetic and epigenetic abnormalities may drive the tumorigenic process and contribute to the development of NPC.
Currently, the mainstay treatments for NPC are radiotherapy and chemoradiotherapy. Despite the high success rate (over 90%) in treating patients in early disease stages, these treatments are less than satisfactory for those with local relapse or distant metastasis[7],[8]. The relapse rate remains high among NPC patients, although intensive efforts are being aimed at optimizing and improving the current chemotherapeutic strategies. It is thus of the utmost importance to identify novel therapeutic approaches for preventing tumor relapse and to develop potential treatments for relapsed tumors. Tumor relapse is believed to result from the failure to eradicate all tumor cells using conventional treatment. In this case, the tumor cells that survive after treatment subsequently develop into a new tumor mass or become metastatic. These drug-resistant cells have been shown to possess stem cell-like properties such as self-renewal and have thus been coined “cancer stem cells/cancer stem-like cells” (CSCs)[9]. The identification of a CD34+CD38− initiating cell population in human myeloid leukemia by Lapidot et al.[10] provided the first and fundamental evidence of the concept of CSCs (or “tumor-initiating cells”). Although the concept of CSC has been debated ever since their discovery, it is currently revolutionizing the field of cancer research[11]. In the past decade, CSCs have been identified in a number of solid tumors and their role in cancer development has been experimentally tested[12]. In NPC, increasing evidence supports the persistence of a small subpopulation of CSCs and the important roles they play in tumor progression. However, controversial findings are noted among the studies on EBV-positive and EBV-negative NPC cell lines. As shown in recent studies, the EBV latent genes, LMP1 and LMP2A, modulate the expression of CSC-associated genes and alter the stem cell properties of epithelial cells. Researches on EBV-positive cell lines and primary tumors may provide the most relevant information on the role of CSCs in NPC. To achieve consistency and maintain clarity on the topic of EBV-associated NPC, we only review the data from studies on EBV-positive tumors. The role of CSCs in NPC development, the interaction between EBV and CSCs, and the therapeutic potential of CSC targeting are also discussed.
The Concept of CSCs
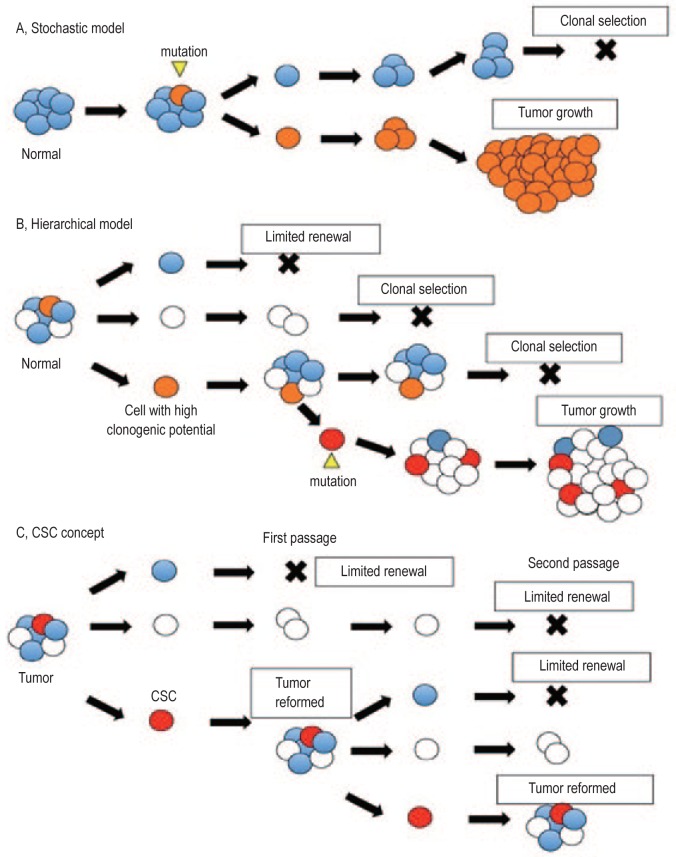
Conventional cancer treatments, such as tumor-targeting radiotherapy and systematic chemotherapy, mainly target the observable mass of tumor cells. However, the origin of cancer, that is, the origin of the first tumor cell and the subsequent sustained tumor growth and renewal, remains elusive. The two main models that have been proposed for cancer development are the stochastic model[13],[14] and the hierarchical model[15]–[17] (Figure 1). In theory, CSCs follow the latter model, which suggests that tumors are hierarchical in structure and heterogeneous in nature. In this model, a subpopulation of cells (CSCs) are said to exhibit high self-renewal capacity and differentiation potential, giving rise to daughter cells with less potential, hence sustaining tumor development. The core stem cell-like properties commonly recognized among CSCs in different types of cancer include the ability to self-renew, the potential to differentiate and give rise to daughter cells, the capacity to sustain tumor propagation in serial transplantation, and the exhibition of drug resistance[12],[13],[18]. These stem cell-like biological features suggest that CSCs are likely to be responsible for the initiation, propagation, and dissemination of metastatic tumor cells and the survival and relapse of tumors. The tumor-initiating role of CSCs also makes them remarkable targets for cancer therapy research.
Figure 1. Cancer models and the concept of cancer stem-like cells (CSCs).
A, the stochastic model demonstrates the equal potency among all cells. Tumors arise from an event, such as a mutation, in a certain cell that enables the cell to out-grow other subpopulations, which leads to clonal selection. B, however, the hierarchical model suggests that there is heterogeneity among the cells and that only subpopulations with high clonogenic potential are able to develop into a tumor mass with the same parental composition. The hierarchical model and the stochastic model may not necessarily be contradictory, as mutations and clonal selection may still occur within the hierarchical model. C, the concept of CSCs follows the hierarchical model in that only a certain subpopulation of cells possesses sufficient potential to propagate and give rise to a tumor with the original composition. Tumors originating from CSCs have exhibited serial transplantation in mice models.
Since the identification of CSCs in leukemia, there has been a plethora of studies on identifying CSCs in solid tumors, such as ovarian cancer[19], renal carcinoma[20], head and neck squamous cell carcinoma[21], and bladder cancer[22]. Direct evidence of tumor regeneration from CSC populations has been demonstrated in studies using genetic tracing[23],[24] and linear tracing[25]. A considerable number of publications have also been published on CSC research in NPC.
CSCs in NPC
In the past few years, numerous studies have sought to identify and characterize the CSC subpopulation in EBV-associated NPC cell lines and primary tumors. A variety of conventional approaches used to examine NPC CSCs were examined in these studies[26]. The expression of potential CSC and embryonic stem cell (ESC) markers in the cell lines and primary tumors were analyzed by multiparameter flow cytometry and immunohistochemical staining, respectively[27],[28]. The anchorage-independent sphere-forming tumor cells or sphe-roids were isolated as a functional subpopulation with CSC properties[29],[30]. The tumor propagating potential of isolated candidate CSCs was validated in immunocompromised mouse models. These experimental methods aimed to determine whether the target tumor cell population exhibited the commonly recognized properties of a CSC population.
By using fluorescence-activated cell sorting (FACS) based on the Hoechst 33342 efflux, Wang et al.[31] were the first group to demonstrate the existence of a CSC side population (SP) in NPC cell lines. However, SP cells were detected in 0.7%-6.8% of EBV-negative NPC cell lines whereas the EBV-positive C666-1 cells contained only a scant amount of SP cells (0.1%). Because the ability of SP cells to efflux out of cells is based on the ATP-binding cassette (ABC) subfamily G, member 2 (ABCG2), the low expression of ABCG2 in CSCs may be responsible for the sparse SP in the EBV-associated NPC cell line. Our recent study showed that there was an absence of ABCG2 up-regulation in either the CD44+ or sphere-forming cells in C666-1 cells[32]. In 2 NPC cases, immunohistochemical staining showed relatively higher ABCG2 expression in recurrent tumors than in corresponding primary tumors[32]. Nevertheless, this study did not provide sufficient evidence of the usefulness of ABCG2 as a CSC marker in NPC. A strategy for using the SP approach to enumerate and isolate CSCs in EBV-associated NPC is still needed to further elucidate a panel of primary tumors.
CSCs in various solid tumors were successfully isolated and characterized using cell surface markers, such as CD24, CD44, CD90, CD117, and CD133. Among these markers, CD44 is a prominent antigen found in CSCs in EBV-positive NPC. As a hyaluronan receptor, CD44 was shown to be a CSC marker in head and neck squamous cell carcinoma[33] and has been shown to be an important molecule for anti-metastasis therapy[34]. In the EBV-positive NPC cell line C666-1, the CD44+ CSCs in NPC were demonstrated to have the exclusive ability to self-renew, differentiate, and initiate tumors in vivo and were shown to have higher resistance to 5-fluorouracil (5-FU) treatment[35],[36]. These findings provide solid evidence for the existence of cancer stem cells in EBV-associated NPC. Using flow cytometry analysis, approximately 8%-20% CD44+ cells were detected in C666-1 cells and patient-derived xenografts[36]. Because identifying the precise CSC subpopulation is crucial for further characterizing their properties and developing effective CSC-targeting therapies, CD44 alone may not be sufficient to define this specific subpopulation[37]. The CSC subpopulation is believed to possess stem-like characteristics. Accordingly, a combination of CD44 with ESC transcription factors may help to delineate the precise CSC subpopulation.
To elucidate the role of ESC transcription factors in NPC CSCs, the expression of multiple ESC transcription factors was examined in sphere-forming cells and the CD44+ subpopulation[24]. Transcription factors such as octamer-binding transcription factor 4 (OCT4) and NANOG are essential for maintaining the pluripotent phenotype of ESCs. These ESC “stem cell markers” are commonly found enriched in CSCs. CD44+ NPC CSCs were found to be enriched in transcription factors OCT4, NANOG, and sex-determining region Y (SRY)-box 2 (SOX2)[36]. SOX2 was found expressed mostly in CD44+ cells, whereas less than 5% of CD44− cells expressed SOX2. SOX2 is a member of the SRY-related high mobility group transcription factor family and is crucial for promoting and maintaining pluripotency in embryonic and adult tissue-specific stem cells[38]. The enriched expression of SOX2 in CD44+ NPC cells suggests this subpopulation of cells possesses pluripotent potential. Furthermore, human NPC primary tumor cells expressing ESC markers were also shown to be able to form secondary tumors in mouse models[39]. CSCs expressing the ESC markers SOX2, OCT4, NANAOG, and NESTIN were also found located at the invasive front in NPC, which were associated with the disease aggressive behavior (T, N, M classification and clinical stage) and patient survival[28]. These findings suggest that CSCs play a major role in tumor progression and invasion.
Aldehyde dehydrogenase isoform 1 (ALDH1) is another recognized marker of CSCs, such as those in breast cancer[40]. In our study on sphere-forming cells, significant enrichment of ALDH1 expression was found[36]. Notably, the ALDH1high cells isolated from the C666-1 cell line demonstrated CSC properties[41]. Coincidentally, strong ALDH1 expression was detected at the NPC invasive front, which was associated with the expression of epithelial-to-mesenchymal transition (EMT)-related biomarkers[42]. In addition, ALDH1-expressing CSCs demonstrated strong association with NPC tumor budding, disease aggressiveness, and poor patient survival[43].
In addition to these markers, several cell surface markers including C-C chemokine receptor type 7 (CCR7) and CD109 were found to be highly up-regulated in the sphere-forming cells using a microarray assay[24]. CCR7 is a chemokine receptor that mediates cell migration in response to its ligand CCL21, whereas CD109 is a glycosyl-phosphatidylinositol-anchored glycoprotein that inhibits tumor growth factor-β1 (TGF-β1)-induced growth. The enrichment of the CCR7+ and CD109+ cell populations in the sphere-forming cells was confirmed by flow cytometry[24]. Importantly, the sphere-forming ability of NPC cells was abolished after treatment with the CCR7-blocking antibody. This finding indicates that CCR7 may contribute to the maintenance of CSCs. The role of CCR7 as a new surface marker of NPC CSCs is currently being validated[36].
Roles of EBV in NPC CSCs
The complex interplay between EBV and genetic aberrations in nasopharyngeal epithelial cells during tumor initiation and progression poses great challenges to NPC research[44]. Our earlier studies demonstrated that several genetic aberrations were involved in the initiation of NPC, which were suggested to predispose these NPC cells to subsequent EBV infection[45]. In EBV-associated NPC and precancerous lesions, the persistence of the clonal EBV genome indicated that EBV latent infection facilitated tumor initiation and the transformation of nasopharyngeal epithelial cells[46],[47]. Recent studies have suggested that EBV-encoded proteins could induce stem cell-like properties in epithelial cells[48]–[50]. In some precancerous lesions, it was also noted that EBV-infected cells only occur in or immediately adjacent to the basal layer of the nasopharyngeal epithelia. It has been speculated that NPC originates from clonal EBV-infected basal stem cells. Therefore, modulating or maintaining the stem cell properties may be a critical function of EBV in NPC initiation[51]. The significant increase in the copy number of EBV DNA and the expression of EBV latent genes in isolated NPC CSCs further support this hypothesis[36].
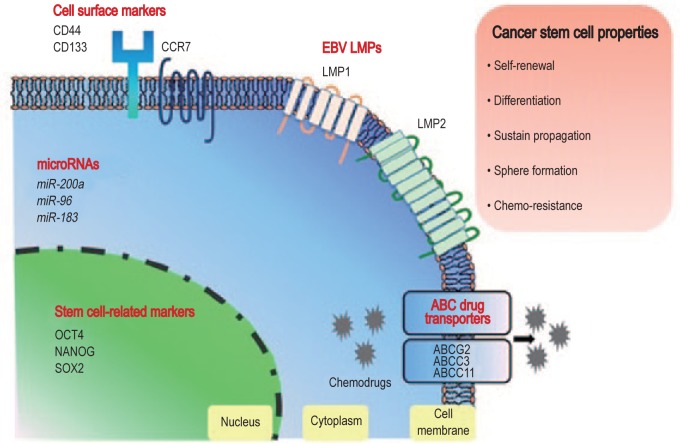
All EBV-infected NPC cells exhibit type II latency, with the expression of Epstein-Barr virus nuclear antigen 1 (EBNA1), LMP1, LMP2A, Epstein-Barr virus-encoded small RNAs (EBERs), BARTs, and a number of EBV-encoded microRNAs (miRNAs)[52],[53]. Among these latent genes, LMP1 encodes a viral oncoprotein, which is responsible for altering multiple cellular mechanisms and signaling pathways in host cells. There is increasing evidence that LMP1 plays a role in acquiring the properties of stem cell-like or progenitor-like cells in NPC. It is well known that LMP1 induces EMT via twist family bHLH transcription factor 1 (TWIST) or snail family zinc finger 1 (SNAIL) in NPC cells. Recently, Kondo et al.[49] demonstrated that LMP1-mediated EMT induced the CD44high CD24low phenotype and the self-renewal properties of nasopharyngeal epithelial cells. Furthermore, the special AT-rich binding protein 1 (SATB1) up-regulated by LMP1 may contribute to cancer stemness by mediating the proper stem cell differentiation and negatively regulating the pluripotent genes[54]. In an early study, LMP1 was proposed to induce a cancer progenitor cell (CPC)-like rather than a CSC-like phenotype due to the lack of induction of pluripotent stem cell-like genes (e.g., OCT4 and NANOG) in LMP1-expressing nasopharyngeal epithelial cells. However, a recent study reported the induction of a SP and some CSC markers such as OCT4, NANAOG, B-cell-specific moloney murine leukemia virus insertion site 1 (BMI-1), and SOX2 in the EBV-negative NPC cell line expressing LMP1. These conflicting findings may be due to the different cell models used. As shown in Figure 2, LMP1 gene is highly expressed in a small subpopulation of cells in most EBV-positive NPC cell lines and primary tumors. To clarify whether LMP1 induces the CSC or CPC phenotypes, further experiments are needed to directly investigate the CSC phenotype in the LMP1-positive subpopulation.
Figure 2. Diagram showing the cell surface and intracellular molecules involved in the regulation of the stemness properties in nasopharyngeal carcinoma (NPC) cells.
EBV, Epstein-Barr virus; LMP, latent membrane protein; CCR7, C-C chemokine receptor type 7; OCT4, octamer-binding transcription factor 4; SOX2, sex-determining region Y (SRY)-box 2; ABC, ATP-binding cassette; ABCG2, ATP-binding cassette subfamily G, member 2; ABCC3, ATP-binding cassette subfamily C, member 3; ABCC11, ATP-binding cassette subfamily C, member 11.
In addition to LMP1, LMP2A is suspected to induce CSCs in EBV-positive NPC due to its function in altering the NOTCH and Hedgehog (Hh) signaling pathways. The predominant LMP2A expression at the invasive tumor front also suggests that it plays a role in promoting tumor invasion and recurrence. Kong et al.[50] demonstrated that LMP2A expression up-regulated the stem cell markers BMI1, NANOG, and SOX2 and increased the size of the SP in EBV-negative NPC cell lines. Notably, the overexpression of LMP2A induced EMT and stem-like characteristics in these cells. Furthermore, the predominant expression of LMP2A at the invasive tumor front also suggested its role as a marker of CSC and for predicting NPC progression.
CSC-associated Signaling Pathways
In addition to the EBV latent genes, several stem cell-related pathways such as the NOTCH and Hh pathways were found to be involved in the survival and functioning of NPC CSCs[55]. The NOTCH signaling pathways are not only important in normal development, cell fate determination, and stem cell renewal, they also play a crucial role in tumorigenesis and contribute to the CSC functioning in various cancer types[56]. In NPC, NOTCH1, NOTCH3, and NOTCH4 are of particular interest. An early study demonstrated that a subpopulation of NPC cells in primary tumors co-expressing SOX2 and OCT4 were involved in an activated NOTCH1 pathway, with increased expression of the downstream effector molecule Hes1[57]. In our study, NOTCH3 was found to be consistently overexpressed in NPC tumor cells and to be responsible for cisplatin resistance[58]. The silencing of NOTCH3 in NPC demonstrated the suppression of stem cell-like properties such as OCT4 expression, spheroid formation, and in vivo tumorigenicity[58]. This finding implies that NOTCH3 is a potential therapeutic target for the depletion of CSCs in NPC. The antitumor effects of NOTCH receptor-specific antibodies and recombinant NOTCH3 peptides have been demonstrated in multiple studies[59]–[62]. Integrating these targeting agents into the current therapeutic strategies may greatly enhance the outcomes for NPC patients. In addition to NOTCH3, enriched NOTCH4 expression was also found in NPC CSCs[24]. NOTCH4 has been identified as a crucial regulator in breast CSCs, and its inhibition was shown to abolish tumor initiation[63]. However, the functional roles of NOTCH4 in NPC CSCs have yet to be elucidated[36].
The Hh signaling pathway is implicated in the maintenance of stem cells and is commonly dysregulated in EBV-associated NPC[48]. Unlike NOTCH3 signaling, the Hh signaling pathway is activated by EBV through autocrine induction of the Sonic Hedgehog (SHH) ligand. Port et al.[48] demonstrated that the EBV-encoded LMP1 and LMP2A proteins induced the expression of various stemness-related gene products (BMI1 and SOX2) and stem cell surface markers (CD44v6 and CD133) via activation of the Hh signaling pathway. Consistent with these findings, our study also noted that the expression of GLI family zinc finger 1 (GLI1), an effector of the Hh signaling pathway, and the EBV latent genes were enriched in NPC CSCs[36]. These findings reveal the detailed mechanisms of EBV-induced CSC phenotypes in NPC.
In NPC, lymph node metastasis is commonly observed. According to the CSC theory, metastasis involves the migration of CSCs from the original site to a distant organ. For chemotaxis of cells to occur, chemokine receptors are required to be present on chemotactic cells along with chemokines in the microenvironment. Among the plethora of chemokines, CCR7 was shown to be related to lymph node metastasis in several types of cancers[64]–[70]. As mentioned above, the up-regulation of CCR7 expression was found in NPC CSCs and may mediate cell migration in response to its ligand CCL21 in lymph nodes[36],[71]. In addition to enhancing lymph node metastasis, CCR7 is crucial for the survival of CSCs. We found that the blocking of this receptor with a neutralizing antibody resulted in a significant reduction in the sphere-forming ability of C666-1 cells. In addition to CCR7, we noted that the chemokine (C-X-C motif) ligand 8 (CXCL8) was highly up-regulated in the CSC subpopulation. Using the inhibitor of CXCL8 or its receptor CXCR2, the contribution of CXCL8 and CXCR2 in the tumor sphere-forming properties via the phosphatidylinositol 3 kinase (PI3K)/AKT signaling pathway in NPC C666-1 cells was determined[72]. These findings indicate the important roles that chemokine-mediated signaling plays in the maintenance of CSCs in NPC.
Aberrant miRNA expression
Aberrant miRNA expression is a key post-transcriptional regulatory mechanism in the tumorigenesis process. It has been reported that miR-200a contributes to the epithelial-mesenchymal to stem-like transition in NPC cells via the targeting of zinc finger E-box-binding homeobox 2 (ZEB2) and catenin (cadherin-associated protein), beta 1 (CTNNB1). The inhibitory effect of miR-200a expression on the properties of stem-like cells, including tumor sphere formation and in vivo tumorigenicity, and on stem cell marker expression was demonstrated in EBV-positive C666-1 cells[73]. Using the microRNA microarray, we also noted the significant repression of multiple stemness-repressing miRNAs, such as miR-96 and miR-183, in the sphere-forming cells (unpublished finding). These findings suggest that miRNAs play a functional role in regulating the properties of CSCs in EBV-associated NPC.
CSCs as Potential Therapeutic Targets
The failure to eradicate drug-resistant CSCs has been proposed to be the core reason for tumor relapse after treatment[74],[75]. The drug-resistant properties of CSCs have been shown to be due to the overexpression of ABC transporters[76], which are responsible for the efflux of therapeutic drugs[77],[78]. ABC transporters have been shown to be overexpressed in other CSCs, such as ABCB5 in hepatocellular carcinoma (HCC) CSCs[79]. In NPC CSCs, several ABC transporters, such as ATP-binding cassette subfamily C, member 3 (ABCC3) and ABCC11, were found to be highly up-regulated[36]. Furthermore, the CD44+ NPC CSCs, which are resistant to cisplatin and doxorubicin, exhibited overexpression of ABCC3 and ABCC11[36]. These findings suggest that the overexpression of these ABC transporters is responsible for the drug resistance in NPC CSCs. Notably, ABCC11 directly induces resistance to 5-FU, which is a main chemotherapeutic drug for treating NPC patients. The overexpression of ABCC11 in NPC CSCs may reduce the survival of this subpopulation during 5-FU treatment and lead to future tumor relapse. The resistance of NPC CSCs to radiotherapy was demonstrated in a study on the therapeutic potential of a mutant vesicular stomatitis virus (VSVΔ51) for treating EBV-positive NPC[80]. The VSVΔ51 effectively eradicated the sphere-forming cells in multiple EBV-positive NPC models and greatly enhanced their sensitivity to treatment with ionizing radiation. Interestingly, depletion of the stem cell-associated gene BMI-1 was also found to significantly sensitize NPC cells to radiation[81]. Furthermore, the aberrant signaling pathways (e.g., NOTCH3 and PI3K/AKT) may promote the survival of the CSC subpopulation in NPC.
Because CSCs resist the conventional treatments for NPC, the development of novel therapies targeting CSCs may provide an efficient strategy for improving patient outcomes. Although few studies have investigated the targeting of the CSCs in EBV-positive NPC, the encouraging findings demonstrate its potential as a new clinical intervention for NPC patients. As mentioned above, NOTCH3 inhibition and the VSVΔ51 treatment greatly enhanced the efficiency of chemotherapy and radiotherapy, respectively, in the in vitro and in vivo EBV-positive NPC models[58],[80],[81]. AT13387, a heat shock protein 90 (Hsp90) inhibitor, is currently undergoing clinical trials for treating various human cancers. A recent study demonstrated its potential effect in targeting the CSC subpopulation in EBV-positive NPC[74]. The AT13387 treatment effectively inhibited the formation of tumor spheres, reduced the expression of CSC markers, and suppressed in vivo tumorigenicity of C666-1 cells. Recently, short hairpin RNA (shRNA) approach has been used to target BMI-1 and enhance the radiotherapy sensitivity of CD44+ NPC CSCs[82]. EBV LMP1 has shown to induce CSC properties and contribute to resistance to radiotherapy[83]. It has been demonstrated that E3-ligase Skp2 and CD24 regulate CSC properties[84],[85]. Thus, a similar shRNA approach[82] can be used to suppress CSC-related makers and signaling molecules[83]–[85]. Notably, the loss of phosphatase and tensin homolog deleted on chromosome ten (PTEN) gene can lead to the emergence and proliferation of CSC clones and reveal the potential of therapeutic targeting of tumors that lack PTEN expression[86]. A similar treatment strategy can be used for NPCs showing reduced IκB kinase α (IKKα) expression[87]. These findings provide preclinical evidence supporting the use of CSC-targeting agents as novel drugs for the systematic treatment of NPC. Further elucidation of the inhibitors of the signaling pathways associated with CSCs, such as the NOTCH, WNT, and Hh signaling pathways, or shRNA targeting CSC markers, including CCR7 and CD44, are necessary for the development of efficient therapeutic approaches for treating NPC.
The application of antisense strategies for NPC therapeutics has been of particular interest, especially the potential therapeutic approach in targeting EBV genes in NPC. Studies have demonstrated that the suppression of LMP1 in C666-1 cells resulted in the inhibition in tumor cell motility, adhesion, and transmembrane invasion ability[88], whereas the suppression of LMP2A in C666-1 cells resulted in the inhibition in cell proliferation and colony formation[89]. These findings further confirmed that EBV-encoded LMPs are potential therapeutic targets for EBV-associated malignancies. A recent study demonstrated that the radiosensitivity of NPC was increased by using DNAzyme 1 (DZ1), a deoxyribozyme that was specifically designed to target LMP1 mRNA[90]. DNAzyme is a small, in vitro selected DNA enzyme that targets on RNA substrates[91]. The study by Cao et al.[90] shed light on the clinical use of targeting LMP1 by DNAzyme in treating NPC patients.
Conclusions
EBV latent infection is not only a critical molecular event in the initiation of non-keratinizing tumors. Its appearance in all tumor cells suggests that it also plays an essential role during the development of NPC. The identification of the CSCs in EBV-associated NPC cell lines reveals that their phenotype is distinct from that in EBV-negative cell lines. The involvement of CSC properties in nasopharyngeal epithelial cells by EBV-encoded LMP1 and LMP2A proteins implies that the induction and maintenance of the CSC subpopulation is an important role of EBV in NPC tumorigenesis. Nevertheless, the precise role of EBV in CSCs still needs to be demonstrated in more EBV-positive NPC models or primary tumors. In most NPC cases, LMP1 and LMP2A were only highly expressed in a subpopulation of tumor cells. Characterizing the CSC properties of the subpopulations with high expression of LMP1 and LMP2A proteins may provide direct evidence of the role of EBV. Furthermore, in addition to unveiling the unique features of CSCs in NPC, studies demonstrated that the targeted CSC subpopulation have the potential to greatly enhance the efficiency of conventional radiotherapy and chemotherapy in the in vitro and in vivo models. These exciting findings indicate a new treatment intervention for NPC patients. However, only a few preclinical studies on CSC-targeting agents have been conducted for EBV-associated NPC. More therapeutic agents or inhibitors targeting the CSC-associated signaling pathways need to be elucidated in preclinical studies and clinical trials involving NPC patients. We envision that collaboration between clinicians and scientists in the fields of EBV, NPC, and stem cell biology will facilitate the quest for a complete cure of NPC.
Acknowledgments
The research of KW Lo was supported by funding from the Focus Investigation Scheme-A of The Chinese University of Hong Kong, the Research Grants Council of Hong Kong, GRF (471211, 470312), CRF (CUHK8/CRF/11R) and AoE NPC (AoE/M-06/08), and the Theme-Based Research Scheme (T12-401/13-R).
References
- 1.Cesarman E. Gammaherpesviruses and lymphoproliferative disorders. Annu Rev Pathol. 2014;9:349–372. doi: 10.1146/annurev-pathol-012513-104656. [DOI] [PubMed] [Google Scholar]
- 2.Young LS, Rickinson AB. Epstein-Barr virus: 40 years on. Nat Rev Cancer. 2004;4:757–768. doi: 10.1038/nrc1452. [DOI] [PubMed] [Google Scholar]
- 3.Powell HF. Tumour of post-nasal space in a man, aged 27. Proc R Soc Med. 1910;3:160–161. [PMC free article] [PubMed] [Google Scholar]
- 4.Epstein MA, Achong BG, Barr YM. Virus particles in cultured lymphoblasts from Burkitt's lymphoma. Lancet. 1964;1:702–703. doi: 10.1016/s0140-6736(64)91524-7. [DOI] [PubMed] [Google Scholar]
- 5.de Schryver A, Friberg S, Jr, Klein G, et al. Epstein-Barr virus-associated antibody patterns in carcinoma of the post-nasal space. Clin Exp Immunol. 1969;5:443–459. [PMC free article] [PubMed] [Google Scholar]
- 6.Pathmanathan R, Prasad U, Chandrika G, et al. Undifferentiated, nonkeratinizing, and squamous cell carcinoma of the nasopharynx. Variants of Epstein-Barr virus-infected neoplasia. Am J Pathol. 1995;146:1355–1367. [PMC free article] [PubMed] [Google Scholar]
- 7.Lee AW, Ng WT, Chan YH, et al. The battle against nasopharyngeal cancer. Radiother Oncol. 2012;104:272–278. doi: 10.1016/j.radonc.2012.08.001. [DOI] [PubMed] [Google Scholar]
- 8.Chan AT. Current treatment of nasopharyngeal carcinoma. Eur J Cancer. 2011;47(Suppl. 3):S302–303. doi: 10.1016/S0959-8049(11)70179-4. [DOI] [PubMed] [Google Scholar]
- 9.Tang C, Ang BT, Pervaiz S. Cancer stem cell: target for anti-cancer therapy. FASEB J. 2007;21:3777–3785. doi: 10.1096/fj.07-8560rev. [DOI] [PubMed] [Google Scholar]
- 10.Lapidot T, Sirard C, Vormoor J, et al. A cell initiating human acute myeloid leukaemia after transplantation into SCID mice. Nature. 1994;367:645–648. doi: 10.1038/367645a0. [DOI] [PubMed] [Google Scholar]
- 11.Dick JE. Looking ahead in cancer stem cell research. Nat Biotech-nol. 2009;27:44–46. doi: 10.1038/nbt0109-44. [DOI] [PubMed] [Google Scholar]
- 12.O'Brien CA, Kreso A, Dick JE. Cancer stem cells in solid tumors: an overview. Semin Radiat Oncol. 2009;19:71–77. doi: 10.1016/j.semradonc.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 13.Nowell PC. The clonal evolution of tumor cell populations. Science. 1976;194:23–28. doi: 10.1126/science.959840. [DOI] [PubMed] [Google Scholar]
- 14.Baylin SB, Jones PA. A decade of exploring the cancer epige-nome—biological and translational implications. Nat Rev Cancer. 2011;11:726–734. doi: 10.1038/nrc3130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dick JE. Stem cell concepts renew cancer research. Blood. 2008;112:4793–4807. doi: 10.1182/blood-2008-08-077941. [DOI] [PubMed] [Google Scholar]
- 16.Shackleton M, Quintana E, Fearon ER, et al. Heterogeneity in cancer: cancer stem cells versus clonal evolution. Cell. 2009;138:822–829. doi: 10.1016/j.cell.2009.08.017. [DOI] [PubMed] [Google Scholar]
- 17.Reya T, Morrison SJ, Clarke MF, et al. Stem cells, cancer, and cancer stem cells. Nature. 2001;414:105–111. doi: 10.1038/35102167. [DOI] [PubMed] [Google Scholar]
- 18.Visvader JE. Cells of origin in cancer. Nature. 2011;469:314–322. doi: 10.1038/nature09781. [DOI] [PubMed] [Google Scholar]
- 19.Zhang S, Balch C, Chan MW, et al. Identification and charac-terization of ovarian cancer-initiating cells from primary human tumors. Cancer Res. 2008;68:4311–4320. doi: 10.1158/0008-5472.CAN-08-0364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bussolati B, Bruno S, Grange C, et al. Identification of a tumor-initiating stem cell population in human renal carcinomas. FASEB J. 2008;22:3696–3705. doi: 10.1096/fj.08-102590. [DOI] [PubMed] [Google Scholar]
- 21.Prince ME, Sivanandan R, Kaczorowski A, et al. Identification of a subpopulation of cells with cancer stem cell properties in head and neck squamous cell carcinoma. Proc Natl Acad Sci U S A. 2007;104:973–978. doi: 10.1073/pnas.0610117104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Chan KS, Espinosa I, Chao M, et al. Identification, molecular characterization, clinical prognosis, and therapeutic targeting of human bladder tumor-initiating cells. Proc Natl Acad Sci U S A. 2009;106:14016–14021. doi: 10.1073/pnas.0906549106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Driessens G, Beck B, Caauwe A, et al. Defining the mode of tumour growth by clonal analysis. Nature. 2012;488:527–530. doi: 10.1038/nature11344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chen J, Li Y, Yu TS, et al. A restricted cell population propagates glioblastoma growth after chemotherapy. Nature. 2012;488:522–526. doi: 10.1038/nature11287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Schepers AG, Snippert HJ, Stange DE, et al. Lineage tracing reveals Lgr5+ stem cell activity in mouse intestinal adenomas. Science. 2012;337:730–735. doi: 10.1126/science.1224676. [DOI] [PubMed] [Google Scholar]
- 26.Tirino V, Desiderio V, Paino F, et al. Cancer stem cells in solid tumors: an overview and new approaches for their isolation and characterization. FASEB J. 2013;27:13–24. doi: 10.1096/fj.12-218222. [DOI] [PubMed] [Google Scholar]
- 27.Greve B, Kelsch R, Spaniol K, et al. Flow cytometry in cancer stem cell analysis and separation. Cytometry A. 2012;81:284–293. doi: 10.1002/cyto.a.22022. [DOI] [PubMed] [Google Scholar]
- 28.Luo W, Li S, Peng B, et al. Embryonic stem cells markers SOX2, OCT4 and Nanog expression and their correlations with epithelial-mesenchymal transition in nasopharyngeal carcinoma. PLoS One. 2013;8:e56324. doi: 10.1371/journal.pone.0056324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Liu JC, Deng T, Lehal RS, et al. Identification of tumorsphere- and tumor-initiating cells in HER2/Neu-induced mammary tumors. Cancer Res. 2007;67:8671–8681. doi: 10.1158/0008-5472.CAN-07-1486. [DOI] [PubMed] [Google Scholar]
- 30.Hirschhaeuser F, Menne H, Dittfeld C, et al. Multicellular tumor spheroids: an underestimated tool is catching up again. J Biotechnol. 2010;148:3–15. doi: 10.1016/j.jbiotec.2010.01.012. [DOI] [PubMed] [Google Scholar]
- 31.Wang J, Guo LP, Chen LZ, et al. Identification of cancer stem cell-like side population cells in human nasopharyngeal carcinoma cell line. Cancer Res. 2007;67:3716–3724. doi: 10.1158/0008-5472.CAN-06-4343. [DOI] [PubMed] [Google Scholar]
- 32.Liang Y, Zhong Z, Huang Y, et al. Stem-like cancer cells are inducible by increasing genomic instability in cancer cells. J Biol Chem. 2010;285:4931–4940. doi: 10.1074/jbc.M109.048397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Joshua B, Kaplan MJ, Doweck I, et al. Frequency of cells expressing CD44, a head and neck cancer stem cell marker: correlation with tumor aggressiveness. Head Neck. 2012;34:42–49. doi: 10.1002/hed.21699. [DOI] [PubMed] [Google Scholar]
- 34.Orian-Rousseau V. CD44, a therapeutic target for metastasising tumours. Eur J Cancer. 2010;46:1271–1277. doi: 10.1016/j.ejca.2010.02.024. [DOI] [PubMed] [Google Scholar]
- 35.Janisiewicz AM, Shin JH, Murillo-Sauca O, et al. CD44(+) cells have cancer stem cell-like properties in nasopharyngeal carcinoma. Int Forum Allergy Rhinol. 2012;2:465–470. doi: 10.1002/alr.21068. [DOI] [PubMed] [Google Scholar]
- 36.Lun SW, Cheung ST, Cheung PF, et al. CD44+ cancer stem-like cells in EBV-associated nasopharyngeal carcinoma. PLoS One. 2012;7:e52426. doi: 10.1371/journal.pone.0052426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Biddle A, Gammon L, Fazil B, et al. CD44 staining of cancer stem-like cells is influenced by down-regulation of CD44 variant isoforms and up-regulation of the standard CD44 isoform in the population of cells that have undergone epithelial-to-mesenchymal transition. PLoS One. 2013;8:e57314. doi: 10.1371/journal.pone.0057314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Driessens G, Blanpain C. Long live sox2: sox2 lasts a lifetime. Cell Stem Cell. 2011;9:283–284. doi: 10.1016/j.stem.2011.09.007. [DOI] [PubMed] [Google Scholar]
- 39.Yang C, Peng J, Jiang W, et al. mTOR activation in immature cells of primary nasopharyngeal carcinoma and anti-tumor effect of rapamycin in vitro and in vivo. Cancer Lett. 2013;341:186–194. doi: 10.1016/j.canlet.2013.08.004. [DOI] [PubMed] [Google Scholar]
- 40.Ginestier C, Hur MH, Charafe-Jauffret E, et al. ALDH1 is a marker of normal and malignant human mammary stem cells and a predictor of poor clinical outcome. Cell Stem Cell. 2007;1:555–567. doi: 10.1016/j.stem.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Yu F, Sim AC, Li C, et al. Identification of a subpopulation of naso-pharyngeal carcinoma cells with cancer stem-like cell properties by high aldehyde dehydrogenase activity. Laryn-goscope. 2013;123:1903–1911. doi: 10.1002/lary.24003. [DOI] [PubMed] [Google Scholar]
- 42.Luo WR, Yao KT. Cancer stem cell characteristics, ALDH1 expression in the invasive front of nasopharyngeal carcinoma. Virchows Arch. 2014;464:35–43. doi: 10.1007/s00428-013-1508-z. [DOI] [PubMed] [Google Scholar]
- 43.Luo WR, Gao F, Li SY, et al. Tumour budding and the expression of cancer stem cell marker aldehyde dehydrogenase 1 in nasopharyngeal carcinoma. Histopathology. 2012;61:1072–1081. doi: 10.1111/j.1365-2559.2012.04350.x. [DOI] [PubMed] [Google Scholar]
- 44.Lo KW, Chung GT, To KF. Deciphering the molecular genetic basis of NPC through molecular, cytogenetic, and epigenetic approaches. Semin Cancer Biol. 2012;22:79–86. doi: 10.1016/j.semcancer.2011.12.011. [DOI] [PubMed] [Google Scholar]
- 45.Lo KW, To KF, Huang DP. Focus on nasopharyngeal carcinoma. Cancer Cell. 2004;5:423–428. doi: 10.1016/s1535-6108(04)00119-9. [DOI] [PubMed] [Google Scholar]
- 46.Pathmanathan R, Prasad U, Sadler R, et al. Clonal proliferations of cells infected with Epstein-Barr virus in preinvasive lesions related to nasopharyngeal carcinoma. N Engl J Med. 1995;333:693–698. doi: 10.1056/NEJM199509143331103. [DOI] [PubMed] [Google Scholar]
- 47.Pak MW, To KF, Lo YM, et al. Nasopharyngeal carcinoma in situ (NPCIS)—pathologic and clinical perspectives. Head Neck. 2002;24:989–995. doi: 10.1002/hed.10161. [DOI] [PubMed] [Google Scholar]
- 48.Port RJ, Pinheiro-Maia S, Hu C, et al. Epstein-Barr virus induction of the Hedgehog signalling pathway imposes a stem cell phenotype on human epithelial cells. J Pathol. 2013;231:367–377. doi: 10.1002/path.4245. [DOI] [PubMed] [Google Scholar]
- 49.Kondo S, Wakisaka N, Muramatsu M, et al. Epstein-Barr virus latent membrane protein 1 induces cancer stem/progenitor-like cells in nasopharyngeal epithelial cell lines. J Virol. 2011;85:11255–11264. doi: 10.1128/JVI.00188-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kong QL, Hu LJ, Cao JY, et al. Epstein-Barr virus-encoded LMP2A induces an epithelial-mesenchymal transition and increases the number of side population stem-like cancer cells in nasopharyngeal carcinoma. PLoS Pathog. 2010;6:e1000940. doi: 10.1371/journal.ppat.1000940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Yoshizaki T, Kondo S, Wakisaka N, et al. Pathogenic role of Epstein-Barr virus latent membrane protein-1 in the development of nasopharyngeal carcinoma. Cancer Lett. 2013;337:1–7. doi: 10.1016/j.canlet.2013.05.018. [DOI] [PubMed] [Google Scholar]
- 52.Young LS, Dawson CW, Clark D, et al. Epstein-Barr virus gene expression in nasopharyngeal carcinoma. J Gen Virol. 1988;69:1051–1065. doi: 10.1099/0022-1317-69-5-1051. [DOI] [PubMed] [Google Scholar]
- 53.Busson P, McCoy R, Sadler R, et al. Consistent transcription of the Epstein-Barr virus LMP2 gene in nasopharyngeal carcinoma. J Virol. 1992;66:3257–3262. doi: 10.1128/jvi.66.5.3257-3262.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Endo K, Shackelford J, Aga M, et al. Upregulation of special AT-rich-binding protein 1 by Epstein-Barr virus latent membrane protein 1 in human nasopharyngeal cells and nasopharyngeal cancer. J Gen Virol. 2013;94:507–513. doi: 10.1099/vir.0.046243-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Karamboulas C, Ailles L. Developmental signaling pathways in cancer stem cells of solid tumors. Biochim Biophys Acta. 2013;1830:2481–2495. doi: 10.1016/j.bbagen.2012.11.008. [DOI] [PubMed] [Google Scholar]
- 56.Espinoza I, Miele L. Deadly crosstalk: Notch signaling at the intersection of EMT and cancer stem cells. Cancer Lett. 2013;341:41–45. doi: 10.1016/j.canlet.2013.08.027. [DOI] [PubMed] [Google Scholar]
- 57.Zhang Y, Peng J, Zhang H, et al. Notch1 signaling is activated in cells expressing embryonic stem cell proteins in human primary nasopharyngeal carcinoma. J Otolaryngol Head Neck Surg. 2010;39:157–166. [PMC free article] [PubMed] [Google Scholar]
- 58.Man CH, Wei-Man Lun S, Wai-Ying Hui J, et al. Inhibition of NOTCH3 signalling significantly enhances sensitivity to cisplatin in EBV-associated nasopharyngeal carcinoma. J Pathol. 2012;226:471–481. doi: 10.1002/path.2997. [DOI] [PubMed] [Google Scholar]
- 59.Rizzo P, Osipo C, Foreman K, et al. Rational targeting of Notch signaling in cancer. Oncogene. 2008;27:5124–5131. doi: 10.1038/onc.2008.226. [DOI] [PubMed] [Google Scholar]
- 60.Wu Y, Cain-Hom C, Choy L, et al. Therapeutic antibody targeting of individual Notch receptors. Nature. 2010;464:1052–1057. doi: 10.1038/nature08878. [DOI] [PubMed] [Google Scholar]
- 61.Li K, Li Y, Wu W, et al. Modulation of Notch signaling by antibodies specific for the extracellular negative regulatory region of NOTCH3. J Biol Chem. 2008;283:8046–8054. doi: 10.1074/jbc.M800170200. [DOI] [PubMed] [Google Scholar]
- 62.Lin L, Mernaugh R, Yi F, et al. Targeting specific regions of the Notch3 ligand-binding domain induces apoptosis and inhibits tumor growth in lung cancer. Cancer Res. 2010;70:632–638. doi: 10.1158/0008-5472.CAN-09-3293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Harrison H, Farnie G, Howell SJ, et al. Regulation of breast cancer stem cell activity by signaling through the Notch4 receptor. Cancer Res. 2010;70:709–718. doi: 10.1158/0008-5472.CAN-09-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Shang ZJ, Liu K, Shao Z. Expression of chemokine receptor CCR7 is associated with cervical lymph node metastasis of oral squamous cell carcinoma. Oral Oncol. 2009;45:480–485. doi: 10.1016/j.oraloncology.2008.06.005. [DOI] [PubMed] [Google Scholar]
- 65.Nakata B, Fukunaga S, Noda E, et al. Chemokine receptor CCR7 expression correlates with lymph node metastasis in pancreatic cancer. Oncology. 2008;74:69–75. doi: 10.1159/000139126. [DOI] [PubMed] [Google Scholar]
- 66.Kodama J, Hasengaowa, Kusumoto T, et al. Association of CXCR4 and CCR7 chemokine receptor expression and lymph node metastasis in human cervical cancer. Ann Oncol. 2007;18:70–76. doi: 10.1093/annonc/mdl342. [DOI] [PubMed] [Google Scholar]
- 67.Cabioglu N, Yazici MS, Arun B, et al. CCR7 and CXCR4 as novel biomarkers predicting axillary lymph node metastasis in T1 breast cancer. Clin Cancer Res. 2005;11:5686–5693. doi: 10.1158/1078-0432.CCR-05-0014. [DOI] [PubMed] [Google Scholar]
- 68.Gunther K, Leier J, Henning G, et al. Prediction of lymph node metastasis in colorectal carcinoma by expression of chemokine receptor CCR7. Int J Cancer. 2005;116:726–733. doi: 10.1002/ijc.21123. [DOI] [PubMed] [Google Scholar]
- 69.Takanami I. Overexpression of CCR7 mRNA in nonsmall cell lung cancer: correlation with lymph node metastasis. Int J Cancer. 2003;105:186–189. doi: 10.1002/ijc.11063. [DOI] [PubMed] [Google Scholar]
- 70.Mashino K, Sadanaga N, Yamaguchi H, et al. Expression of chemokine receptor CCR7 is associated with lymph node metastasis of gastric carcinoma. Cancer Res. 2002;62:2937–2941. [PubMed] [Google Scholar]
- 71.Forster R, Davalos-Misslitz AC, Rot A. CCR7 and its ligands: balancing immunity and tolerance. Nat Rev Immunol. 2008;8:362–371. doi: 10.1038/nri2297. [DOI] [PubMed] [Google Scholar]
- 72.Lo MC, Yip TC, Ngan KC, et al. Role of MIF/CXCL8/CXCR2 signaling in the growth of nasopharyngeal carcinoma tumor spheres. Cancer Lett. 2013;335:81–92. doi: 10.1016/j.canlet.2013.01.052. [DOI] [PubMed] [Google Scholar]
- 73.Xia H, Cheung WK, Sze J, et al. miR-200a regulates epithelial-mesenchymal to stem-like transition via ZEB2 and beta-catenin signaling. J Biol Chem. 2010;285:36995–37004. doi: 10.1074/jbc.M110.133744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Frank NY, Schatton T, Frank MH. The therapeutic promise of the cancer stem cell concept. J Clin Invest. 2010;120:41–50. doi: 10.1172/JCI41004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Garvalov BK, Acker T. Cancer stem cells: a new framework for the design of tumor therapies. J Mol Med. 2011;89:95–107. doi: 10.1007/s00109-010-0685-3. [DOI] [PubMed] [Google Scholar]
- 76.Holohan C, Van Schaeybroeck S, Longley DB, et al. Cancer drug resistance: an evolving paradigm. Nat Rev Cancer. 2013;13:714–726. doi: 10.1038/nrc3599. [DOI] [PubMed] [Google Scholar]
- 77.Lou H, Dean M. Targeted therapy for cancer stem cells: the patched pathway and ABC transporters. Oncogene. 2007;26:1357–1360. doi: 10.1038/sj.onc.1210200. [DOI] [PubMed] [Google Scholar]
- 78.Dean M. ABC transporters, drug resistance, and cancer stem cells. J Mammary Gland Biol Neoplasia. 2009;14:3–9. doi: 10.1007/s10911-009-9109-9. [DOI] [PubMed] [Google Scholar]
- 79.Cheung ST, Cheung PF, Cheng CK, et al. Granulin-epithelin precursor and ATP-dependent binding cassette (ABC)B5 regulate liver cancer cell chemoresistance. Gastroenterology. 2011;140:344–355. doi: 10.1053/j.gastro.2010.07.049. [DOI] [PubMed] [Google Scholar]
- 80.Alajez NM, Mocanu JD, Shi W, et al. Efficacy of systemically administered mutant vesicular stomatitis virus (VSVDelta51) combined with radiation for nasopharyngeal carcinoma. Clin Cancer Res. 2008;14:4891–4897. doi: 10.1158/1078-0432.CCR-07-4134. [DOI] [PubMed] [Google Scholar]
- 81.Alajez NM, Shi W, Hui AB, et al. Targeted depletion of BMI1 sensitizes tumor cells to P53-mediated apoptosis in response to radiation therapy. Cell Death Differ. 2009;16:1469–1479. doi: 10.1038/cdd.2009.85. [DOI] [PubMed] [Google Scholar]
- 82.Xu XH, Liu XY, Su J, et al. ShRNA targeting Bmi-1 sensitizes CD44(+) nasopharyngeal cancer stem-like cells to radiotherapy. Oncol Rep. 2014;32:764–770. doi: 10.3892/or.2014.3267. [DOI] [PubMed] [Google Scholar]
- 83.Yang CF, Peng LX, Huang TJ, et al. Cancer stem-like cell characteristics induced by EB virus-encoded LMP1 contribute to radioresistance in nasopharyngeal carcinoma by suppressing the p53-mediated apoptosis pathway. Cancer Lett. 2014;344:260–271. doi: 10.1016/j.canlet.2013.11.006. [DOI] [PubMed] [Google Scholar]
- 84.Wang J, Huang Y, Guan Z, et al. E3-ligase Skp2 predicts poor prognosis and maintains cancer stem cell pool in nasopharyngeal carcinoma. Oncotarget. 2014;5:5591–5601. doi: 10.18632/oncotarget.2149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Yang CH, Wang HL, Lin YS, et al. Identification of CD24 as a cancer stem cell marker in human nasopharyngeal carcinoma. PLoS One. 2014;9:e99412. doi: 10.1371/journal.pone.0099412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Ciuffreda L, Falcone I, Cesta Incani U, et al. PTEN expression and function in adult cancer stem cells and prospects for therapeutic targeting. Adv Biol Regul. 2014 Jul 19;pii:S2212-4926(14)00029-3. doi: 10.1016/j.jbior.2014.07.002. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 87.Yan M, Zhang Y, He B, et al. IKKα restoration via EZH2 suppression induces nasopharyngeal carcinoma differentiation. Nat Commun. 2014;5:3661. doi: 10.1038/ncomms4661. [DOI] [PubMed] [Google Scholar]
- 88.Li XP, Li G, Peng Y, et al. Suppression of Epstein-Barr virus-encoded latent membrane protein-1 by RNA interference inhibits the metastatic potential of nasopharyngeal carcinoma cells. Biochem Biophys Res Commun. 2004;315:212–218. doi: 10.1016/j.bbrc.2004.01.045. [DOI] [PubMed] [Google Scholar]
- 89.Ying X, Zhang R, Wang H, et al. Lentivirus-mediated RNAi knockdown of LMP2A inhibits the growth of nasopharyngeal carcinoma cell line C666-1 in vitro. Gene. 2014;542:77–82. doi: 10.1016/j.gene.2014.03.020. [DOI] [PubMed] [Google Scholar]
- 90.Cao Y, Yang L, Jiang W, et al. Therapeutic evaluation of Epstein-Barr virus-encoded latent membrane protein-1 targeted DNAzyme for treating of nasopharyngeal carcinomas. Mol Ther. 2014;22:371–377. doi: 10.1038/mt.2013.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Santoro SW, Joyce GF. A general purpose RNA-cleaving DNA enzyme. Proc Natl Acad Sci U S A. 1997;94:4262–4266. doi: 10.1073/pnas.94.9.4262. [DOI] [PMC free article] [PubMed] [Google Scholar]